Abstract
Modulation of the sonic hedgehog (SHH) pathway is a crucial factor in cerebellar morphogenesis. Stimulation of granule neuron progenitor (GNP) proliferation is a central function of SHH signalling, but how this is controlled locally is not understood. We show that two sequentially expressed members of the contactin (CNTN) family of adhesion molecules, TAG1 and F3, act antagonistically to control SHH-induced proliferation: F3 suppresses SHH-induced GNP proliferation and induces differentiation, whereas TAG1 antagonises F3. Production of GNPs in TAG1-null mice is delayed and reduced. F3 and TAG1 colocalise on GNPs with the related L1-like adhesion molecule NrCAM, and F3 fails to suppress the SHH-induced proliferation of NrCAM-deficient GNPs. We show that F3 and SHH both primarily affect a group of intermediate GNPs (IPs), which, though actively dividing, also express molecules associated with differentiation, including β-tubulin III (TuJ1) and TAG1. In vivo, intermediate progenitors form a discrete layer in the middle of the external germinal layer (mEGL), while F3 becomes expressed on the axons of postmitotic granule neurons as they leave the inner EGL (iEGL). We propose, therefore, that F3 acts as a localised signal in the iEGL that induces SHH-stimulated cells in the overlying mEGL to exit cell cycle and differentiate. By contrast, expression of TAG1 on GNPs antagonises this signal in the mEGL, preventing premature differentiation and sustaining GNP expansion in a paracrine fashion. Together, these findings indicate that CNTN and L1-like proteins play a significant role in modulating SHH-induced neuronal precursor proliferation.
Keywords: Sonic hedgehog, Cerebellum, Neural cell adhesion molecule, Neuronal progenitor, Mouse
INTRODUCTION
The developing cerebellum is a robust system in which to study both neurogenesis and morphogenesis, because it comprises relatively few cell types, yet assumes a complex but stereotyped architecture during a major expansion after birth in mammals (Goldowitz and Hamre, 1998; Chédotal, 2010). This expansion is largely due to the proliferation of granule neuron precursors (GNPs) at the cortical surface of the cerebellum, the external germinal layer (EGL), that subsequently differentiate as granule cell neurons (GNs) and migrate radially past the underlying Purkinje cells (PCs) to the inner granular layer (IGL) (Altman and Bayer, 1997). This proliferation is regulated by the sonic hedgehog (SHH) and Notch signalling pathways, disruptions of which have been implicated in medulloblastoma in mammals (Behesti and Marino, 2009). How these signals are regulated is crucial to our understanding of both normal development and disease.
Several signals are suggested to control GNP responses to the mitogenic effects of SHH, which is made by PCs and presumably diffuses into the overlying EGL (Dahmane and Ruiz-i-Altaba, 1999; Wallace, 1999; Wechsler-Reya and Scott, 1999), including FGFs (Pons et al., 2001; Wechsler-Reya and Scott, 1999), BMPs (Rios, 2004) and protease nexin 1 (Vaillant et al., 2007). However, since these are secreted it is unclear how their effects are confined to the inner EGL (iEGL) where GNPs become postmitotic. Indeed, all proliferating GNPs appear to respond to SHH (Corrales et al., 2004) and although signals from the overlying meninges potentiate SHH signalling in the outer EGL (oEGL) (Blaess et al., 2004), it remains unclear why GNPs closest to the SHH source are the ones first to become postmitotic. Although GNPs in the iEGL come into contact with vitronectin expressed on parallel fibres in the underlying molecular layer (ML), which is known to suppress SHH mitogenesis in vitro (Pons et al., 2001), the integrin receptor for vitronectin, αvβ3, is expressed only on cells in the iEGL (Pons et al., 2001), which are believed to be already postmitotic. Moreover, loss of vitronectin in vivo has no effect on cerebellar development (Zheng et al., 1995).
A further candidate for modulating GNP cell cycle exit is the immunoglobulin-like adhesion molecule F3/contactin (F3), one of six glycosyl phosphatidyl inositol (GPI)-linked contactins (CNTNs) that, together with their cognate and related partners, the L1-like proteins (Brummendorf and Rathjen, 1996), form the L1-CNTN family of cell-adhesion molecules (Herron et al., 2009; Katidou et al., 2008). Like vitronectin, F3 is strongly expressed on postmitotic GNs and their axons (Faivre-Sarrailh et al., 1992; Virgintino et al., 1999; Yoshihara et al., 1995). In addition, loss of F3 leads to postnatal lethality and severely disrupts parallel fibre development (Berglund et al., 1999). Premature expression of F3 in GNPs in transgenic TAGF3 mice, using the promoter of the related TAG1 gene (Cntn2) (which is normally expressed upon cycling as well as on postmitotic GNPs), suppresses GNP proliferation and leads to transient delay of cerebellar growth and reduced proliferative potential in vitro (Bizzoca et al., 2003). However, whether F3 affects SHH-induced GNP proliferation and whether its effects are direct or indirect has not been tested, although receptors for F3 are expressed on GNPs, including L1 and the related NrCAM, which are redundantly required for GNP survival (Brummendorf and Rathjen, 1996; Hu et al., 2003; Sakurai et al., 2001; Solecki et al., 2001).
To understand better how F3 affects granule neuron development, we studied its effects on purified GNPs stimulated with SHH in culture. We demonstrate that F3 directly inhibits SHH-induced GNP proliferation, affecting GNPs mainly at an intermediate stage of maturation. We show that TAG1 protein antagonises effects of F3 and that GNP proliferation is reduced in specific regions of the cerebellum of mice lacking TAG1. Finally, we present evidence that F3 and TAG1 bind to NrCAM on GNPs and that NrCAM is required to mediate the suppression of SHH-induced proliferation by F3. Together, these findings indicate that antagonism between different CNTN proteins plays a critical role in modulating neuronal precursor differentiation for which the function of L1-like proteins is required.
MATERIALS AND METHODS
Antibodies
Anti-BrdU [monoclonal (mAb), BD Biosciences]; anti-Ki67 [polyclonal (pAb), Novocastra]; anti-Math-1 (pAb) (Helms and Johnson, 1998); anti-NeuN (mAb, Chemicon); anti-NrCAM (pAb 838) (Sakurai et al., 2001); anti-TAG1 [mAb 4D7, pAb anti-TAG (Dodd et al., 1988; Denaxa et al., 2001)]; anti-TuJ1 (mAb, Covance); anti-L1 (mAb 324; Chemicon) (Lindner et al., 1983); anti-Pax6 (mAb, DSHB); anti-GFAP (pAb; DAKO); goat anti-human immunoglobulin-fc domain (Sigma) and anti-phosphorylated Histone H3 (mAb, pH3, Upstate) were used.
Animals
Production, characterisation and maintenance of TAG1- and NrCAM-null mutant mice was as reported previously (Law et al., 2008; Sakurai et al., 2001). Math1-lacZ mice have been described previously (Helms and Johnson, 1998). Mice were maintained on C57Bl/6 background. Animals were generated and maintained with UK Home Office and Local Ethical Committee approval.
DNA constructs and proteins
F3-fc, TAG1-fc and L1-fc fusion proteins were produced in HEK293T cells (Law et al., 2008) with F3-fc, TAG1-fc or L1-fc expression vectors; L1 and TAG1 are human cDNAs (De Angelis et al., 2002; Tsiotra, 1996); F3 is a mouse cDNA (Buttiglione et al., 1998). Secreted proteins were purified on ProteinG sepharose (GE Healthcare). Purified fusion proteins (6-10 μg/ml) were crosslinked with anti-fc (10 μg/ml) for 1.5 hours at 37°C before addition to cultures. For binding studies, fusion proteins were crosslinked as above, were added to cells for 15 minutes at 37°C and were processed for immunodetection. SHH was produced as described (Ohyama et al., 2005) and titrated to give maximal GNP proliferation response. GPI-linked proteins were cleaved using phosphatidylinositol-specific phospholipase C (PI-PLC) (Law et al., 2008).
Cerebellar granule neuron cultures
Whole cerebellae were dissected at postnatal day 5 (P5) and GNPs were purified over two-step Percoll gradient as described previously (Solecki et al., 2001). GNPs were then plated on PDL-coated coverslips in serum-free medium (BME with N2 supplement). Each experiment used cells harvested from at least five pups of the relevant genotype. Additions of SHH and/or purified proteins (F3-fc, TAG1-fc, L1-fc) were made 1.5 hours after plating. Cells fixed and immunostained at 48 hours. For BrdU labelling, BrdU was added to medium up to 10 μM for the last 24 hours of culture.
Quantitation of granule neuron subclasses
GNP proliferation was quantitated by calculating the percentage of BrdU-labelled cells per field (at least 10 fields per experimental point); values plotted are mean±s.e.m. from at least three separate experiments. Significant differences between treatments were assayed by Student's t-test. Proportions of different GNP subpopulations (see Results) were determined in vivo by counting and classifying the cells from three sagittal sections per animal in the vermis at P5, or at least 1000 cells per condition per in vitro experiment. Results reported are mean±s.e.m. from at least three animals or from three separate culture experiments. Significant differences between subpopulations were assayed by ANOVA analysis with Bonferroni post-treatment.
