Abstract
In humans, haploinsufficiency of either SOX2 or PAX6 is associated with microphthalmia, anophthalmia or aniridia. In this study, through the genetic spatiotemporal specific ablation of SOX2 on both wild-type and Pax6-haploinsufficent backgrounds in the mouse, we have uncovered a transcriptionally distinct and developmentally transient stage of eye development. We show that genetic ablation of SOX2 in the optic cup results in complete loss of neural competence and eventual cell fate conversion to non-neurogenic ciliary epithelium. This cell fate conversion is associated with a striking increase in PAX6, and genetically ablating SOX2 on a Pax6-haploinsufficient background partially rescues the Sox2-mutant phenotype. Collectively, these results demonstrate that precise regulation of the ratio of SOX2 to PAX6 is necessary to ensure accurate progenitor cell specification, and place SOX2 as a decisive factor of neural competence in the retina.
Keywords: Multipotential retinal progenitors, Ciliary epithelium, SOX2, PAX6, Gene dosage, Mouse
INTRODUCTION
The vertebrate eye, which is composed of neurogenic and non-neurogenic structures, arises from a single progenitor pool in the optic vesicle. The eye, therefore, provides a useful and accessible model for studying potential interactions of signaling pathways in the specification of distinct cell fates. At around embryonic day (E) 10.5 of mouse development, the optic vesicle receives signals from the surface ectoderm telling it to invaginate, forming the bilayered optic cup. The inner layer of the optic cup gives rise to the neural retina (NR) and the outer layer becomes the retinal pigment epithelium (RPE). The interface of these two domains is the peripheral optic cup margin, which gives rise to the non-pigmented ciliary body epithelium (CE) and the inner iris epithelium. Progenitor cells at the boundary between prospective NR and CE make a binary cell fate decision to become either neurogenic (NR) or non-neurogenic (CE) (Beebe, 1986). The molecular and cellular mechanisms regulating this cell fate decision are poorly understood, in part because cells fated to become CE exhibit pervasive expression of the retinal progenitor transcription factors Rax, Chx10 (Vsx2 – Mouse Genome Informatics) and Pax6 (Cho and Cepko, 2006; Fuhrmann et al., 2000; Furukawa et al., 1997; Hodgkinson et al., 1993; Kubo and Nakagawa, 2008; Liu et al., 2006; Liu et al., 2003; Pittack et al., 1997; Rowan and Cepko, 2004).
A number of cell-extrinsic signaling pathways have been implicated in CE fate specification. One report showed that CE markers are found at the edges of tissue that ectopically expresses fibroblast growth factor (FGF), suggesting that the ciliary body is specified in the optic vesicle where bone morphogenetic protein (BMP) and FGF signals overlap (Dias da Silva et al., 2007). In addition, studies in multiple species have shown canonical Wnt signaling to be a potent regulator of peripheral eye structures (Cho and Cepko, 2006; Liu, H. et al., 2007; Tomlinson, 2003). A role for Wnt signaling in specifying CE fate in the mouse comes from the observation that constitutive activation of β-catenin in optic cup progenitor cells results in ectopic expression of CE-specific genes at the expense of NR-specific genes (Liu, H. et al., 2007). However, these ectopic CE-like cells fail to express Pax6 and Chx10, both of which are normally maintained in the prospective CE of control eyes at early stages. In the adult, Pax6 is maintained in the CE of the iris and ciliary body.
The reduction of Pax6 expression upon activated Wnt signaling is surprising given that PAX6 is a positive regulator of peripheral eyecup development (Davis-Silberman et al., 2005). A member of the paired-box and homeobox-containing family of transcription factors, PAX6 has been shown to be required for iris specification, optic cup morphogenesis, lens formation and retinal neuronal differentiation (Baumer et al., 2002; Davis-Silberman and Ashery-Padan, 2008; Davis-Silberman et al., 2005; Grindley et al., 1997; Marquardt et al., 2001; Philips et al., 2005; Smith et al., 2009; Xu et al., 1999). These developmental processes require a critical threshold of PAX6 as demonstrated by the fact that heterozygous carriers of PAX6 deletions (Davis-Silberman et al., 2005; Hill et al., 1991; Hogan et al., 1986; Ton et al., 1991) and transgenic mice with increased levels of PAX6 (Ericson et al., 1997; Schedl et al., 1996) display eye abnormalities (Favor et al., 2001; Hack et al., 2004; Heins et al., 2002; Kim and Lauderdale, 2008; Manuel et al., 2007). Humans with mutations in PAX6 exhibit aniridia (no iris) and often have smaller ciliary bodies (reviewed by Hanson and Van Heyningen, 1995; Hayashi et al., 2004; Okamoto et al., 2004; Prosser and van Heyningen, 1998). Mice that are haploinsufficient for Pax6 exhibit reduced size of the optic cup margin, implicating a shift in the boundary between NR and CE (Davis-Silberman et al., 2005).
Here, we test the hypothesis that there is an antagonistic relationship between transcription factors that are restricted to the prospective NR and those that, like PAX6, span the boundary between prospective NR and CE. One of these potential regulators of NR specification is the high mobility group (HMG)-containing transcription factor SOX2. Conditional deletion of Sox2 in the developing mouse retina results in the loss of competence to undergo neuronal differentiation, and mice that are hypomorphic for Sox2 exhibit reduced eye size (Taranova et al., 2006). Moreover, ∼10% of human individuals with anophthalmia (lack of eye) or severe microphthalmia (small eye) carry a SOX2 mutation (Fantes et al., 2003; Hagstrom et al., 2005; Hanson and Van Heyningen, 1995; Ragge et al., 2005a; Ragge et al., 2005b; Zenteno et al., 2005; Zenteno et al., 2006) (for a review, see Hever et al., 2006).
Although both SOX2 and PAX6 have been shown to be essential for the maintenance of multipotent retinal progenitor cells (RPCs) (Marquardt et al., 2001; Taranova et al., 2006; Xu et al., 1999) and studies in mouse illustrate that changes in SOX2 and PAX6 dosage result in developmental defects of the eye, no study has yet addressed their epistatic relationship in the developing optic cup. To examine the relationship between Sox2 and Pax6 in the optic cup, we performed genetic analysis in the mouse and uncovered a mechanism through which the eyecup is regionalized into NR and CE. We show that SOX2 and PAX6 are expressed in an inverse gradient in the developing optic cup and find that ablation of SOX2 in multipotent optic cup progenitor cells biases them towards a non-neurogenic CE fate. The immediate molecular readout of this cell fate conversion is the upregulation of PAX6. Accordingly, the deletion of Sox2 on a Pax6-haploinsufficient background (Pax6Sey/+) significantly rescues the Sox2-mutant phenotype. Therefore, in the absence of SOX2, multipotent RPCs cannot maintain neuronal differentiation capacity (i.e. NR identity) and undergo cell fate conversion to CE. These results identify SOX2 as a crucial factor defining multipotent neural retinal progenitor identity (Taranova et al., 2006) and suggest a model of dosage-dependent transcriptional regulation of cell fate in the optic cup.
MATERIALS AND METHODS
Mouse breeding
Sox2cond/+ mice (Taranova et al., 2006) were crossbred to αP0CREiresGFP (Dr P. Gruss, Max-Planck-Institute of Biophysical Chemistry, Germany) (Marquardt et al., 2001) or Chx10CreGFP (Jackson Laboratories, Bar Harbor, ME, USA) (Rowan and Cepko, 2004) to generate Sox2cond/+; αP0CREiresGFP and Sox2cond/+; Chx10CreGFP mouse lines. These lines were then backcrossed to the Sox2cond line to generate homozygous mutant genotypes. Lineage tracing was carried out using Rosa26Reporter (R26R) mice (Jackson Laboratories) (Soriano et al., 1987). Pax6Sey/+ mice (Dr A. LaMantia, The George Washington University, DC, USA) (Hill et al., 1991) were bred to Sox2cond/+; αP0CREiresGFP mice to obtain Sox2cond/+; αP0CREiresGFP; Pax6Sey/+ mice and then backcrossed to Sox2cond/+ mice to yield the Sox2cond/cond; Pax6Sey/+; αP0CREiresGFPdouble mutant. β-cateninactivated mice (Dr R. Wechsler-Reya, Duke University, Durham, NC, USA) (Harada et al., 1999) were crossed with the αP0CREiresGFP to obtain the constitutively activated genotype β-cateninactivated; αP0CREiresGFP. Primer sequences for all of the alleles mentioned can be found in Table S1 in the supplementary material. It was necessary to genotype for the Sox2Δcond allele to eliminate animals in which germline recombination occurred. All animal work was carried out in accordance with University of North Carolina at Chapel Hill IACUC and DLAM approval.
