Abstract
Physical activity has been shown to benefit cancer survivors' physical functioning, emotional well-being, and symptoms. Physical activity may be of particular benefit to survivors of endometrial cancer because they are more likely to be obese and sedentary than the general population, as these are risk factors for the disease, and thus experience a number of related co-morbid health problems. However, there is little research systematically studying mechanisms of physical activity adherence in cancer survivor populations. This paper describes the design of the Steps to Health study, which applies a Social Cognitive Theory-based model of endometrial cancer survivors' adoption and maintenance of exercise in the context of an intervention to increase walking or other moderate intensity cardiovascular activity. In Steps to Health we will test the influence of self-efficacy and outcome expectations on adherence to exercise recommendations, as well as studying the determinants of self-efficacy. Endometrial cancer survivors who are at least 6 months post-treatment are provided with an intervention involving print materials and telephone counseling, and complete assessments of fitness, activity, self-efficacy and outcome expectations, and determinants of self-efficacy every two months for a six month period. In addition to testing an innovative model, the Steps to Health study employs multiple assessment methods, including ecological momentary assessment, implicit tests of cognitive variables, and ambulatory monitoring of physical activity. The study results can be used to develop more effective interventions for increasing physical activity in sedentary cancer survivors by taking into account the full complement of sources of self-efficacy information and outcome expectations.
Physical activity benefits cancer survivors' physical and psychological functioning, as evidenced by the growing body of research on this topic (Ahmed, Thomas, Yee, & Schmitz, 2006; Basen-Engquist et al., 2006; Courneya, 2001, 2003; Courneya & Christine M. Friedenreich, 1999; Courneya, Keats, & Turner, 2000; Courneya et al., 2007; Daley et al., 2007; Fairey, Courneya, Field, & Mackey, 2002; Friedenreich & Courneya, 1996; Galvao & Newton, 2005; Knols, Aaronson, Uebelhart, Fransen, & Aufdemkampe, 2005; Ohira, Schmitz, Ahmed, & Yee, 2006; Pinto & Maruyama, 1999; Schmitz et al., 2005). Epidemiologic research indicates that physical activity is associated with a lower risk of cancer recurrence (Holmes, Chen, Feskanich, Kroenke, & Colditz, 2005; Meyerhardt, Heseltine et al., 2006) and increased survival (Holmes et al., 2005; Meyerhardt, Giovannucci et al., 2006; Meyerhardt, Heseltine et al., 2006; Pierce et al., 2007) in breast and colon cancer, as well as a decreased risk of developing other chronic diseases (Devogelaer & de Deuxchaisnes, 1993; Helmrich, Ragland, Leung, & Paffenbarger, 1991; LaCroix, Leveille, Hecht, Grothaus, & Wagner, 1996). Other physical benefits include increased muscle strength (Ahmed et al., 2006; K. S. Courneya et al., 2007; R. J. Segal et al., 2003), improved physical functioning (Mock et al., 1997; R. Segal et al., 2001), controlled body weight (Segal et al., 2001) or body fat (K. S. Courneya et al., 2007), improved endurance (Basen-Engquist et al., 2006), reduced fatigue (Mock et al., 1997; Segal et al., 2003; Vallance, Courneya, Plotnikoff, Yutaka, & Mackey, 2007), and fewer symptoms during and after treatment (Basen-Engquist et al., 2006; MacVicar & Winningham, 1986; Mock et al., 1997). Exercise also increases emotional well-being and quality of life (QOL) in cancer patients and survivors (Courneya et al., 2000) and is associated with improvements in distress (Dimeo, Stieglitz, Novelli-Fischer, Fetscher, & Keul, 1999; MacVicar & Winningham, 1986; Segar et al., 1998), body image (Pinto, Clark, Maruyama, & Feder, 2003), depression (Mock et al., 1994), and anxiety (Mock et al., 1997).
While these studies document the benefits of activity, evidence regarding the mechanisms underlying adoption and maintenance of a physically active lifestyle for cancer survivors is only beginning to emerge. One cross-sectional survey applying the Theory of Planned Behavior to explaining exercise behavior in endometrial cancer survivors found that intention to exercise and self-efficacy were the most important correlates of exercise, and that self-efficacy and attitude were correlated with the intention to exercise. However, self-efficacy was related to intention for obese survivors only (Karvinen et al., 2007). Several exercise intervention studies for cancer survivors show that self-efficacy, either at baseline or changes over time, was related to exercise behavior at the end of the study (Bennett, Lyons, Winters-Stone, Nail, & Scherer, 2007; Jones, Courneya, Fairey, & Mackey, 2005; Mosher et al., 2008; Pinto, Rabin, & Dunsiger, 2009; Vallance, Courneya, Plotnikoff, & Mackey, 2008), but this research has so far not shown convincing evidence that the interventions influence self-efficacy, indicating that more research is needed on variables that affect self-efficacy so that interventions can effectively influence this important exercise determinant.
It is possible that the process of exercise adoption and maintenance for individuals facing chronic disease is more complex than for healthy individuals, further strengthening the need for research in this area. For example, cancer survivors are more likely to suffer from fatigue and other quality of life problems than people who have not had cancer (Cella, Lai, Chang, Peterman, & Slavin, 2002), and these symptoms are associated with lower self-efficacy for exercise (Perkins, Baum, Taylor, & Basen-Engquist, 2009; Rogers, McAuley, Courneya, & Verhulst, 2008). Research is needed to help us determine how interventions to increase physical activity need to be tailored for cancer survivors to obtain optimal effectiveness.
The Steps to Health study investigates mechanisms of physical activity adoption and maintenance in a sample of endometrial cancer survivors receiving a telephone counseling and print material intervention to help them adopt and maintain regular physical activity. Endometrial cancer is estimated to affect 40,100 women in the United States this year (Jemal et al., 2008); women in the US have a 2.62% lifetime risk of being diagnosed with this disease (Ries et al., 2003). Early stage endometrial cancer is generally treated with surgery or surgery plus radiation therapy, with these treatments often producing a cure. For stage I disease, recurrence rates of 0 to 14% have been reported (Aalders, Abeler, Kolstad, & Onsrud, 1980; Creutzberg et al., 2000; Eltabbakh, Piver, Hempling, & Shin, 1997; Morrow et al., 1991). Because endometrial cancer is often diagnosed at an early stage (Ries et al., 2003) and has a high cure rate (DiSaia & Creasman, 2002), survival rates are high and methods to enhance the quality of survivorship after cancer treatment are a relevant area of study.
Several risk factors and co-morbidities associated with endometrial cancer make physical activity a particularly appropriate intervention for this population. First, endometrial cancer is usually diagnosed in older women; incidence is highest among women aged 60 to 84 (Ries et al., 2003) and the median age at diagnosis is 61 (DiSaia & Creasman, 2002). As people age, they become less active and suffer declines in physical functioning (Ware Jr., Snow, Kosinski, & Gandek, 1997). However, studies of physical activity in elderly populations have shown that declines in fitness and physical functioning can be prevented or even reversed when regular physical activity is adopted (Dubbert, Cooper, Kirchner, Meydrech, & Bilbrew, 2002; King, Haskell, Taylor, Kraemer, & DeBusk, 1991; Lavie & Milani, 1995; Morey & Zhu, 2003; Nelson, Fisher, Dilmanian, Dallal, & Evans, 1991; Posner et al., 1992).
