Abstract
Objective
To measure clinically relevant change in Alzheimer's disease (AD) using a family member completed dementia severity rating scale (DSRS) questionnaire.
Background
Measuring rate of change provides important clinical information. Most neuropsychological scores change nonlinearly, complicating their use as a predictor of change throughout the illness.
Methods
DSRS and Mini Mental State (MMS) scores were prospectively collected on 702 patients with AD from first evaluation until they became too impaired to return to clinic.
Results
DSRS score increased an average of 4.48 points per year (95% CI 4.14 - 4.82) throughout the entire range of severity. In contrast, the MMS declined an average of 2.15 points per year (95% CI 1.85-2.46) during the first two years, accelerated to 3.83 points per year (95% CI 3.28-4.38) during the subsequent three years and then slowed to an annual decline of 1.63 points during the last two years (95% CI 0.21-3.05). A younger age of symptom onset was associated with an increased rate of DSRS change (p=0.03).
Conclusions
The DSRS provides a clinical measure of functional impairment in AD that increases about 4.48 points per year from the earliest symptomatic stage until patients become too severely impaired to return to clinic.
Keywords: Dementia severity rating scale, Alzheimer's disease, Rate of decline
1. Introduction
Measures that mark change in Alzheimer's disease (AD) symptom severity provide information useful for patient care management, in both assessing treatment efficacy and providing predictions for future care needs. Using standardized and validated measures, such as the Washington University Clinical Dementia Rating (CDR) scale1 or the Alzheimer's Disease Cooperative Studies Activities of Daily Living questionnaire2 to reliably assess a patient's current functional status requires considerable training and time. Development of a valid and reliable functional abilities assessment measure that has optimal performance characteristics and can be used with minimal staff involvement, could assist clinicians by identifying patients with AD who are progressing unusually fast and by providing some prognostication of the future rate of decline and care needs.3
The Dementia Severity Rating Scale (DSRS) is a multiple-choice questionnaire that collects information from a knowledgeable informant on impairment severity in twelve cognitive and functional domains. It is based on the CDR, with modifications to expand the severity range of the functional abilities assessed and changes to the method used to collect the information. In addition, the numeric range defining the functional status was expanded to express finer increments of change. The DSRS measures the full range of severity, from no impairment (a total score of 0) to extreme impairment for each category assessed (a total score of 54).4 The performance characteristics, including the affect of using individuals with a variety of clinical training to administer the questionnaire, have been reported.4
In this study we assessed the ability of the DSRS to measure change in symptom severity over time. While many tools can mark severity at a single point, measuring change over time is easier to use in clinical management if the severity scores change linearly throughout the course of the disease. The most commonly used measures of severity in AD have been shown to follow nonlinear trajectories of cognitive decline.5-7 This has also implications for the analysis of data from clinical trials8 because the statistical analysis of differential rates of decline is more difficult if the rate of change is not linear over time.7, 9
In the present study, we estimated the annual rate of change in 702 patients with a clinical diagnosis of AD using the DSRS and the Mini Mental State exam (MMS).10 We examined several factors that may modify the rate of decline in DSRS and MMS.
2. Methods
2.1. Study cohort
The study cohort included 702 consecutive patients with probable or possible AD by National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association Criteria.11 These subjects were evaluated in the Penn Memory Center (PMC) of the University of Pennsylvania's Alzheimer's Disease Center. They returned for follow-up visits every six months. Patients entered the study at various time points (also called staggered entry). Some patients had had only an initial visit before the study ended because they were new PMC patients. Thus, patients who had shorter or no follow-up time are not necessarily drop-outs. There were 455 subjects who had had at least two clinic visits during the study period. The average number of follow-up visits is five among those who had follow-ups. Patients who were in the earliest symptomatic phase of the illness at the time of their first visit were initially given a pre-dementia diagnosis such as mild cognitive impairment. Consent, or when appropriate, assent, to use information collected during their assessment for research purposes was obtained from each patient using an Institutional Review Board approved protocol. Informed consent was also obtained from each patient's self-designated decision maker.
2.2. Measures
At each clinic visit, a knowledgeable informant (typically a spouse or adult child) completed the multiple choice DSRS.
The DSRS includes questions on memory, speech and language, recognition of family members, orientation to time and space, ability to make decisions, social and community activities, home activities, personal care, eating, incontinence (related to AD) and ability to get from place to place. It covers all of the topics addressed in the full five-point version of the CDR scale.12, 13 The patient's score was determined by adding the numeric value assigned to each response chosen.
