Summary
TCR signaling leads to the activation of kinases such as Itk, a key regulatory protein in T lymphocyte activation and function. The homolog of Itk in B cells is Btk, previously shown to bind and phosphorylate the transcription factor TFII-I. TFII-I plays major roles in transcription and signaling. Our purpose herein was two-fold: first, to identify some of the molecular determinants involved in TFII-I activation downstream of receptor crosslinking in T cells; and second, to uncover the existence of Itk-TFII-I signaling in T lymphocytes. We report for the first time that TFII-I is tyrosine phosphorylated upon TCR, TCR/CD43, and TCR/CD28 co-receptor engagement in human and/or murine T cells. We show that Itk physically interacts with TFII-I and potentiates TFII-I-driven c-fos transcription. We demonstrate that TFII-I is phosphorylated upon co-expression of wild type, but not kinase-dead, or kinase-dead/R29C mutant Itk, suggesting these residues are important for TFII-I phosphorylation, presumably via an Itk-dependent mechanism. Structural analysis of TFII-I-Itk interactions revealed that the first 90 residues of TFII-I are dispensable for Itk binding. Mutations within Itk’s kinase, pleckstrin-homology and proline-rich regions did not abolish TFII-I-Itk binding. Our results provide an initial step in understanding the biological role of Itk-TFII-I signaling in T cell function.
Keywords: T cell signaling, T cell receptors, Co-stimulation, Itk, Transcription factor TFII-I
Introduction
T lymphocyte activation through the TCR and other co-stimulatory molecules results in the initiation of downstream signaling cascades which involve a vast array of protein kinases, phosphatases, adaptor molecules and ultimately, transcription factors. The selectivity or redundancy of given signaling pathways demands a tight and coordinated spatio-temporal regulation in order to ensure proper T cell function. One key regulatory enzyme which is activated upon TCR engagement is the hematopoietic non-receptor tyrosine kinase Itk, a protein recognized for its importance in T lymphocyte development, activation and function [1, 2]. Itk is a member of the Tec family of kinases and is expressed in T cells, NKT cells and mast cells [1, 3]. Of the three members expressed in αβ TCR+ T cells (Itk, Rlk and Tec), Itk exhibits the highest level of expression [1, 3].
The homolog of Itk in B cells is Btk, and contrary to what is known about Btk – where Btk mutations are responsible for the X-linked immunodeficiencies XLA in humans (X-linked agammaglobulinemia) [4, 5], and Xid in mice [6, 7] – no naturally occurring mutations have been described for Itk. However, mice deficient in Itk have impaired thymic development, T cell proliferation, cytokine production, and exhibit defects in T cell activation processes downstream of the TCR [1, 3].
Previous work has demonstrated that Btk [8–11] and c-Src kinase [11, 12] are binding partners for the transcription factor TFII-I. Using overexpression studies and upon immunoglobulin crosslinking in B cells, it has been shown that Btk phosphorylates TFII-I on tyrosine residues, and furthermore, that it associates physically and functionally with TFII-I in vitro and in vivo [8–11]. Interestingly, Btk-TFII-I signaling is deregulated in Xid mice, where TFII-I accumulates abnormally and constitutively in the nuclei of resting B cells, suggesting that Btk-TFII-I signaling might play an important role in B cell development and/or function in vivo [9].
TFII-I is a ubiquitous multifunctional transcription factor with broad biological roles. Containing a helix-loop-helix, a DNA binding domain, a leucine zipper and six unique I-repeats, TFII-I is capable of partnering with a vast array of both cytoplasmic and nuclear factors, thus affecting diverse signal transduction cascades and modulating the expression of various genes [13–23]. One of the modes by which TFII-I is regulated is via phosphorylation at both serine and tyrosine residues, and tyrosine phosphorylation is critical for the transcriptional activity of TFII-I [24]. In fact, it has been shown that in response to different extracellular signals in various cell types, TFII-I is tyrosine phosphorylated, undergoes a regulated translocation into the nucleus and modulates gene transcription [9, 12, 24].
One of our first questions was whether TFII-I function and signaling might be similar in T cell and B cell lineages. We specifically addressed whether TFII-I could be activated upon receptor crosslinking in T cells, and whether that paralleled TFII-I activation in B cells. We focused primarily on signals emanating from the TCR as well as from the co-stimulatory molecule CD43, which is one of the most abundant cell-surface receptors on T cells [25], previously implicated in the activation of various downstream signaling targets, including ZAP-70 and the ζ chain, Fyn, Vav, Cbl, and ERK, and modulating gene transcription events [26–31].
In this study, we demonstrate that TFII-I is indeed tyrosine phosphorylated upon TCR or CD43 receptor crosslinking in T cells and that this induction occurs rapidly, consistent with the TFII-I activation pattern observed in B cells. Moreover, our results reveal that the strongest tyrosine phosphorylation of TFII-I occurred when both TCR and CD43 were simultaneously engaged. In addition, TFII-I tyrosine phosphorylation was also rapidly induced upon CD3/CD28 stimulation in Jurkat cells and splenic murine T cells. We further reasoned that because Itk and Btk exhibit a significant degree of structural similarity to each other and to Src family kinases, that Itk-TFII-I protein complexes could be uncovered in T cells, and presumably, be of relevance for T lymphocyte function. We provide evidence that all tested isoforms of TFII-I physically interact with Itk in a constitutive manner. Furthermore, we show that Itk co-expression potentiates the TFII-I-driven transcriptional activity of a model promoter in cell culture conditions. By using an overexpression system in fibroblasts, we also demonstrate that TFII-I is tyrosine phosphorylated in the presence of wild type, but not kinase-dead Itk, suggesting that TFII-I could be an Itk-dependent phosphorylation target. TFII-I phosphorylation was completely abrogated in the presence of a double mutant of Itk, harboring both the kinase-dead mutation and a mutation in the pleckstrin homology domain (Xid mutation R29C), suggesting that in addition to the kinase domain, the R29 residue may be important for TFII-I phosphorylation. Structural analysis of TFII-I and Itk interaction domains demonstrated that – unlike the requirements for Btk-TFII-I interactions – the first 90 N-terminal residues of TFII-I are not critical for Itk binding. Furthermore, point mutations in Itk’s kinase, pleckstrin homology (PH) or proline rich (PR) regions did not affect TFII-I-Itk binding.
To our knowledge, the present work provides some of the first results of TFII-I activation in T cells. Moreover, the data suggest that Itk could function as one of the kinases responsible for TFII-I activation in T cells, further illustrating the remarkable degree of similarity between B and T cell signaling pathways. Our results lay down a platform which will allow further elucidation of the functional importance of the Itk-TFII-I pathway in T cell signaling and gene expression.