Second, obesity and physical inactivity are risk factors for endometrial cancer (Cust, Armstrong, Friedenreich, Slimani, & Bauman, 2007; Furberg & Thune, 2003; Goodman et al., 1997; Kaaks, Lukanova, & Kurzer, 2002). Goodman and colleagues reported a four-fold increased odds ratio for endometrial cancer for women in the highest quartile of BMI versus the lowest quartile (Goodman et al., 1997). Diabetes and hypertension have also been implicated as risk factors, particularly among the most obese women (Furberg & Thune, 2003). Surveys of endometrial cancer survivors show they have higher rates of obesity, as well as lower rates of physical activity, than the general population (Basen-Engquist et al., 2009; Courneya et al., 2005). While no studies exist demonstrating a link between physical activity and endometrial cancer recurrence, their higher rates of obesity and sedentary behavior, as well as co-morbid disease risk, increase the importance of a physical activity intervention because it increases their risk of second cancers and other chronic diseases.
Taken together, these data support the idea that endometrial cancer survivors may benefit from increasing their physical activity. Data on quality of life of endometrial cancer survivors indicate that they face a number of lingering symptoms and side effects (Bye, Trope, Loge, Hjermstad, & Kaasa, 2000; Huguenin, Baumert, Lutolf, Wight, & Glanzmann, 1999; Juraskova et al., 2003; Klee & Machin, 2001; Li, Samsioe, & Iosif, 1999), in addition to problems caused by premorbid conditions, such as obesity or diabetes. Some of these quality of life problems, such as fatigue, pain, hot flashes, and sleep disturbance, may be ameliorated by increasing physical activity; others, such as incontinence and diarrhea, may indicate a need to tailor messages about physical activity to meet the specific needs of endometrial cancer survivors.
Social Cognitive Theory
To study the mechanisms of physical activity adoption and maintenance in endometrial cancer survivors we are applying a model based on Bandura's Social Cognitive Theory (Bandura, 1977, 1986, 1997). Previous studies have applied Social Cognitive Theory to evaluate and increase physical activity in other populations, but this theory has not been systematically applied to study the mechanisms of exercise adoption and maintenance in cancer survivors. Furthermore, most studies have been limited to exploring the effect of self-efficacy on adherence. This study will test a more complete model, probing the influence of the sources of self-efficacy information on self-efficacy and the relationship of both self-efficacy and outcome expectations to exercise adherence, as described by Bandura (Bandura, 1986, 1997).
Social Cognitive Theory puts forth that our cognitions or beliefs influence our behavior. A person's self-efficacy, or confidence that he or she can successfully perform a behavior, is a key determinant of behavior. Previous studies have demonstrated relationships between self-efficacy related to exercise and exercise adherence (Moore, Dolansky, Ruland, Pashkow, & Blackburn, 2003; Plotnikoff, Hotz, Birkett, & Courneya, 2001; Rejeski et al., 2003; Steptoe, Rink, & Kerry, 2000). Self-efficacy is most predictive of behavior when it is measured in specific domains. For example, exercise researchers have studied both self-efficacy for actually performing the exercise (e.g., a person's confidence that he/she can walk briskly for 30 minutes) and for overcoming the barriers to ongoing exercise adherence (e.g., confidence that one can exercise even with the weather is bad) (McAuley, Jerome, Marquez, Elavsky, & Blissmer, 2003). A person's self-efficacy about a particular behavior is developed using four sources of information (Bandura, 1986, 1997): mastery experiences (the successful enactment of a behavior); vicarious experience, or modeling (observing another's success at performing a behavior); verbal persuasion and feedback (support from others highlighting one's capabilities or specific feedback on performance); and physiological and affective states while performing the behavior. For physical activity, this can include the experience and interpretation of somatic sensations such as increased heart rate and respiration, muscle soreness, and fatigue. While these are common sensations in response to moderate or vigorous activity, individuals can attend to and interpret them differently. While some people may dwell on somatic states, others discount them.
In addition to self-efficacy, an individual's perception of the likely outcomes of engaging in physical activity (outcome expectations) influences his or her subsequent levels of activity, i.e., a heightened perception of positive outcomes leads to increases in physical activity. However, an expectancy violation effect can occur as well; for example, if the individual expects certain benefits, and these are not realized after increasing activity levels, this can decrease adherence to physical activity recommendations (Brassington, Atienza, Perczek, DiLorenzo, & King, 2002; Sears & Stanton, 2001).
Steps to Health Model
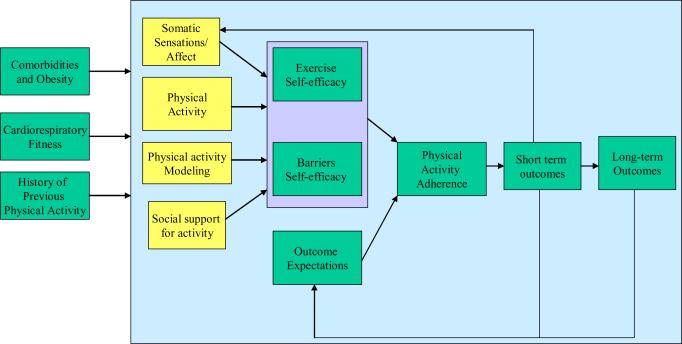
This study tests a model of endometrial cancer survivors' physical activity adoption and maintenance based on Social Cognitive Theory (see Figure 1). In this model, self-efficacy and outcome expectations are hypothesized to predict adherence to a physical activity recommendation. Two types of self-efficacy are assessed. The first, exercise self-efficacy, refers to the participant's confidence that she is capable of engaging in exercise at a particular intensity and duration. For example, we assess confidence in walking at a moderate intensity for periods of 2 minutes up to 60 minutes. This form of self-efficacy is expected to be related to levels of physical activity in the early months of the intervention (months 2 and 4), rather than to adherence to regular physical activity over the six months of the program (McAuley, 1992; McAuley, Courneya, Rudolph, & Lox, 1994). The second, self-regulatory, or barriers, self-efficacy, refers to a participant's confidence that she will be able to adhere to regular physical activity, despite daily challenges. This form of self-efficacy is expected to predict physical activity throughout the intervention period (months 2, 4, and 6) (Brassington et al., 2002; Litt, Kleppinger, & Judge, 2002; Rejeski et al., 2003; Rhodes, Martin, & Taunton, 2001).
Figure 1.
Social Cognitive Theory-based model of physical activity adherence.
Outcome expectations are also expected to influence physical activity adherence. However, cancer survivors may value different outcomes than healthy individuals. In a study of breast and colorectal cancer survivors, Courneya and colleagues identified the following outcome expectations: distraction from cancer and treatment: feeling better and improving well-being, maintaining a normal lifestyle, coping with the stress of cancer and treatment, gaining a sense of control over cancer and my life, recovering from surgery and treatment, and controlling weight (Courneya & Friedenreich, 1997; Courneya & Friedenreich, 1999). For breast cancer survivors, all outcome expectations correlated with the exercise intentions and all except for maintaining weight correlated with exercise behavior (in a cross-sectional study). Consistent with Social Cognitive Theory, we also hypothesize that an expectancy violation effect will occur; we predict that participants whose expectations about physical activity outcomes are not met will subsequently engage in less physical activity.