Tissue preparation, immunohistochemistry and in situ hybridization
Mouse embryos were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS). Tissue was immersed sequentially in 10%, 20% and 30% sucrose in DEPC PBS overnight and then embedded and frozen in OCT (optimal cutting temperature) medium (Tissue-Tek). Horizontal cryostat sections (14 μm) were blocked in 1% goat serum and 0.1% Triton X-100 in PBS and incubated with primary antibodies at 4°C overnight and secondary antibodies for one hour at room temperature. The following antibodies were used in this study: rabbit anti-cleaved caspase 3 (1:300, Cell Signaling), chicken anti-GFP (1:2000, Abcam), rabbit anti-Ki67 (1:1000, Abcam), mouse anti-PAX6 (1:100, Hybridoma), rabbit anti-SOX2 (1:3000, Millipore), mouse anti-β-tubulin III (1:1000, Covance), Alexa Fluor 488-conjugated anti-chicken (1:2000, Molecular Probes), Alexa Fluor 546-conjugated anti-mouse (1:2000, Molecular Probes) and Alexa Fluor 546-conjugated anti-rabbit (1:2000, Molecular Probes). In situ hybridization was performed on cryostat sections (20 μm) using digoxygenin (DIG)-labeled antisense probes followed by enzymatic detection according to manufacturer's protocols (Roche). The following in situ probes were employed in this study: Axin2 [Dr F. Costantini (Jho et al., 2002)], Bmp4 [Dr A. LaMantia (Bhasin et al., 2003], Bmp7 [Dr B. Hogan (Lyons et al., 1995)], Chx10 [Dr R. McInnes (Horsford et al., 2005)], Hes5 [Dr E. Anton (Chenn and Walsh, 2002)], Lef1 [Dr R. Grosschedl (Galceran et al., 1999)], Msx1 [Dr Y. Liu (Liu et al., 2006)], NeuroD1 [Dr J. Lee (Lee et al., 1995)], Notch1 [Dr U. Lendahl (Lardelli and Lendahl, 1993)], Rax [Dr C. Cepko (Furukawa et al., 1997)], Sfrp2 [Dr J. Nathans (Rattner et al., 1997)], Otx1 [Dr J. P. Martinez (Simeone et al., 1992)], Pax6 [Dr A. LaMantia (Anchan et al., 1997)] and Zic1 [Dr K. Millen (Aruga et al., 1994)]. For β-galactosidase (β-gal) staining, slides were washed with PBS and immersed in β-gal staining solution [final concentrations: 5 mM K3Fe(CN)6 (Sigma), 5 mM K4Fe(CN)6.3H2O (Sigma), 2 mM MgCl2 (Mallinckrodt), 0.02% Igepal CA-630 (Sigma), 0.01% sodium-deoxycholate (Sigma) and X-gal (1:50, Promega)] overnight at 37°C. Fluorescent and light microscopy images were taken on a Leica inverted microscope (Leica DMIRB) using a Q-imaging Retiga-4000RV digital CCD camera (Vashaw Scientific, Raleigh, NC, USA).
BrdU labeling
Pregnant mothers were weighed and injected with 6 μl/g of 15 mg/ml BrdU (Sigma B5002) in PBS 2 hours before dissection. Embryos were sectioned and prepared for immunohistochemistry as stated above and stained overnight with mouse anti-BrdU (1:500, BD Biosciences).
PAX6 immunofluorescence intensity analysis
PAX6 immunofluorescence intensity was measured in tissue sections taken from the central-most portion of E14.5 eyes. To exclude intensity variations caused by the appearance of different cell sections (and correspondingly, different volumes) at the focus of the objective, optical sectioning was performed to generate the maximum image projection of the resulting slices. To capture the whole cell volume, initial optical sectioning was conducted to determine the objective position at which the fluorescence intensity in the selected region of interest (ROI) would be maximal. The ROI (at least 20 cells per ROI) was selected at similar locations in each sample. The maximum intensity plane served as a reference point. For the final imaging for intensity calculations, a stack of 13 μm (6.5 μm above and below the reference point) was collected. Twenty cells from each region of the eyecup (the lens epithelium, prospective CE, retinal ganglion cells, SOX2-positive neural progenitors and SOX2-ablated neural progenitors) were selected from the final 8-bit image for fluorescence intensity calculations and the intensity was plotted using the Olympus Fluoview 2.1c. All intensity measurements were conducted on an Olympus Fluoview FV1000 confocal microscope with a 40× NA0.6 objective (Olympus Corp.). Statistics calculations were performed using Excel software (Microsoft, Redmond, WA, USA).
RESULTS
The central NR and the peripheral optic cup margin are defined by an inverse gradient of SOX2 and PAX6
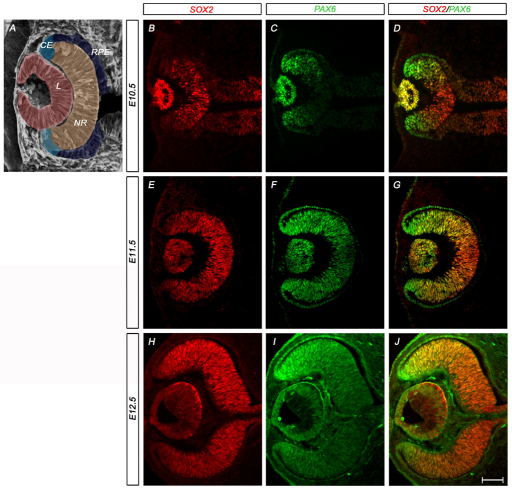
To establish the functional interaction between SOX2 and PAX6 in the specification of optic cup progenitor cells, we first compared their expression patterns using immunohistochemistry. SOX2 and PAX6 are co-expressed in the anterior neural plate and throughout the optic vesicle prior to optic cup formation (Rex et al., 1997; Uchikawa et al., 2003; Uwanogho et al., 1995). However, in the early optic cup (E10.5), SOX2 and PAX6 began to exhibit inverse expression gradients. Whereas SOX2 was highly expressed in the prospective NR (Fig. 1A,B) in a graded centralhigh-to-distallow pattern, PAX6 was highly expressed in prospective CE and RPE (Fig. 1A,C) in a gradient from centrallow-to-distalhigh (Baumer et al., 2002; Grindley et al., 1997; Kamachi et al., 2001; Walther et al., 1991). These inverse expression patterns were maintained throughout early optic cup development (Fig. 1D,G,J). Consequently, in the adult, SOX2 appeared to be excluded from the non-neural CE and RPE (see Fig. S1A,C in the supplementary material), whereas PAX6 was maintained in the CE (see Fig. S1B,C in the supplementary material).
Fig. 1.
The neural retina and optic cup margin are defined by an inverse gradient of SOX2 and PAX6. (A) Schematic of an E12.5 eye indicating the boundaries of the mouse neural retina (NR), lens (L), prospective ciliary body epithelium (CE) and retinal pigment epithelium (RPE). (B-D) Immunohistochemistry on horizontal sections of wild-type embryos shows high SOX2 expression (red) in the central optic cup and high PAX6 expression (green) in the peripheral optic cup margin. (E-J) As the eye develops, the inverse gradients of SOX2 and PAX6 expression are maintained. Scale bar: 100 μm.
To determine whether the SOX2-PAX6 gradient is concurrent with the early divergence of cell fate into central prospective NR and peripheral prospective CE, we examined a repertoire of established optic cup markers at E13.5, before the NR and CE become morphologically distinguishable. We found that in the prospective NR, SOX2 and PAX6 (Fig. 2A,B) were co-expressed with the NR-specific genes Notch1 (Lardelli and Lendahl, 1993), Hes5 (Chenn and Walsh, 2002) and NeuroD1 (Neurod1 – Mouse Genome Informatics) (Lee et al., 1995) (Fig. 2D,E,F) and with the multipotent optic cup progenitor genes Rax and Chx10 (Fig. 2G,H). By contrast, SOX2 was downregulated in the prospective CE (Fig. 2A,C, box), where Msx1 (Liu et al., 2006), Otx1 (Simeone et al., 1992), Bmp4 (Zhao et al., 2002) (Fig. 2J,K,L) and Zic1 (data not shown) (Trimarchi et al., 2009) were preferentially expressed. PAX6 was highly maintained in the prospective CE (Fig. 2B,I,box).
Fig. 2.
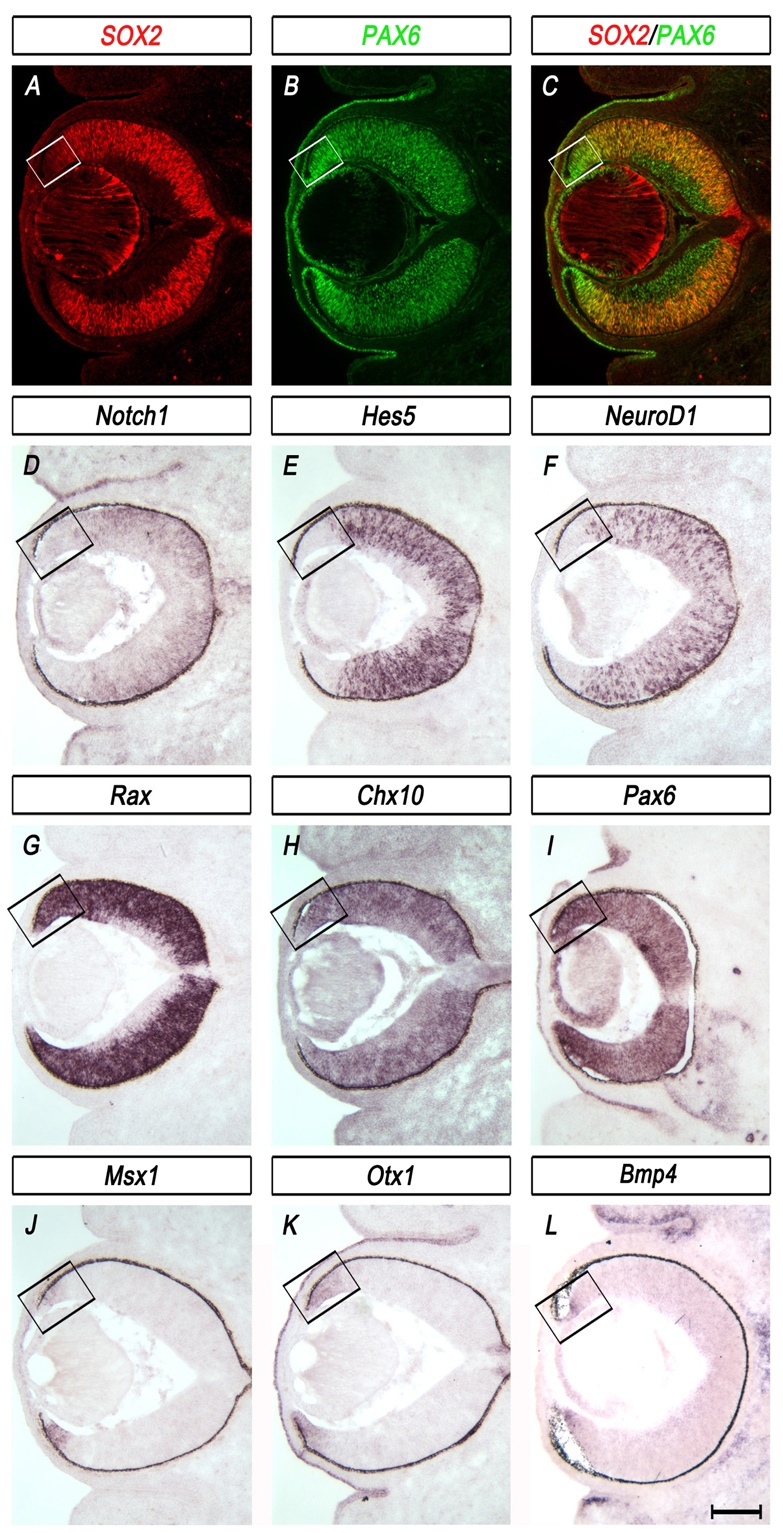
Expression profiles of the central neural retina and peripheral optic cup margin at E13.5 reflect the inverse expression patterns of SOX2 and PAX6. (A-C) Immunohistochemistry for SOX2 (red) and PAX6 (green) on horizontal sections of wild-type mouse eye illustrates inverse gradients of expression, with SOX2 highly expressed in the central neural retina (NR) and PAX6 highly expressed in the peripheral optic cup margin. (D-F) In situ hybridization (ISH) of NR-specific genes shows that Notch1 (D), Hes5 (E) and NeuroD1 (F) are co-expressed with SOX2 in the central NR. (G-I) ISH of the optic cup progenitor transcription factors Rax (G), Chx10 (H) and Pax6 (I) shows expression in both the prospective NR and CE. (J-L) ISH of Msx1 (J), Otx1 (K) and Bmp4 (L) shows expression in the optic cup margin. Boxes delineate the optic cup margin. Scale bar: 200 μm.