Results
TFII-I is rapidly phosphorylated on tyrosine residues upon CD3 or CD43 receptor crosslinking in Jurkat cells
The role of TFII-I induction in T lymphocytes has only been studied in the context of its function in transcriptional regulation. For instance, TFII-I modulates transcription and gene expression of T-cell specific Vβ8.2 [15], and CD3δ [32] promoters. Only recently, reports by one group have revealed that TFII-I, in combination with USF proteins, is required for chromosomally integrated HIV-1 LTR (long-term repeat) induction in activated T cells and that TFII-I is phosphorylated upon PMA stimulation [33–35]. Nevertheless, the signal transduction events leading to TFII-I activation, and the possibility that TFII-I is phosphorylated downstream of the TCR and/or other co-stimulatory molecules has not been examined.
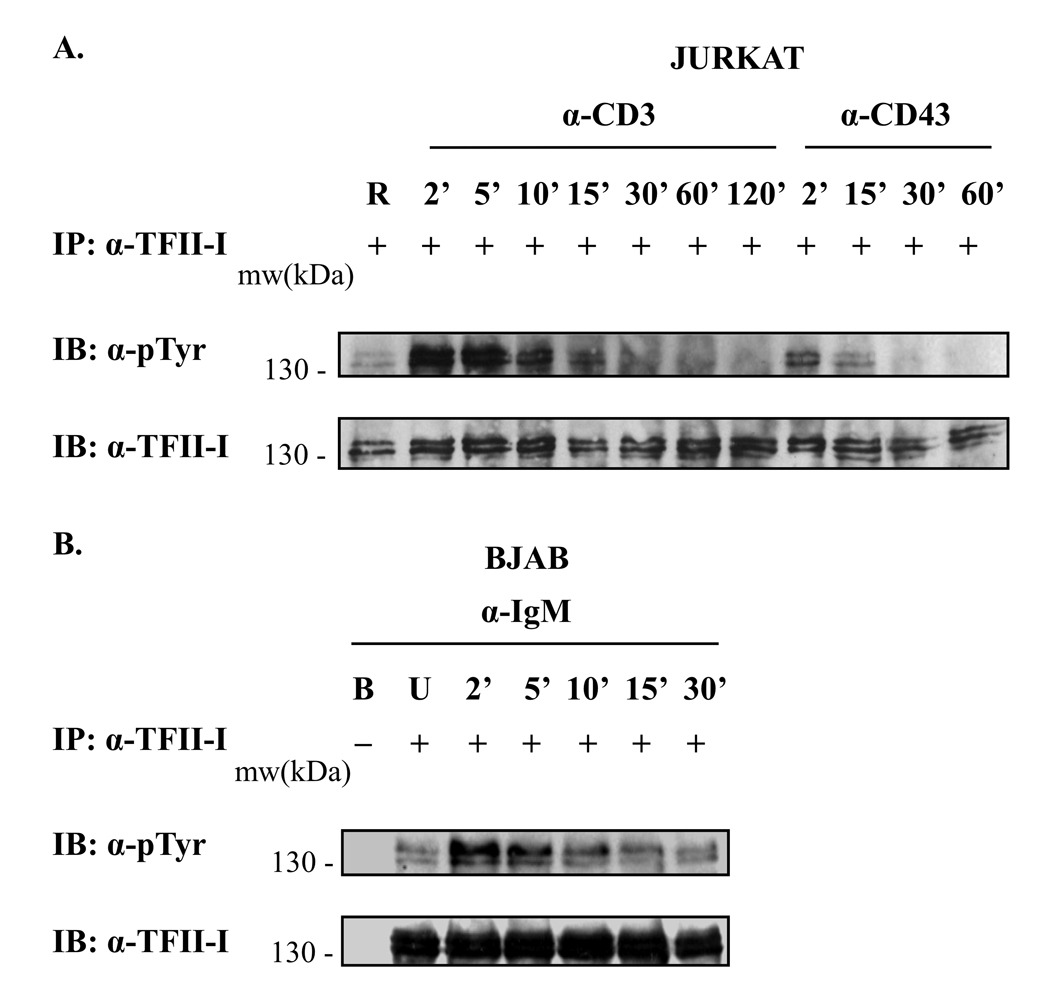
Aside from the downstream signals emanating from the TCR, we have focused on the co-receptor molecule CD43, which has been shown to provide intracellular signals independently, as well as synergistically, with those of the TCR [36, 37]. Thus, we comparatively tested whether TFII-I could be activated upon TCR, and upon CD43 engagement. Jurkat cells were stimulated via anti-CD3 (TCR) or via anti-CD43 for the times indicated (Fig. 1A). Analysis of immunoprecipitated (IP) TFII-I revealed a rapid and strong induction of tyrosine phosphorylation of TFII-I at 2’ following anti-CD3 stimulation when compared to basal levels (RaMIg “mock” stimulation). Phosphotyrosine levels were highest at 2’ to 5’ post-stimulation and remained robust for up to 10’ or 15’, decreasing thereafter and returning to near basal levels after 1 h post-stimulation. TFII-I was also tyrosine phosphorylated within 2’ of anti-CD43 stimulation (Fig. 1A). The induced tyrosine phosphorylation returned to basal levels faster (by 15’– 30’) than upon anti-CD3 stimulation. Thus, TFII-I induction occurs downstream of TCR and of CD43 engagement in T cells.
Figure 1. TFII-I is rapidly phosphorylated on tyrosine residues upon receptor crosslinking in T and B cells.
(A) Jurkat T cells were serum-starved and stimulated during a kinetic time-course experiment with anti-CD3 + RaMIg Abs, anti-CD43 + RaMIg Abs, or RaMIg crosslinking Ab alone (R lane). Cell lysates were subjected to immunoprecipitation (IP) with anti-TFII-I Abs. Phosphotyrosine levels of TFII-I were detected by immunoblotting (IB) using an anti-p-Tyr Ab (upper panels). Blots were stripped and re-probed with anti-TFII-I Ab to monitor TFII-I protein levels (lower panels). (B) BJAB cells were serum-starved and stimulated with anti-IgM Ab during a kinetic time-course experiment. Cell lysates were processed as in (A). U lane: unstimulated cells. B lane: protein A/G beads alone were incubated with whole cell lysates in the absence of Ab and included as a negative control for the IP assay. The absence or presence of Ab in the IPs is indicated by a (−) or (+) respectively. Data are representative of at least two experiments for both (A) and (B).