The participant's experience of the four sources of efficacy information is assessed to empirically test their relationship to both types of self-efficacy. First, the study evaluates whether the participants' exposure to modeling of physical activity, as reported daily using handheld computers, is related to self-efficacy. While previous studies have used modeling as an intervention tool to promote physical activity (McAuley et al., 1994), no identified research has attempted to measure exposure to modeling of physical activity in a person's natural environment and test its relationship to self-efficacy. We hypothesize that the frequency of observing physical activity modeling will predict both types of self-efficacy. Second, verbal persuasion, in the form of social support for physical activity, is also hypothesized to have a positive impact on self-efficacy (Duncan & McAuley, 1993). Cancer survivors may receive support from loved ones to engage in regular exercise, or experience negative feedback and discouragement from their friends, family, and health care providers, because of common misconceptions about the inappropriateness of physical activity after cancer. The extent to which they experience exercise encouragement also will be measured daily via handheld computers. Third, self-efficacy is influenced by mastery experiences; therefore, we hypothesize that physical activity at baseline and earlier time points in the study will predict self-efficacy at later timepoints. Previous physical activity has been shown to influence exercise and barriers self-efficacy (McAuley et al., 2003).
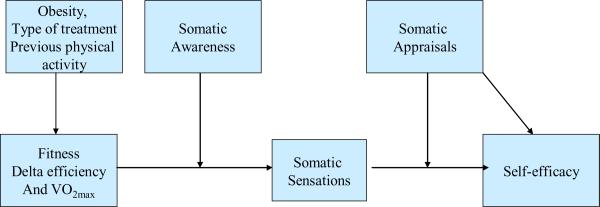
Finally, this study places special emphasis on the relationship between physiologic feedback (somatic sensations during exercise) and self-efficacy (see Figure 2). Physiologic feedback has been the least studied determinant of exercise self-efficacy, although one study with adolescent girls found perceived exertion during exercise to be a predictor of self-efficacy (Pender, Bar-Or, Wilk, & Mitchell, 2002). Another study of older adults found that perceptions of pain and shortness of breath during exercise were associated with lower self-efficacy (Clark & Nothwehr, 1999). Somatic sensations during exercise have not been studied as a predictor of self-efficacy in cancer survivors, but studies of survivors have shown that symptoms such as fatigue and pain have been correlated with exercise self-efficacy (Perkins et al., 2009; Rogers et al., 2008). Individual differences in the effect of exercise-related somatic sensations on self-efficacy may exist for a number of reasons. The most obvious is that people with low levels of aerobic fitness may experience more sensations such as fatigue, shortness of breath, and muscle discomfort during activity. Although there is compelling evidence that exercising at the recommended levels will improve VO2 max, a measure of cardiorespiratory fitness, there is insufficient information on how cardiorespiratory fitness relates to somatic sensations and self-efficacy. While a few studies have shown weak associations between VO2 max and self-efficacy (McAuley et al., 1999; Pender et al., 2002), the relationships among cardiorespiratory fitness, somatic sensations, and self-efficacy have not been systematically studied.
Figure 2.
Model for relationships of somatic sensations and fitness with self-efficacy
Bandura also theorizes that people differ in their attention to and appraisal of somatic sensations, and that these predispositions affect the influence of somatic sensations on self-efficacy (Bandura, 1997). Health psychology literature on symptom perception demonstrates that individuals vary in their degree of attention to physical sensations (Martin, Ahles, & Jeffery, 1991). This characteristic, referred to as somatic awareness, body consciousness, or somatosensory amplification, has been related to increased symptom reporting, particularly when attentional processes and anxiety increase the likelihood that the somatic sensation will be processed affectively (Martin et al., 1991).
The mere detection of somatic sensations is not sufficient to affect self-efficacy; a person's actual interpretation of the sensation is critical to determining its effect (Cioffi, 1991). Survivors who are becoming more physically active may interpret muscle soreness during walking either as a sign that they are experiencing a good work-out, or that they are not physically `up to' the challenge of exercise. Obviously these two interpretations have different implications for proximal self-efficacy judgments. As cancer survivors, our participants may be more likely to attend to their somatic sensations, and to develop more threatening interpretations of these sensations (Benyamini, McClain, Leventhal, & Leventhal, 2003). We hypothesize that somatic sensations will be related to self-efficacy, but that this relationship will be moderated by the appraisal of the somatic sensations.
Steps to Health study design
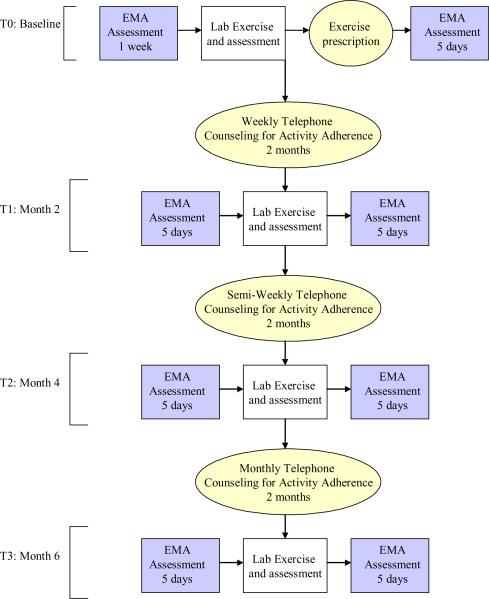
This longitudinal study examines the process of physical activity adoption and maintenance in endometrial cancer survivors (see Figure 3 for a schematic of the design). Endometrial cancer survivors have to be at least 6 months from the completion of treatment (to allow for sufficient healing time). We focused on Stage I – IIIa cancers because these patients have the highest probability of surviving cancer, and are less likely to recur. Participants need physician approval to participate, and are excluded from the study if they have any medical condition that would make sub-maximal fitness testing or home-based exercise unsafe (American College of Sports Medicine, 2006). We also exclude survivors who are already engaging in programmatic physical activity at moderate or greater intensity on five or more days per week for 30 minutes or more, or vigorous intensity activity for 20 minutes or more at least 3 days per week, and have maintained this activity for 6 months. This was done because we wanted to study the adoption and maintenance process, and thus studying survivors who are already maintaining an exercise program that meets or exceeds public health guidelines would not provide us with this information. Potentially eligible survivors at M. D. Anderson are identified using medical record data, and are either approached about the study at their next appointment or receive a letter inviting them to participate. We also recruit patients at a private gynecologic oncology practice in Houston, where physician assistants identify and recruit participants. Screening of participants takes place in two steps. First, the recruiting project staff member verifies information about tumor type and diagnosis date and assessed English-speaking ability, orientation, and current physical activity level. A medical eligibility checklist is given to the patient's physician to complete. The physician confirms diagnosis information, indicates whether medical exclusion criteria apply, and signs the checklist if he/she agreed the patient is eligible.
Figure 3.
