Abstract
Hematopoietic Stem Cells (HSCs) are used clinically to treat human blood-related genetic diseases and leukemias, but the availability of this therapy is constrained by the limiting number of transplantable HSCs. Previous strategies to expand HSCs in vitro resulted in precocious differentiation and reduced transplant efficiency. In vivo analysis of the mechanisms utilized by fetal and adult niches to control HSCs can be mimicked for therapeutic use. This review summarizes the latest research on the in vivo HSC niche and the clinical applications of this knowledge to promote ex vivo expansion and direct de novo generation of HSCs.
Introduction
The in vivo potential of hematopoietic stem cells (HSCs) is evident by the faithful maintenance of the entire blood system of an organism throughout its lifetime. Clinically, HSC transplantation is a commonly used therapy for the treatment of patients suffering from a variety of hematologic disorders. A major limitation to this therapy is the shortage of immune matched donors and the paucity of cells available from more accessible sources such as cord blood. In vitro expansion has proven elusive as removal of these cells from their in vivo home leads to a decrease in self-renewing and multipotent characteristics. Derivation of functional HSCs from embryonic stem cells (ESCs) has enormous clinical implications, but hematopoietic progenitors obtained from in vitro differentiation display poor self-renewal capacity, and thus are not ideal for therapeutic use [1]. Understanding how the in vivo microenvironments of HSCs direct self-renewal and differentiation will improve these therapies.
Developmental HSC Niche
Throughout ontogeny, HSCs reside in various cellular niches that direct their fate decisions. The first event in HSC specification is the induction of mesoderm to a hematopoietic tissue. The first embryonic or primitive HSCs form in the extraembryonic yolk sac in mammals and chicken [2]. In zebrafish and Xenopus, primitive hematopoiesis begins within the embryo, although the genetic control is highly conserved with that of mammals [3]. The differentiated blood cells produced by primitive HSCs are unique to embryonic development and are absent in adults. The next wave of HSC formation is termed adult or definitive hematopoiesis, as these HSCs will produce all blood cell types in adult animals. In all vertebrates including mammals, chicken, Xenopus, and zebrafish, definitive HSC specification occurs in specific vascular regions within the developing embryo including the aorta-gonad-mesonephros (AGM) and placenta [2, 3]. HSCs in these fetal microenvironments are the first to display self-renewal as well as lymphoid and myeloid differentiation abilities, suggesting it is the signals from these embryonic niches that direct these stem cell characteristics. HSCs then migrate into the fetal liver where they undergo a massive expansion. At birth, the liver no longer serves as a hematopoietic organ, but can become reactivated in certain disease states [4]. In mammals and chickens, HSCs also home to fetal bone where they will reside for the remainder of the life of the animal. In zebrafish, HSCs seed the intrarenal spaces of the kidney, which provides a similar supportive environment for long-term adult hematopoiesis [5]. Within the adult bone marrow, HSCs remain in a relatively dormant state, but are revived by hematopoietic stress, such as massive hemorrhage or infection [6, 7].
Microenvironment for HSC Formation
Recent studies have begun to inspect how the cellular, chemical, and mechanical environment within the vasculature of the developing embryo promotes adult HSC formation. Within the AGM and placenta, HSCs arise from the endothelium within the major vessels including the dorsal aorta, vitelline, and umbilical arteries [2, 8-10]. It has been noted for years that the birth of adult HSCs is coincident with the start of circulation, but the signaling function of blood flow in this process was only recently tested. Studies in the zebrafish and mouse embryo revealed a dependency of HSC formation on the mechanics of blood flow that is mediated in part through nitric oxide (NO) signaling [11, 12].
Derivation of HSCs from ESCs offers great clinical potential, but the existing differentiation protocols rely on genetic manipulation or co-culture with murine stromal cell lines, which are unsafe procedures for deriving cells for clinical use [1]. The use of mechanical shear stress, which mimicked blood flow, or exposure to L-NAME, an activator of NO signaling, was sufficient to induce hematopoietic progenitor formation from ESCs without additional genetic alterations [11]. This represents a leap forward in developing protocols for the successful derivation of transplantable HSCs from human ESCs (hESCs). Additionally, these studies highlight the potential therapeutic importance of understanding how the microenvironment can promote HSC formation.