The rapid kinetics of TFII-I induction upon receptor engagement are similar in T and B cells
It is possible that TFII-I signaling and function in T and B cell lineages are similar, or alternatively, that this transcription factor plays differential roles in the two lineages. Thus, because we wished to establish a comparison between the kinetics of tyrosine phosphorylation of TFII-I in T and B cells, we used the BJAB B cell line, and performed a similar kinetic IP experiment as conducted in Jurkat cells (Fig. 1B). BJAB cells also exhibited a rapid induction of TFII-I tyrosine phosphorylation above basal levels (unstimulated cells) at 2’ post-treatment with anti-IgM antibody (Ab) (Fig. 1B). The tyrosine phosphorylation of TFII-I returned to basal levels at 15’ to 30’. A similar temporal pattern of TFII-I tyrosine phosphorylation was observed using the human Ramos B cell line (data not shown). Collectively, our findings demonstrated that TFII-I activation followed a similar rapid phosphorylation pattern (2 – 5’) in T and B cells. Interestingly, whereas the T cell responses were prolonged past 15’ of anti-CD3 stimulation, B cells responses were curtailed within 10’ post-stimulation (Fig. 1A and 1B, and data not shown).
Simultaneous CD3 and CD43 crosslinking in T cells results in stronger TFII-I phosphorylation than with CD3 or CD43 crosslinking alone
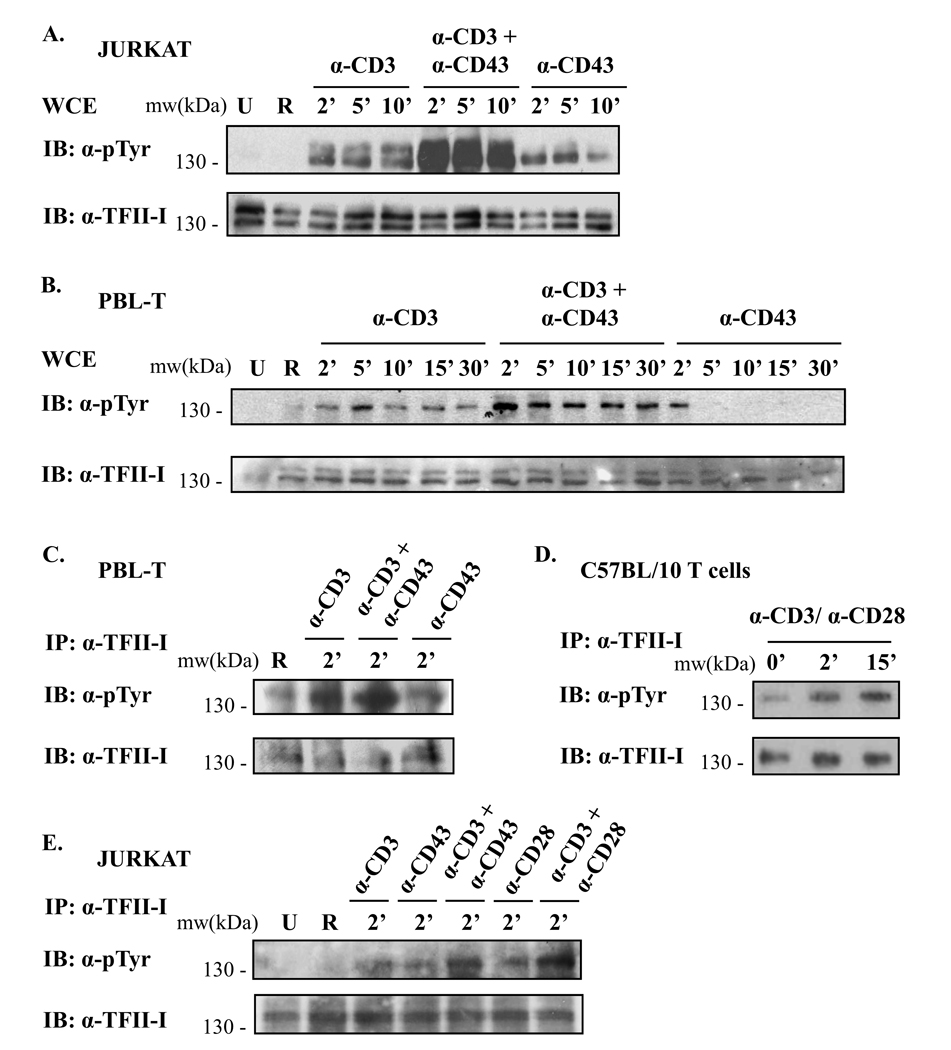
With the precedent that the timing of CD43 ligation greatly influences the outcome of T cell activation via the TCR, and that simultaneous CD43/TCR engagement augments T cell responses [31], we conducted kinetic time-course experiments to evaluate TFII-I tyrosine phosphorylation upon TCR and/or CD43 stimulation in Jurkat cells (Fig. 2A) and human PBL-Ts (Fig. 2B and 2C). Results from Jurkat whole cell extracts (WCE) (Fig. 2A) and PBL-Ts (Fig. 2B) revealed rapid tyrosine phosphorylation of TFII-I, within 2’ – 5’ following TCR and/or CD43 ligation, relative to unstimulated or RaMIg-treated cells. Noteworthy was the dramatic induction of tyrosine phosphorylation when CD3 and CD43 were jointly crosslinked (highest tyrosine phosphorylation levels peaking at 2’ – 5’ post-stimulation) in both Jurkat cells and PBL-Ts. CD43 engagement also resulted in TFII-I tyrosine phosphorylation, albeit not nearly as strong as that resulting from CD3 or CD3/CD43 crosslinking. In addition, a more transient phosphorylation pattern of TFII-I was observed upon CD43 stimulation in PBL-Ts relative to Jurkat cells.
Figure 2. Simultaneous crosslinking of two receptors results in stronger TFII-I phosphorylation than crosslinking receptors singly.
(A) Jurkat cells and (B) human peripheral blood lymphocytes (PBL-Ts) were serum-starved and stimulated with either anti-CD3 + RaMIg, anti-CD3 + anti-CD43 + RaMIg, anti-CD43 + RaMIg Abs or RaMIg alone (R lane) for the times specified. Phosphotyrosine levels of TFII-I in whole cell extracts (WCE) were detected by immunoblotting using an anti-p-Tyr Ab (upper panels). Blots were stripped and re-probed with anti-TFII-I Ab to reveal TFII-I protein levels (lower panels). U lane: unstimulated cells. (C) PBLTs, (D) splenic T cells from C57BL/10 mice and (E) Jurkat cells were serum-starved and stimulated either as described in (A, B) but for 2’ or with anti-CD3 + anti-CD28 Abs for the times indicated. WCE were then subjected to IP with anti-TFII-I Abs. Phosphotyrosine levels of TFII-I were detected by immunoblotting (IB) using an anti-pTyr Ab. Blots were stripped and re-probed to reveal TFII-I protein levels using anti-TFII-I Ab. Data are representative of at least two independent experiments for (A –E).