Ablation of SOX2 results in neurogenic to non-neurogenic cell fate conversion
We previously generated mice carrying a conditional floxed allele of Sox2 (Sox2cond/+) (Taranova et al., 2006). To conditionally ablate SOX2 from multipotent cells throughout the peripheral optic cup, we used αP0CREiresGFP transgenic mice, in which CRE expression is driven by the Pax6 retina-specific enhancer α and minimal promoter P0 beginning at E10.0 (Kammandel et al., 1999; Marquardt et al., 2001). This mouse line can be used as a transgenic reporter of α enhancer-driven Pax6 expression in the optic cup (Baumer et al., 2002). We previously crossed Sox2cond/+ mice with Sox2cond/+; αP0CREiresGFP mice and showed that ablation of SOX2 results in severe ocular deformities, including extremely reduced eye size (Taranova et al., 2006). Here, we determine the fate of Sox2-mutant optic cup progenitor cells by comparing gene expression, proliferation and cell death in mutant (Sox2cond/cond; αP0CREiresGFP) eyes with that of control (Sox2cond/+; αP0CREiresGFP) eyes.
We first examined Sox2 and Pax6 expression at E16.5, when the prospective NR and CE initially become morphologically distinguishable. In control eyes, the inverse SOX2-PAX6 gradient was maintained throughout the optic cup, with PAX6 expression (Fig. 3B) highest in the distal tips and SOX2 expression (Fig. 3C) highest in the central optic cup. By contrast, SOX2-ablated cells (Fig. 3I) throughout the prospective NR exhibited an increase in αP0CREiresGFP reporter (Fig. 3G), PAX6 protein (Fig. 3H) and Pax6 mRNA expression (Fig. 3U). To confirm this increase in PAX6 protein, we quantified PAX6 immunofluorescence intensity in different regions of the eyecup of control and mutant embryos (see Fig. S2 in the supplementary material). PAX6 was significantly upregulated exclusively in CRE-positive, SOX2-ablated progenitor cells of the central optic cup when compared with wild-type SOX2-positive central progenitor cells (P<0.0001).
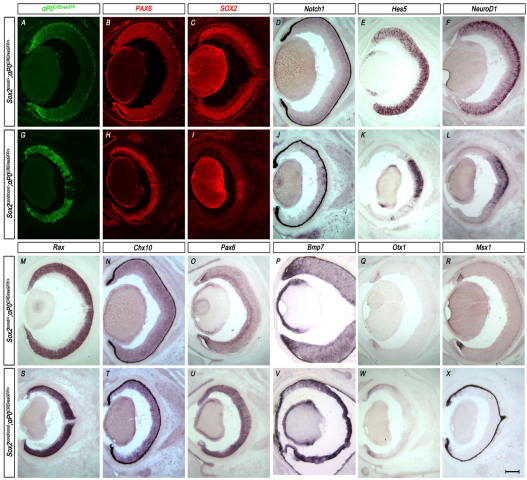
Fig. 3.
Ablation of SOX2 in peripheral optic cup progenitor cells results in loss of neural characteristics, maintenance of optic cup progenitor transcription factors and central expansion of the optic cup margin. (A-X) Horizontal sections through the eyes of E16.5 mouse embryos. (A,G) αP0CREiresGFP reporter expression in control (Sox2cond/+; αP0CREiresGFP) and mutant (Sox2cond/cond; αP0CREiresGFP) eyes as indicated by CRE-GFP expression (green). αP0CREiresGFP is centrally expanded in mutant embryos (G) compared with controls (A). (B,C,H,I) Immunohistochemistry of SOX2 and PAX6 indicates upregulation of PAX6 (H) throughout the central eyecup in which SOX2 has been ablated (I) when compared with PAX6 expression (B) in SOX2-positive cells (C) of wild-type controls. (D-F,J-L) In situ hybridization (ISH) of the NR-specific genes Notch1 (N,J), Hes5 (E,K) and NeuroD1 (F,L) indicates loss of expression of members of the Notch1 signaling pathway, Notch1 (J), Hes5 (K) and NeuroD1 (L), in regions where SOX2 has been ablated (J-L) when compared with wild-type controls (D-F). (M-O,S-U) ISH of Rax (M,S), Chx10 (N,T) and Pax6 (O,U) shows no change in expression of Rax (S) or Chx10 (T) but increased expression of Pax6 (U) in SOX2-ablated eyes (S-U) when compared with wild-type controls (M-O). (P,Q,V,W) ISH shows the expansion of the CE markers Bmp7 (P,V) and Otx1 (Q,W) into the prospective NR of Sox2-mutant eyes. (R,X) ISH of the CE marker Msx1 shows little change in expression between the control (R) and mutant eye (X). Scale bar: 200 μm.
To determine the identity of these Sox2-mutant cells, we examined the expression of NR-specific genes. Sox2-mutant cells failed to express the NR markers Notch1 (Fig. 3D,J) and Hes5 (Fig. 3E,K) and markers of postmitotic neurons, including NeuroD1 (Fig. 3F,L) and β-tubulin III (Tubb3 – Mouse Genome Informatics) (data not shown).
We next examined whether this loss of neural characteristics is specific to the deletion of Sox2 or is associated with decreased expression of other optic cup progenitor transcription factors, Rax and Chx10. In contrast to the upregulation of Pax6 upon SOX2 ablation (Fig. 3H,U) Rax (Fig. 3M,S) and Chx10 (Fig. 3N,T) remained unchanged between controls and mutants. Given that Rax and Chx10 are expressed in CE progenitors, we hypothesized that Sox2-mutant cells might gain CE characteristics, so we examined the expression of established CE markers. Our data indicate that some Sox2-mutant cells ectopically express a subset of genes normally restricted to the prospective CE, including Bmp7 (Fig. 3P,V) and Raldh2 (Aldh1a2 – Mouse Genome Informatics; data not shown). Otx1 (Fig. 3Q,W), which is normally restricted to the distal tips of the optic cup, exhibited slight central expansion in Sox2-mutant eyes. However, most Sox2-mutant cells failed to express other prospective CE genes, including Msx1 (Fig. 3R,X) and Mitf (data not shown) at E16.5.
The Wnt/β-catenin signaling pathway has been implicated in the specification of CE fate (Cho and Cepko, 2006; Liu, F. et al., 2007; Liu et al., 2003). We examined components of this pathway to test the hypothesis that the Wnt signaling domain is expanded upon Sox2 deletion. Axin2, an endogenous readout of Wnt activity (Fuhrmann et al., 2009; Jho et al., 2002), was increased in Sox2-mutant cells compared with wild-type controls (see Fig. S3F,M in the supplementary material), but Lef1 expression appeared to be unchanged (see Fig. S3E,L in the supplementary material). Sfrp2, a Wnt signaling antagonist expressed in NR progenitors (Liu et al., 2003), was centrally shifted (see Fig. S3D,K in the supplementary material). Taken together, these data suggest that the active Wnt signaling domain is centrally expanded upon SOX2 ablation.
Forced expression of a stabilized form of β-catenin in optic cup progenitors has been used previously to model the role of Wnt signaling in CE induction (Liu, H. et al., 2007). We therefore compared expression of prospective NR and CE genes between Sox2-mutant embryos and embryos with constitutive activation of Wnt signaling. Consistent with the previous results of Liu et al. (Liu, H. et al., 2007), regions with stabilized β-catenin (βcateninactivated; αP0CREiresGFP) exhibited upregulation of Lef1 and Axin2 (see Fig. S3S,T in the supplementary material) and failed to express the NR markers Sox2, Hes5 and Sfrp2 (see Fig. S3O-R in the supplementary material). However, in contrast to Sox2-mutant eyes, which exhibited central expansion of CRE and increased Pax6 expression, regions that constitutively express β-catenin did not show expansion of αP0CREiresGFP and did not express Pax6 (see Fig. S3I,N,P,U in the supplementary material). Thus, the loss of SOX2 parallels activation of β-catenin at E16.5 in the expansion of the Axin2-positive domain and the loss of NR characteristics.