The placenta has emerged as a newly defined HSC niche during development [13]. Functional HSCs are not found in the placenta until after the onset of circulation, precluding the ability to distinguish if the cells arose through de novo generation or migration from the AGM. To decipher these possibilities, Rhodes et al. evaluated the level of HSCs in the placenta of Ncx knockout mice, which lack a heartbeat and thus lacks circulation [14]. HSCs were found within the placenta in the absence of blood flow, although at a diminished rate, suggesting de novo generation occurs in this tissue. Similarly, AGM HSCs were greatly reduced in the zebrafish mutant, silent heart, which lacks blood flow, indicating a common dependency of HSC specification on circulation [12]. An alternative interpretation of the data is that the HSCs in the placenta migrate from the AGM in a circulation-independent fashion. Studies in zebrafish embryos demonstrated AGM HSCs could travel to the thymus and kidney by migration through the mesenchyme without entry into circulation [15, 16]. Further characterization of the placenta as a site of HSC generation versus a reservoir for pre-existing HSCs need to be performed to distinguish these possibilities.
To identify other factors within the embryonic microenvironment important for HSC induction, gene expression profiling of murine AGM stromal cell lines that support HSCs in vitro was performed, identifying high transcript levels of BMP4 (a TGFβ family member), βNGF (a neurotrophic factor), and MIPγ (a C-C chemokine) [17]. In vitro exposure of AGM explants with any of these three factors increased HSC frequency, indicating they can enhance AGM HSC function. BMP4 is expressed in human and murine AGM [18]. BMP4 treatment of murine ESCs augmented hematopoietic progenitor formation [20, 21]. It was also shown that ex vivo treatment of adult human hematopoietic progenitor cells with BMP4 enhanced transplant efficiency into immune-compromised mice. Together these data indicate the conservation of BMP4 activity in mammalian HSC induction and expansion [19].
Since these murine AGM stromal cell lines could support hematopoiesis, Ledran et al. assayed their ability, as well as a fetal liver derived stromal cell line, to induce HSC production from hESCs [22]. Co-culture of hESCs with these stromal cell lines resulted in an increased frequency of blood cells as well as the precursors to HSCs, the hemangioblasts. Moreover, co-culture enhanced self-renewal capacity of these cells demonstrated by increased serial transplantation efficiency into immune deficient mice, the most rigorous test for human HSC function. Identification of the exact inductive signals supplied by these stromal co-cultures will provide a better paradigm for therapeutic derivation of human HSC.
Microenvironment for HSC Expansion
After the cells migrate from the AGM and placenta, they seed the fetal liver. As the fetal liver is a site of great HSC growth, defining the cell types and signals increasing HSCs within this environment may identify factors for ex vivo HSC expansion. Several fetal liver cell populations have been assessed for their ability to support and expand HSCs ex vivo. Transcriptional profiling of HSC-supportive vs. non-supportive fetal liver cells uncovered a large number of secreted and cell-bound factors differentially expressed [23, 24]. Angiopoietin-like 2 and 3 were discovered as orphan ligands highly expressed in murine fetal liver CD3+ niche cells that could increase HSC frequency up to 20-fold when added to bone marrow cultures [24]. Treatment of human cord blood cells with either Angiopoietin-like 5 or Insulin Growth Factor Binding Protein-2 expanded long term HSCs and maintained their self-renewal capacity [25]. These factors hold promise in expanding human cells for use in HSC transplantation.
Microenvironment for HSC maintenance
The final destination for mammalian adult HSCs is the bone marrow. The bone niche maintains long-term self-renewal and multilineage differentiation potential. Although HSCs can be detected in the liver, spleen, and peripheral blood of adult animals under certain stressful conditions, the bone marrow is unique in its ability to maintain life-long hematopoiesis [4]. During steady state, HSCs are relatively dormant, but can be stimulated following acute injuries, such as radiation, chemotherapy, or severe blood loss. The bone marrow niche regulates the balance between these resting and active states. Rapid recovery after injury is critical not only for patients suffering from hematologic disorders, but also those receiving myeloablative treatments that diminish their hematopoietic output. Thus, greater insight into the mechanisms that the niche employs to regulate HSC quiescence and activation will improve recovery from these therapies.
To define the cells within the developing bone that are sufficient to generate a niche de novo, Chan et al. developed an in vivo assay to assess adult HSC niche formation [26]. Implants of fetal bone-forming cells, or osteoblasts, into the subcapsular region of the kidney, an area normally devoid of resident HSCs, was sufficient to form ectopic marrow cavities containing functional long term HSCs. Endochondral ossification, the production of bone through a cartilage intermediate, was a requisite step for the formation of an ectopic HSC niche. Recruitment of vasculature through VEGF secretion was necessary both for endochondral ossification and HSC niche formation, suggesting a vital connection between bone and vessels in the adult HSC niche. This assay can be utilized to characterize factors that enhance or suppress HSC niche function.