To obtain direct proof of TFII-I tyrosine phosphorylation, we immunoprecipitated TFII-I from PBL-T (Fig. 2C) and Jurkat (Fig. 2E) cell extracts following anti-CD3, anti-CD43, or anti-CD3 plus anti-CD43 stimulation for 2’ (Fig. 2C). The results were consistent with our previous findings (Fig. 1A and Fig 2A and 2B), revealing higher phosphotyrosine levels of TFII-I upon stimulation (with all antibodies) relative to the RaMIg control (R lane), (Fig. 2C, 2E) or unstimulated cells (Fig 2E). A more robust phosphotyrosine induction occurred when cells were simultaneously co-stimulated with anti-CD3 plus anti-CD43 relative to anti-CD3 or anti-CD43 stimulation alone (approximately 2-fold). Intermediate levels of TFII-I tyrosine phosphorylation were observed with anti-CD3 stimulation, while the weakest response was seen with anti-CD43 stimulation. Taken together, these findings indicate that the kinetics of TFII-I tyrosine phosphorylation, as well as the observed strong synergy of CD3 plus CD43 co-stimulation are reproducible and universal throughout T cells.
CD3/CD28 co-stimulation in murine T cells and Jurkat cells also results in rapid TFII-I phosphorylation
We additionally examined TFII-I tyrosine phosphorylation ex vivo. As we wanted to ensure strong T cell activation that would likely result in robust TFII-I induction, we stimulated murine splenic T cells via simultaneous anti-CD3 and anti-CD28 ligation [1] (Fig. 2D). This would also allow us to investigate whether engagement of other co-receptor molecules could induce TFII-I activation. Results revealed a strong increase in tyrosine phosphorylation of immunoprecipitated TFII-I upon CD3/CD28 crosslinking (1.7-fold at 2’ and 2.6-fold at 15’) relative to basal levels (0’).
To further examine the role of CD28 and CD28/CD3-mediated induction of TFII-I tyrosine phosphorylation, we activated Jurkat cells with anti-CD28 and/or anti-CD3 for 2’ in the experiment shown in Fig. 2E, where cells were also stimulated with anti-CD43 and/or anti-CD3, in parallel. CD28 engagement resulted in an increase in tyrosine phosphorylation of TFII-I relative to unstimulated, or RaMIg-treated cells. Similar to CD3/CD43, CD3/CD28 engagement resulted in a remarkable increase in phosphotyrosine levels of TFII-I relative to RaMIg-treated cells (6 or 8-fold, respectively), and relative to CD3 or CD28 crosslinking alone (2.4 and 2.6-fold, respectively). CD28 crosslinking resulted in TFII-I phosphotyrosine levels that were comparable to those obtained with CD3 crosslinking. Taken together, results from Fig. 2 indicate that induction of TFII-I phosphorylation upon extracellular stimulation is reproducible in human and murine T cells, both for CD3/CD43, and for CD3/CD28 co-stimulation.
Itk interacts constitutively with TFII-I in T lymphocytes
Previous studies have shown that Btk phosphorylates the transcription factor TFII-I in tyrosine residues, and furthermore, that it associates physically and functionally with TFII-I [8, 9, 11]. Btk and Itk proteins exhibit striking structural similarities [38, 39] – which in addition to common features shared by other Tec kinase family members (Rlk, Tec, and Bmx) bearing SH3, SH2, and kinase domains – consist namely of the presence of a pleckstrin homology (PH) domain, a Btk homology domain (BH) and a proline rich region (PR) [1]. While the kinase domain bears catalytic activity for transferring phosphate groups onto tyrosine residues of substrate proteins, the SH3, SH2, PH, BH and PR domains all mediate protein-protein interactions regulating the binding partners of Tec kinases [1]. Because of the conserved similarity between Itk and Btk, we argued that with strong likelihood, Itk could physically interact with TFII-I in T lymphocytes, and that this interaction could be important for T cell function.
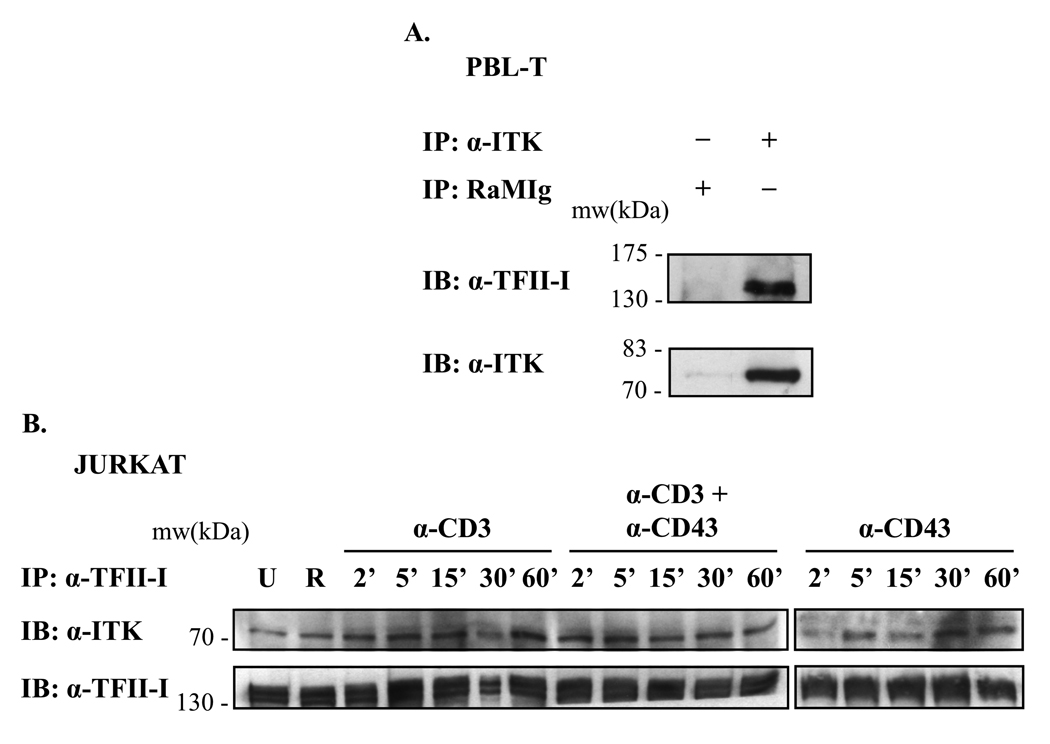
To examine the ability of these two proteins to form protein complexes endogenously, we conducted Itk and TFII-I immunoprecipitations on human PBL-T and Jurkat whole cell lysates. We determined that TFII-I could associate with Itk in resting PBL-Ts (Fig. 3A) and in Jurkat T cells stimulated via CD3 and/or CD43 (Fig. 3B). Itk-TFII-I interactions were visualized immunoprecipitating Itk with an anti-Itk Ab combination, and then immunoblotting with anti-TFII-I Ab (Fig. 3A, upper panel). To ensure appropriate efficiency of the IP, Itk protein levels were also examined (Fig. 3A, lower panel). As a negative control, we included an IP with an irrelevant Ab, RaMIg (R lane), and showed that as expected, no background TFII-I-Itk interaction band could be observed in this sample (Fig. 3A, upper panel). When reverse experiments were undertaken – immunoprecipitating TFII-I from resting or activated (Fig. 3B) Jurkat cells and PBL-Ts (data not shown) – and subsequently probing with anti-Itk Abs, we confirmed the presence of endogenous co-immunoprecipitated Itk-TFII-I protein complexes.