Immunodetection
Immunodetection was carried out as described previously (Cohen et al., 1998). Cells/sections were analysed using epifluorescence, GRID or confocal microscopy, as indicated. For BrdU labelling, cells/sections were incubated in 2 N HCl solution for 35 minutes prior to antibody application. DAPI was used in all cells/sections to counterstain nuclei. β-Galactosidase activity was visualised as described previously (Helms et al., 2000).
Nissl staining and cluster quantitation
Paraffin-embedded 10 μm sections were Nissl stained using standard procedures. NIH image software used to quantify the number of clusters. Four coronal sections were quantitated per animal (see Fig. S1 in the supplementary material for details).
In vivo BrdU labelling and quantitation
To birthdate ectopic granule neuron clusters, mice were injected intraperitoneally with BrdU (50 μg/g) at P6 and P12 and analysed at P30. To quantitate proliferation and migration at P12, mice were injected as above at 0 and 8 hours, culled at 24 hours, perfused with PBS and 4% PFA, and processed for anti-BrdU immunodetection. One to three sections per animal from the pre-pyramidal fissure (lobule 7) were imaged and the proportion of BrdU labelled cells/DAPI nuclei determined in each cortical layer (∼900 cells counted per section). Four animals were analysed per genotype; values plotted are mean±s.e.m. Differences were assayed for significance using a t-test.
RESULTS
F3/contactin acts directly on cerebellar granule neuron precursors to suppress sonic hedgehog-induced proliferation
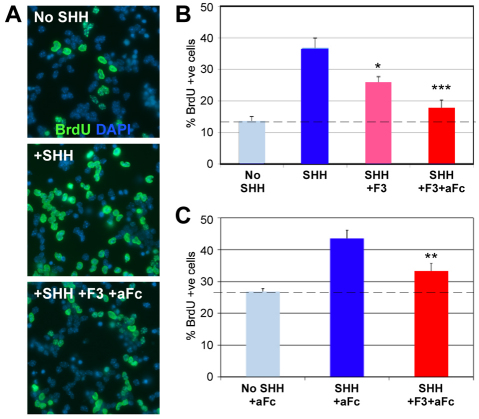
Misexpression of F3/contactin (F3) in the developing cerebellum of TAGF3 mice delays the growth of the cerebellar cortex in the first two postnatal weeks (Bizzoca et al., 2003). However, it was unclear whether this was a direct effect of F3 on GNPs or due to F3 acting indirectly through other cell types. To address this, we tested the effect of purified F3 protein on cultures of GNPs isolated from whole P5 cerebellae (Hatten, 1985). We cultured cells at low density in the presence of the GNP mitogen SHH (Fig. 1) (Dahmane and Ruiz-i-Altaba, 1999; Wallace, 1999; Wechsler-Reya and Scott, 1999). SHH increased GNP proliferation, but this increase was significantly reduced when purified F3 protein fused to the fc domain of human immunoglobulin (F3-fc) was also added to the cultures, and almost completely eliminated if F3-fc was first crosslinked with anti-fc antibody (Fig. 1B) or presented as a substrate (not shown). By contrast, addition of anti-fc, or anti-fc and fc alone, or of the related molecule L1-fc (Dihne et al., 2003) had no effect (Fig. 1B,C; not shown). Because these cultures are over 95% Pax6+ and under 1% GFAP+, this indicates that F3 protein acts directly on GNPs to suppress SHH-induced proliferation.
Fig. 1.
F3/contactin suppresses Shh-induced proliferation of granule neuron precursors. (A) GNPs from P5 mice were purified and cultured with no additions (no SHH), with SHH (SHH), with SHH and F3-fc (SHH+F3) or with SHH and F3-fc pre-incubated with anti-fc (SHH+F3-fc+afc), then labelled at 48 hours with anti-BrdU and DAPI. (B) Quantification of a typical experiment demonstrating that anti-fc crosslinking of F3-fc produces a stronger effect (***P<0.0005, t-test) than F3-fc alone, which nonetheless gives significantly less proliferation (*P<0.05, t-test) than SHH alone. (C) Quantification of five experiments: crosslinked F3-fc is compared with addition of SHH plus anti-fc (SHH+afc) and anti-fc alone (no SHH+afc). Crosslinked F3-fc consistently suppresses the SHH-induced proliferation (**P<0.01, t-test). Anti-fc was added in all conditions. Results are mean±s.e.m.
F3 induces GNP differentiation in the presence of SHH
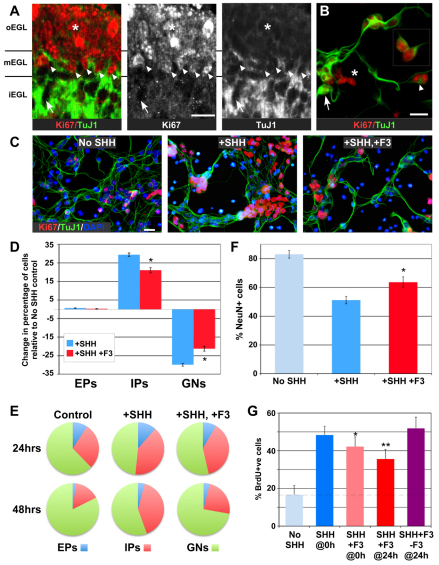
Failure to see an increase in BrdU incorporation in the presence of F3 could indicate that GNPs are induced to exit cell cycle and differentiate, as observed for FGF added with SHH (Wechsler-Reya and Scott, 1999), but may also indicate increased cell death or arrest of cells in an immature state. As we did not see increased apoptosis (not shown), we monitored the state of maturation of GNPs under different culture conditions. Despite being over 95% Pax6+, our cultures nonetheless contain GNPs at different stages of differentiation. Using the proliferation marker Ki67 (Scholzen and Gerdes, 2000) and TuJ1, which identifies neuron-specific β-tubulin III in immature neurons (Yaginuma et al., 1990), we classified cells as early progenitors (EPs: Ki67+/TuJ1–), intermediate progenitors (IPs: Ki67+/TuJ1+) and postmitotic granule neurons (GNs: Ki67–/TuJ1+). In vivo, EPs define the oEGL (Fig. 2A), whereas GNs are found in the iEGL and IGL. However, the EGL also contains IPs in a region of overlap between the oEGL and iEGL which we term the middle EGL (mEGL; Fig. 2A). At P5, we find the proportion of IPs to be 0.17 (±0.01 s.e.m.; compare with EPs 0.48±0.06 and GNs 0.36±0.05; see Materials and methods). The mEGL is probably equivalent to the PCNA+/p27+ region identified previously (Miyazawa et al., 2000).
Fig. 2.
SHH and F3 primarily affect intermediate GNPs. (A) GRID confocal images of cryosections from P5 mice immunolabelled with Ki67 and Tuj1 identify early progenitors (EPs; Ki67+/TuJ1–; asterisk) in the oEGL, intermediate progenitors (IPs; Ki67+/TuJ1+; arrowheads) in the mEGL and postmitotic GNs (Ki67–/TuJ1+; arrow) in the iEGL. Ki67 and TuJ1 are also shown separately in grayscale. See also Fig. S2 in the supplementary material. (B) The same populations (EPs, asterisk; IPs, arrowhead; GNs, arrow) are present in in vitro cultures of P5 GNPs. Inset shows isolated pair of IPs. (C-E) Treatment of GNP cultures with SHH (+SHH) or SHH and F3-fc (+SHH, +F3) alters the proportion of each population (C), as quantified in D and illustrated in E. F3-fc induces a significant increase in GNs (*P=0.0001, ANOVA) and corresponding decrease in IPs (*P<0.0005, ANOVA) relative to SHH alone. (F) A similar increase in GN numbers is seen with NeuN (*P<0.05, t-test). (G) The main effect of F3-fc addition was evident in the second 24-hour period: although both treatments significantly suppressed SHH-induced proliferation, addition of F3-fc 24 hours after SHH (SHH+F3@24h) was more effective (**P<0.0001, t-test) than addition at the same time (SHH+F3@0h; *P<0.005, t-test), whereas removal after 24 hours (SHH+F3-F3@24h) had no significant effect. Anti-fc was added in all conditions. Results are mean±s.e.m. Scale bars: 50 μm.
Each of these developmental stages was also found in vitro (Fig. 2B), including Ki67+ cells with neurites clearly labelled by TuJ1 (IPs, e.g. inset), consistent with previous observations (Wolf et al., 1997; Memberg and Hall, 1995). However, the proportion of each stage changed according to the culture conditions. Notably, addition of SHH increased the proportion of IPs at the expense of differentiated GNs, while simultaneous addition of F3-fc reduced this effect (Fig. 2C-E). Consistently, SHH treatment also reduced the proportion of cells expressing the neuronal differentiation marker NeuN, whereas, again, F3-fc antagonised this effect (Fig. 2F). Although the effects of F3 and SHH were detectable after 24 hours, the strongest reversal of the SHH effect by F3 occurred during the second 24 hours when IPs are most abundant (Fig. 2E,G). Indeed, the F3 effect was stronger if added at 24 hours than at 0 hours, whereas it had no effect if removed after 24 hours (Fig. 2G).