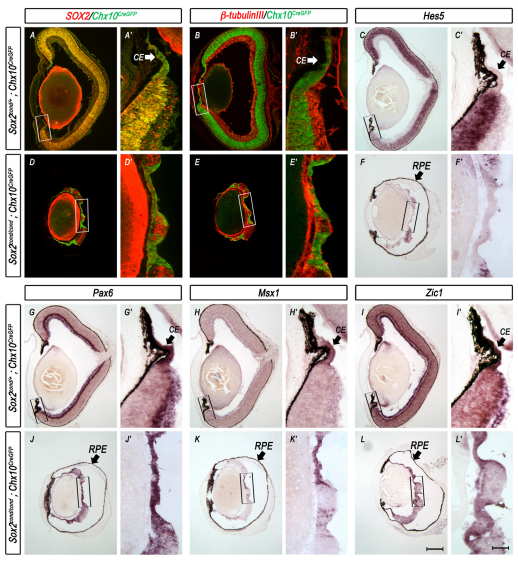
Based on the central expansion of some genes known to be involved in specifying CE fate, we hypothesized that the loss of SOX2 induces a transient liminal or `in-between' state in which a maturation period is required for cells to fully adopt CE identity. To test this hypothesis, we examined the expression of NR- and CE-specific genes in control and Sox2-mutant eyes at postnatal day (P) 0. In control eyes, the NR exhibited laminar morphology and αP0CREiresGFP expression in the inner nuclear layer and retinal ganglion cell layer (Fig. 4A,A′). The CE contained a single layer of cuboidal cells with high αP0CREiresGFP expression (Fig. 4A, box and 4A′, arrow). The NR-specific gene Hes5 was expressed throughout the laminar NR, marking an abrupt boundary between the neurogenic retina and non-neurogenic CE (Fig. 4C,C′). The neurogenic and non-neurogenic regions were also distinguishable by the edge of β-tubulin III expression (Fig. 4B,B′). In stark contrast to control eyes, Sox2-mutant eyes showed expanded αP0CREiresGFP expression throughout the central eyecup (Fig. 4D,D′). Similar to what was observed in the eyes of mutant embryos, postnatal Sox2-mutant cells failed to express genes specific to the NR, including Hes5 (Fig. 4C,C′,F,F′), and lost neuronal differentiation capacity as demonstrated by the mutual exclusivity of β-tubulin III and αP0CREiresGFP expression (Fig. 4B,B′,E,E′). In addition to the upregulation of αP0CREiresGFP, Sox2-mutant cells gained expression of genes that were preferentially expressed in the CE at P0, including Pax6, Msx1 and Zic1 (Fig. 4G-I′). These ectopic CE-like regions exhibited the thin single-layered morphology characteristic of wild-type CE. By contrast, the SOX2-positive NR regions, which presumably developed from cells that did not undergo CRE-mediated recombination, exhibited proper thickness and laminar morphology when compared with the NR of control eyes. These results suggest that Sox2-mutant progenitors autonomously undergo cell fate conversion from neurogenic retina to non-neurogenic CE.
Fig. 4.
Ablation of SOX2 by αP0CREiresGFP results in cell fate conversion of the neurogenic retina to non-neurogenic CE. (A-L′) Low (A-L) and high magnification (A′-L′) images of horizontal sections through the eyes of P0 control (Sox2cond/+; αP0CREiresGFP) and mutant (Sox2cond/cond; αP0CREiresGFP) mouse pups. Boxes indicate the magnified regions. αP0CREiresGFP expression is in the distal tips as well as in the inner nuclear layer and retinal ganglion cell layer of control eyes (A,A′). By contrast, αP0CREiresGFP expression expands throughout the prospective NR of Sox2-mutant eyes (D,D′). β-tubulin III antibody staining (B,B′,E,E′) and in situ hybridization (ISH) for Hes5 (C,C′,F,F′) are present only throughout the SOX2-positive neural retina (NR) of mutant eyes and not in SOX2-ablated regions when compared with controls. ISH of CE-specific genes, including Pax6 (G,G′,J,J′), Msx1 (H,H′,K,K′) and Zic1 (I,I′,L,L′), indicates upregulation in mutant eyes when compared with controls. CE, ciliary epithelium; RPE, retinal pigment epithelium. Scale bars: in L, 400 μm for A-L; in L′, 100 μm for A′-L′.
Previous studies have shown that the development of the CE monolayer results from a decrease in cell division. Indeed, the optic cup margin exhibits a lower proliferation rate than does the prospective NR (Beebe, 1986; Cho and Cepko, 2006; Kubota et al., 2004). Examination of proliferation markers revealed that many SOX2-ablated cells, particularly in the peripheral region of the eyecup, did not incorporate BrdU (see Fig. S4B,F in the supplementary material) or express Ki67 (see Fig. S4C,G in the supplementary material). However, many SOX2-ablated cells in the central eyecup did. These results suggest that upon the deletion of Sox2 by αP0CREiresGFP, there is decrease in the number of proliferating cells, particularly throughout the peripheral eyecup. By contrast, there was no significant change in apoptosis as indicated by cleaved caspase-3 expression (see Fig. S4D,H in the supplementary material).
Optic cup progenitor transcription factors PAX6, CHX10 and RAX are not sufficient to maintain neuronal differentiation capacity in the absence of SOX2
The previous data suggest that ablation of SOX2 in multipotent peripheral progenitor cells that can give rise to both NR and CE results in their eventual restriction to CE fate. To test further the hypothesis that cells that are specified to become NR will convert to CE upon loss of SOX2, we used the Chx10CreGFP mouse line to ablate SOX2 in a mosaic pattern of progenitor cells throughout the whole optic cup beginning at E11.0. Thus, SOX2 was removed from alternating patches of cells that had been specified to become NR, and neighboring wild-type cells could serve as internal controls (Fig. 5B,C,H-I′) (Donovan and Dyer, 2004; Jadhav et al., 2006; Oron-Karni et al., 2008; Rowan et al., 2004; Zhang et al., 2004). In Sox2cond/cond; Chx10CreGFP mutant eyes, SOX2 was specifically ablated in CRE-expressing cells marked by GFP (Fig. 5B,G-H′). As a consequence of SOX2 loss, mutant cells did not undergo neuronal differentiation, as shown by the mosaic expression of β-tubulin III (Fig. 5C,I,I′) and NeuroD1 (Fig. 5F,L) in a pattern mutually exclusive of Chx10CreGFP expression (Fig. 5I, arrowhead). Moreover, SOX2-ablated cells failed to express the NR-specific genes Notch1, Hes5 and Sfrp2 (Fig. 5D,E,J,K,M,S).
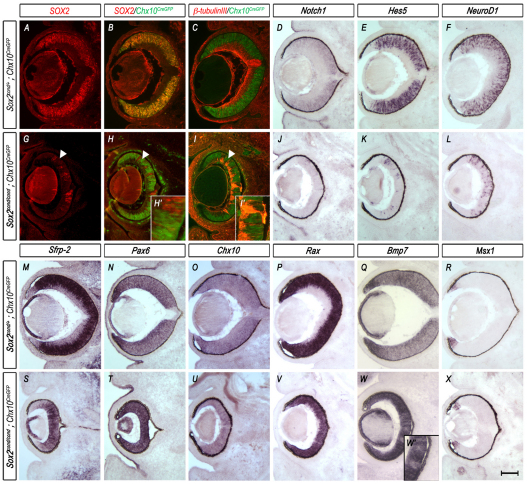
Fig. 5.
Mosaic ablation of SOX2 in neural progenitor cells using Chx10CreGFP results in loss of neuronal differentiation, maintenance of optic cup progenitor genes and central expansion of the optic cup margin. (A-X) Horizontal sections through the eyes of E14.5 control (Sox2cond/+; Chx10CreGFP) and mutant (Sox2cond/cond; Chx10CreGFP) mouse embryos. (A-C,G-I′) Chx10CreGFP reporter (B,C,H-I′; green) double labeled with SOX2 (A,B,G-H′; red) or β-tubulin III (C,I,I′; red) shows expression throughout the whole optic cup in control eyes. In mutant eyes, CRE-GFP is mutually exclusive of SOX2 (H) and β-tubulin III (I). Arrowheads indicate regions in which SOX2 has been ablated. (D-F,J-X) In situ hybridization (ISH) of Notch1 (D,J), Hes5 (E,K), NeuroD1 (F,L), Sfrp2 (M,S) and the optic cup progenitor genes Pax6 (N,T), Chx10 (O,U) and Rax (P,V) shows loss of expression of members of the Notch1 signaling pathway (J-L), central restriction of the Wnt antagonist Sfrp2 (S), upregulation of Pax6 (T) and maintenance of the progenitor markers Chx10 (U) and Rax (V) in regions where SOX2 has been ablated (G) when compared with wild-type controls (D-F,M-P). ISH of the CE marker Bmp7 (Q,W,W′) shows expansion into the prospective NR of mutant embryos, whereas Msx1 (R,X) shows little difference in expression between the control and mutant eye. Scale bar: 200 μm.
To determine if these Sox2-mutant progenitor cells undergo cell fate conversion to CE, we examined the localization of genes normally expressed in the prospective CE at E14.5. In regions of SOX2 ablation, Pax6 was upregulated (Fig. 5N,T) and Chx10 (Fig. 5O,U) and Rax (Fig. 5P,V) were maintained. Moreover, Sox2-mutant cells expressed some prospective CE markers, including Bmp7 (Fig. 5Q,W,W′), but not others, including Msx1 (Fig. 5R,X). This expression profile recapitulates that of the early Sox2cond/cond; aP0CREiresGFP mutant cells described above.
The upregulation of Pax6 and Bmp7 suggests that ablation of SOX2 by Chx10CreGFP induces a liminal state similar to that observed in Sox2cond/cond; αP0CREiresGFP mutants. At P0, mosaic regions of Chx10CreGFP expression/SOX2-ablation were clearly distinguishable from neighboring Chx10CreGFP-negative/SOX2-postive regions (Fig. 6A,A′,D,D′). In mutant eyes, SOX2-ablated regions lacked expression of the NR gene Hes5 (Fig. 6C,C′,F,F′) and the postmitotic neuronal marker β-tubulin III (Fig. 6B,B′,E,E′). Conversely, Sox2-mutant regions upregulated Pax6 (Fig. 6G,G′,J,J′) and cell-autonomously gained expression of the CE markers Msx1 and Zic1 (Fig. 6H-I′,K-L′). These data support our finding that loss of SOX2 in multipotent progenitor cells results in a temporary liminal state typifying their maturation to CE. In addition, at P0, Sox2-mutant regions exhibited thin, single-layered morphology that starkly contrasts with neighboring control regions, which exhibited proper NR laminar morphology. Therefore, Sox2-mutant progenitors fated to become NR lose neuronal differentiation capacity and undergo cell fate conversion to CE (Cho and Cepko, 2006; Liu, H. et al., 2007).