Figure 3. TFII-I interacts with Itk in T lymphocytes, and its interaction is constitutive.
(A) PBL-T WCE were subjected to IP with anti-Itk Ab, followed by immunoblotting (IB) with anti-TFII-I Ab (upper panel). Blots were stripped and re-probed with anti-Itk Ab to monitor Itk protein levels (lower panel). RaMIg: Rabbit anti-mouse Ig negative IP control. The absence or presence of Ab in the IPs is indicated by a (−) or (+) respectively. (B) Jurkat cells were serum-starved and stimulated with either anti-CD3 + RaMIg, anti-CD3 + anti-CD43 + RaMIg, anti-CD43 + RaMIg Abs or RaMIg crosslinking Ab alone (R lane) during a kinetic time-course. WCE were subjected to IP with anti-TFII-I Abs, followed by immunoblotting (IB) with anti-Itk Ab (upper panels). Blots were stripped and re-probed with anti-TFII-I Ab to monitor TFII-I IP levels (lower panels). U lane: unstimulated cells. Data are representative of at least two experiments for (A) and (B).
One of the models of Btk-TFII-I signaling stipulates that a portion of the TFII-I pool which is tethered to Btk complexes in the cytoplasm is released following B cell activation, after which TFII-I translocates into the nucleus to modulate transcription [9, 11]. Moreover, because our kinetic results (Fig. 1) suggested that there might be a more transient tyrosine phosphorylation pattern in B cells than in T cells, we sought to determine whether the endogenous Itk-TFII-I interactions we observed remained stable under stimulating conditions. To this end, Jurkat cells (Fig. 3B) and PBL-Ts (data not shown) were stimulated either via CD3, and/or CD43 crosslinking at different times. Whole cell lysates were subjected to an IP with anti-TFII-I Ab, and protein complexes visualized using anti-Itk Abs (Fig. 3B, upper panels). TFII-I IP protein levels are also shown (Fig. 3B, lower panels). We determined that relative to unstimulated cells, Itk-TFII-I interactions remained unchanged for up to 60’ of CD3 and/or CD43 stimulation (Fig. 3B and data not shown). Similar results were obtained performing reverse IP experiments using anti-Itk Abs in Jurkat cells and PBL-Ts (data not shown). Thus, single or co-stimulated receptor engagement (CD3 and/or CD43) did not destabilize Itk-TFII-I protein interactions at the times tested. Overall, it appears that some characteristics of Itk-TFII-I signaling differ from those of Btk-TFII-I signaling in B cells.
TFII-I interacts with Itk in an ectopic expression system in fibroblasts
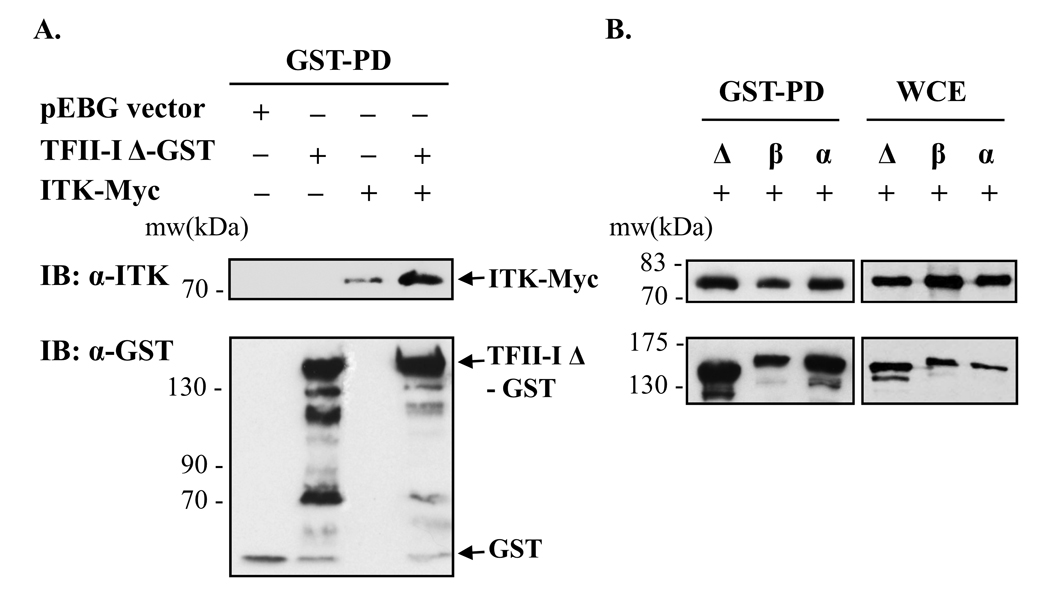
To further validate our findings that TFII-I interacts with Itk, we used an expression system in COS-7 fibroblasts to analyze the ability of Itk to associate with ectopic TFII-I. An important advantage of this system is that COS-7 cells do not express endogenous Itk. We initially tested the most widely studied TFII-I isoform, TFII-I-Δ (delta) [40, 41]. We expressed GST-tagged-TFII-I-Δ, in the presence or absence of Myc-tagged Itk in COS-7 cells and performed whole cell extract GST pull-down assays. When probing with anti-Itk Abs, an Itk-TFII-I protein interaction was distinctly observed when TFII-I and Itk were co-expressed (Fig. 4A and Supporting Fig. S1). Equivalent pulled-down TFII-I-Δ-GST levels are shown (Fig. 4A, lower panel, and Fig. S1). Whole cell lysates used in the pull-down assays were also monitored as a control for equivalent protein expression levels of TFII-I-Δ-GST and Itk-Myc (Fig. S1). In agreement with our findings in COS-7 cells, when ectopic TFII-I-Δ-GST and Itk-Myc proteins were co-expressed in Jurkat cells, GST-pull-down assays demonstrated that the two proteins also co-immunoprecipitated (data not shown). It should be noted that some non-specific binding of Itk-Myc to glutathione agarose beads could be appreciated in samples expressing Itk-Myc alone (Fig. 4A, upper panel, and Fig. S1A and B), or Itk-Myc, in the presence of GST-tagged pEBG vector (Fig. S1B), but both anti-Itk and anti-Myc Abs confirmed the Itk-TFII-I protein interaction (Fig. 4A and Fig. S1). The artifactual band was not observed with the anti-Myc antibody (Fig. S1A). Also, the intensity of the apparent band (Itk-Myc lane) was much lower than that of the protein complex band observed with Itk-Myc and TFII-I-GST protein co-expression (Fig. 4A and S1). In addition, detection of negligible non-specific binding of GST to Itk-Myc fusion proteins has been previously observed using the 10B2 anti-Itk Ab as well [42].