Our analysis indicates that the IP population in vitro is expanded relative to that in vivo, especially in the presence of SHH. This suggests that, in vivo, the IP population may be kept in check by factors that induce their terminal differentiation (perhaps F3 itself), which are either not present or reduced in effect in vitro. Alternatively, IP expansion in vitro could be due to removal of a constraint that normally inhibits the differentiation of EPs into IPs. However, this constraint cannot simply be lack of exposure to SHH because in vivo EPs already express markers that indicate SHH pathway activation [Gli1 (Corrales et al., 2004); Ptc1 (Lewis et al., 2004)]. This implies that other factors would be involved.
In principle, given these scenarios, F3 could act on IPs or EPs. However, as F3-fc addition in vitro does not significantly affect EP proliferation (Fig. 2D-E) and its strongest effects occur coincident with increasing IP, not EP abundance, the most parsimonius interpretation of our data argues that F3-fc suppresses the SHH-induced expansion of Ki67+/TuJ1+ IPs by inducing cell cycle exit and promoting GN differentiation (see also below).
TAG1 is expressed on proliferating cerebellar granule neuron precursors
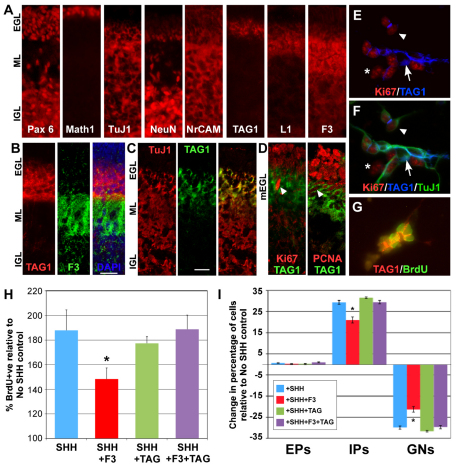
In vivo, high level expression of F3 on GNPs is restricted to postmitotic cells of the iEGL and IGL, coincident with NeuN, whereas the related molecule TAG1 (Cntn2) appears more superficially in the EGL, largely non-overlapping with F3, but not on the Math1+ cells of the oEGL (Bizzoca et al., 2003; Virgintino et al., 1999) (Fig. 3A,B). This suggests TAG1 marks the mEGL. Indeed, its expression overlaps with TuJ1 superficially (Fig. 3A,C, see also Fig. S2 in the supplementary material) and with proliferation markers in some cells in vivo and in vitro (Fig. 3D-G, see also Fig. S2 in the supplementary material). These results confirm TAG1 expression on proliferating and post-mitotic GNPs (Bizzoca et al., 2003) and define mEGL progenitors as TAG1+/TuJ1+/NeuN-/Math1–/Ki67+ (Fig. 3C,F).
Fig. 3.
TAG1 antagonises F3 suppression of SHH-induced proliferation. (A-D) Immunolabelled P5 cryosections (Pax6, Math1, TuJ1, NeuN, NrCAM, TAG1, L1, F3 antibodies) visualised by epifluorescence (A) or GRID microscopy (B-D). TAG1 expression in the lower EGL is largely mutually exclusive with that of F3 (B), overlaps TuJ1 superficially (C) and is expressed on Ki67+ and PCNA+ cells (arrowheads) in the mEGL (D; see Fig. S2 in the supplementary material). (E-G) TAG1+/Ki67+ GNPs are found in cultures (arrowhead; E), along with TAG1–/Ki67+ (asterisk) and TAG1+/Ki67– (arrow) cells, corresponding to IPs, EPs and GNs respectively, as defined by co-labelling with TuJ1 (F). BrdU also co-labels some TAG1+ cells (G). (H,I) P5 GNPs cultured with SHH, SHH+F3, SHH+TAG or SHH+F3+TAG. Cells immunolabelled at 48 hours with anti-BrdU (H), or Ki67 and TuJ1 (I), and quantified as before. Significant repression of proliferation (*P<0.05, t-test, H) and stimulation of differentiation (*P<0.001, ANOVA, I) is reversed by TAG-fc. TAG-fc alone has no effect. Anti-fc was added in all conditions. Results are mean±s.e.m. Scale bars: 50 μm.
TAG1 antagonises F3 induction of GNP differentiation
Both misexpression of F3 in vivo (Bizzoca et al., 2003) and F3-fc treatment in vitro inhibit GNP proliferation and disrupt differentiation. As TAG1 and F3 expression is largely mutually exclusive (Fig. 3A,B) and these molecules are known to be antagonistic in promoting axon outgrowth (Buttiglione et al., 1998), we tested whether TAG1 antagonises F3 function on GNPs. Addition of crosslinked TAG1-fc to GNP cultures with or without SHH had no significant effect on proliferation (Fig. 3H). However, addition of TAG1-fc at the same time as F3-fc completely negated its inhibitory effect (Fig. 3H). This occurred whether the proteins were crosslinked together or separately, indicating this was not due to TAG1-fc reducing the dosage of crosslinked F3-fc. As purified TAG1-fc does not bind F3-fc directly (Pavlou et al., 2002), it is also unlikely to be due to TAG1-fc neutralising F3-fc through direct binding. TAG1-fc also negated the effects of F3-fc on differentiation (Fig. 3I). Thus, TAG1 antagonises the effects of F3 on GNP proliferation and differentiation in vitro.
Loss of TAG1 affects granule neuron development
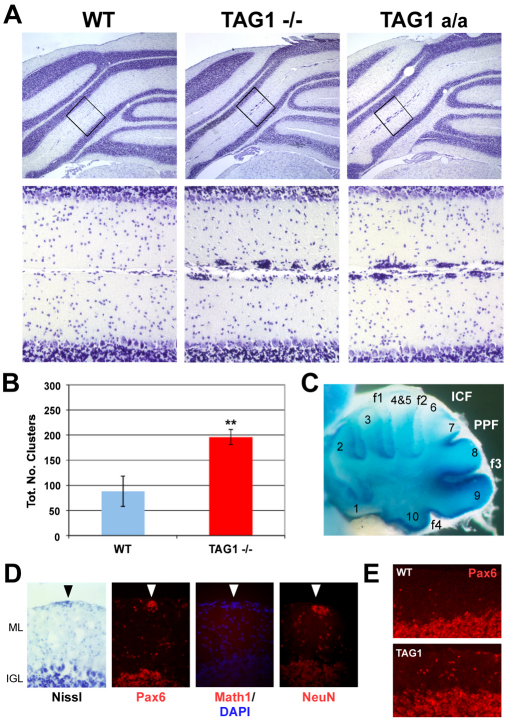
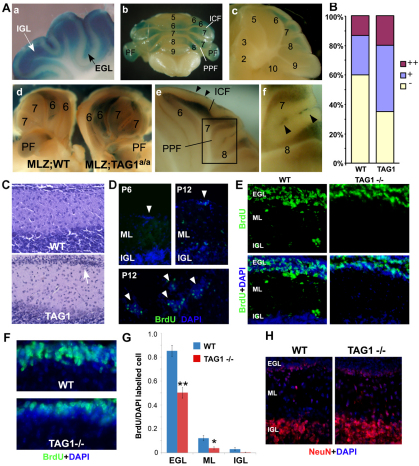
We looked for evidence that TAG1 is required in vivo for regulation of GNP proliferation. Brain and cerebellar morphology is largely unaffected by loss of TAG1 (Fukamauchi et al., 2001; Poliak et al., 2003) (Fig. 4A). However, we noticed ectopic clusters of small granule neuron-like cells at the pial surface of the cerebellum of adult TAG1 mutant mice (Fig. 4A). Clusters were present in both TAG1-null (Poliak et al., 2003) and TAG1a/a hypomorph (Law et al., 2008) animals (Fig. 4A,B), and in both cases were more prevalent in posterior lobules, notably in the intercrural fissure, paraflocculus, pre-pyramidal fissure and third fissure. Interestingly, this is coincident with higher TAG1 expression in this region at P2 (Fig. 4C). Overall, TAG1 mutants had more than twice the number of clusters seen in wild-type animals (Fig. 4B). Cells in clusters expressed Pax6 and NeuN, but not Math1, or the mitotic marker phospho-histone H3 (Fig. 4D), suggesting these are GNs that failed to migrate from the EGL.
Fig. 4.