Steps to Health design: assessment and intervention schema. (EMA: Ecological Momentary Assessment)
Because spontaneous adoption of a physically active lifestyle is expected to be low (Pinto, Trunzo, Reiss, & Shiu, 2002), all participants receive a Social Cognitive Theory-based physical activity intervention utilizing methods and strategies with demonstrated efficacy. We did not use a randomized controlled design because our goal is to study the process of physical activity adoption and adherence, not to test intervention efficacy. All participants receive this 6-month behavioral intervention consisting of an exercise prescription, telephone counseling, and written materials to increase their physical activity. Based on Social Cognitive Theory (Bandura, 1986, 1997), Steps to Health facilitates mastery experiences by helping participants set and reach realistic goals, and provides verbal persuasion and social support for increasing physical activity, realistic information on benefits of physical activity to encourage the formation of reasonable outcome expectations, modeling of the adoption and maintenance of physical activity, training in behavioral self-regulatory skills (e.g., goal-setting, self-monitoring, problem solving for overcoming barriers, time management), and feedback on progress and problems encountered as participants become more active. The telephone counseling intervention is delivered by one masters-level health educator and one doctoral-level health educator. These individuals use a call session guide to standardize the content of their calls. At each session participants are asked about their exercise since the last session, barriers to exercising and how to overcome, health and medication changes, and goals for next week. During each session there is a brief `special topic activity' to amplify content that is provided in the newsletter for that week. These sections (and the newsletters) are used to teach behavioral skills and provide modeling of exercise through role model stories (stories of cancer survivors who adopted exercise programs). The participants are given an exercise recommendation with specific weekly goals based on their fitness level; these goals are modified as needed by the telephone counselor based on their ability to meet the goals each week. Although participants are informed that we want them to progress to at least 150 minutes of moderate intensity physical activity per week, any increase in activity is considered a positive outcome.
Participants complete assessments every two months for a total of four assessments periods. Each assessment period has both a laboratory and an at-home component. Laboratory assessments include fitness testing; questionnaire assessments, and cognitive assessments of somatic awareness and outcome expectations (expectancy accessibility). The fitness test is a graded submaximal exercise test conducted on an electronic rate-limited cycle ergometer. Participants exercise at 20 watts (W) for 2 minutes, followed by increases of 20 W every 2 minutes until they reach 85% of predicted maximum heart rate, a respiratory exchange ratio equal to 1, or volitional fatigue. Breath-by-breath measurements of Watts, VO2 (ml/kg/min), VO2 (L), VCO2 (L), VE (L), VE/VO2 (L), VE/VCO2 (L), METs, and caloric expenditure (kcal/min) are ascertained continuously throughout each test using a metabolic measurement cart. The domains measured by questionnaires include quality of life [Medical Outcomes Study Short Form-36 (McHorney, Ware, & Raczek, 1993; Ware et al., 1995; Ware Jr. et al., 1997); Quality of Life in Adult Cancer Survivors scale (Avis et al., 2005)], psychological distress [Brief Symptom Inventory-18 (Derogatis, 2000), Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983)], sleep quality [Pittsburgh Sleep Quality Index (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989; Smyth, 1999)], physical activity [Community Health Activities Model Program for Seniors questionnaire (Harada, Chiu, King, & Stewart, 2001; Stewart et al., 2001)] and Social Cognitive Theory determinants [based on scales by McAuley (Duncan & McAuley, 1993; McAuley, 1992; McAuley et al., 1994; McAuley et al., 2003) and Marcus (Marcus & Owen, 1992; Marcus, Rakowski, & Rossi, 1992; Marcus, Selby, Niaura, & Rossi, 1992)]. Before and after these laboratory assessments, participants complete 5 days of field measures using ecological momentary assessment (EMA) to measure their physical activity, self-efficacy, and sources of self-efficacy (somatic sensations and appraisals, modeling, social support for exercise). They also wear an accelerometer (Actigraph GT1M, Actigraph L.L.C., Pensacola, FL) during this time to provide an objective measure of home-based physical activity.
A unique feature of this project is its multi-method measurement approach; in addition to traditional retrospective self-report questionnaires, we use EMA to measure physical activity, self-efficacy, and exposure to modeling and social support. EMA captures real-time phenomena in a person's natural environment, which makes the data less subject to recall biases compared with longer-term assessments (Shiffman, 2000). For example, in answering retrospective questionnaires, highly salient or unusual events are more likely to be recalled, and retrospective reports of psychological states are influenced by the respondent's current psychological state (Stone, Shiffman, Atienza, & Nebeling, 2007). EMA assessment for this study is done for 5 days before and after each laboratory assessment session. Participants are prompted to complete brief assessment of exercise self-efficacy and outcome expectations in the morning, assessments of somatic sensations at 3 random times throughout the day (to provide a comparison to somatic sensations during exercise), and assessment of physical activity, modeling, and social support in the evening. Furthermore, each time the participant exercises they are asked to input the starting and ending times of their exercise, and to answer questions regarding somatic sensations and affect before and after exercising. In addition, the participants wear an accelerometer to measure physical activity on the days they are doing the EMA.
An additional method we are employing to help overcome some of the limitations and response biases in self-reported questionnaires is the assessment of implicit cognitions related to outcome expectations and somatic awareness. Questionnaire measures that require participants to introspect and report on their mental states are considered “explicit” cognitive measures (Fazio & Olson, 2003). While self-report measures are easy to administer, and much is known about their psychometric properties, they also have limitations (Nisbett & Wilson, 1977) including a dependency on participants' willingness to report private knowledge and their ability to accurately report it (Hammersley, 1994), as well as a susceptibility to artifacts such as impression management and demand characteristics (Greenwald et al., 2002). It is generally believed that cognitive processes unavailable to conscious introspection can influence behavior (Schacter, 1992). We are using computerized reaction time tasks to measure relevant implicit cognitions, defined as “introspectively unidentified (or inaccurately identified) traces of past experience that mediate feeling, thought, or action” (Greenwald & Banaji, 1995). The expectancy accessibility task assesses the speed with which participants endorse outcomes related to exercise. Two individuals may both endorse the same outcome expectancy (e.g., Exercise makes me…..HEALTHY), but they may differ in the time taken to endorse these outcomes. Research in addiction has demonstrated that there is dependence-relevant information in reaction times that is incremental to information derived from self-report measures of outcome expectations (Palfai, 2002; Palfai, Monti, Ostafin, & Hutchinson, 2000). Response times contain information about the accessibility of cognitive representations (how quickly expectancies “come to mind”) that may not be possible to assess using self-report measures, which focus on the content of expectancies (what the expectancies are). A modified Stroop task (a widely used cognitive task that assesses individuals' tendency to attend to certain stimuli) is being used as an implicit assessment of the participant's tendency to attend to threatening physiologic sensations. Research using the modified Stroop task has shown that this cognitive measure can predict important behavioral outcomes when controlling for relevant self-report measures (Waters, Sayette, & Wertz, 2003; Waters, Shiffman, Bradley, & Mogg, 2003; Waters, Shiffman, Sayette et al., 2003).
Analysis of data
We propose a sample size of 200, based on the number of participants needed to to detect Pearson's correlation coefficients of ρ = .20 or higher with 80% power, using a significance leve of α = .05. For the multiple regression analyses, we are using hierarchical modeling. In these analyses, demographic and medical characteristics are first entered into the models for a given outcome measure, followed by the social cognitive measures of interest. If approximately 4 to 8 demographic and medical characteristics are first entered in the models, combining to explain no more than 15 percent of the variance in the outcome measures (multiple R of .4), a sample of 200 participants provides 80 percent power to detect an improvement in the model R2 of .032 for each additional social cognitive variable added to the model.