The original form of the DSRS included 11 multiple-choice questions and had a maximum score of 51.4 It was later revised to reduce the reading level and improve comprehension. To avoid confusion, the question on orientation in time and space was divided into two separate questions. The maximum score on memory was reduced from 7 to 6. The question on social activities was expanded to five categories to include complete lack of responsiveness to the caregiver. The new form has a maximum score of 54 (see Appendix).
The original scoring was made comparable to the new form by doubling the score on the orientation question, reducing a memory score of 7 to 6, and increasing the social activity score of 4 to 5. The latter changes in memory and social activities only affect patients at the most extreme levels of severity that were rarely observed in patients still able to return to clinic (the illness period used for this analysis).
Other clinical and demographic measures collected included the MMS score, gender, race, age at symptom onset of the patient, and the number of years of school completed, their occupation, and their apolipoprotein E (ApoE) genotype. Education was classified as less than 8 years, 8-12 years, and greater than 12 years. Occupation was coded as: Professional, Technical, and Labor. ApoE was coded as 3 categories: (1) at least one e4; (2) no e4; (3) missing (including e2/e4 or information not available).
2.3. Statistical Analysis
A piece-wise linear mixed-effect model (proc mixed program, SAS v9.1, SAS Institute, Cary, NC) was used to estimate the rate of change14, 15 for each instrument: DSRS or MMS score, over seven years from the first clinic visit. This statistical procedure accounts for the within-subject correlations in this longitudinal analysis that are due to the repeated measurements of DSRS or MMS over time in the same patients and accounts for missing data. Follow-up time was treated as both a random effect and a fixed effect, and a random intercept was also included in the mixed-effect model. Demographic variables such as age and education were treated as fixed effects. The model included three piece-wise linear terms in order to test for changes in the rate of change for each instrument. The three time segments corresponding to the three linear terms were 0-2, 2-5, and 5-7 years since the first clinic evaluation. The corresponding annual rate of change in each time segment was then estimated from the linear terms. Covariates (gender, race, age at symptom onset, education, occupation, and ApoE genotype) were also considered in the mixed-effect model to investigate if the rate of change differed by different level of covariate values.
Although the DSRS covers the full range of AD severity, for analysis purposes the data were truncated at the first DSRS score of 40 or more (score of 40 were included in the analysis). Patients with scores over 40 are very likely to be in a long-term care nursing facility and have a very high risk of death. Based on our current database of AD patients, we estimated that only 3% of AD patients who come back to clinic when their DSRS scores are above 40. Near this level of severity, the MMS is typically zero.
Test-retest reliability of the DSRS was evaluated by comparing scores that were collected within three months of each other. These pairs of scores occurred naturally when a patient was scheduled for two visits within a three month period. This generally occurred following an initial entry visit but covered a wide range of initial DSRS scores. The reliability coefficient was used to compare the total scores.16 The individual items were evaluated using kappa statistics and Spearman correlation coefficients. All statistical tests were two-sided. Statistical significances were set at the 0.05 level.
3. Results
The analysis cohort included 702 subjects. Table 1 presents the demographic information for the whole cohort and by initial diagnosis [mild cognitive impairment (MCI) or AD]. The sample contained more females (65%) than males (35%). Eighty percent of the subjects were white and 20% were African American. About 50% of the subjects had 8-12 years of education. The ApoE allele frequencies were: e2=5%, e3=58%, e4=38%. These frequencies are very close to what would be expected in a sample of cases with the observed composition by race.17 The age at symptom onset ranged from 44 to 95 years with an average of 72 years. The mean of MMS at initial visit was 18. There were no significant differences of sex, race, education, ApoE genotype between subjects who had only one visit (n=247) and subjects who had at least two visits (n=455). The mean age of onset among subjects with only one visit (mean=73, SD=8.3) was significantly higher (p= 0.004) than that among subjects with at least two visits (mean=71, SD=8.4).