Ectopic granule neurons in TAG1 mutants. (A) Nissl-stained sections of adult wild-type (WT), TAG1-null (–/–) and TAG1 a/a mice showing clusters, with boxed areas shown at higher magnification in the lower panels. (B) Quantification of clusters (see Materials and methods, and Fig. S1 in the supplementary material) in wild-type and TAG1–/– mice. TAG1–/– have significantly more clusters than wild type (**P<0.05, t-test). Results are mean±s.e.m. (C) β-Gal staining of TAG1-lacZ expression at P2 in TAG1 lacZ knock-in mice (Poliak et al., 2003). Clusters in adult TAG1 mutants are observed in lobules 6-10, notably in the intercrural (ICF), pre-pyramidal (PPF) and third fissures (f3), coincident with higher TAG1 expression at P2, but not in lobules 1-5. (D) Clusters (arrowheads) in a TAG1 mutant at P30, identified by Nissl and DAPI staining, contain Pax6 and NeuN, but not Math1. (E) More granule neurons (Pax6+) were present in the ML of TAG1 mutants.
Consistent with a perturbation in GN migration, there appeared to be more Pax6+ cells in the ML of adult TAG1 mutant animals (Fig. 4E). To examine this in more detail, we made use of a transgenic line (MLZ) that expresses lacZ from the Math1 promoter (Helms and Johnson, 1998). Although Math1 normally marks proliferating oEGL cells, β-galactosidase activity persists in MLZ GNs as they migrate radially (Helms et al., 2001), so that at P15 both the EGL and IGL are labelled (Fig. 5Aa). However, by P22, labelling persists only in the late-developing lobules (Fig. 5A, parts b,c) (Altman and Bayer, 1997), and then only in the IGL (Fig. 5A, part c). When the MLZ gene was crossed into the TAG1a/a mutant background (the TAG1 null allele contains a tau-lacZ reporter, precluding its use in this context) (Poliak et al., 2003), we consistently observed more labelling in animals lacking wild-type TAG1 (MLZ;TAG1a/a) than in littermates with wild-type TAG1 (MLZ;WT, Fig. 5A, part d, B), including labelling in the EGL (compare Fig. 5A, part c with part e), particularly in lobules 6-10, where many cells are born after P11 (Altman and Bayer, 1997). A persistent EGL was also observed in TAG1 mutants, most strikingly in the hemispheric PPF at P15 (Fig. 5C), but not obviously at earlier times or at any time in the earlier developing lobules at the midline (not shown). Birth-dating with bromodeoxyuridine (BrdU) confirmed that the ectopic clusters in TAG1 mutants included cells born at P12, but not at P6 (Fig. 5D), consistent with loss of TAG1 particularly affecting lobules where GN production is prolonged (Altman and Bayer, 1997). Together, these data suggested that loss of TAG1 perpetuated the differentiation of GNPs, particularly in the late-developing hemispheric lobules.
Fig. 5.
Perturbed granule neuron development in TAG1 mutant mice. (A) (a) Whole-mount sagittal section of P15 cerebellum. β-Gal activity (blue) in MLZ mice persists in GNs in the IGL. (b) Posterior view of P22 MLZ cerebellum shows differing intensities of staining especially in lobules 6-9, which include the ICF, PPF and paraflocculus (PF). (c) Sagittal section of b. β-Gal persists only in the IGL of lobules 6-8. (d) Compared with MLZ mice (MLZ;WT), MLZ;TAG1a/a mutant cerebellae exhibited more β-gal staining at P22 (whole-mount view of cerebellar halves developed together). (e,f) Sagittal section of MLZ;TAG1a/a P22 cerebellum reveals prominent staining in the EGL (arrowheads) and IGL, (compare with c). (B) Classification of MLZ;WT versus MLZ;TAG1a/a cerebellae. β-Gal labelling was classified by a `blinded' observer as strong (++), moderate (+) or unlabelled (–) in 23 cerebellae from each genotype. (C) Representative Nissl-stained sections from the PPF of wild-type and TAG1a/a mutants at P15. EGL was consistently thicker in the hemispheres of mutants (arrow), but not at the midline (not shown) of TAG1 mutants. (D) TAG1 mutant animals injected with BrdU at P6 and P12 and analysed at P30. DAPI counterstain reveals clusters (arrowheads) are BrdU labelled after P12, but not after injection at P6. The lower panel is a higher magnification of a section from an animal injected after P12. (E-G) Twenty-four hours after injection of BrdU at P12, significantly fewer labelled cells were found in the EGL (**P<0.005, t-test) and ML (*P<0.05, t-test) of the PPF of TAG1–/– animals compared with wild type (WT) (G). Results are mean±s.e.m. BrdU label in TAG1–/– (E) was largely restricted to oEGL, whereas it is found throughout EGL in wild type (F). (H) NeuN+ cells are no more abundant in TAG1–/– EGL than in wild type.
A persistent EGL in older mutant animals might be due to a failure of GNs to migrate from the EGL or it might result from a reduction in the rate at which cells are produced, perhaps causing production to continue for longer in compensation. To examine whether GN radial migration is affected, we injected BrdU at P12 and examined the distribution of labelled cells 24 hours later, focussing upon the pre-pyramidal fissure (PPF). Although we found significantly fewer labelled cells in the ML and IGL of TAG1-null mice than wild type, we also found an ∼40% reduction in the proportion of labelled cells in the EGL (Fig. 5E-G). As we did not see a significant accumulation of differentiated NeuN+ GNs in the iEGL (Fig. 5H), this indicates that the main effect of loss of TAG1 was a reduction in GN production rather than a delay in radial migration. Interestingly, most of the BrdU labelling in the TAG1 mutant was in the outer layer of the EGL, whereas that in wild type was also present in the deeper layers (Fig. 5E,F), consistent with the idea that TAG1 is indeed required for proliferation of IPs in the mEGL. Thus, given that antibodies to TAG1 do not disrupt radial migration in vitro (Fishell and Hatten, 1991) and that TAG1 antagonises the suppressive effect of F3 on GNP proliferation in vitro, these data support that TAG1 expression is required on GNPs for the sustained production of GNs in the later developing lobules of the cerebellum.
F3 and TAG1 appear to compete for binding to a common receptor on GNPs
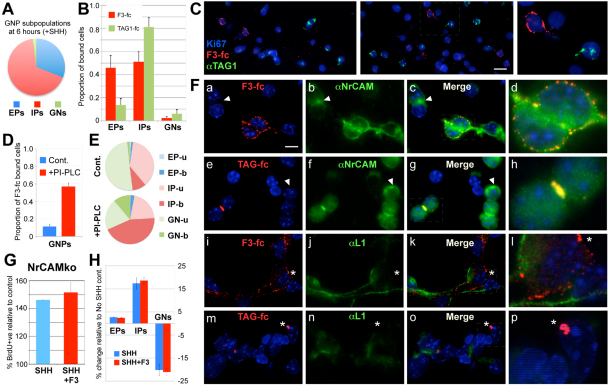
To begin to elucidate the mechanism by which F3 and TAG1 have their antagonistic effects on GNP proliferation, we sought to identify which cells bind these proteins in our cultures. At 6 hours, ∼10% of all cells bound F3-fc, whereas somewhat fewer (∼5%) bound TAG1-fc. Consistent with the overall composition of the culture at this time (31%±4.7% EPs, 67%±4.7% IPs and 2%±0.2% GNs; Fig. 6A), the majority of the cells bound (at least 95%) were proliferating (Ki67+) progenitors, but in the case of F3-fc roughly half were EPs and half were IPs. The majority of cells bound by TAG1-fc were IPs (Fig. 6B). Fewer than 5% were post-mitotic GNs in either case.
Fig. 6.
NrCAM colocalises with F3 on GNPs and is required for F3 suppression of SHH-induced proliferation. (A) Overall composition of GNP cultures at 6 hours (compare with Fig. 2E). (B) Distribution of cells at 6 hours that bind either F3-fc or TAG-fc among the GNP subpopulations. The total number of cells binding F3-fc and TAG-fc represents ∼10% and ∼5% of all cells at 6 hours, respectively. Results are mean±s.e.m. (C) F3-fc does not bind to TAG1-expressing cells. Left panels: two different fields of GNPs co-labelled with F3-fc, anti-TAG1 and Ki67. Dashed area in right panel shown at higher magnification on the right. Scale bar: 50 μm. (D) The proportion of SHH-treated GNPs that bind F3-fc at 24 hours increases at least fivefold after addition of PI-PLC at 18 hours (PI-PLC+), compared with SHH-treated alone (Cont.). Results are mean±s.e.m. (E) Although all classes of GNP bind more F3-fc (EP-b, IP-b, GN-b) after PI-PLC treatment (compare with Cont.), the major increase is in the IP-bound component (IP-b). Moreover, PI-PLC treatment increases the overall proportion of IPs in the culture [i.e. IP-b + IP-unbound (IP-u)], apparently at the expense of the GN component (GN-b + GN-u; see text). (F) Micrographs of GNPs binding either F3-fc (a-d,i-l) or TAG-fc (e-h,m-p) and double stained with anti-NrCAM (a-h) or anti-L1 (i-q), and counterstained with DAPI (blue). Scale bar: 20 μm. The boxed areas of the merged panels are shown at higher magnification on the right (d,h,m,q). Asterisks, lack of colocalisation with L1; arrowheads, NrCAM-expressing cells that were not labelled with fusion protein. (G) GNPs from NrCAM-null mice (NrCAMko) proliferate in response to SHH (relative to no SHH control), but proliferation is not repressed by F3-fc. (H) NrCAM-null GNPs are not induced to differentiate by F3-fc. In contrast to wild type (Fig. 2C), addition of F3-fc with SHH (SHH+F3) does not alter the proportions of cells in each of the GNP subpopulations relative to SHH alone (SHH). Anti-fc added in all conditions. Results are mean±s.e.m.