Exercise adherence is measured separately using the accelerometer and EMA assessments during the 5-day periods before and after the laboratory assessment. For within day analyses of the EMA data we calculate the number of minutes of physical activity in a particular day. For the other analyses these measures are collapsed across the 10-day period to create simple measures representing total minutes of moderate or more intense physical activity. If the physical activity data are right-skewed (as is often the case), normalizing transformations, such as YP (0<P<1) or log(Y+1), will be computed as appropriate. The relationships specified in the model will be examined using Pearson's Product Moment correlations and hierarchical linear regression. In the hierarchical regression models, participant demographic and medical characteristics will be entered first, followed by the social cognitive measures of interest. Partial R's and R2's will be computed for the social cognitive measures and will represent the correlation and proportion variance explained, respectively, of the social cognitive variables with respect to the outcome variables after adjustment for the demographic, medical and baseline exercise variables. These analyses will be repeated for the data obtained at each assessment period. In addition, an overall model examining the relationship among these variables across all assessment periods will be fitted using hierarchical linear mixed models. A mixed models approach will be used for these analyses because the measurements obtained on each person from different assessments are expected to be correlated.
For exploring mediation hypotheses we will also incorporate structural equation modeling. In order to examine the effect of the mediating variables specified in the hypothesis, researchers typically use the four criteria proposed by Baron and Kenny (Baron & Kenny, 1986). Though these four criteria represent a valid approach to establishing mediation, a recent paper by MacKinnon, et al. (MacKinnon, Lockwood, Hoffma, West, & Sheets, 2002) comparing a total of 14 different methods of testing for mediation showed that this approach has very low Type I error rate and low power unless the effect or sample size was large, indicating a high probability of not detecting existing mediation. Their suggested alternative was a joint test of criteria 2 and 3 above, which they showed provided better Type I error rates and better power, while providing a valid test of mediation. The mediation effect estimate itself will be computed according to MacKinnon (MacKinnon, 1994) as the product of the effect of the intervention dose on the mediators and the mediators on physical activity adherence controlling for the intervention dose. This will be examined in simultaneous model and parameter tests using structural equation modeling software (EQS). SEM provides a flexible approach to modeling means, covariance, and correlation structures that yields relevant effect estimates (directional and non-directional; direct, indirect, and total) and standard errors for all of the parameters of interest in mediator analyses (Bollen, 1987; MacKinnon, 1994). In addition, it provides estimates for the combined mediation effect of all of the mediators, as well as individual effects for the individual mediators. Beyond providing tests of model parameters, SEM also provides global measures of model fit (Hu & Bentler, 1999); and permits the incorporation of latent variables into mediator models when appropriate (Bollen, 1989; Finch, West, & MacKinnon, 1997). In addition, recent versions of software such as EQS permit robust model and parameter assessment under varied distributional conditions (West, Finch, & Curran, 1995).
Moving the field forward
The Steps to Health study will elucidate how cancer survivors form self-efficacy expectations about physical activity, including how cardiorespiratory fitness and somatic sensations during exercise influence self-efficacy. In addition we will identify the types of self-efficacy that are most influential at different points in the exercise adoption and maintenance processes. It also will help us understand the influence of outcome expectations and the expectancy violation effect in a cancer survivor sample.
The unique assessment methodologies will help move the field forward, as we explore the relationship between Social Cognitive Theory variables measured with traditional retrospective self-report questionnaires, measurements made using EMA, and implicit assessments, and identify to what extent the measures contribute to the understanding of predictors of exercise adoption and maintenance.
The study results can be used to develop more effective interventions for increasing physical activity in sedentary cancer survivors by taking into account the full complement of sources of self-efficacy information and outcome expectations. For example, results of the study will indicate whether interventions should include components addressing appropriate appraisal of somatic sensations, fostering realistic expectations about the outcomes of increased activity, or formalizing networks of similar individuals to serve as models and provide social support for increasing activity. Results may also be used to identify individual differences in the predictors of physical activity adoption, which would enable the tailoring of interventions on these variables, such as somatic awareness. The development of more effective interventions to help cancer survivors adopt a more active lifestyle and maintain it over longer periods of time time will enable a more cancer survivors to experience health and quality of life benefits.
Acknowledgments
Supported in part by NIH Grant R01 CA109919, Karen Basen-Engquist, Ph.D., Principal Investigator, a cancer prevention fellowship from the National Cancer Institute grant R25 CA57730, Robert M. Chamberlain, Ph.D., Principal Investigator, and P30 CA16672, John Mendelsohn, MD, Principal Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aalders J, Abeler V, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: Clinical and histopathologic study of 540 patients. Obstetrics & Gynecology. 1980;56(4):419–427. [PubMed] [Google Scholar]
- Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol. 2006;24(18):2765–2772. doi: 10.1200/JCO.2005.03.6749. [DOI] [PubMed] [Google Scholar]
- American College of Sports Medicine . Guidelines for Exercise Testing and Prescription. 7th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. [Google Scholar]
- Avis NE, Smith KW, McGraw S, Smith RG, Petronis VM, Carver CS. Assessing quality of life in adult cancer survivors (QLACS) Qual Life Res. 2005;14(4):1007–1023. doi: 10.1007/s11136-004-2147-2. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social Foundations of Thought and Action: A Social-Cognitive Theory. Prentice-Hall; Englewood Cliffs, NJ: 1986. [Google Scholar]
- Bandura A. Self-Efficacy: The Exercise of Control. W. H. Freeman and Company; New York, NY: 1997. [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Basen-Engquist K, Scruggs S, Jhingran A, Bodurka DC, Lu K, Ramondetta L, et al. Physical activity and obesity in endometrial cancer survivors: associations with pain, fatigue, and physical functioning. Am J Obstet Gynecol. 2009;200(3):288, e281–288. doi: 10.1016/j.ajog.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basen-Engquist K, Taylor CC, Rosenblum C, Smith MA, Shinn EH, Greisinger A, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Education and Counseling. 2006;64(1–3):225–234. doi: 10.1016/j.pec.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Bennett JA, Lyons KS, Winters-Stone K, Nail LM, Scherer J. Motivational interviewing to increase physical activity in long-term cancer survivors: a randomized controlled trial. Nurs Res. 2007;56(1):18–27. doi: 10.1097/00006199-200701000-00003. [DOI] [PubMed] [Google Scholar]