Figure 4. TFII-I-Δ, -β or -α interact with Itk in an ectopic expression system in fibroblasts.
(A) GST-tagged TFII-I-Δ was expressed in the presence or absence of Myc-tagged wild type Itk in COS-7 cells and subjected to whole cell extract GST pull-down assays (GST-PD). For control purposes, the GST-tagged pEBG backbone vector, TFII-I-Δ-GST and ITK-Myc were also singly expressed. The presence or absence of TFII-I-Δ-GST and/or ITK-Myc vectors is indicated by (+) or (−). The presence of Itk in the GST-PD complexes was detected by immunoblotting (IB) using anti-Itk Ab (upper panels). Membranes were stripped and re-probed with anti-GST Ab to reveal TFII-I-GST protein levels (lower panels). Bands recognized by the anti-GST Ab at a lower molecular weight than TFII-I represent protein degradation products. (B) GST-tagged TFII-I-Δ, TFII-I-β or TFII-I-α were co-expressed with Myc-tagged Itk in COS-7 cells and subjected to GST pull-down, followed by IB with anti-Itk Ab. GST-PD are shown in the left panels and WCE without GST-PD in the right panels. Data are representative of at least three experiments for (A) and (B).
Itk interacts with spliced variants of TFII-I
There are at least four splice variants of TFII-I naturally occurring in mammals [40, 41, 43]. The α-isoform is not expressed in murine cells but is expressed in human cells; the β-isoform is expressed in both murine and human cells; the Δ-isoform is expressed in both human and murine cells and is the most widely studied TFII-I variant [40, 41]. Because the γ-isoform is predominantly, if not exclusively, expressed in neuronal cells [40, 41], we did not include this isoform in our studies. Previous work has shown that TFII-I-α, TFII-I-β and TFII-I-Δ isoforms participate in homomeric as well as heteromeric interactions with each other [43], and they also appear to play different roles during certain cellular responses [16]. We have demonstrated in earlier studies that these three TFII-I isoforms are all capable of interacting with Btk in a comparable manner [11]. This prompted us to test whether different isoforms of TFII-I could bind to Itk. TFII-I-α, TFII-I-β and TFII-I-Δ isoforms were expressed in COS-7 cells as GST fusion proteins in the presence of Itk-Myc. A GST-pull down assay (Fig. 4B) was performed in the same manner as in Fig. 4A and Fig. S1. WCE were included as controls for input level comparison with pull-down samples (right panels). Results revealed that the Δ, α, and β isoforms of TFII-I could all associate with Itk to a similar extent. For simplicity purposes, based on the data obtained using three isoforms of TFII-I, we focused solely on TFII-I-Δ in subsequent experiments.
Structural analysis of TFII-I-Itk interactions
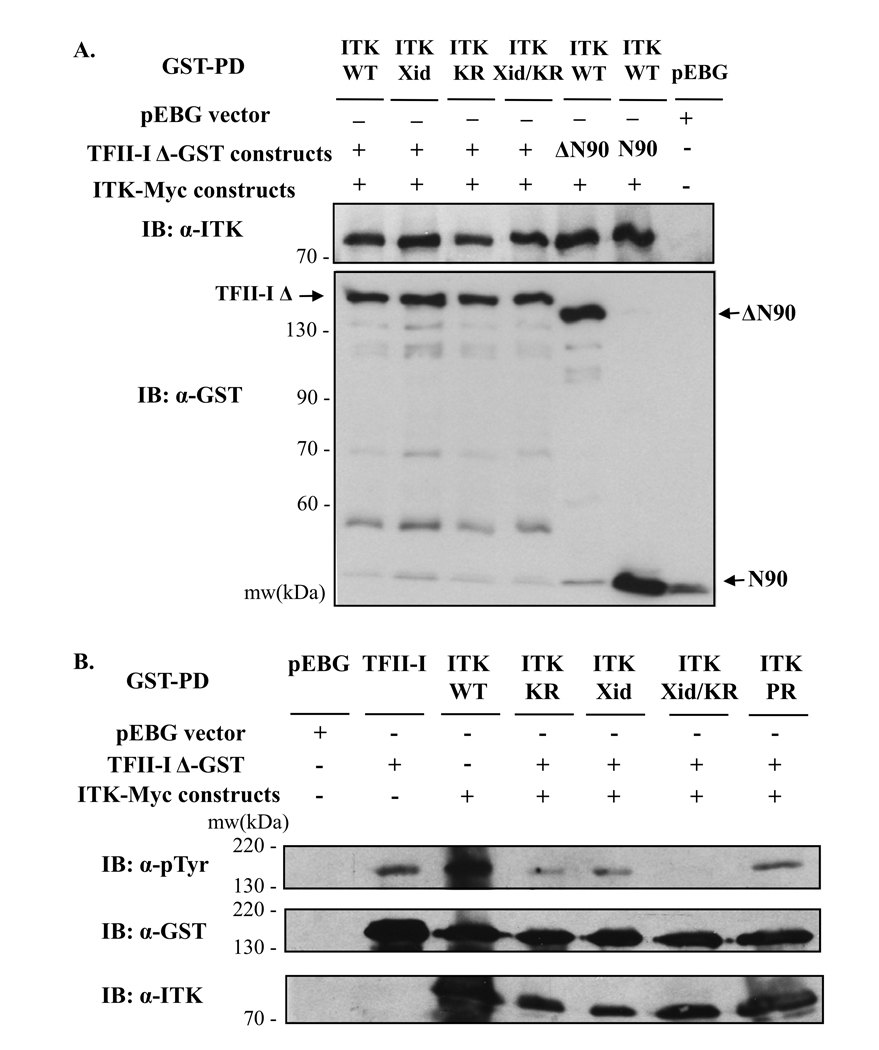
To gain some insight into the domains via which Itk and TFII-I interact, we expressed a panel of previously characterized Itk-Myc and TFII-I-GST mutants in COS-7 cells, and compared the ability of these mutants to form Itk-TFII-I complexes relative to wild type Itk and TFII-I, in GST pull-down assays (Figure 5). The N90 mutant consists of the first 90 N-terminal amino acids of TFII-I-Δ, and the ΔN90 mutant consists of the entire TFII-I-Δ sequence, yet lacks the first 90 N-terminal amino acids [11, 43, 44]. Because previous work has demonstrated that these first N-terminal residues are required for TFII-I-Btk binding [11, 43, 44], we questioned whether the same residues were critical in mediating TFII-I-Itk interactions. Protein associations were visualized using anti-Itk Ab, and overall pull-down levels, monitored, using anti-GST Ab (Fig. 5A). The molecular weight position of wild type and mutant proteins on gel is also indicated (Fig. 5A). Both N90 and ΔN90 bound Itk as readily as wild type TFII-I, demonstrating that – unlike certain requirements for TFII-I-Btk interactions – the first N-terminal 90 amino acids of TFII-I, although utilized, are not critical for Itk binding. This represents another distinguishing feature between TFII-I-Btk and TFII-I-Itk protein interactions.