We were surprised that F3-fc bound relatively few cells at 6 hours and considered whether this binding might increase with time, given the stronger effect of F3-fc addition at 24 hours (Fig. 2G). In fact, binding remained similar at 24 hours (∼10%), although by this time the majority of cells binding F3-fc were IPs (∼85%, compare with ∼50% at 6 hours). We noticed, however, that F3-fc never bound to cells expressing endogenous TAG1 (Fig. 6C), consistent with previous observations (Pavlou et al., 2002), and suggesting that TAG1 expression may prevent the binding of F3-fc to GNPs. To test this, we treated SHH-induced GNPs at 18 hours with PI-PLC, which cleaves the tails of GPI-anchored proteins, and then assayed F3-fc binding at 24 hours. PI-PLC treatment resulted in a fivefold increase in the proportion of cells that bind F3-fc (Fig. 6D), suggesting that a GPI-linked endogenous protein normally blocks binding of F3-fc to GNPs. Binding increases significantly to IPs and GNs, with the major part accounted for by IPs (Fig. 6E). Interestingly, PI-PLC treatment at 18 hours also increases the overall proportion of IPs in the culture (65±4% after SHH and PI-PLC, compared with 46±3% with SHH alone), at the expense of GNs (Fig. 6E). As most GNs in our cultures (but not IPs) themselves express F3 by 24 hours (not shown), these data are consistent with the idea that contact of IPs with F3-expressing GNs induces differentiation, that TAG1 normally inhibits F3 binding to GNPs and that when both GPI-linked proteins are absent, the number of proliferating IPs increases, while differentiation is inhibited.
NrCAM is required on GNPS to mediate the effects of F3
To identify the receptor mediating the effects of F3 on GNPs, we considered especially L1 and NrCAM, as each binds both F3 and TAG1 (Brummendorf and Lemmon, 2001; De Angelis et al., 2002; Sakurai et al., 2001) and is expressed in the EGL during the first postnatal week (Fig. 3A) (Sakurai et al., 2001). A priori NrCAM appeared more likely because we found it expressed on cells in the oEGL (Fig. 3A, see also Fig. S3A,B in the supplementary material), albeit at lower levels than the high level expression previously reported in the iEGL and ML (Sakurai et al., 2001), whereas L1 is not present in this region (Fig. 3A).
When co-labelled with anti-NrCAM, both F3-fc and TAG1-fc bound only to NrCAM-expressing cells and were substantially colocalised with bright puncta of endogenous NrCAM staining (F3-fc: Fig. 6F, parts a-d; TAG1-fc: Fig. 6F, parts e-h). However, in each case NrCAM-expressing cells were found that were not labelled with fusion protein (Fig. 6F, parts a-h, arrowheads), suggesting that molecules in addition to NrCAM may be required for binding or that NrCAM binding sites may be masked in some GNPs.
In contrast to NrCAM, both fusion proteins could be found bound to cells that did not express L1; moreover, where the proteins did bind L1-expressing cells, there was a marked lack of colocalisation with L1 (Fig. 6F, parts i-k,n-p, asterisks). Thus, both F3-fc and TAG1-fc bound only cells expressing NrCAM and colocalised with NrCAM, whereas neither protein colocalised with L1 and both could be found bound to cells not expressing L1. Because L1 was expressed only on postmitotic (Ki67-) cells, this is consistent with F3-fc and TAG1-fc fusion proteins having their effects by binding to NrCAM on proliferating GNPs.
To confirm that NrCAM is the relevant F3-binding receptor, we tested the ability of F3-fc to bind GNPs from NrCAM-null mutants (Sakurai et al., 2001). Binding was reduced by ∼50% compared with wild type, mainly through loss of binding to IPs, but persisted on ∼5% of GNPs, mainly EPs, and was not colocalised with L1 (see Fig. S4 in the supplementary material).
To determine the functional significance of the loss of F3-fc binding, we tested the ability of F3-fc to suppress SHH-induced proliferation of NrCAM-null GNPs. As with wild-type cells, SHH substantially increased the proportion of proliferating cells (Fig. 6G). However, simultaneous addition of F3-fc failed to suppress this increase in proliferation (Fig. 6G), indicating that NrCAM is required to mediate the F3 effect. Consistently, F3-fc also failed to induce the differentiation of NrCAM-null GNPs (Fig. 6H; cf. Fig. 3G). Importantly, this result also shows that F3-fc is not producing its effect on normal cells simply by binding directly to SHH to reduce its effective concentration. Together, these data strongly indicate that NrCAM is required on GNPs for F3-fc to suppress SHH-induced proliferation, and suggest that it is the binding of F3-fc to IPs, not to EPs, that is required for these effects.
DISCUSSION
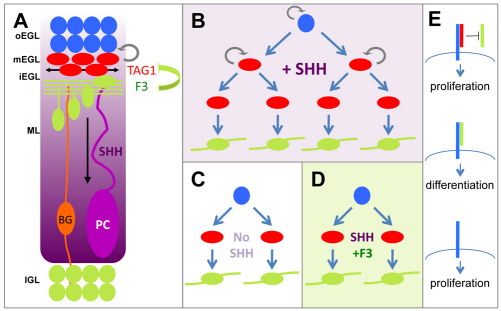
The mechanisms that control the coordinated growth of a tissue remain poorly understood, but in cerebellar morphogenesis SHH signalling has emerged as a crucial factor affecting the complexity of foliation (Corrales et al., 2004; Corrales et al., 2006; Lewis, 2004). A key effect of SHH is stimulation of cerebellar GNP proliferation, but exactly which progenitors are affected and how the effects are controlled locally is not known. Here, we show that two sequentially expressed members of the CNTN family of adhesion molecules, TAG1 and F3, act antagonistically to control SHH-induced proliferation: in vitro F3 protein suppresses proliferation and instead induces differentiation, but this is reversed by TAG1. Correspondingly, loss of TAG1 in vivo leads to reduced and delayed production of GNPs in the EGL, notably in the later developing lobules. F3 and TAG1 both colocalise with NrCAM on GNPs, and F3 fails to suppress SHH-induced proliferation of NrCAM null GNPs. The main effect of F3 is on the IP subpopulation, which is also the subpopulation expanded by SHH and that to which TAG1 primarily binds. In vivo, IPs in the mEGL express TAG1, whereas F3 becomes expressed on the axons of differentiated GNs as they leave the iEGL. We propose, therefore, that F3 acts as a localised signal in the iEGL that induces SHH-stimulated cells in the overlying mEGL to exit cell cycle and differentiate (Fig. 7A). By contrast, expression of TAG1 on GNPs antagonises this signal, inhibiting differentiation while sustaining GNP expansion. Dissociation of GNPs in vitro releases IPs from localised inhibition by F3 so that these cells proliferate in a SHH-dependent manner (Fig. 7B,C), which in turn can be suppressed by delocalised addition of F3-fc protein (Fig. 7D).
Fig. 7.
Model of action of F3 and TAG1 in GNP development. (A) IPs expressing TAG1 (red) respond to SHH synthesised by Purkinje cells (PC) by proliferating, but exit cell cycle and differentiate when they contact postmitotic GNs expressing F3 (green). (B-D) In vitro, SHH expands the pool of proliferating IPs expressing TAG1, whereas without SHH, or when F3 is added with SHH, their proliferation is suppressed and they differentiate. (E) When TAG1 is present on the GNP cell surface, F3 cannot bind and NrCAM (blue) promotes proliferation. Without TAG1, F3 binds and promotes differentiation. In the absence of both TAG1 and F3, NrCAM promotes proliferation.
SHH and CNTNs affect intermediate GNP proliferation and differentiation
The identification of IPs as a major target of the mitogenic effects of SHH and the modulatory effects of F3 and TAG1 is consistent with previous observations: an identifiable, albeit thin, EGL remains when SHH is blocked by antibodies or conditionally removed from Purkinje cells (Dahmane and Ruiz-i-Altaba, 1999; Wechlser-Reya and Scott, 1999; Lewis et al., 2004). In the latter, it is clear that while the number of Math1+/TuJ1– cells in the oEGL remains near normal, there is major loss of TAG1+ cells and depletion of mature GNs in the IGL. This suggests that a major function of SHH is to expand the IP population, while F3 and TAG1 modulate this expansion, and that SHH is not required for the survival of EPs, at least not during the postnatal period. Although we see little effect of SHH on EP proliferation in vitro, we cannot rule out that it is required in vivo, as early removal of SHH (using Pax2-cre) does deplete the EP layer (Lewis et al., 2004). However, it is not clear whether this is due to the effects of loss of SHH on EPs directly, or to effects on other cell types, as Pax2 is expressed throughout the midbrain-hindbrain region before the EGL is formed.