Fig. 6.
At P0 mosaic regions of SOX2 ablation by Chx10CreGFP in the central eyecup indicate cell fate conversion to CE. (A-L′) Low (A-L) and high magnification (A′-L′) images of horizontal sections through the eyes of P0 control (Sox2cond/+; Chx10CreGFP) and mutant (Sox2cond/cond; Chx10CreGFP) mouse pups. Boxes indicate magnified regions. Immunohistochemistry for SOX2 (A,A′,D,D′; red), β-tubulin III (B,B′,E,E′; red) and GFP (A-E′; green) in control and mutant eyes shows thinning of the retinal neuroepithelium to ciliary epithelial (CE)-like morphology (D-E′) and loss of neuronal differentiation capacity (E,E′) in mosaic regions of SOX2 ablation (D,D′) throughout the mutant eyecup (D-E′) when compared with wild-type controls (A-B′). In situ hybridization shows the loss of expression of the NR marker Hes5 (F,F′), upregulation of Pax6 (J,J′), and gain of expression of the CE markers Msx1 and Zic1 (K-L′) in SOX2-ablated regions (D,D′) when compared with wild-type controls (C,C′,G-I′). CE, ciliary epithelium; RPE, retinal pigment epithelium. Scale bars: in L, 400 μm for A-L; in A′, 100 μm for A′-L′.
Fate mapping of Sox2 mutant cells depicts loss of neural progenitor capacity in the retina
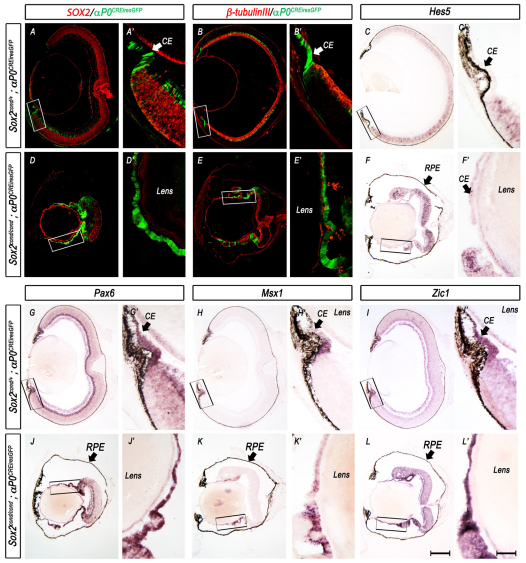
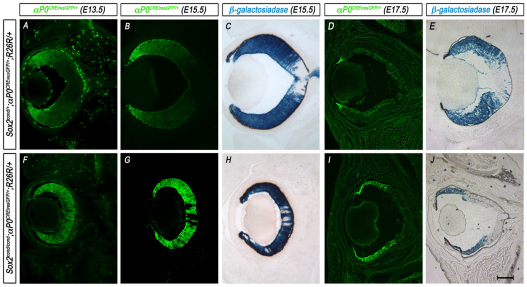
To establish directly whether ablation of SOX2 results in an autonomous cell fate change from NR to CE, we genetically fate mapped Sox2-mutant cells using the αP0CREiresGFP mouse line. Sox2cond/+; αP0CREiresGFP mice were crossed with mice carrying the Rosa26R CRE reporter allele (Sox2cond/+; R26R/+), which expresses β-galactosidase (β-gal) following CRE-mediated excision of a translational stop cassette, permanently marking the progeny of CRE-expressing cells (Soriano et al., 1987). Therefore, cells that express CRE at the time of analysis can be detected using GFP fluorescence, whereas all the progeny of CRE-positive cells express β-gal. In control Sox2cond/+; αP0CREiresGFP; R26R/+ embryos, αP0CREiresGFP was initially expressed throughout the peripheral optic cup (Fig. 7A). By E15.5, it became restricted to the distal tips (Fig. 7B), maintaining high expression in the CE by E17.5 (Fig. 7D). However, at E15.5 and E17.5, β-gal was detected throughout both the laminar NR (αP0CREiresGFP-negative) and the distal CE (Fig. 7C,E), leaving only a portion of central NR cells unmarked. This β-gal expression pattern confirms that αP0CREiresGFP-expressing progenitor cells can give rise to both NR and CE. By contrast, as previously shown, Sox2cond/cond; αP0CREiresGFP; R26R/+ mutant eyes exhibited expanded αP0CREiresGFP expression into the central optic cup (Fig. 7F,G,I), and all αP0CREiresGFP-positive cells appeared to express β-gal (Fig. 7H,J). By E17.5, these β-gal-positive regions exhibited thin morphology when compared with the SOX2-postive β-gal-negative regions in the same eye (Fig. 7J).
Fig. 7.
Fate mapping Sox2-mutant progenitor cells using αP0CREiresGFP and R26R. (A,F) αP0CREiresGFP (green) is expressed in peripheral progenitor cells in control (Sox2cond/+; αP0CREiresGFP; R26R) eyes at E13.5, whereas αP0CREiresGFP reporter expression in mutant (Sox2cond/cond; αP0CREiresGFP; R26R) eyes is expanded into the central prospective neural retina (NR). (B,D,G,I) As the wild-type eye develops, αP0CREiresGFP reporter expression (green) is further restricted to the peripheral eyecup at E15.5 (B) and serves as a marker of CE by E17.5 (D). In the mutant eye, αP0CREiresGFP reporter expression remains an indicator for all recombined cells throughout the NR and prospective ciliary epithelium (CE; G,I). (C,E,H,J) β-gal reporter assay on E15.5 and E17.5 eyes indicates that αP0CREiresGFP-positive progenitor cells are capable of giving rise to both prospective NR and CE in control eyes but appear to be restricted in Sox2-mutant eyes, in which β-gal colocalizes with αP0CREiresGFP. Scale bar: 200 μm.
Sox2 and Pax6 genes interact to coordinate eye development
Based on the increase in PAX6 expression upon SOX2 ablation, we hypothesized that proper regionalization of the optic cup depends on a fine balance of SOX2 and PAX6 dosage. To test this hypothesis directly, we performed genetic epistasis analysis of SOX2 and PAX6 in the developing optic cup. To modulate PAX6 dosage, we used the Pax6Sey/+ mouse line in which a spontaneous mutation in the Pax6 gene produces a truncated protein that lacks a DNA-binding homeodomain and the C-terminal transactivation domain. This truncated PAX6 is considered to be functionally inactive and is widely used as a Pax6-null allele (Hill et al., 1991; Hogan et al., 1986; Osumi et al., 2008). Compared with Sox2cond/+ or Sox2cond/cond mice, which display normal eye development (Taranova et al., 2006), Pax6Sey/+ mice exhibit reduced external eye size, iris hypoplasia and small lens (Hill et al., 1991).
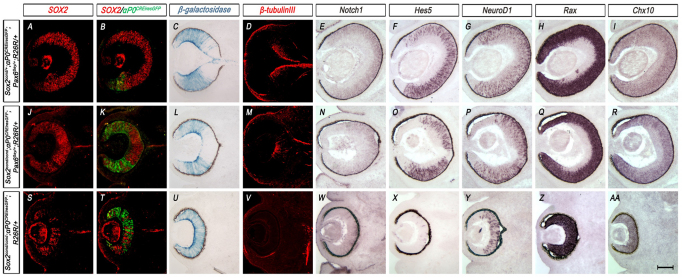
To ablate specifically SOX2 in peripheral progenitors on this Pax6-haploinsufficient background, we crossed Sox2cond/+; αP0CREiresGFP mice with Sox2cond/+; Pax6Sey/+mice. We then compared the resulting Sox2cond/cond; αP0CREiresGFP; Pax6Sey/+ (Sox2 Pax6 double mutant) embryos with Sox2cond/cond; αP0CREireGFP (Sox2 single mutant) embryos and Sox2cond/+; αP0CREiresGFP; Pax6Sey/+ (Pax6 single mutant) control embryos. Sox2 Pax6 double mutant eyes, which have reduced levels of both SOX2 and PAX6, were significantly normalized compared with Sox2 single mutant eyes, which are wild type for Pax6 but lose SOX2 upon CRE-mediated ablation (Fig. 8J-AA). The central expansion of αP0CREiresGFP seen in Sox2 single mutant eyes was significantly rescued in Sox2 Pax6 double mutant eyes (Fig. 8J-AA). Lineage tracing analysis using the Rosa26R CRE reporter showed that the domain of αP0CREiresGFP activity, as marked by β-gal expression, is peripherally restricted in Sox2 Pax6 double mutants compared with Sox2 single mutants. These data indicate that αP0CREiresGFP expression in Sox2 Pax6 double mutants is restored to an expression pattern that more closely resembles that of controls (Fig. 8B,K). Nevertheless, αP0CREiresGFP expression co-localized with β-gal activity in Sox2 Pax6 double mutants (Fig. 8B,C,K,L), which is consistent with the maintenance of αP0CREiresGFP in SOX2-ablated cells as described above (Fig. 7). The ablation of SOX2 in the Pax6-haploinsufficient background significantly rescued neuronal differentiation capacity, as illustrated by normalization of β-tubulin III (Fig. 8D,M,V), Notch1 (Fig. 8E,N,W), Hes5 (Fig. 8F,O,X) and NeuroD1 (Fig. 8G,P,Y). Modulating SOX2 or PAX6 levels did not affect Rax (Fig. 8H,Q,Z) or Chx10 (Fig. 8I,R,AA) expression.
Fig. 8.
Pax6-haploinsufficiency significantly rescues the Sox2-mutant NR. (A,B,J,K,S,T) Comparison of SOX2 immunohistochemistry (red) with αP0CREiresGFP expression (green) in Pax6 single mutant (Sox2cond/+; αP0CREiresGFP; Pax6Sey/+), Sox2 Pax6 double mutant (Sox2cond/cond; αP0CREiresGFP; Pax6Sey/+) and Sox2 single mutant (Sox2cond/cond; αP0CREiresGFP) eyes indicating little to no SOX2 expression in the Sox2 single mutant compared with the other two genotypes. (C,L,U) β-gal activity illustrating the progeny of all αP0CREiresGFP-expressing cells indicates rescue of αP0CREiresGFP expression in Sox2 Pax6 double mutants. (D,M,V) β-tubulin III (red) shows maintenance of neuronal differentiation capacity in Pax6 single mutants and Sox2 Pax6 double mutants but not in Sox2 single mutants. (E-I,N-R,W-AA) In situ hybridization of Notch1 (E,N,W), Hes5 (F,O,X), NeuroD1 (G,P,Y), Rax (H,Q,Z) and Chx10 (I,R,AA) shows maintenance of prospective NR markers in the Sox2 Pax6 double mutants. Scale bar: 200 μm.