Visualization of the adult HSC niche in vivo has been hampered by many technical limitations, but recent advances in dual-photon confocal microscopy, computer processing speeds, and biologically compatible labeling procedures has finally provided a platform for high resolution real time imaging (Figure 1) [27]. Intravital microscopy of the vasculature within the central sinus and adjacent marrow cavity within the mouse skull revealed a frequent and close juxtaposition of osteoblasts and vasculature within 20um of each other >90% of the time [28]. Engrafted HSCs were also found in close proximity to osteoblasts. These data indicate the osteoblasts and vasculature within the marrow space may represent a single functional niche.
Figure 1. Imaging Hematopoietic Stem Cell Niche.
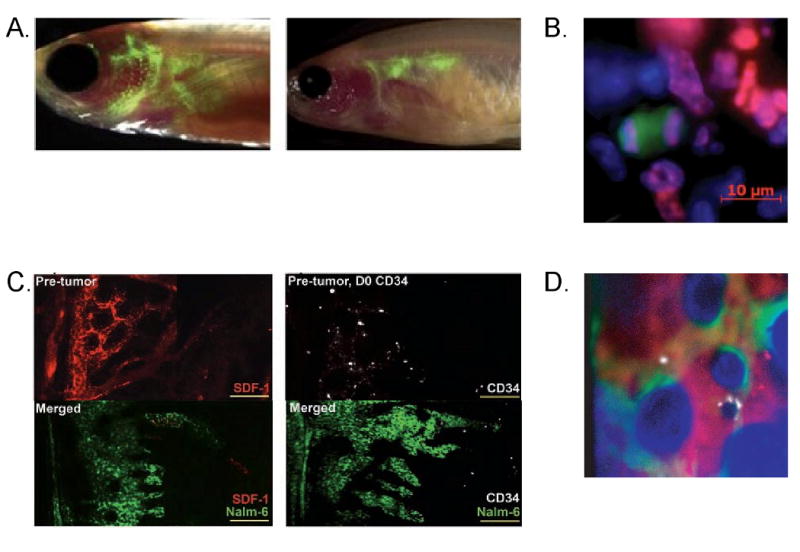
A. Green fluorescent-labeled blood cells in transparent zebrafish at 2 weeks and 4 weeks post-transplant (left and right, respectively). B. Image of dividing cells following transplantation into irradiated mice. Green cell is HSC and purple is marking the dividing nucleus. C. Changes in CXCL12 levels (red) and HSC (white) localization within bone marrow following transplantation with leukemic cells (green). Left shows decreased CXCL12 levels where the leukemic cells are present; right shows the mislocalization of transplanted human HSC. D. Localization of HSC (white), osteoblasts (green), and vasculature (red).
While the calvarium is the most accessible bone marrow niche for in vivo imaging of HSCs, this bone is not a standard source for HSCs in transplantation-based therapies, thus the observations in the calvarium need to be corroborated in more commonly studied bones. In a parallel study utilizing ex vivo imaging of HSCs within mouse femurs, HSCs were observed in similar positions as within the calvarium, demonstrating the similarities of HSC niche in different bones [29]. The localization of HSCs to the endosteum correlated with robust engraftment and full lineage differentiation potential [28]. In aged animals that display diminished hematopoietic output, HSCs were found more distal to the endosteum compared to young HSC [30]. Together, these results indicate a supportive role of the endosteum on in vivo HSC function throughout life.
Although imaging of the HSC niche is in its infancy, the potential to uncover novel regulation is already evident. For the first time, dynamic movements of HSCs within the bone marrow niche were captured showing that even “dormant” HSC are actively dividing and locally motile [28-30]. Imaging of leukemic cells within the HSC niche showed that even before a leukemic clone has overcrowded the HSC niche, the malignant cells alter the cytokine and chemokine milieu changing the response of HSC to mobilizing drugs [31]. These data underscore the possibility to gain novel insight into complex biological systems with imaging approaches that was not possible in the past.