- Benyamini Y, McClain CS, Leventhal EA, Leventhal H. Living with the worry of cancer: Health perceptions and behaviors of elderly people with self, vicarious, or no history of cancer. Psycho-Oncology. 2003;12:161–172. doi: 10.1002/pon.637. [DOI] [PubMed] [Google Scholar]
- Bollen KA. Total, direct, and indirect effects in structural equation models. In: Clogg CC, editor. Sociological Methodology. American Sociological Association; Washington DC: 1987. pp. 37–69. [Google Scholar]
- Bollen KA. Structural Equations with Latent Variables. Wiley; New York, NY: 1989. [Google Scholar]
- Brassington GS, Atienza AA, Perczek RE, DiLorenzo TM, King AC. Intervention-related cognitive versus social mediators of exercise adherence in the elderly. American Journal of Preventive Medicine. 2002;23(2S):80–86. doi: 10.1016/s0749-3797(02)00477-4. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Bye A, Trope C, Loge JH, Hjermstad M, Kaasa S. Health-related quality of life occurrence of intestinal side effects after pelvic radiotherapy. Evaluation of long-term effects of diagnosis and treatment. Acta Oncologica. 2000;39(2):173–180. doi: 10.1080/028418600430734. [DOI] [PubMed] [Google Scholar]
- Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- Cioffi D. Beyond attentional strategies: A cognitive-perceptual model of somatic interpretation. Psychological Bulletin. 1991;109(1):25–41. doi: 10.1037/0033-2909.109.1.25. [DOI] [PubMed] [Google Scholar]
- Clark DO, Nothwehr F. Exercise self-efficacy and its correlates among socioeconomically disadvantaged older adults. Health Education and Behavior. 1999;26(4):535–546. doi: 10.1177/109019819902600410. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein RF. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Courneya KS. Exercise interventions during cancer treatment: biopsychosocial outcomes. Exerc Sport Sci Rev. 2001;29(2):60–64. doi: 10.1097/00003677-200104000-00004. [DOI] [PubMed] [Google Scholar]
- Courneya KS. Exercise in cancer survivors: an overview of research. Med Sci Sports Exerc. 2003;35(11):1846–1852. doi: 10.1249/01.MSS.0000093622.41587.B6. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Friedenreich CM. Determinants of exercise during colorectal cancer treatment: An application of the theory of planned behavior. Oncology Nursing Forum. 1997;24(10):1715–1717. [PubMed] [Google Scholar]
- Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Annals of Behavioral Medicine. 1999;21(2):171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Friedenreich CM. Utility of the Theory of Planned behavior for understanding exercise during breast cancer treatment. Psycho-Oncology. 1999;8(2):112–122. doi: 10.1002/(SICI)1099-1611(199903/04)8:2<112::AID-PON341>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Karvinen KH, Campbell KL, Pearcey RG, Dundas G, Capstick V, et al. Associations among exercise, body weight, and quality of life in a population-based sample of endometrial cancer survivors. Gynecol Oncol. 2005;97(2):422–430. doi: 10.1016/j.ygyno.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Keats MR, Turner AR. Social cognitive determinants of hospital-based exercise in cancer patients following high-dose chemotherapy and bone marrow transplantation. International Journal of Behavioral Medicine. 2000;7(3):189–203. [Google Scholar]
- Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- Creutzberg CL, van Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for pateints with state-1 endometrial carcinoma: multicentre randomised trial. PORTEC Study Group. Post Operative Radiation Therapy in Endometrial Carcinoma. Lancet. 2000;355(9213):1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- Cust AE, Armstrong BK, Friedenreich CM, Slimani N, Bauman A. Physical activity and endometrial cancer risk: a review of the current evidence, biologic mechanisms and the quality of physical activity assessment methods. Cancer Causes Control. 2007;18(3):243–258. doi: 10.1007/s10552-006-0094-7. [DOI] [PubMed] [Google Scholar]
- Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. J Clin Oncol. 2007;25(13):1713–1721. doi: 10.1200/JCO.2006.09.5083. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. Brief Symptom Inventory 18: Administration, Scoring, and Procedures Manual. National Computer Systems; Minneapolis, MN: 2000. [Google Scholar]
- Devogelaer JP, de Deuxchaisnes CN. Therapy and the 1990s: Osteoporosis. British Journal of Rheumatology. 1993;32:48–55. doi: 10.1093/rheumatology/32.suppl_4.48. [DOI] [PubMed] [Google Scholar]
- Dimeo FC, Stieglitz R, Novelli-Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999;85(10):2273–2277. [PubMed] [Google Scholar]
- DiSaia PJ, Creasman WT. Clinical Gynecologic Oncology. Sixth Edition ed. Mosby, Inc.; 2002. [Google Scholar]
- Dubbert PM, Cooper KM, Kirchner KA, Meydrech EF, Bilbrew D. Effects of nurse counseling on walking for exercise in elderly primary care patients. Jornal of Gerontol A Biol Sci Med Sci. 2002;57(11):M733–M740. doi: 10.1093/gerona/57.11.m733. [DOI] [PubMed] [Google Scholar]
- Duncan TE, McAuley E. Social support and efficacy cognitions in exercise adherence: A latent growth curve analysis. Journal of Behavioral Medicine. 1993;16(2):199–218. doi: 10.1007/BF00844893. [DOI] [PubMed] [Google Scholar]
- Eltabbakh GH, Piver MS, Hempling RE, Shin KH. Excellent long-term survivoral and absence of vaginal recurrences in 332 pateints with low-risk stage I endometrial adenocarcinoma treated with hysterectomy and vaginal brachytherapy without formal staging lymph node sampling: Report of a prospective trial. International Journal of Radiation Oncology, Biology, Physics. 1997;38(2):373–380. doi: 10.1016/s0360-3016(97)00040-0. [DOI] [PubMed] [Google Scholar]
- Fairey AS, Courneya KS, Field CJ, Mackey JR. Physical exercise and immune system function in cancer survivors. Cancer. 2002;94(2):539–551. doi: 10.1002/cncr.10244. [DOI] [PubMed] [Google Scholar]
- Fazio RH, Olson MA. Implicit measure of social cognition research: Their meaning and use. Annual Review Psychology. 2003;54:297–327. doi: 10.1146/annurev.psych.54.101601.145225. [DOI] [PubMed] [Google Scholar]
- Finch JF, West SG, MacKinnon DP. Effects of sample size and nonnormality on the estimation of mediated effects in latent variables models. Structural Equation Modeling. 1997;4:87–107. [Google Scholar]
- Friedenreich CM, Courneya KS. Exercise as rehabilitation for cancer patients. Clinical Journal of Sport Medicine. 1996;6(4):237–244. doi: 10.1097/00042752-199610000-00006. [DOI] [PubMed] [Google Scholar]
- Furberg AS, Thune I. Metabolic abnormalities (hypertension, hyperglycemia and overweight), lifestyle (high energy intake and physical inactivity) and endometrial cancer risk in a Norwegian cohort. International Journal of Cancer. 2003;104:669–676. doi: 10.1002/ijc.10974. [DOI] [PubMed] [Google Scholar]
- Galvao DA, Newton RU. Review of exercise intervention studies in cancer patients. Journal of Clinical Oncology. 2005;23(4):899–909. doi: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- Goodman MT, Hankin JH, Wilkens LR, Lyu LC, McDuffie K, Liu LQ, et al. Diet, body size, physical activity, and the risk of endometrial cancer. Cancer Research. 1997;57:5077–5085. [PubMed] [Google Scholar]
- Greenwald AG, Banaji MR. Implicit social cognition -- Attitudes, self-esteem and sterotypes. Psychological Review. 1995;102:4–27. doi: 10.1037/0033-295x.102.1.4. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Banaji MR, Rudman LA, Farnham SD, Nosek BA, Mellot DS. A unified theory of implicit attitudes, stereotypes, self-esteem, and self-concept. Psychological Review. 2002;109:3–25. doi: 10.1037/0033-295x.109.1.3. [DOI] [PubMed] [Google Scholar]
- Hammersley R. A digest of memory phenomena for addiction research. Addiction. 1994;89:283–293. doi: 10.1111/j.1360-0443.1994.tb00890.x. [DOI] [PubMed] [Google Scholar]
- Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Medicine & Science in Sports & Exercise. 2001;33(6):962–970. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- Helmrich SP, Ragland DR, Leung RW, Paffenbarger RS. Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. The New England Journal of Medicine. 1991;325(3):147–152. doi: 10.1056/NEJM199107183250302. [DOI] [PubMed] [Google Scholar]
- Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. Journal of the American Medical Association. 2005;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- Hu L-H, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Huguenin P, Baumert B, Lutolf UM, Wight E, Glanzmann C. Curative radiotherapy in elderly patients with endometrial center. Patterns of relapse, toxicity and quality of life. Strahlenther Onkol. 1999;175(7):309–314. doi: 10.1007/pl00002298. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jones LW, Courneya KS, Fairey AS, Mackey JR. Does the theory of planned behavior mediate the effects of an oncologist's recommendation to exercise in newly diagnosed breast cancer survivors? Results from a randomized controlled trial. Health Psychol. 2005;24(2):189–197. doi: 10.1037/0278-6133.24.2.189. [DOI] [PubMed] [Google Scholar]
- Juraskova I, Butow P, Robertson R, Sharpe L, McLeod C, Hacker N. Post-treatment sexual adjustment following cervical and endometrial cancer: A qualitative insight. Psycho-Oncology. 2003;12:267–279. doi: 10.1002/pon.639. [DOI] [PubMed] [Google Scholar]
- Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: A synthetic review. Cancer Epidemiology, Biomarkers and Prevention. 2002;11(12):1531–1543. [PubMed] [Google Scholar]
- Karvinen KH, Courneya KS, Campbell KL, Pearcey RG, Dundas G, Capstick V, et al. Correlates of exercise motivation and behavior in a population-based sample of endometrial cancer survivors: an application of the Theory of Planned Behavior. Int J Behav Nutr Phys Act. 2007;4:21. doi: 10.1186/1479-5868-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Haskell WL, Taylor B, Kraemer HC, DeBusk RF. Group-vs home-based exercise training in healthy older men and women: A community-based clinical trial. Journal of the American Medical Association. 1991;266:1535–1542. [PubMed] [Google Scholar]
- Klee M, Machin D. Health-related quality of life of patients with endometrial cancer who are disease-free following external irradiation. Acta Oncologica. 2001;40(7):816–824. doi: 10.1080/02841860152703436. [DOI] [PubMed] [Google Scholar]
- Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. Journal of Clinical Oncology. 2005;23(16):3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- LaCroix AZ, Leveille SG, Hecht JA, Grothaus LC, Wagner EH. Does walking decrease the risk of cardiovascular disease hospitalizations and death in older adults? Journal of the American Geriatrics Society. 1996;44:113–120. doi: 10.1111/j.1532-5415.1996.tb02425.x. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Milani RV. Effects of cardiac rehabilitation programs on exercise capacity, coronary risk factors, behavioral characteristics, and quality of life in a large elderly cohort. The American Journal of Cardiology. 1995;76:177–179. doi: 10.1016/s0002-9149(99)80054-x. [DOI] [PubMed] [Google Scholar]
- Li C, Samsioe G, Iosif C. Quality of life in endometrial cancer survivors. Maturitas. 1999;31:227–236. doi: 10.1016/s0378-5122(98)00106-6. [DOI] [PubMed] [Google Scholar]
- Litt MD, Kleppinger A, Judge JO. Initiation and maintenance of exercise behavior in older women: Predictors from the social learning model. Journal of Behavioral Medicine. 2002;25(1):83–97. doi: 10.1023/a:1013593819121. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP. Analysis of mediating variables in prevention and intervention research. NIDA Research Monographs. 1994;139:127–153. [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Hoffma JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychological Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar MG, Winningham ML. Promoting the functional capacity of cancer patients. The Cancer Bulletin. 1986;38(5):235–239. [Google Scholar]
- Marcus BH, Owen N. Motivational readiness, self-efficacy and decision making for exercise. Journal of Applied Social Psychology. 1992;22:3–16. [Google Scholar]
- Marcus BH, Rakowski W, Rossi JS. Assessing motivational readiness and decision making for exercise. Health Psychology. 1992;11(4):257–261. doi: 10.1037//0278-6133.11.4.257. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Research Quarterly for Exercise and Sport. 1992;63(1):60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- Martin JB, Ahles TA, Jeffery R. The role of private body consciousness and anxiety in the report of somatic symptoms during magnetic resonance imaging. Journal of Behav. Ther. & Exp. Psychiat. 1991;22(1):3–7. doi: 10.1016/0005-7916(91)90027-3. [DOI] [PubMed] [Google Scholar]
- McAuley E. The role of efficacy cognitions in the prediction of exercise behavior in middle-aged adults. Journal of Behavior Medicine. 1992;15(1):65–87. doi: 10.1007/BF00848378. [DOI] [PubMed] [Google Scholar]
- McAuley E, Courneya KS, Rudolph DL, Lox CL. Enhancing exercise adherence in middle-aged males and females. Preventive Medicine. 1994;23:498–506. doi: 10.1006/pmed.1994.1068. [DOI] [PubMed] [Google Scholar]
- McAuley E, Jerome GJ, Marquez DX, Elavsky S, Blissmer B. Exercise self-efficacy in older adults: Social, affective, and behavioral influences. Annals of Behavioral Medicine. 2003;25(1):1–7. doi: 10.1207/S15324796ABM2501_01. [DOI] [PubMed] [Google Scholar]
- McAuley E, Katula J, Mihalko SL, Blissmer B, Duncan TE, Pena M, et al. Mode of physical activity and self-efficacy in older adduts: A latent growth curve analysis. Journal of Gerontology: Psychological Sciences. 1999;54B(5):283–292. doi: 10.1093/geronb/54b.5.p283. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24(22):3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24(22):3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- Mock V, Burke MB, Sheehan P, Creaton EM, Winningham ML, McKenney-Tedder S, et al. A nursing rehabilitation program for women with breast cancer receiving adjuvant chemotherapy. Oncology Nursing Forum. 1994;21(5):899–908. [PubMed] [Google Scholar]
- Mock V, Dow KH, Meares CJ, Grimm PM, Dienemann JA, Haisfield-Wolfe ME, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncology Nursing Forum. 1997;24(6):991–1000. [PubMed] [Google Scholar]
- Moore SM, Dolansky MA, Ruland CM, Pashkow FJ, Blackburn GG. Predictors of women's exercise maintenance after cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation. 2003;23:40–49. doi: 10.1097/00008483-200301000-00008. [DOI] [PubMed] [Google Scholar]
- Morey MC, Zhu CW. Improved fitness narrows the symptom-reporting gap between older men and women. Journal of Women's Health. 2003;12(4) doi: 10.1089/154099903765448899. [DOI] [PubMed] [Google Scholar]
- Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: A Gynecologic Oncology Group study. Gynecologic Oncology. 1991;40(1):55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- Mosher CE, Fuemmeler BF, Sloane R, Kraus W, Lobach D, Snyder D, et al. Self-efficacy partially mediates the effects of the FRESH START Intervention on Cancer Survivors' Dietary Outcomes. Psycho-Oncology. 2008 doi: 10.1002/pon.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Fisher EC, Dilmanian FA, Dallal GE, Evans WJ. A 1-Y walking program and increased dietary calcium in postmenopausal women: Effects on bone. American Journal of Clinical Nutrition. 1991;53:1304–1311. doi: 10.1093/ajcn/53.5.1304. [DOI] [PubMed] [Google Scholar]
- Nisbett RE, Wilson TD. Telling more than we can know: Verbal reports on mental processes. Psychological Review. 1977;84:231–259. [Google Scholar]