Table 1. Mean (±S.D., range) Demographic Characteristics of Participants#.
| Characteristics | Whole Cohort (N=702) | Initial Diagnosis: MCI (n=46) | Initial Diagnosis: AD (n=607) |
|---|---|---|---|
| Male, n (%) | 243 (35) | 15 (33) | 211 (35) |
| Female, n (%) | 459 (65) | 31 (67) | 396 (65) |
| White, n (%) | 559 (80) | 42 (91) | 475 (78) |
| African American, n (%) | 138 (20) | 4 (9) | 127 (21) |
| Other race, n (%) | 5 (1) | 0 (0) | 5 (1) |
| Education < 8 years, n (%)* | 103 (15) | 2 (4) | 96 (16) |
| Education 8-12 years, n (%)* | 344 (49) | 22 (48) | 302 (50) |
| Education >12 years, n (%)* | 249 (36) | 22 (48) | 204 (34) |
| ApoE: no e4, n (%)† | 211 (30) | 15 (33) | 181 (30) |
| ApoE: at least one e4, n (%)† | 320 (46) | 27 (59) | 273 (45) |
| ApoE: missing, n (%)† | 171 (24) | 4 (8) | 153 (25) |
| Age at symptom onset (years)‡ | 72 ± 8 (44-95) | 72 ± 7 (51-82) | 72 ± 8 (44-95) |
| Age at initial visit (years) | 76 ± 8 (49-96) | 74 ± 7 (53-86) | 76 ± 8 (50-97) |
| MMS at initial visit | 18 ± 7 (0-30) | 25 ± 4 (9-29) | 17 ± 7 (0-30) |
| Length of follow up time (years) | 2 ± 2 (0-7) | 3 ± 2 (0-7) | 2 ± 2 (0-7) |
Out of 702 subjects, 49 subjects had initial diagnosis that were not MCI or AD.
Six subjects had missing values in education.
Missing values include e2/e4 or information not available in ApoE.
Fifteen subjects had missing values in age at symptom onset.
Table 2 displays the characteristics of informants. About 87% of the informants were either spouse or child. Seventy-six percent of the informants had contact with the patients five or more days per week.
Table 2. Characteristics of Informants.
| Characteristics | N (%) | |
|---|---|---|
| Relationship to patients* | spouse | 272 (40) |
| child | 317 (47) | |
| sibling | 22 (3) | |
| other relative | 48 (7) | |
| friend/neighbor | 12 (2) | |
| paid caregiver/provider | 8 (1) | |
| Amount Contact† | less than 1 day/week | 4 (4) |
| 1 day/week | 7 (7) | |
| 2 days/week | 8 (8) | |
| 3-4 days/week | 4 (4) | |
| 5 or more days per week | 75 (76) | |
| all the time | 1 (1) |
23 subjects did not have this information available.
603 subjects did not have this information available.
The rates of change in DSRS during 0-2, 2-5, and 5-7 years were estimated and no significant differences among the three rates were found (p>0.73). The estimated annual rate of change difference between 0-2 and 2-5 years is -0.09 (95% confidence interval [CI]: -1.04 - 0.86). The estimated annual rate of change difference between 2-5 and 5-7 years is -0.41 (95% CI: -2.76 - 1.94). The estimated annual rate of change difference between 0-2 and 5-7 years is -0.50 (95% CI: -2.79 - 1.79). These confidence intervals all contained 0 and were not wide clinically. Thus, an overall rate of change can be generated. The results indicate that the DSRS increased by an average of 4.48 (95% CI: 4.14 - 4.82) points per year throughout the observation period. Accounting for the random effects, the 95% prediction interval for this annual rate of decline is 2.03 – 6.93. Because some DSRS values were missing due to the truncation of score 40, we performed a sensitivity analysis to examine if our conclusion changed when we didn't truncate the DSRS values. We found that there were no significant differences of the three rates of decline corresponding to 0-2, 2-5, and 5-7 years (p > 0.42) when we didn't truncate DSRS values. The overall annual rate of change for the entire period is 5.05 (95% CI: 4.67 – 5.42) in this situation. Thus, the annual expected rates of declines were similar when truncating or not truncating at score 40 of DSRS.
In contrast, the MMS score declined by an average of 2.15 (95% CI: 1.85 - 2.46) points per year during the first two years of observation and then accelerated to an annual decline of 3.83 (95% CI: 3.28 - 4.38) points per year during the subsequent three years before slowing to an average decline of 1.63 (95% CI: 0.21 - 3.05) points per year during the last two years of observation. The annual rates of MMS change in the three time intervals were significantly different (p< 0.0001 when comparing the annual rate in the first two years to the annual rate in years 2-5; p=0.0107 when comparing the annual rate in years 2-5 to the annual rate in years 5-7).