While the in vivo position of HSCs near both osteoblasts and vasculature has been observed, more studies have focused on the endogenous role of osteoblasts as HSC supportive cells [32]. Upon myelosuppressive damages induced by chemotherapy or high dose radiation, not only are blood cells lost, but also the integrity of the marrow sinusoidal vessels [28, 33]. Regeneration of this vascular network is required for the recovery of endogenous blood cells or engraftment of exogenous HSCs, showing the importance of these cells in vivo for hematopoietic regeneration [33]. This study provides evidence that the vasculature within the regenerating bone marrow acts as an endogenous HSC supportive niche.
Zebrafish as in vivo imaging tool for embryonic HSC niche
As the genetic program controlling vertebrate hematopoiesis is highly conserved, zebrafish has emerged as an ideal model for the study of embryonic hematopoiesis due to its high fecundity and external development. Additionally, various lineage tracing procedures and fluorescent transgenic zebrafish lines that label hematopoietic and vascular cell types permit real time visualization of the birth and migration of HSC within the developing embryo. Studying HSC movement is essential as the delivery of these cells to specific sites is critical for their clinical use.
Cells born in the AGM and placenta migrate to and seed the fetal liver, thymus and bone marrow in mammals, and the caudal hematopoietic tissue (CHT), thymus and kidney in zebrafish. There are two hypotheses on the way these cells traffic in the embryo: either via circulation or by crawling through the mesenchyme. The former theory is the favored model as HSCs are born within vessels, and can easily enter circulation. In support of the latter model, HSCs are detected in the mesenchyme below vessels, suggesting these cells extravasate out of the vessel into these sub-vascular spaces [2]. These cells possess transplant ability, but the endogenous capacity of these cells to seed distal hematopoietic organs and contribute to adult hematopoiesis was unknown. Fate tracing and real time imaging in the zebrafish shed light on these theories. AGM HSCs did move to the CHT via circulation, but could travel to the thymus and kidney via circulation or by migration through mesenchyme [15, 16, 34, 35]. These data show both modalities can be utilized in vivo.
These studies highlight the utility of zebrafish to image and model the movement of HSCs within the embryo. Our lab recently developed a transparent adult zebrafish to facilitate imaging of HSC movements within adults (Figure 1) [36]. Homing and engraftment of transplanted fluorescent blood cells into irradiated transparent recipient fish was monitored over time, revealing the migration of hematopoietic progenitors into several hematopoietic tissues including blood vessels, thymus, and ultimately the kidney. This imaging paradigm permits simultaneous visualization of cells throughout the organism, not just a single predetermined spot in the animal. Reducing the time to engraftment in patients following transplantation is critical to decreasing morbidity, thus highlighting the importance of understanding the events following transplantation.
Conclusions
Basic research on developmental and adult niche has increased significantly over the past decade, resulting in several discoveries that have the potential to improve transplantation-based therapies. One example of a successful therapy targeting the HSC-niche interaction is the use of Plexifor, a CXCR4 antagonist, for the mobilization of HSC for use as donor cells for transplantation [37]. Studies in the 1990's uncovered an essential role for the chemokine CXCL12 and its receptor CXCR4 in the migration of HSCs during embryogenesis [38]. Subsequent analyses in adult bone marrow demonstrated CXCL12 is a key factor for retention of HSCs within the adult niche, and that disruption of the CXCL12-CXCR4 interaction results in mobilization of HSCs into the peripheral blood. More recently, two NIH-funded clinical trials are currently underway to determine the ability to ex vivo expand cord blood HSCs using information acquired from the study of in vivo HSC control (Table1). These projects indicate the clinical importance of pursuing basic research on defining the HSC niche throughout development. This knowledge and insight gained from the study of HSC niche both during embryogenesis and in adults will guide the in vitro expansion of human HSC and direct the de novo production of transplantable HSC for clinical use.
Table 1.