To determine whether this rescue phenotype is maintained postnatally, we examined Sox2 Pax6 double mutants at P0 and found a slightly expanded region of αP0CREiresGFP-positive thin CE-like cells compared with Pax6 single mutant controls (see Fig. S5A-H in the supplementary material, brackets). This result contrasts with the single-layered morphology and CE gene expression present throughout the center of the eyecup in Sox2 single mutants (see Fig. S5I-L in the supplementary material). Moreover, the NR of Sox2 Pax6 double mutants exhibited proper laminar morphology and neuronal differentiation capacity as indicated by β-tubulin III and Hes5 expression, and Msx1 expression was restricted to the distal tips (see Fig. S5F-H in the supplementary material). Therefore, Sox2 Pax6 double mutants more closely resembled Pax6 single mutants than Sox2 single mutants.
DISCUSSION
SOX2 maintains neuorgenic fate of proliferating neuorepithelial progenitor cells
Here, we have demonstrated for the first time that the genetic ablation of SOX2 causes a neurogenic-to-non-neurogenic cell fate conversion. These results place SOX2 as a crucial factor defining neurogenic identity in retinal neuroepithelium. In early stage mouse embryos, SOX2 expression marks the region fated to become neural ectoderm and its appearance in chicken embryos coincides with the onset of neural fate specification (Wood and Episkopou, 1999). Inhibition of SOX2 function in Xenopus embryos blocks neural differentiation, and SOX2 signaling in chick has been shown to promote proliferation and inhibit neuronal differentiation (Bylund et al., 2003; Graham et al., 2003; Kishi et al., 2000). In the retina, unlike in other regions of the CNS, SOX2 is expressed exclusively of the highly related SOXB1 factors, SOX1 and SOX3. Thus, the optic cup, which retains the unique capacity to generate non-neurogenic structures, provides an excellent model for studying the transcriptional role of SOX2 in maintaining neurogenic identity.
SOX2 ablation initiates gradual cell fate conversion from NR to CE
Secreted molecules known to affect neurogenic versus non-neurogenic optic cup cell fate include FGFs, BMPs and Wnts. A previous report showed that converging FGF and BMP signals might define the prospective NR-CE boundary at optic vesicle stages of the chick (Dias da Silva et al., 2007). BMP signaling appears to be required for Otx1 and Msx1 expression in the prospective CE (Zhao et al., 2002), and Msx1 expression has been shown to be induced by activated Wnt signaling (Willert et al., 2002). Thus, overlapping FGF, BMP and Wnt signals might coordinate the cell-intrinsic gene expression necessary for CE development. However, at least one study has demonstrated induced ectopic CE marker expression without the onset of Wnt2b expression (Dias da Silva et al., 2007). Interestingly, it has been proposed that extrinsic signals that direct neurogenic versus non-neurogenic fate converge at the regulation of SOX proteins (Wilson et al., 2001). Here, we have investigated the cell-intrinsic role of SOX2 and PAX6 in setting up the NR-CE boundary in the optic cup.
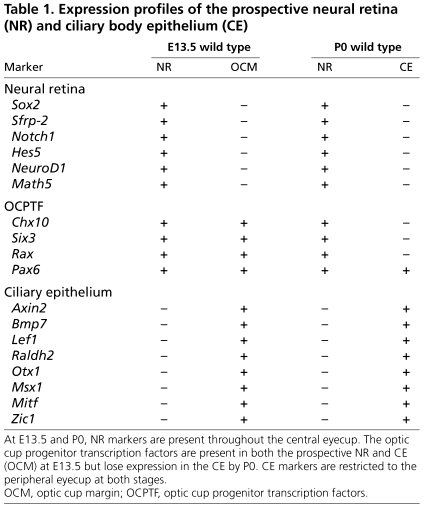
Using genetic fate mapping, we have demonstrated that removal of a transcription factor, SOX2, converts prospective NR to CE. Our results are consistent with previous data showing that neural RPCs maintain multipotent differentiation capacity (i.e. the capacity to form CE) at a developmental time point (E11.0) subsequent to the division of NR and CE fate (Fekete et al., 1994; Turner and Cepko, 1987; Turner et al., 1990). The gradual cell fate conversion upon Sox2 deletion is characterized by: (1) loss of the NR markers Notch1, Hes5, NeuroD1 and Sfrp2 (see Table 1, neural retina markers); (2) maintenance of the multipotent progenitor genes Chx10, Rax and Pax6 (see Table 1, optic cup progenitor transcription factors); (3) expansion of Wnt and BMP signals; and (4) decreased proliferation with progressive thinning of the neuroepithelium. These characteristics define a liminal or in-between state that culminates in the expression of CE markers, including Otx1, Zic1 and Msx1 (see Table 1, ciliary epithelium markers). A similar delay in the consolidation of CE identity in response to ectopic Wnt signaling has been previously demonstrated (Cho and Cepko, 2006). In both instances, the delay could be due to the presence of competing signals in the eyecup: Wnt signals promote non-neurogenic fate but multipotent progenitor genes and Wnt antagonists promote neurogenic fate. Intriguingly, activation of β-catenin in the eyecup was shown to produce a stronger and more rapid onset of CE characteristics than did Wnt2b overexpression, suggesting that stabilized β-catenin bypasses Wnt antagonists in the prospective NR to cause a more immediate increase in CE gene expression (Cho and Cepko, 2006). Thus, the presence of Wnt antagonists might explain why consolidation of CE fate is gradual upon SOX2 ablation but rapid upon β-catenin activation (Liu, F. et al., 2007).
Table 1.
Expression profiles of the prospective neural retina (NR) and ciliary body epithelium (CE)
Sox2 and Pax6 interact to regionalize the optic cup
A major difference between SOX2 loss-of-function and β-catenin gain-of-function in the prospective retina is the effect on Pax6. Ablation of SOX2 leads to an immediate increase in Pax6 expression, whereas activation of β-catenin diminishes Pax6 expression. In humans, haploinsufficiency of PAX6 is associated with anterior eye formations, including defects of the iris and ciliary body (for reviews, see Hever et al., 2006; Hill et al., 1991). In the mouse, deletion of one copy of Pax6 in the distal optic cup resulted in the loss of CE precursors and a distal shift in the boundary between prospective NR and CE (Davis-Silberman et al., 2005). Moreover, transgenic overexpression of Pax6 resulted in abnormalities of the ciliary body, the iris and the cornea (Schedl et al., 1996). These studies demonstrate the importance of regulating appropriate levels of PAX6 for the proper development of peripheral eye structures. Here, we have described increased Pax6 expression in the optic cup upon the removal of a potential repressor.
Our genetic epistasis analyses provide evidence for a mechanism of PAX6 regulation in the developing eyecup. The dramatic increase in PAX6 upon Sox2 deletion and the subsequent NR-to-CE cell fate conversion suggest that SOX2 normally antagonizes PAX6 signaling to maintain NR identity. Moreover, the genetic rescue of eye development by lowering PAX6 levels while deleting Sox2 indicates a functional antagonism between the two genes. These data raise the possibility that neurogenic versus non-neurogenic fate in the optic cup is extremely sensitive to the ratio of PAX6 to SOX2.
We propose a model (see Fig. S6 in the supplementary material) in which SOX2 levels are high enough to antagonize Pax6 expression in central optic cup progenitor cells, thereby maintaining neurogenic capacity. Conversely, in the peripheral optic cup, SOX2 levels are low, perhaps allowing PAX6 to activate its own expression via the α enhancer. In fact, previous studies have shown that PAX6 can directly bind a conserved site in α, and that lowering PAX6 levels decreases α-driven CRE-GFP expression (Baumer et al., 2002; Schwarz et al., 2000). Further studies are needed to address the question of whether SOX2 and PAX6 coordinate Pax6 expression through the α enhancer. Indeed, SOX2 and PAX6 have been shown to co-regulate expression of the δ-crystallin gene in the lens, where they form a complex at the DC5 enhancer that is stabilized by both protein-protein and protein-DNA interactions (Kamachi et al., 2001). A similar co-regulation of α enhancer activity might control Pax6 expression in the optic cup given that the α enhancer has been suggested to mediate the proximallow-to-distalhigh PAX6 gradient (Baumer et al., 2002; Davis-Silberman et al., 2005).
Although the role of secreted signaling molecules in positioning the boundary between prospective NR and CE has been studied extensively, little is known about the cell-intrinsic mechanisms responsible for setting up or maintaining this boundary. Our results suggest a tightly coordinated dosage-dependent transcriptional mechanism directing NR versus CE cell fate. We have shown that the functional antagonism between SOX2 and PAX6 is necessary for proper patterning of the eyecup, such that in the absence of SOX2, PAX6 is not sufficient to maintain NR identity, and cells originally fated to become retinal neurons instead take on a peripheral non-neurogenic cell fate.