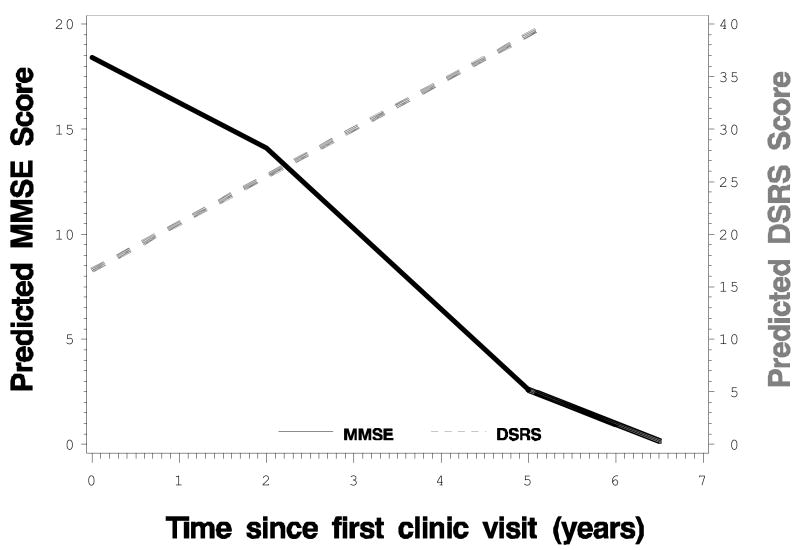
Figure 1 displays the predicted DSRS and MMS values from the piece-wise linear mixed-effect model which produced three estimated rates corresponding to the three time periods (0-2, 2-5, 5-7 years). The DSRS changed almost linearly throughout the study period, while the MMS changed in a non-linear fashion.
Figure 1. Predicted DSRS and MMS Values over Time from the Piece-wise Mixed-effect Models*.
Note: * The models included three piece-wise linear terms corresponding to three time periods (0-2, 2-5, 5-7 years since first evaluation).
The annual rates of change in both the DSRS and the MMS differed significantly by age at symptom onset. The annual rate of change in the DSRS slowed by 0.045 points for each one-year increase in the age at onset (p=0.03). Therefore, the estimated annual rate of change dropped from about 4.47 points for age at onset of 70 years to 4.02 points for age at onset of 80 years. The annual rate of change in the MMS also slowed by 0.076 points for each one-year increase in the age at onset (p<0.0001).
We performed the mixed-effect analysis of DSRS and MMS by examining the interactions between duration of follow-up time and disease severity at baseline [MCI, mild AD (MMS >=18), moderate AD (MMS 11-17, inclusive), severe AD (MMS <=10)]. We found that the interaction term was not significant (p=0.12 for DSRS and p=0.11 for MMS) and thus dementia severity at baseline did not significantly influence the annual rate of decline for both DSRS and MMS. For DSRS, this implies that we can expect a patient to decline at a rate of about 4.48 points in a 12 month period no matter whether they started with MCI or probable AD.
There were no significant differences at the 0.05 level in the annual rate of change for the DSRS or the MMS by sex, education, ApoE genotype, race or occupation.
There were 196 patients for whom there were two DSRS scores collected from the same respondent within three months of each other. The mean interval between scores was 43 days. The mean score on the first test was 18.69 compared to 18.65 for the second. The mean absolute difference between the two scores was 3.06 with a maximum of 12 points. The reliability coefficient for the total score was 0.95. Kappa statistics for the individual items ranged from 0.54 to 0.71. All were significantly different from 0 at the 0.05 level. Spearman correlation coefficients for the individual items ranged from 0.66 to 0.82, and all were significantly different from 0 at the 0.05 level.
4. Discussion
The DSRS was previously shown to be a valid and reliable measure of severity of AD through the entire course of the disease. The high reliability is demonstrated here for a much larger sample. The estimated reliability (0.95) is excellent and substantially better than the cognitive functioning subscale of the Relative's Assessment of Global Symptomatology-Elderly (RAGS-E) which has a reliability of 0.79.6
Measures based on caregiver reports have advantages.18 Caregiver perception of the severity of disease can play a central role in clinical trials, studies of caregiver burden and research on factors that predict nursing home placement. Several studies have demonstrated the value of information provided by caregivers. Informant reporting of memory has been shown to correctly identify 86% of individuals with CDR scores of 0 and 84% of those with a CDR of 0.5.19 The DSRS measures caregiver perception of the core aspects of the disease that are central to a clinician's evaluation of the severity of AD as codified in the CDR. In fact, a clinician's evaluation generally relies in part on information provided by a caregiver about many aspects of the disease incorporated in the DSRS.3 Since the DSRS is a simple 12 item questionnaire filled out by a caregiver, it requires very little staff time during a clinic visit. It can be administered at late stages of the disease and thus can reduce the chance of missing data due to the extremes of impairment.