Nonetheless, Gli1 and Ptc1 expression is elevated throughout the EGL in the early postnatal period, indicating that oEGL cells respond to SHH (Corrales et al., 2004; Lewis et al., 2004). The minimal response of EPs in vitro compared with IPs may indicate that dissociation of the cells has removed some component required for EP proliferation, or it may reflect that EPs normally respond to SHH by becoming proliferating IPs. Although it is possible that the IP expansion seen is due entirely to an effect of SHH on the EPs, the elevation of Ptc1/Gli1 expression in all EGL cells indicates that SHH also elicits responses in IPs.
The role of SHH in expanding IPs may be analogous to the role of Hedgehog (Hh) in the Drosophila eye, where it initiates a final round of mitosis by retinal precursor cells, as part of their terminal differentiation program. Whether SHH induces just one round of mitosis in IPs is unclear, but GNP proliferation in culture is limited, while differentiation continues, even when SHH is present (Miyazawa et al., 2000) (this study). Hh induces final mitosis by activating Notch through elevated Delta expression (Baonza and Freeman, 2005). Notch signalling also controls GNP proliferation (Solecki et al., 2001) and medulloblastoma arising from SHH pathway activation is Notch pathway dependent (Hallahan et al., 2004). How these two pathways interact in vertebrates remains unclear (Behesti and Marino, 2009), but knowing that GNP subpopulations respond differentially to SHH should aid their dissection.
TAG1 modulates the effects of F3 on neuronal progenitors
IPs that are expanded in response to SHH express TAG1. We suggest that TAG1 is only required in this context to counteract the effects of F3. Treatment of GNPs with TAG1 alone does not induce proliferation, but when added together with F3 suppresses its effects. F3 does not bind GNPs that express TAG1 and when GPI-linked proteins are removed by PI-PLC, F3-binding sites increase in number, especially on IPs (which normally express TAG1 not F3), suggesting that TAG1 prevents F3 binding. Intriguingly, PI-PLC also increases the number of IPs at the expense of differentiated GNs, indicating that in the absence of either protein IPs proliferate rather than differentiate (Fig. 7E). What finally allows F3 to induce differentiation is not clear, although the apparently graded expression of F3 in the EGL (Fig. 3A) suggests the balance between TAG1 and F3 expression may be important. How TAG1 expression is regulated in the cerebellum is not known, although it has been shown to switch from surface to secreted expression as spinal neurons mature (Karagogeos et al., 1991). Whatever the mechanism, our data agree with the notion that TAG1 modulates the generation of postmitotic neurons (Ma et al., 2008) and together these studies provide clear evidence that CNTNs affect neural differentiation before cell cycle exit.
There is considerable evidence that CNTNs function at later stages of differentiation (Katidou et al., 2008; Law et al., 2008; Osterfield et al., 2008), and indeed TAG1 and F3 are reported to act antagonistically to control GN axon outgrowth (Buttiglione et al., 1998). It is possible that apparent effects on axon growth may in fact be due to effects on progenitors; experiments with GNs in culture, for example, often only quantify axon length and do not necessarily quantitate what proportion of cells extend axons (a measure of differentiation).
Similarly, the appearance of aberrant axonal projections into the EGL after ablation of the TAG1 homolog axonin-1 by RNA interference (RNAi) in chick was interpreted to indicate that axonin-1 is required for parallel fibre guidance (Baeriswyl and Stoeckli, 2008). Although we see no evidence of major parallel fibre disruption in TAG1 null mice (L.Y., unpublished), the RNAi electroporation method used by Baeriswyl results in mosaic knockdown of axonin-1 (Baeriswyl and Stoeckli, 2008), which may be significant: if loss of axonin-1 in individual cells in the proliferating mEGL results in those cells exiting cell cycle and differentiating precociously, these may also begin premature radial migration, leaving behind an axon, as normally occurs in the deeper iEGL. Examined retrospectively, this would give the impression of axons extending into the EGL. This interpretation is consistent with the failure of anti-TAG1 antibodies to inhibit radial migration in vitro (Fishell and Hatten, 1991), but leaves unresolved the issue of whether the absence of tangentially extending axons noted by Baeriswyl and Stoeckli (Baeriswyl and Stoeckli, 2008) is due to a requirement for axonin-1 in this extension (or indeed the preceding tangential migration of the GNP cell body) (Komuro et al., 2001), or because extension is not initiated if GNPs differentiate prematurely in the abnormal environment of the mEGL.
NrCAM is required for anti-proliferative effects of F3
Our evidence indicates NrCAM is required in GNPs for F3 to suppress SHH-induced proliferation and induce differentiation. This is consistent with our observations that NrCAM-null GNs do not extend neurites on F3 as a substrate (Sakurai et al., 2001), but suggests the F3-NrCAM interaction is required for GNPs to exit cell cycle and differentiate, rather than it necessarily being involved in neurite extension per se. In vivo, the cerebellum of NrCAM-null mice is mildly hypoplastic, an effect exacerbated when combined with loss of L1 (Sakurai et al., 2001), indicating L1 can compensate for NrCAM loss. Consistent with our observations, the IGL of L1/NrCAM double mutants, is severely depleted. Previously, since EGL thickness and TAG1 expression was largely unaffected, and because TAG1 was thought a post-mitotic marker, this was interpreted to mean that NrCAM and L1 promote survival of differentiated IGL GNs. As TAG1 is clearly expressed by cycling GNPs (this study) (Bizzoca et al., 2003), a simpler explanation is that few post-mitotic GNs are produced in these mice.
Our results suggest, however, that L1 is not normally involved in modulating SHH-induced proliferation, because we do not find it expressed on Ki67+ cells, whereas NrCAM clearly overlaps the proliferating population. Moreover, the loss of response of NrCAM-null GNPs to F3, and the fact that F3 does not bind L1 in the absence of NrCAM, suggest that L1 does not compensate this function in NrCAM knockouts. NrCAM is also redundant with CHL1 (Heyden et al., 2008) and loss of CHL1 alone results in reduced GNP proliferation. However, CHL1 is expressed only on mature GNs in the IGL and effects on proliferation appear to be an indirect consequence of its loss from Purkinje cells (Jakovcevski et al., 2009).
The lack of compensation for NrCAM loss in vitro suggests that compensation in vivo is due to redundancy with a distinct system that is not operating in our GNP cultures. Vitronectin has also been shown to antagonise SHH in vitro, yet its loss in vivo has no apparent effect (Pons et al., 2001), suggesting that vitronectin function may overlap with that of F3 and NrCAM in some way. However, given that F3 has multiple known ligands – including Notch1, RPTPβ, PTPα, neurofascin and tenascins (Katidou et al et al., 2008), most of which are expressed in the postnatal cerebellum (Berglund et al., 1999; Hu et al., 2003) – it is also possible that the binding of F3 to one of these is sufficient to compensate for NrCAM in vivo. Clearly, further studies will be required to distinguish these possibilities.
Exactly how NrCAM is involved in the modulation of SHH-induced proliferation is not clear. NrCAM is known to interact with a number of intracellular binding partners, including: FERM-domain proteins; the PDZ-containing proteins SAP102 and SAP97; members of the membrane-associated guanylate kinases (MAGUK) family; and GIPC proteins (Herron et al., 2009). SAP102 is expressed in GNs and a form of NrCAM lacking the C-terminal PDZ-binding motif suppressed GN neurite outgrowth (Davey et al., 2005); however, effects on proliferation were not assessed. Indeed, most studies of L1-like protein signalling have focussed on postmitotic events and so have not addressed directly effects on mitogenesis. The proposed involvement of L1- and CNTN-like proteins in a range of cancers (Katidou et al., 2008) and our observation that CNTN proteins can modulate SHH-induced mitogenesis, indicates that understanding these pathways may have clinical relevance, especially for medulloblastoma (Behesti and Marino, 2009).
CNTNs, L1-like molecules and cerebellar morphogenesis
Our results suggest a model in which TAG1 and F3 compete to modulate the function of NrCAM, which in some way controls when cells exit cell cycle and differentiate. The expression of F3 by differentiated GNs can be seen as a surface-bound feedback signal to less mature GNPs to coordinate their timely differentiation (Fig. 7A). That crosslinked or substrate-bound F3-fc is more effective than `free' F3-fc is consistent with this idea, as is the reduction in cerebellar size seen in our in vivo gain-of-function experiment (Bizzoca et al., 2003). However, although loss of F3 clearly causes major disruption of GN development, the F3-null cerebellum is also reduced in size (Berglund et al., 1999). Although this may not fit with F3 simply being a brake on proliferation, it is possible that the NrCAM-F3 interaction is required, not only to trigger differentiation, but also for cell survival, as suggested by Sakurai et al. (Sakurai et al., 2001). Alternatively, as F3 is also expressed in Purkinje cells and widely outside of the cerebellum (Virgintino et al., 1999), cerebellar growth may be impeded by dysfunctions in other neuronal types. Further investigation of F3 using conditional mutations will be required to resolve these possibilities.