NIH-funded Clinical Trials on in vitro cord blood expansion
| Pros | Cons | Clinical Trial Coordinator | Refs | |
|---|---|---|---|---|
| Expansion with 16,16 dimethyl prostaglandin E2 treatment | Treatment with a single quality-assured drug | Variable drug response among cord blood samples | Dana-Farber Cancer Institute PI: Corey Cutler Boston, MA Phase I 2009-201 | [39] |
| Expansion by co-culture with human mesenchymal cells | Exposure to secreted factors and direct contact with supportive stroma | Variable mesenchymal cell preparations | M.D. Anderson Houston, TX Phase I 2007-2010 | [40] |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKinney-Freeman SL, Daley GQ. Towards hematopoietic reconstitution from embryonic stem cells: a sanguine future. Curr Opin Hematol. 2007;14:343–347. doi: 10.1097/MOH.0b013e3281900edd. [DOI] [PubMed] [Google Scholar]
- 2.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertrand JY, Traver D. Hematopoietic cell development in the zebrafish embryo. Curr Opin Hematol. 2009;16:243–248. doi: 10.1097/MOH.0b013e32832c05e4. [DOI] [PubMed] [Google Scholar]
- 4.Lataillade JJ, et al. Does primary myelofibrosis involve a defective stem cell niche? From concept to evidence. Blood. 2008;112:3026–3035. doi: 10.1182/blood-2008-06-158386. [DOI] [PubMed] [Google Scholar]
- 5.Traver D, et al. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- 6.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 7.Wilson A, et al. Dormant and self-renewing hematopoietic stem cells and their niches. Ann N Y Acad Sci. 2007;1106:64–75. doi: 10.1196/annals.1392.021. [DOI] [PubMed] [Google Scholar]
- 8.Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 10.Lancrin C, et al. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adamo L, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009 doi: 10.1038/nature08073. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.North TE, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–3744. doi: 10.1242/dev.02568. [DOI] [PubMed] [Google Scholar]
- 14.Rhodes KE, et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2:252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertrand JY, Kim AD, Teng S, Traver D. CD41+ cmyb+ precursors colonize the zebrafish pronephros by a novel migration route to initiate adult hematopoiesis. Development. 2008;135:1853–1862. doi: 10.1242/dev.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kissa K, et al. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood. 2008;111:1147–1156. doi: 10.1182/blood-2007-07-099499. [DOI] [PubMed] [Google Scholar]
- 17.Durand C, et al. Embryonic stromal clones reveal developmental regulators of definitive hematopoietic stem cells. Proc Natl Acad Sci U S A. 2007;104:20838–20843. doi: 10.1073/pnas.0706923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall CJ, Kinnon C, Thrasher AJ. Polarized expression of bone morphogenetic protein-4 in the human aorta-gonad-mesonephros region. Blood. 2000;96:1591–1593. [PubMed] [Google Scholar]
- 19.Bhatia M, et al. Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J Exp Med. 1999;189:1139–1148. doi: 10.1084/jem.189.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lengerke C, et al. BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell. 2008;2:72–82. doi: 10.1016/j.stem.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 21.Nostro MC, Cheng X, Keller GM, Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2008;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ledran MH, et al. Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell. 2008;3:85–98. doi: 10.1016/j.stem.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Hackney JA, et al. A molecular profile of a hematopoietic stem cell niche. Proc Natl Acad Sci U S A. 2002;99:13061–13066. doi: 10.1073/pnas.192124499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang CC, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang CC, Kaba M, Iizuka S, Huynh H, Lodish HF. Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood. 2008;111:3415–3423. doi: 10.1182/blood-2007-11-122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan CK, et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature. 2009;457:490–494. doi: 10.1038/nature07547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeder T. Imaging stem-cell-driven regeneration in mammals. Nature. 2008;453:345–351. doi: 10.1038/nature07043. [DOI] [PubMed] [Google Scholar]
- 28.Lo Celso C, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie Y, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457:97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 30.Kohler A, et al. Altered cellular dynamics and endosteal location of aged early hematopoietic progenitor cells revealed by time-lapse intravital imaging in long bones. Blood. 2009 doi: 10.1182/blood-2008-12-195644. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colmone A, et al. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322:1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 32.Raaijmakers MH, Scadden DT. Evolving concepts on the microenvironmental niche for hematopoietic stem cells. Curr Opin Hematol. 2008;15:301–306. doi: 10.1097/MOH.0b013e328303e14c. [DOI] [PubMed] [Google Scholar]
- 33.Hooper AT, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4:263–274. doi: 10.1016/j.stem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin H, Xu J, Wen Z. Migratory path of definitive hematopoietic stem/progenitor cells during zebrafish development. Blood. 2007;109:5208–5214. doi: 10.1182/blood-2007-01-069005. [DOI] [PubMed] [Google Scholar]
- 35.Murayama E, et al. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25:963–975. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 36.White RM, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–189. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiPersio JF, Uy GL, Yasothan U, Kirkpatrick P. Plerixafor. Nat Rev Drug Discov. 2009;8:105–106. doi: 10.1038/nrd2819. [DOI] [PubMed] [Google Scholar]
- 38.Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol. 2008;15:49–58. doi: 10.1097/MOH.0b013e3282f29012. [DOI] [PubMed] [Google Scholar]
- 39.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson SN, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37:359–366. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]