The linear change in the DSRS also makes it a valuable tool for research. Estimates of the speed of decline in Alzheimer's disease form the foundation of treatment trials designed to demonstrate disease modifying effects. In addition, information on the rate of decline is crucial in studies of clinical and biologic factors that influence the disease process and in helping families know what to expect in the future. However, interpretations of the estimated rates of decline are often complicated by three issues present among most of the scales used to measure change.
First, floor and ceiling effects and changes in the rate of decline during the course of the disease hinder analysis and interpretation.20 Second, estimates of the rate of change of many scales have very low reliability for periods of observation of a year or less. The variance of the estimated rate of change depends on the ratio of the rate of change to the reliability of the scale. The usefulness of most of the standard clinical measures is limited by one or more of these problems.21 The more statistical adjustments required to adjust for these limitations, the more difficult it is to interpret the results.22 Third, most clinical measures require a clinic visit and use of staff time for assessment. Since clinic visits are more difficult at later stages of the disease, missing data may be more common for those patients who progress rapidly.23
Most neuropsychological measures are also confounded by nonlinearity.7 In particular, this is true for the MMS,8, 24 the Dementia Rating Scale,8 and the Alzheimer's Disease Assessment Scale-cognitive.25 In the present study, we demonstrated that the DSRS changed at a very steady rate through the course of the disease from the earliest stages of memory impairment (0-4) to very high levels of impairment often associated with nursing home placement (35-40). The annual rate of change for DSRS does not depend on disease severity. For example, subjects with a baseline of MMS score of 25 will have the same expected annual rate of decline (4.48) as the subjects with a baseline of MMS score of 12. Also, the rate of decline for DSRS does not depend on duration of follow-up. For example, subjects with 7 year follow-up will have the same expected annual rate of decline (4.48) as subjects with 2 year follow-up. So it does not matter whether a clinical trial is 2 years long or 7 years long and the rate of decline for DSRS remains constant for either short clinical trials or long clinical trials. Although the rate of change varies by age at onset, the differences are only 1% faster or slower for each one-year difference in age at onset. This linearity has significant advantages for clinical trials. Treatment effects in a trial would be seen in the DSRS as a slowing of the linear rate of change during some or all stages of the disease. This is much easier than evaluating estimated treatment effects in a measure that does not change linearly to begin with.
This constancy of change for the DSRS is contrasted here with the non-linear changes in the MMS. The annual rate of MMS change during the middle observation years (durations 2-5 years) is about twice as fast as the annual rate during earlier and later years. This pattern might appear as a quadratic rate of change in a data set that is dominated by observations at the early years of durations of observation. However, this quadratic pattern would be misleading at the later years of durations of observation. For a data set that covers the full range of MMS values, a quadratic term would probably not be significant and the change would appear to be linear.
Thus the DSRS provides a tool that can be implemented with minimal staff training to quickly measure global disease severity throughout the entire clinical course of AD. Because the DSRS increases by an average of 4.48 points per year throughout the course of AD, a clinician can easily compare an individual's rate of decline to this average (expected) rate. In addition, this property should be helpful in assessing response to treatment and as an endpoint measure in the treatment trials.
Supplementary Material
Acknowledgments
This work was supported in part by funds from National Institute of Health/National Institute on Aging, Grant no. P30 AG 10124 and P30 AG12836.
Footnotes
Portions of this work were presented at 9th International Conference on Alzheimer's Disease and Related Disorders.