- Ohira T, Schmitz KH, Ahmed RL, Yee D. Effects of weight training on quality of life in recent breast cancer survivors: the Weight Training for Breast Cancer Survivors (WTBS) study. Cancer. 2006;106(9):2076–2083. doi: 10.1002/cncr.21829. [DOI] [PubMed] [Google Scholar]
- Palfai TP. Positive outcome expectancies and smoking behavior: The role of expectancy accessibility. Cognitive Therapy and Research. 2002;26:317–333. [Google Scholar]
- Palfai TP, Monti PM, Ostafin B, Hutchinson K. Effects of nicotine deprivation on alcohol-related information processing and drinking behavior. Journal of Abnormal Psychology. 2000;109(1):96–105. doi: 10.1037//0021-843x.109.1.96. [DOI] [PubMed] [Google Scholar]
- Pender NJ, Bar-Or O, Wilk B, Mitchell S. Self-efficacy and perceived exertion of girls during exercise. Nursing Res. 2002;51(2):86–91. doi: 10.1097/00006199-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Perkins HY, Baum GP, Taylor CL, Basen-Engquist KM. Effects of treatment factors, comorbidities and health-related quality of life on self-efficacy for physical activity in cancer survivors. Psychooncology. 2009;18(4):405–411. doi: 10.1002/pon.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce JP, Stefanick ML, Flatt SW, Natarajan L, Sternfeld B, Madlensky L, et al. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. Journal of Clinical Oncology. 2007;25(17):2345–2351. doi: 10.1200/JCO.2006.08.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto BM, Clark MM, Maruyama NC, Feder SI. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psycho-Oncology. 2003;12:118–126. doi: 10.1002/pon.618. [DOI] [PubMed] [Google Scholar]
- Pinto BM, Maruyama NC. Exercise in the rehabilitation of breast cancer survivors. Journal of Psycho-Oncology. 1999;8:191–206. doi: 10.1002/(SICI)1099-1611(199905/06)8:3<191::AID-PON355>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Pinto BM, Rabin C, Dunsiger S. Home-based exercise among cancer survivors: adherence and its predictors. Psychooncology. 2009;18(4):369–376. doi: 10.1002/pon.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto BM, Trunzo J, Reiss P, Shiu S. Exercise participation after diagnosis of breast cancer: Trends and effects on mood and quality of life. Psycho-Oncology. 2002;11:389–400. doi: 10.1002/pon.594. [DOI] [PubMed] [Google Scholar]
- Plotnikoff RC, Hotz SB, Birkett NJ, Courneya KS. Exercise and the Transtheoretical Model: A longitudinal test of a population sample. Preventive Medicine. 2001;33:441–452. doi: 10.1006/pmed.2001.0914. [DOI] [PubMed] [Google Scholar]
- Posner JD, Gorman KM, Windsor-Landsberg, Larsen J, Bleiman M, Shaw C, et al. Low to moderate intensity endurance training in healthy older adults: Physiological responses after four months. Journal of the American Geriatric Society. 1992;40:1–7. doi: 10.1111/j.1532-5415.1992.tb01820.x. [DOI] [PubMed] [Google Scholar]
- Rejeski WJ, Brawley LR, Ambrosius WT, Brubaker PH, Focht BC, Foy CG, et al. Older adults with chronic disease: Benefits of group-mediated counseling in the promotion of physically active lifestyles. Health Psychology. 2003;22(4):414–423. doi: 10.1037/0278-6133.22.4.414. [DOI] [PubMed] [Google Scholar]
- Rhodes RE, Martin AD, Taunton JE. Temporal relationships of self-efficacy and social support as predictors of adherence in a 6-month strength-training program for older women. Perceptual and Motor Skills. 2001;93:693–703. doi: 10.2466/pms.2001.93.3.693. [DOI] [PubMed] [Google Scholar]
- Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, et al., editors. SEER Cancer Statistics Review, 1975–2000. National Cancer Institute; Bethesda, MD: 2003. [Google Scholar]
- Rogers LQ, McAuley E, Courneya KS, Verhulst SJ. Correlates of physical activity self-efficacy among breast cancer survivors. Am J Health Behav. 2008;32(6):594–603. doi: 10.5555/ajhb.2008.32.6.594. [DOI] [PubMed] [Google Scholar]
- Schacter DL. Implicit knowledge- new perspectives on unconscious processes. Proceedings of the National Academy of Sciences. 1992;89:11113–11117. doi: 10.1073/pnas.89.23.11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz KH, Holtzman J, Courneya KS, Masse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiology, Biomarkers & Prevention. 2005;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. [DOI] [PubMed] [Google Scholar]
- Sears SR, Stanton AL. Expectancy -value constructs and expectancy violation as predictors of exercise adherence in previously sedentary women. Health Psychology. 2001;20(5):326–333. [PubMed] [Google Scholar]
- Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, et al. Structured exercise improves physical functioning in women with stage I and II breast cancer: Results of a randomized controlled trial. Journal of Clinical Oncology. 2001;19(3):657–665. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- Segal RJ, Reid RD, Courneya KS, Malone SC, Parliament MB, Scott CG, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. Journal of Clinical Oncology. 2003;21(9):1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- Segar ML, Katch VL, Roth RS, Garcia AW, Portner TI, Glickman SG, et al. The effect of aerobic exercise on self-esteem and depressive and anxiety symptoms among breast cancer survivors. Oncology Nursing Forum. 1998;25(1):107–113. [PubMed] [Google Scholar]
- Shiffman S. Real-time self-report of momentary states in the natural environment: Computerized Ecological Momentary Assessment. In: Stone A, Turkkan JS, Bachrach CA, Jobe JB, Kurtzman HS, Cain VS, editors. The Science of Self-Report: Implications for Research and Practice. Lawrence Erlbaum Associates; Mahwah: 2000. pp. 277–296. [Google Scholar]
- Smyth C. The Pittsburgh Sleep Quality Index (PSQI) Journal of Gerontological Nursing. 1999;25:10–11. doi: 10.3928/0098-9134-19991201-10. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Rink E, Kerry S. Psychosocial predictors of changes in physical activity in overweight sedentary adults following counseling in primary care. Preventive Medicine. 2000;31:183–194. doi: 10.1006/pmed.2000.0688. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS Physical activity questionnaire for older adults: Outcomes for interventions. Medicine & Science in Sports & Exercise. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Stone A, Shiffman S, Atienza AA, Nebeling L. The Science of Real-time Data Capture: Self-Reports in Health Research. Oxford University Press; New York, NY: 2007. [Google Scholar]
- Vallance JK, Courneya KS, Plotnikoff RC, Mackey JR. Analyzing theoretical mechanisms of physical activity behavior change in breast cancer survivors: results from the activity promotion (ACTION) trial. Ann Behav Med. 2008;35(2):150–158. doi: 10.1007/s12160-008-9019-x. [DOI] [PubMed] [Google Scholar]
- Vallance JK, Courneya KS, Plotnikoff RC, Yutaka Y, Mackey JR. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. Journal of Clinical Oncology. 2007;25(17):2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the Medical Outcomes Study. Medical Care. 1995;33(4):AS264–AS279. [PubMed] [Google Scholar]
- Ware JE, Jr., Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. The Health Institute, New England Medical Center; Boston, MA: 1997. [Google Scholar]
- Waters AJ, Sayette M, Wertz J. Carry-over effects can modulate emotional Stroop effects. Cognition and Emotion. 2003;17:501–509. doi: 10.1080/02699930143000716. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Bradley BP, Mogg K. Attentional shifts to smoking cues in smokers. Addiction. 2003;98:1409–1417. doi: 10.1046/j.1360-0443.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychology. 2003;22(378–387) doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West SG, Finch JF, Curran PJ. Structural equations with non-normal variables: Problems and remedies. In: Hoyle R, editor. Structural Equation Modeling: Issues and Applications. Sage; Newbury Park, CA: 1995. pp. 56–75. [Google Scholar]