Figure 5. Characterization of ITK-TFII-I domain interactions and TFII-I tyrosine phosphorylation.
(A, B) GST-tagged wild type or mutant (ΔN90, N90) TFII-I-Δ constructs were expressed in the presence or absence of Myc-tagged wild type (WT) or mutant (kinase-dead K390R (KR), R29C (Xid) or the P158A, P159A double mutation (PR)) Itk in COS-7 cells and subjected to whole cell extract GST pull-down assays (GST-PD). The presence or absence of TFII-I-GST and/or ITK-Myc vectors is indicated by (+, ΔN90, N90) or (−) respectively. The control pEBG vector was used as a negative control. (A) The presence of Itk in the GST-PD complexes was detected by immunoblotting (IB) using anti-Itk Ab (upper panel). The membrane was stripped and re-probed with anti-GST Ab to reveal TFII-I-GST protein levels (lower panel). Fainter bands recognized by the anti-GST Ab at lower molecular weights than TFII-I represent protein degradation products, or co-migration with the gel running front (bottom). (B) Following GST-PD, TFII-I-Δ phosphotyrosine levels were assessed by immunoblotting with anti-p-Tyr Ab (upper panel). The presence of Itk in the GST-PD complexes was detected by immunoblotting (IB) using anti-Itk Ab (bottom panel). The membrane was stripped and re-probed with anti-GST Ab to reveal TFII-I-GST protein levels (middle panel). Data are representative of at least two experiments for (A) and (B).
In addition, we analyzed the effect of TFII-I binding to Myc-tagged Itk mutants encompassing specific point mutations on various Itk domains [42, 45, 46]. We examined a version of Itk harboring a lysine to arginine substitution (K390R) which renders the protein kinase-dead (Itk-KR); an “Xid” mutation (R29C) corresponding to a mutation on Btk (at R28), affecting the phosphatidylinositol 3,4,5-trisphosphate binding pocket of Itk’s pleckstrin homology domain (PH), which commonly results in XLA or Xid disease; a mutant bearing both Xid and KR mutations (Xid/KR); and a mutant with an inactivated proline rich (PR) region (P158A, P159A) within Itk, affecting protein-protein interactions [42, 45, 46]. Results from GST pull-down assays revealed that mutations in the kinase, PH (Xid) and PR domains did not impair TFII-I binding to Itk (Fig. 5A upper panel, and 5B, lower panel). Our findings parallel previous studies demonstrating that TFII-I-Btk associations remain unaffected upon inactivation of the kinase domain [42, 45, 46], but differ from data showing that the Xid mutation impairs TFII-I-Btk binding [42, 45, 46].
TFII-I is tyrosine phosphorylated upon co-expression of Itk, but not kinase-dead Itk
TFII-I is a binding partner and phosphorylation substrate of Btk and c-Src [8, 9, 11, 12]. Thus, we investigated whether TFII-I, in addition to interacting with Itk, could potentially be its tyrosine phosphorylation target. Using the COS-7 ectopic expression system, we examined the phosphotyrosine status of TFII-I when in the absence or presence of Itk (Fig. 5B). GST pull-down assays indicated that the basal phosphorylation of TFII-I in the absence of Itk was minimal relative to the increase observed when wild type Itk was co-expressed with TFII-I (Fig 5B and Supporting Fig. S2A, upper panels). TFII-I-GST protein levels were if anything, lower in the TFII-I plus Itk lanes than in the TFII-I lanes, supporting a bona fide increase in tyrosine phosphorylation when both proteins were co-expressed (Fig 5B and S2A, middle panels). No tyrosine phosphorylation band was observed when the pEBG vector was expressed.
To further determine whether Itk was responsible for the induction of tyrosine phosphorylation of TFII-I, COS-7 cells were transfected with TFII-I and the kinase-dead Itk-Myc mutant. This resulted in dramatically reduced TFII-I phosphotyrosine levels (ITK-KR) relative to those observed upon wild type Itk co-expression (ITK-WT) (Fig. 5B, and S2B, upper panels). Furthermore, we compared the phosphotyrosine levels of TFII-I when the Itk-Myc mutants tested in Fig. 5A, Xid, Xid/KR and PR, were co-expressed with TFII-I (Fig. 5B). Diminished tyrosine phosphorylation of TFII-I was observed in the presence of Xid and PR mutants, albeit not as strongly as in the presence of kinase-dead Itk. Strikingly, co-expression of TFII-I with the Itk mutant harboring both Xid and kinase-dead mutations resulted in a complete loss of TFII-I tyrosine phosphorylation, suggesting that aside from the catalytic kinase domain, the Xid residue may be important for TFII-I tyrosine phosphorylation (Fig. 5B). The data hence suggest that a functional Itk is required to induce tyrosine phosphorylation of TFII-I above basal levels. The levels of TFII-I-GST were equivalent in the pull-down for all samples (Fig. 5B middle panel, S2B left lower panel, and S2C upper panel). Moreover, by monitoring Itk and TFII-I-GST levels in these pull-down assays (WCE and pull-down samples), we also determined that mutant Itk-Myc expression levels were not lower than those of ITK-WT (Fig. 5, and supporting figures S2 and S3). Thus, the inability of these mutants – particularly KR and Xid/KR— to induce tyrosine phosphorylation of TFII-I, was not due to their inability to associate with TFII-I (Fig. 5B and S2), corroborating our findings in Fig. 5A. In addition, by immunoprecipitating TFII-I from COS-7 whole cell extracts obtained from the same GST pull-down assays in Fig. 5, we confirmed that WT, KR, Xid, Xid/KR and PR Itk were all capable of interacting with TFII-I (Supporting Figure S4). Thus, protein complex formation of Itk with TFII-I does not depend on Itk’s ability to function as a kinase.