Supplementary Material
Acknowledgments
Work in the L.H.P. laboratory is supported by NIH (NIMH – R01MH071822 and NEI – R01EY018261), in the UNC Neuroscience in situ Core by NIH (NINDS – P30-S045892-02) and in the UNC Neuroscience Multiphoton and Confocal Imaging Core by NIH (5P30NS045892). We thank UNC Neuroscience Core Facility Directors Megumi Aita and Vladimir Gukassyan for their help and expertise. We thank the members of the Pevny Lab for their helpful discussions and comments on the manuscript. Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.055178/-/DC1
References
- Anchan R. M., Drake D. P., Haines C. F., Gerwe E. A., LaMantia A. S. (1997). Disruption of local retinoid-mediated gene expression accompanies abnormal development in the mammalian olfactory pathway. J. Comp. Neurol. 379, 171-184 [PubMed] [Google Scholar]
- Aruga J., Yokota N., Hashimoto M., Furuichi T., Fukuda M., Mikoshiba K. (1994). A novel zinc finger protein, zic, is involved in neurogenesis, especially in the cell lineage of cerebellar granule cells. J. Neurochem. 63, 1880-1890 [DOI] [PubMed] [Google Scholar]
- Baumer N., Marquardt T., Stoykova A., Ashery-Padan R., Chowdhury K., Gruss P. (2002). Pax6 is required for establishing naso-temporal and dorsal characteristics of the optic vesicle. Development 129, 4535-4545 [DOI] [PubMed] [Google Scholar]
- Beebe D. C. (1986). Development of the ciliary body: a brief review. Trans. Ophthalmol. Soc. UK 105, 123-130 [PubMed] [Google Scholar]
- Bhasin N., Maynard T. M., Gallagher P. A., LaMantia A. S. (2003). Mesenchymal/epithelial regulation of retinoic acid signaling in the olfactory placode. Dev. Biol. 261, 82-98 [DOI] [PubMed] [Google Scholar]
- Bylund M., Andersson E., Novitch B. G., Muhr J. (2003). Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 6, 1162-1168 [DOI] [PubMed] [Google Scholar]
- Chenn A., Walsh C. A. (2002). Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297, 365-369 [DOI] [PubMed] [Google Scholar]
- Cho S. H., Cepko C. L. (2006). Wnt2b/beta-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development 133, 3167-3177 [DOI] [PubMed] [Google Scholar]
- Davis-Silberman N., Ashery-Padan R. (2008). Iris development in vertebrates; genetic and molecular considerations. Brain. Res. 1192, 17-28 [DOI] [PubMed] [Google Scholar]
- Davis-Silberman N., Kalich T., Oron-Karni V., Marquardt T., Kroeber M., Tamm E. R., Ashery-Padan R. (2005). Genetic dissection of Pax6 dosage requirements in the developing mouse eye. Hum. Mol. Genet. 14, 2265-2276 [DOI] [PubMed] [Google Scholar]
- Dias da Silva M., Tiffin N., Mima T., Mikawa T., Hyer J. (2007). FGF-mediated induction of ciliary body tissue in the chick eye. Dev. Biol. 304, 272-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan S. L., Dyer M. A. (2004). Developmental defects in Rb-deficient retinae. Vision Res. 44, 3323-3333 [DOI] [PubMed] [Google Scholar]
- Ericson J., Rashbass P., Schedl A., Brenner-Morton S., Kawakami A., van Heyningen V., Jessell T. M., Briscoe J. (1997). Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell 90, 169-180 [DOI] [PubMed] [Google Scholar]
- Fantes J., Ragge N. K., Lynch S. A., McGill N. I., Collin J. R., Howard-Peebles P. N., Hayward C., Vivian A. J., Williamson K., van Heyningen V., et al. (2003). Mutations in SOX2 cause anophthalmia. Nat. Genet. 33, 461-463 [DOI] [PubMed] [Google Scholar]
- Favor J., Peters H., Hermann T., Schmahl W., Chatterjee B., Neuhauser-Klaus A., Sandulache R. (2001). Molecular characterization of Pax6(2Neu) through Pax6(10Neu): an extension of the Pax6 allelic series and the identification of two possible hypomorph alleles in the mouse Mus musculus. Genetics 159, 1689-1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete D. M., Perez-Miguelsanz J., Ryder E. F., Cepko C. L. (1994). Clonal analysis in the chicken retina reveals tangential dispersion of clonally related cells. Dev. Biol. 166, 666-682 [DOI] [PubMed] [Google Scholar]
- Fuhrmann S., Levine E. M., Reh T. A. (2000). Extraocular mesenchyme patterns the optic vesicle during early eye development in the embryonic chick. Development 127, 4599-4609 [DOI] [PubMed] [Google Scholar]
- Fuhrmann S., Riesenberg A. N., Mathiesen A. M., Brown E. C., Vetter M. L., Brown N. L. (2009). Characterization of a transient TCF/LEF-responsive progenitor population in the embryonic mouse retina. Invest. Ophthalmol. Vis. Sci. 50, 432-440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Kozak C. A., Cepko C. L. (1997). rax, a novel paired-type homeobox gene, shows expression in the anterior neural fold and developing retina. Proc. Natl. Acad. Sci. USA 94, 3088-3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galceran J., Farinas I., Depew M. J., Clevers H., Grosschedl R. (1999). Wnt3a–/–like phenotype and limb deficiency in Lef1(–/–)Tcf1(–/–) mice. Genes Dev. 13, 709-717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham V., Khudyakov J., Ellis P., Pevny L. (2003). SOX2 functions to maintain neural progenitor identity. Neuron 39, 749-765 [DOI] [PubMed] [Google Scholar]
- Grindley J. C., Hargett L. K., Hill R. E., Ross A., Hogan B. L. (1997). Disruption of PAX6 function in mice homozygous for the Pax6Sey-1Neu mutation produces abnormalities in the early development and regionalization of the diencephalon. Mech. Dev. 64, 111-126 [DOI] [PubMed] [Google Scholar]
- Hack M. A., Sugimori M., Lundberg C., Nakafuku M., Gotz M. (2004). Regionalization and fate specification in neurospheres: the role of Olig2 and Pax6. Mol. Cell. Neurosci. 25, 664-678 [DOI] [PubMed] [Google Scholar]
- Hagstrom S. A., Pauer G. J., Reid J., Simpson E., Crowe S., Maumenee I. H., Traboulsi E. I. (2005). SOX2 mutation causes anophthalmia, hearing loss, and brain anomalies. Am. J. Med. Genet. 138A, 95-98 [DOI] [PubMed] [Google Scholar]
- Hanson I., Van Heyningen V. (1995). Pax6: more than meets the eye. Trends Genet. 11, 268-272 [DOI] [PubMed] [Google Scholar]
- Harada N., Tamai Y., Ishikawa T., Sauer B., Takaku K., Oshima M., Taketo M. M. (1999). Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 18, 5931-5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Kao W. W., Kohno N., Nishihara-Hayashi M., Shiraishi A., Uno T., Yamaguchi M., Okamoto S., Maeda M., Ikeda T., et al. (2004). Expression patterns of sialylated epitope recognized by KL-6 monoclonal antibody in ocular surface epithelium of normals and dry eye patients. Invest. Ophthalmol. Vis. Sci. 45, 2212-2217 [DOI] [PubMed] [Google Scholar]
- Heins N., Malatesta P., Cecconi F., Nakafuku M., Tucker K. L., Hack M. A., Chapouton P., Barde Y. A., Gotz M. (2002). Glial cells generate neurons: the role of the transcription factor Pax6. Nat. Neurosci. 5, 308-315 [DOI] [PubMed] [Google Scholar]
- Hever A. M., Williamson K. A., van Heyningen V. (2006). Developmental malformations of the eye: the role of PAX6, SOX2 and OTX2. Clin. Genet. 69, 459-470 [DOI] [PubMed] [Google Scholar]
- Hill R. E., Favor J., Hogan B. L., Ton C. C., Saunders G. F., Hanson I. M., Prosser J., Jordan T., Hastie N. D., van Heyningen V. (1991). Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature 354, 522-525 [DOI] [PubMed] [Google Scholar]
- Hodgkinson C. A., Moore K. J., Nakayama A., Steingrimsson E., Copeland N. G., Jenkins N. A., Arnheiter H. (1993). Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell 74, 395-404 [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Horsburgh G., Cohen J., Hetherington C. M., Fisher G., Lyon M. F. (1986). Small eyes (Sey): a homozygous lethal mutation on chromosome 2 which affects the differentiation of both lens and nasal placodes in the mouse. J. Embryol. Exp. Morphol. 97, 95-110 [PubMed] [Google Scholar]
- Horsford D. J., Nguyen M. T., Sellar G. C., Kothary R., Arnheiter H., McInnes R. R. (2005). Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development 132, 177-187 [DOI] [PubMed] [Google Scholar]
- Jadhav A. P., Mason H. A., Cepko C. L. (2006). Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development 133, 913-923 [DOI] [PubMed] [Google Scholar]
- Jho E. H., Zhang T., Domon C., Joo C. K., Freund J. N., Costantini F. (2002). Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 22, 1172-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y., Uchikawa M., Tanouchi A., Sekido R., Kondoh H. (2001). Pax6 and SOX2 form a co-DNA-binding partner complex that regulates initiation of lens development. Genes Dev. 15, 1272-1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammandel B., Chowdhury K., Stoykova A., Aparicio S., Brenner S., Gruss P. (1999). Distinct cis-essential modules direct the time-space pattern of the Pax6 gene activity. Dev. Biol. 205, 79-97 [DOI] [PubMed] [Google Scholar]
- Kim J., Lauderdale J. D. (2008). Overexpression of pairedless Pax6 in the retina disrupts corneal development and affects lens cell survival. Dev. Biol. 313, 434-454 [DOI] [PubMed] [Google Scholar]
- Kishi M., Mizuseki K., Sasai N., Yamazaki H., Shiota K., Nakanishi S., Sasai Y. (2000). Requirement of Sox2-mediated signaling for differentiation of early Xenopus neuroectoderm. Development 127, 791-800 [DOI] [PubMed] [Google Scholar]
- Kubo F., Nakagawa S. (2008). Wnt signaling in retinal stem cells and regeneration. Dev. Growth Differ. 50, 245-251 [DOI] [PubMed] [Google Scholar]
- Kubota R., McGuire C., Dierks B., Reh T. A. (2004). Identification of ciliary epithelial-specific genes using subtractive libraries and cDNA arrays in the avian eye. Dev. Dyn. 229, 529-540 [DOI] [PubMed] [Google Scholar]