Although the effects of its loss suggest a central role for F3, the relatively mild effects of single gene knockouts of TAG1 and NrCAM indicate their roles are more subtle. Although this is, in part, explained by redundancy between different members of the family, it is intriguing that the knockouts examined so far appear to affect different subregions of the cerebellum (Fransen et al., 1998; Sakurai et al., 2001; Heyden et al., 2008) (this study). Although there is little regional variation in the expression of the L1-like molecules, there is considerable variation in the expression levels of the CNTNs (Sakurai et al., 2001; Takeda et al., 2003; Virgintino et al., 1999; Yoshihara et al., 1995) (this study). Indeed, it is striking that regions with high level F3 expression are coincident with regions of low level SHH signalling (cf. Virgintino et al.,1999; Corrales et al., 2004). Given our evidence that TAG1 and F3 can modulate SHH-induced proliferation and the evidence that SHH signalling may affect the complexity of the foliation of the cerebellum (Corrales et al., 2004; Corrales et al., 2006), it will be interesting to discover whether the patchwork of CNTN expression contributes to regional variations in cerebellar morphogenesis.
Supplementary Material
Acknowledgments
We thank Sunny Sunshine for Fig. 5Aa (P15 cerebellum) and Heather Yeoman for Fig. 4C; Jin Nakahara for early counting of TAG1 mutant clusters; Catherine Faivre-Sarrailh, Ido Horresh, Tom Jessell, Jane Johnson, Domna Karagogeos, Sue Kenwrick, Vance Lemmon and Elior Peles for DNAs and antibodies; Jane Johnson for the Math1-LacZ mouse; Verdon Taylor for critically reading the manuscript. D.X. thanks Mary-Beth Hatten and David Solecki for instruction in granule cell cultures. This work was supported by grants to A.J.W.F. from BBSRC (50/G19549; BB/D014867) and Yorkshire Cancer Research. I.B.M. was supported by a CRUK studentship grant (C7795/A7237). T.S. is a Seaver Fellow. The Biomedical Science Light Microscopy Facility is supported by the Wellcome Trust (GR077544AIA). Deposited in PMC for release after 6 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.051912/-/DC1
References
- Altman J., Bayer S. A. (1997). Development of the cerebellar system in relation to its evolution, structure and functions. Boca Raton, FL: CRC Press; [Google Scholar]
- Baeriswyl T., Stoeckli E. T. (2008). Axonin-1/TAG1 is required for pathfinding of granule cell axons in the developing cerebellum. Neural Dev. 3, 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baonza A., Freeman M. (2005). Control of cell proliferation in the Drosophila eye by Notch signaling. Dev. Cell 8, 529-539 [DOI] [PubMed] [Google Scholar]
- Behesti H., Marino S. (2009). Cerebellar granule cells: Insights into proliferation, differentiation, and role in medulloblastoma pathogenesis. Int. J. Biochem. Cell Biol. 41, 435-445 [DOI] [PubMed] [Google Scholar]
- Berglund E. O., Murai K. K., Fredette B., Sekerková G., Marturano B., Weber L., Mugnaini E., Ranscht B. (1999). Ataxia and abnormal cerebellar microorganization in mice with ablated contactin gene expression. Neuron 24, 739-750 [DOI] [PubMed] [Google Scholar]
- Bizzoca A., Virgintino D., Lorusso L., Buttiglione M., Yoshida L., Polizzi A., Tattoli M., Cagiano R., Rossi F., Kozlov S., et al. (2003). Transgenic mice expressing F3/contactin from the TAG1 promoter exhibit developmentally regulated changes in the differentiation of cerebellar neurons. Development 130, 29-43 [DOI] [PubMed] [Google Scholar]
- Blaess S., Graus-Porta D., Belvindrah R., Radakovits R., Pons S., Littlewood-Evans A., Senften M., Guo H., Li Y., Miner J., et al. (2004). Beta1-integrins are critical for cerebellar granule cell precursor proliferation. J. Neurosci. 24, 3402-3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummendorf T., Rathjen F. G. (1996). Structure/function relationships of axon-associated adhesion receptors of the immunoglobulin superfamily. Curr. Opin. Neurobiol. 6, 584-593 [DOI] [PubMed] [Google Scholar]
- Brummendorf T., Lemmon V. (2001). Immunoglobulin superfamily receptors: cis-interactions, intracellular adapters and alternative splicing regulate adhesion. Curr. Opin. Cell Biol. 13, 611-618 [DOI] [PubMed] [Google Scholar]
- Buttiglione M., Revest J., Pavlou O., Karagogeos D., Furley A., Rougon G., Faivre-Sarrailh C. (1998). A functional interaction between the neuronal adhesion molecules TAG1 and F3 modulates neurite outgrowth and fasciculation of cerebellar granule cells. J. Neurosci. 18, 6853-6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chédotal A. (2010). Should I stay or should I go? Becoming a granule cell. Trends Neurosci. 33, 163-172 [DOI] [PubMed] [Google Scholar]
- Cohen N. R., Taylor J. S., Scott L. B., Guillery R. W., Soriano P., Furley A. J. (1998). Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr. Biol. 8, 26-33 [DOI] [PubMed] [Google Scholar]
- Corrales J. D., Rocco G. L., Blaess S., Guo Q., Joyner A. L. (2004). Spatial pattern of sonic hedgehog signaling through Gli genes during cerebellum development. Development 131, 5581-5590 [DOI] [PubMed] [Google Scholar]
- Corrales J. D., Blaess S., Mahoney E. M., Joyner A. L. (2006). The level of sonic hedgehog signaling regulates the complexity of cerebellar foliation. Development 133, 1811-1821 [DOI] [PubMed] [Google Scholar]
- Dahmane N., Ruiz-i-Altaba A. (1999). Sonic hedgehog regulates the growth and patterning of the cerebellum. Development 126, 3089-3100 [DOI] [PubMed] [Google Scholar]
- Davey F., Hill M., Falk J., Sans N., Gunn-Moore F. J. (2005). Synapse associated protein 102 is a novel binding partner to the cytoplasmic terminus of neurone-glial related cell adhesion molecule. J. Neurochem. 94, 1243-1253 [DOI] [PubMed] [Google Scholar]
- De Angelis E., Watkins A., Schüfer M., Brümmendorf T., Kenwrick S. (2002). Disease-associated mutations in L1 CAM interfere with ligand interactions and cell-surface expression. Hum. Mol. Genet. 11, 1-12 [DOI] [PubMed] [Google Scholar]
- Denaxa M., Chan C., Schachner M., Parnavelas J., Karagogeos D. (2001). The adhesion molecule TAG1 mediates the migration of cortical interneurons from the ganglionic eminence along the corticofugal fiber system. Development 128, 4635-4644 [DOI] [PubMed] [Google Scholar]
- Dihne M., Bernreuther C., Sibbe M., Paulus W., Schachner M. (2003). A new role for the cell adhesion molecule L1 in neural precursor cell proliferation, differentiation, and transmitter-specific subtype generation. J. Neurosci. 23, 6638-6650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J., Morton S., Karagogeos D., Yamamoto M., Jessell T. (1988). Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron 1, 105-116 [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C., Gennarini G., Goridis C., Rougon G. (1992). F3/F11 cell surface molecule expression in the developing mouse cerebellum is polarized at synaptic sites and within granule cells. J. Neurosci. 12, 257-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G., Hatten M. E. (1991). Astrotactin provides a receptor system for CNS neuronal migration. Development 113, 755-765 [DOI] [PubMed] [Google Scholar]
- Fransen E., D'Hooge R., Camp G. V., Verhoye M., Sijbers J., Reyniers E., Soriano P., Kamiguchi H., Willemsen R., Koekkoek S., et al. (1998). L1 knockout mice show dilated ventricles, vermis hypoplasia and impaired exploration patterns. Hum. Mol. Genet. 7, 999-1009 [DOI] [PubMed] [Google Scholar]
- Fukamauchi F., Aihara O., Wang Y., Akasaka K., Takeda Y., Horie M., Kawano H., Sudo K., Asano M., Watanabe K., et al. (2001). TAG1-deficient mice have marked elevation of adenosine A1 receptors in the hippocampus. Biochem. Biophys. Res. Commun. 281, 220-226 [DOI] [PubMed] [Google Scholar]
- Goldowitz D., Hamre K. (1998). The cells and molecules that make a cerebellum. Trends Neurosci. 21, 375-382 [DOI] [PubMed] [Google Scholar]
- Hallahan A. R., Pritchard J. I., Hansen S., Benson M., Stoeck J., Hatton B. A., Russell T. L., Ellenbogen R. G., Bernstein I. D., Beachy P. A., et al. (2004). The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog-induced medulloblastomas. Cancer Res. 64, 7794-7800 [DOI] [PubMed] [Google Scholar]