Reference List
- 1.Berg L. Clinical dementia rating (CDR) Psychopharmacol Bull. 1988;25:639. [PubMed] [Google Scholar]
- 2.Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. Alzheimer Dis Assoc Disord. 1997;11 2:S33–S39. [PubMed] [Google Scholar]
- 3.Gelb DJ. Measurement of progression in Alzheimer's disease: a clinician's perspective. Stat Med. 2000;19(11-12):1393–400. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1393::aid-sim431>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Clark CM, Ewbank DC. Performance of the dementia severity rating scale: a caregiver questionnaire for rating severity in Alzheimer disease. Alzheimer Dis Assoc Disord. 1996;10(1):31–9. [PubMed] [Google Scholar]
- 5.Hoyt BD, Massman PJ, Schatschneider C, et al. Individual growth curve analysis of APOE epsilon 4-associated cognitive decline in Alzheimer disease. Arch Neurol. 2005;62(3):454–9. doi: 10.1001/archneur.62.3.454. [DOI] [PubMed] [Google Scholar]
- 6.Ippen CG, Olin JT, Schneider LS. Can caregivers independently rate cognitive and behavioral symptoms in Alzheimer's disease patients?: A longitudinal analysis. Am J Geriatr Psychiatry. 1999;7(4):321–30. [PubMed] [Google Scholar]
- 7.Milliken JK, Edland SD. Mixed effect models of longitudinal Alzheimer's disease data: a cautionary note. Stat Med. 2000;19(11-12):1617–29. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1617::aid-sim450>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 8.Galasko DR, Gould RL, Abramson IS, et al. Measuring cognitive change in a cohort of patients with Alzheimer's disease. Stat Med. 2000;19(11-12):1421–32. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1421::aid-sim434>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 9.Plassman BL, Breitner JC. Apolipoprotein E and cognitive decline in Alzheimer's disease. Neurology. 1996;47(2):317–20. doi: 10.1212/wnl.47.2.317. [DOI] [PubMed] [Google Scholar]
- 10.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS- ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 12.Burke WJ, Miller JP, Rubin EH, et al. Reliability of the Washington University Clinical Dementia Rating. Arch Neurol. 1988;45:31–2. doi: 10.1001/archneur.1988.00520250037015. [DOI] [PubMed] [Google Scholar]
- 13.Berg G, Edwards D, Danzinger W, et al. Longitudinal change in three brief assessments of SDAT. J Am Geriatr Soc. 1987;35:205–12. doi: 10.1111/j.1532-5415.1987.tb02310.x. [DOI] [PubMed] [Google Scholar]
- 14.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 15.Neter J, Wasserman W, Kutner MH. Applied linear statistical models: regression, analysis of variance, and experimental designs. 3rd. Homewood: Irwin; 1990. [Google Scholar]
- 16.Streiner DL, Norman GR. Health measurement scales A practical guide to their development and use. Oxford: Oxford University Press; 1989. [Google Scholar]
- 17.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–56. [PubMed] [Google Scholar]
- 18.Jorm AF. The value of informant reports for assessment and prediction of dementia. J Am Geriatr Soc. 2003;51(6):881–2. doi: 10.1046/j.1365-2389.2003.51276.x. [DOI] [PubMed] [Google Scholar]
- 19.Carr DB, Gary S, Baty J, et al. The value of informant versus individual's complaints of memory impairment in early dementia. Neurology. 2000;55:1724–7. doi: 10.1212/wnl.55.11.1724. [DOI] [PubMed] [Google Scholar]
- 20.Mohs RC, Schmeidler J, Aryan M. Longitudinal studies of cognitive, functional and behavioural change in patients with Alzheimer's disease. Stat Med. 2000;19(11-12):1401–9. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1401::aid-sim432>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 21.van Belle G, Uhlmann RF, Hughes JP, et al. Reliability of estimates of changes in mental status test performance in senile dementia of the Alzheimer type. J Clin Epidemiol. 1990;43(6):589–95. doi: 10.1016/0895-4356(90)90163-j. [DOI] [PubMed] [Google Scholar]
- 22.Drachman DA, Leber P. Treatment of Alzheimer's disease -- searching for a breakthrough, settling for less. N Engl J Med. 1997;336(17):1245–7. doi: 10.1056/NEJM199704243361710. [DOI] [PubMed] [Google Scholar]
- 23.Thomas RG, Berg JD, Sano M, et al. Analysis of longitudinal data in an Alzheimer's disease clinical trial. Stat Med. 2000;19(11-12):1433–40. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1433::aid-sim435>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 24.Mendiondo MS, Ashford JW, Kryscio RJ, et al. Modelling mini mental state examination changes in Alzheimer's disease. Stat Med. 2000;19(11-12):1607–16. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1607::aid-sim449>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25.Stern RG, Mohs RC, Davidson M, et al. A longitudinal study of Alzheimer's disease: measurement, rate, and predictors of cognitive deterioration. Am J Psychiatry. 1994;151(3):390–6. doi: 10.1176/ajp.151.3.390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.