- Lardelli M., Lendahl U. (1993). Motch A and motch B-two mouse Notch homologues coexpressed in a wide variety of tissues. Exp. Cell Res. 204, 364-372 [DOI] [PubMed] [Google Scholar]
- Lee J. E., Hollenberg S. M., Snider L., Turner D. L., Lipnick N., Weintraub H. (1995). Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science 268, 836-844 [DOI] [PubMed] [Google Scholar]
- Liu F., Thirumangalathu S., Gallant N. M., Yang S. H., Stoick-Cooper C. L., Reddy S. T., Andl T., Taketo M. M., Dlugosz A. A., Moon R. T., et al. (2007). Wnt-beta-catenin signaling initiates taste papilla development. Nat. Genet. 39, 106-112 [DOI] [PubMed] [Google Scholar]
- Liu H., Thurig S., Mohamed O., Dufort D., Wallace V. A. (2006). Mapping canonical Wnt signaling in the developing and adult retina. Invest. Ophthalmol. Vis. Sci. 47, 5088-5097 [DOI] [PubMed] [Google Scholar]
- Liu H., Xu S., Wang Y., Mazerolle C., Thurig S., Coles B. L., Ren J. C., Taketo M. M., van der Kooy D., Wallace V. A. (2007). Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Dev. Biol. 308, 54-67 [DOI] [PubMed] [Google Scholar]
- Liu J., Wilson S., Reh T. (2003). BMP receptor 1b is required for axon guidance and cell survival in the developing retina. Dev. Biol. 256, 34-48 [DOI] [PubMed] [Google Scholar]
- Lyons K. M., Hogan B. L., Robertson E. J. (1995). Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech. Dev. 50, 71-83 [DOI] [PubMed] [Google Scholar]
- Manuel M., Georgala P. A., Carr C. B., Chanas S., Kleinjan D. A., Martynoga B., Mason J. O., Molinek M., Pinson J., Pratt T., et al. (2007). Controlled overexpression of Pax6 in vivo negatively autoregulates the Pax6 locus, causing cell-autonomous defects of late cortical progenitor proliferation with little effect on cortical arealization. Development 134, 545-555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt T., Ashery-Padan R., Andrejewski N., Scardigli R., Guillemot F., Gruss P. (2001). Pax6 is required for the multipotent state of retinal progenitor cells. Cell 105, 43-55 [DOI] [PubMed] [Google Scholar]
- Okamoto F., Nakano S., Okamoto C., Hommura S., Oshika T. (2004). Ultrasound biomicroscopic findings in aniridia. Am. J. Ophthalmol. 137, 858-862 [DOI] [PubMed] [Google Scholar]
- Oron-Karni V., Farhy C., Elgart M., Marquardt T., Remizova L., Yaron O., Xie Q., Cvekl A., Ashery-Padan R. (2008). Dual requirement for Pax6 in retinal progenitor cells. Development 135, 4037-4047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi N., Shinohara H., Numayama-Tsuruta K., Maekawa M. (2008). Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells 26, 1663-1672 [DOI] [PubMed] [Google Scholar]
- Philips G. T., Stair C. N., Young Lee H., Wroblewski E., Berberoglu M. A., Brown N. L., Mastick G. S. (2005). Precocious retinal neurons: Pax6 controls timing of differentiation and determination of cell type. Dev. Biol. 279, 308-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittack C., Grunwald G. B., Reh T. A. (1997). Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development 124, 805-816 [DOI] [PubMed] [Google Scholar]
- Prosser J., van Heyningen V. (1998). PAX6 mutations reviewed. Hum. Mutat. 11, 93-108 [DOI] [PubMed] [Google Scholar]
- Ragge N. K., Brown A. G., Poloschek C. M., Lorenz B., Henderson R. A., Clarke M. P., Russell-Eggitt I., Fielder A., Gerrelli D., Martinez-Barbera J. P., et al. (2005a). Heterozygous mutations of OTX2 cause severe ocular malformations. Am. J. Hum. Genet. 76, 1008-1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragge N. K., Lorenz B., Schneider A., Bushby K., de Sanctis L., de Sanctis U., Salt A., Collin J. R., Vivian A. J., Free S. L., et al. (2005b). SOX2 anophthalmia syndrome. Am. J. Med. Genet. A 135, 1-7 [DOI] [PubMed] [Google Scholar]
- Rattner A., Hsieh J. C., Smallwood P. M., Gilbert D. J., Copeland N. G., Jenkins N. A., Nathans J. (1997). A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc. Natl. Acad. Sci. USA 94, 2859-2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex M., Orme A., Uwanogho D., Tointon K., Wigmore P. M., Sharpe P. T., Scotting P. J. (1997). Dynamic expression of chicken Sox2 and Sox3 genes in ectoderm induced to form neural tissue. Dev. Dyn. 209, 323-332 [DOI] [PubMed] [Google Scholar]
- Rowan S., Cepko C. L. (2004). Genetic analysis of the homeodomain transcription factor Chx10 in the retina using a novel multifunctional BAC transgenic mouse reporter. Dev. Biol. 271, 388-402 [DOI] [PubMed] [Google Scholar]
- Rowan S., Chen C. M., Young T. L., Fisher D. E., Cepko C. L. (2004). Transdifferentiation of the retina into pigmented cells in ocular retardation mice defines a new function of the homeodomain gene Chx10. Development 131, 5139-5152 [DOI] [PubMed] [Google Scholar]
- Schedl A., Ross A., Lee M., Engelkamp D., Rashbass P., van Heyningen V., Hastie N. D. (1996). Influence of PAX6 gene dosage on development: overexpression causes severe eye abnormalities. Cell 86, 71-82 [DOI] [PubMed] [Google Scholar]
- Schwarz M., Cecconi F., Bernier G., Andrejewski N., Kammandel B., Wagner M., Gruss P. (2000). Spatial specification of mammalian eye territories by reciprocal transcriptional repression of Pax2 and Pax6. Development 127, 4325-4334 [DOI] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Gulisano M., Stornaiuolo A., Boncinelli E. (1992). Nested expression domains of four homeobox genes in developing rostral brain. Nature 358, 687-690 [DOI] [PubMed] [Google Scholar]
- Smith A. N., Miller L. A., Radice G., Ashery-Padan R., Lang R. A. (2009). Stage-dependent modes of Pax6-Sox2 epistasis regulate lens development and eye morphogenesis. Development 136, 2977-2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Gridley T., Jaenisch R. (1987). Retroviruses and insertional mutagenesis in mice: proviral integration at the Mov 34 locus leads to early embryonic death. Genes Dev. 1, 366-375 [DOI] [PubMed] [Google Scholar]
- Taranova O. V., Magness S. T., Fagan B. M., Wu Y., Surzenko N., Hutton S. R., Pevny L. H. (2006). SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev. 20, 1187-1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson A. (2003). Patterning the peripheral retina of the fly: decoding a gradient. Dev. Cell 5, 799-809 [DOI] [PubMed] [Google Scholar]
- Ton C. C., Hirvonen H., Miwa H., Weil M. M., Monaghan P., Jordan T., van Heyningen V., Hastie N. D., Meijers-Heijboer H., Drechsler M., et al. (1991). Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell 67, 1059-1074 [DOI] [PubMed] [Google Scholar]
- Trimarchi J. M., Cho S. H., Cepko C. L. (2009). Identification of genes expressed preferentially in the developing peripheral margin of the optic cup. Dev. Dyn. 238, 2327-2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner D. L., Cepko C. L. (1987). A common progenitor for neurons and glia persists in rat retina late in development. Nature 328, 131-136 [DOI] [PubMed] [Google Scholar]
- Turner D. L., Snyder E. Y., Cepko C. L. (1990). Lineage-independent determination of cell type in the embryonic mouse retina. Neuron 4, 833-845 [DOI] [PubMed] [Google Scholar]
- Uchikawa M., Ishida Y., Takemoto T., Kamachi Y., Kondoh H. (2003). Functional analysis of chicken Sox2 enhancers highlights an array of diverse regulatory elements that are conserved in mammals. Dev. Cell 4, 509-519 [DOI] [PubMed] [Google Scholar]
- Uwanogho D., Rex M., Cartwright E. J., Pearl G., Healy C., Scotting P. J., Sharpe P. T. (1995). Embryonic expression of the chicken Sox2, Sox3 and Sox11 genes suggests an interactive role in neuronal development. Mech. Dev. 49, 23-36 [DOI] [PubMed] [Google Scholar]
- Walther C., Guenet J. L., Simon D., Deutsch U., Jostes B., Goulding M. D., Plachov D., Balling R., Gruss P. (1991). Pax: a murine multigene family of paired box-containing genes. Genomics 11, 424-434 [DOI] [PubMed] [Google Scholar]
- Willert J., Epping M., Pollack J. R., Brown P. O., Nusse R. (2002). A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev. Biol. 2, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. I., Rydstrom A., Trimborn T., Willert K., Nusse R., Jessell T. M., Edlund T. (2001). The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature 411, 325-330 [DOI] [PubMed] [Google Scholar]
- Wood H. B., Episkopou V. (1999). Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mech. Dev. 86, 197-201 [DOI] [PubMed] [Google Scholar]
- Xu H. E., Rould M. A., Xu W., Epstein J. A., Maas R. L., Pabo C. O. (1999). Crystal structure of the human Pax6 paired domain-DNA complex reveals specific roles for the linker region and carboxy-terminal subdomain in DNA binding. Genes Dev. 13, 1263-1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenteno J. C., Gascon-Guzman G., Tovilla-Canales J. L. (2005). Bilateral anophthalmia and brain malformations caused by a 20-bp deletion in the SOX2 gene. Clin. Genet. 68, 564-566 [DOI] [PubMed] [Google Scholar]
- Zenteno J. C., Perez-Cano H. J., Aguinaga M. (2006). Anophthalmia-esophageal atresia syndrome caused by an SOX2 gene deletion in monozygotic twin brothers with markedly discordant phenotypes. Am. J. Med. Genet. 140A, 1899-1903 [DOI] [PubMed] [Google Scholar]
- Zhang J., Gray J., Wu L., Leone G., Rowan S., Cepko C. L., Zhu X., Craft C. M., Dyer M. A. (2004). Rb regulates proliferation and rod photoreceptor development in the mouse retina. Nat. Genet. 36, 351-360 [DOI] [PubMed] [Google Scholar]
- Zhao S., Chen Q., Hung F. C., Overbeek P. A. (2002). BMP signaling is required for development of the ciliary body. Development 129, 4435-4442 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.