- Hatten M. (1985). Neuronal regulation of astroglial morphology and proliferation in vitro. J. Cell Biol. 100, 384-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms A. W., Johnson J. E. (1998). Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development 125, 919-928 [DOI] [PubMed] [Google Scholar]
- Helms A., Abney A., Ben-Arie N., Zoghbi H., Johnson J. (2000). Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development 127, 1185-1196 [DOI] [PubMed] [Google Scholar]
- Helms A. W., Gowan K., Abney A., Savage T., Johnson J. E. (2001). Overexpression of MATH1 disrupts the coordination of neural differentiation in cerebellum development. Mol. Cell. Neurosci. 17, 671-682 [DOI] [PubMed] [Google Scholar]
- Herron L. R., Hill M., Davey F., Gunn-Moore F. J. (2009). The intracellular interactions of the L1 family of cell adhesion molecules. Biochem. J. 419, 519-531 [DOI] [PubMed] [Google Scholar]
- Heyden A., Angenstein F., Sallaz M., Seidenbecher C., Montag D. (2008). Abnormal axonal guidance and brain anatomy in mouse mutants for the cell recognition molecules close homolog of L1 and NgCAM-related cell adhesion molecule. Neuroscience 155, 221-233 [DOI] [PubMed] [Google Scholar]
- Hu Q., Ang B., Karsak M., Hu W., Cui X., Duka T., Takeda Y., Chia W., Sankar N., Ng Y., et al. (2003). F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell 115, 163-175 [DOI] [PubMed] [Google Scholar]
- Jakovcevski I., Siering J., Hargus G., Karl N., Hoelters L., Djogo N., Yin S., Zecevic N., Schachner M., Irintchev A. (2009). Close homologue of adhesion molecule L1 promotes survival of Purkinje and granule cells and granule cell migration during murine cerebellar development. J. Comp. Neurol. 513, 496-510 [DOI] [PubMed] [Google Scholar]
- Karagogeos D., Morton S. B., Casano F., Dodd J., Jessell T. M. (1991). Developmental expression of the axonal glycoprotein TAG-1: differential regulation by central and peripheral neurons in vitro. Development 112, 51-67 [DOI] [PubMed] [Google Scholar]
- Katidou M., Vidaki M., Strigini M., Karagogeos D. (2008). The immunoglobulin superfamily of neuronal cell adhesion molecules: Lessons from animal models and correlation with human disease. Biotechnol. J. 3, 1564-1580 [DOI] [PubMed] [Google Scholar]
- Komuro H., Yacubova E., Rakic P. (2001). Mode and tempo of tangential cell migration in the cerebellar external granular layer. J. Neurosci. 21, 527-540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law C. O., Kirby R. J., Aghamohammadzadeh S., Furley A. J. W. (2008). The neural adhesion molecule TAG1 modulates responses of sensory axons to diffusible guidance signals. Development 135, 2361-2371 [DOI] [PubMed] [Google Scholar]
- Lewis P. (2004). Sonic hedgehog signaling is required for expansion of granule neuron precursors and patterning of the mouse cerebellum. Dev. Biol. 270, 393-410 [DOI] [PubMed] [Google Scholar]
- Lindner J., Rathjen F. G., Schachner M. (1983). L1 mono- and polyclonal antibodies modify cell migration in early postnatal mouse cerebellum. Nature 305, 427-430 [DOI] [PubMed] [Google Scholar]
- Ma Q. H., Futagawa T., Yang W. L., Jiang X. D., Zeng L., Takeda Y., Xu R. X., Bagnard D., Schachner M., Furley A. J., et al. (2008). A TAG1-APP signalling pathway through Fe65 negatively modulates neurogenesis. Nat. Cell Biol. 10, 283-294 [DOI] [PubMed] [Google Scholar]
- Memberg S. P., Hall A. K. (1995). Dividing neuron precursors express neuron-specific tubulin. J. Neurobiol. 27, 26-43 [DOI] [PubMed] [Google Scholar]
- Miyazawa K., Himi T., Garcia V., Yamagishi H., Sato S., Ishizaki Y. (2000). A role for p27/Kip1 in the control of cerebellar granule cell precursor proliferation. J. Neurosci. 20, 5756-5763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K., Ellis P., Kimura S., Placzek M. (2005). Directed differentiation of neural cells to hypothalamic dopaminergic neurons. Development 132, 5185-5197 [DOI] [PubMed] [Google Scholar]
- Osterfield M., Egelund R., Young L. M., Flanagan J. G. (2008). Interaction of amyloid precursor protein with contactins and NgCAM in the retinotectal system. Development 135, 1189-1199 [DOI] [PubMed] [Google Scholar]
- Pavlou O., Theodorakis K., Falk J., Kutsche M., Schachner M., Faivre-Sarrailh C., Karagogeos D. (2002). Analysis of interactions of the adhesion molecule TAG1 and its domains with other immunoglobulin superfamily members. Mol. Cell Neurosci 20, 367-381 [DOI] [PubMed] [Google Scholar]
- Poliak S., Salomon D., Elhanany H., Sabanay H., Kiernan B., Pevny L., Stewart C. L., Xu X., Chiu S. Y., Shrager P., et al. (2003). Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG1. J. Cell Biol. 162, 1149-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons S., Trejo J., Martinez-Morales J., Marti E. (2001). Vitronectin regulates Sonic hedgehog activity during cerebellum development through CREB phosphorylation. Development 128, 1481-1492 [DOI] [PubMed] [Google Scholar]
- Rios I. (2004). Bmp2 antagonizes sonic hedgehog-mediated proliferation of cerebellar granule neurones through Smad5 signalling. Development 131, 3159-3168 [DOI] [PubMed] [Google Scholar]
- Sakurai T., Lustig M., Babiarz J., Furley A. J., Tait S., Brophy P. J., Brown S. A., Brown L. Y., Mason C. A., Grument M. (2001). Overlapping functions of the cell adhesion molecules Nr-CAM and L1 in cerebellar granule cell development. J. Cell Biol. 154, 1259-1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T., Gerdes J. (2000). The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182, 311-322 [DOI] [PubMed] [Google Scholar]
- Solecki D., Liu X., Tomoda T., Fang Y., Hatten M. (2001). Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron 31, 557-568 [DOI] [PubMed] [Google Scholar]
- Takeda Y., Akasaka K., Lee S., Kobayashi S., Kawano H., Murayama S., Takahashi N., Hashimoto K., Kano M., Asano M., et al. (2003). Impaired motor coordination in mice lacking neural recognition molecule NB-3 of the contactin/F3 subgroup. J. Neurobiol. 56, 252-265 [DOI] [PubMed] [Google Scholar]
- Tsiotra P., Theodorakis K., Papamatheakis J., Karagogeos D. (1996). The fibronectin domains of the neural adhesion molecule TAX-1 are necessary and sufficient for homophilic binding. J. Biol. Chem. 271, 29216-29222 [DOI] [PubMed] [Google Scholar]
- Vaillant C., Michos O., Orolicki S., Brellier F., Taieb S., Moreno E., Té H., Zeller R., Monard D. (2007). Protease nexin 1 and its receptor LRP modulate SHH signalling during cerebellar development. Development 134, 1745-1754 [DOI] [PubMed] [Google Scholar]
- Virgintino D., Ambrosini M., D'Errico P., Bertossi M., Papadaki C., Karagogeos D., Gennarini G. (1999). Regional distribution and cell type-specific expression of the mouse F3 axonal glycoprotein: a developmental study. J. Comp. Neurol. 413, 357-372 [DOI] [PubMed] [Google Scholar]
- Wallace V. A. (1999). Purkinje-cell-derived Sonic hedgehog regulates granule neuron precursor cell proliferation in the developing mouse cerebellum. Curr. Biol. 9, 445-448 [DOI] [PubMed] [Google Scholar]
- Wechsler-Reya R. J., Scott M. P. (1999). Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 22, 103-114 [DOI] [PubMed] [Google Scholar]
- Wolf E., Wagner J. P., Black I. B., DiCicco-Bloom E. (1997). Cerebellar granule cells elaborate neurites before mitosis. Brain Res. Dev. Brain Res. 102, 305-308 [DOI] [PubMed] [Google Scholar]
- Yaginuma H., Shiga T., Homma S., Ishihara R., Oppenheim R. W. (1990). Identification of early developing axon projections from spinal interneurons in the chick embryo with a neuron specific beta-tubulin antibody: evidence for a new `pioneer' pathway in the spinal cord. Development 108, 705-716 [DOI] [PubMed] [Google Scholar]
- Yoshihara Y., Kawasaki M., Tamada A., Nagata S., Kagamiyama H., Mori K. (1995). Overlapping and differential expression of BIG-2, BIG-1, TAG1, and F3: four members of an axon-associated cell adhesion molecule subgroup of the immunoglobulin superfamily. J. Neurobiol. 28, 51-69 [DOI] [PubMed] [Google Scholar]
- Zheng X., Saunders T. L., Camper S. A., Samuelson L. C., Ginsburg D. (1995). Vitronectin is not essential for normal mammalian development and fertility. Proc. Natl. Acad. Sci. USA 92, 12426-12430 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.