Itk potentiates the TFII-I-driven transcriptional activity of the c-fos promoter
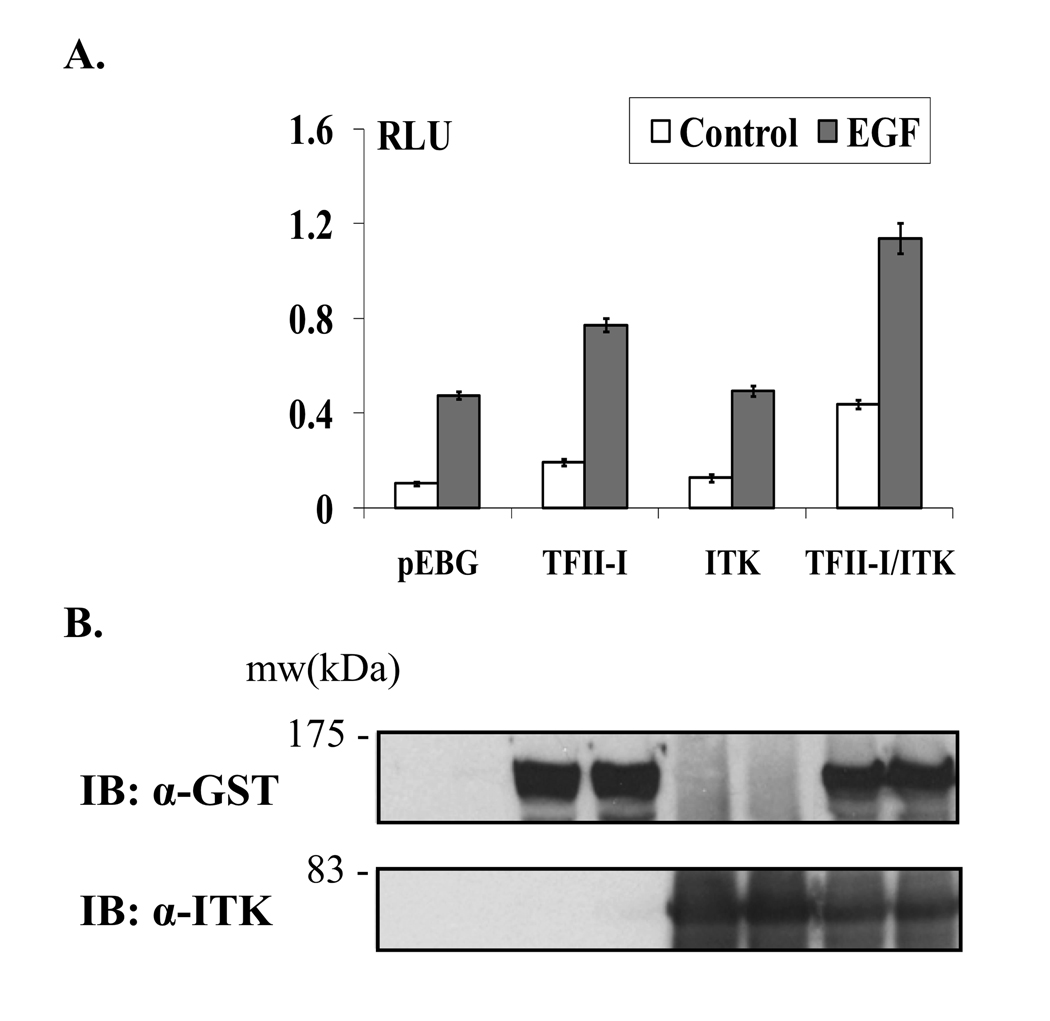
To begin evaluating the biological role of Itk-mediated phosphorylation of TFII-I, we examined the effects of Itk and TFII-I co-expression on the transcriptional regulation of the c-fos promoter. This model promoter has been previously used in TFII-I transcription studies [11, 12, 19]. Wild type TFII-I-Δ-GST was expressed in COS-7 cells in the absence or presence of Myc-tagged Itk. Subsequently, we assessed in reporter assays, the effect of Itk on wild type TFII-I-mediated transcription of the c-fos promoter, in the absence or presence of EGF stimulation [11, 12, 19] (Fig. 6A and 6B). Results indicated that as expected [11, 12, 19], TFII-I induced the transcriptional activity of the c-fos promoter, particularly under stimulating conditions. Expression of Itk did not drive c-fos transcription above control levels (empty vector). Importantly, TFII-I-mediated basal and activated transcription of the c-fos promoter was potentiated upon co-expression of Itk above the effect generated by TFII-I expression alone. Whole cell lysates of samples used in the luciferase reporter were analyzed via Western blotting in order to monitor TFII-I and Itk protein expression levels (Fig. 6B). These results implicate the Itk-TFII-I pathway in gene regulation and further suggest that these two proteins directly or indirectly cooperate to activate and regulate transcription.
Figure 6. Itk potentiates the TFII-I-driven transcriptional activity of the c-fos promoter under basal and growth factor-stimulating conditions.
(A) Wild type TFII-I-Δ-GST was expressed in the presence or absence of wild type ITK-Myc in COS-7 cells, along with a c-fos promoter luciferase construct and the levels of transcription measured by a luciferase reporter assay. The pEBG empty vector was used as a negative control in the assay. Transfected cells were either stimulated with EGF for 4 h (EGF columns) or remained untreated in serum-free medium (control columns). RLU: relative luciferase units. Transfections were performed in triplicate and the results presented as mean ± SD. (B) Western blot analysis of equivalent volumes (50µl) of the COS-7 whole cell lysates used in the luciferase reporter assay. TFII-I-Δ-GST protein levels were visualized using anti-GST Ab (top panel). The membrane was stripped and re-probed using anti-Itk Ab to monitor Itk-Myc protein levels (bottom panel). The results are representative of at least two experiments for (A) and (B).
Discussion
Activation of Tec family kinases in T cells occurs predominantly downstream of the TCR, and results in the regulation of various cellular processes such as lymphoid development, T cell activation and optimal T cell effector functions [1, 2]. Itk is the principal Tec kinase in T cells, and its activation is necessary to ensure proper immune responses [1]. Nevertheless, many of the pathways connecting Itk-mediated signaling to immune-specific gene expression are not fully understood. The transcription factor TFII-I is a target of Btk signaling in B cells [8–11], and thus we questioned whether TFII-I might also serve as a downstream target of Itk signaling in T lymphocytes. We focused our efforts into determining first, if TFII-I could be activated upon TCR and co-receptor signaling in T cells; and second, into uncovering some of the molecular determinants of Itk-TFII-I signaling in T cells.
Here, we provide evidence that TFII-I is tyrosine phosphorylated within 2’ of TCR and/or CD43 crosslinking in human T cells, as well as post-CD3/CD28 ligation in murine T lymphocytes and Jurkat cells (Fig. 1 and 2). Strong and sustained TFII-I tyrosine phosphorylation was observed when both TCR and CD43, or TCR and CD28 were simultaneously engaged, relative to TCR, CD43, or CD28 ligation alone. These findings are in agreement with recent data showing that relative to single crosslinking, simultaneous crosslinking of CD43 and TCR [31], or CD28 and TCR receptors [47], resulted in stronger and prolonged ERK phosphorylation, enhanced Zap70 and ζ-chain phosphorylation, accompanied by high levels of IL-2 production and cell proliferation in human PBL-Ts. Consistent with this work, our current results show that the intensity of TFII-I tyrosine phosphorylation varied according to the extracellular stimulus involved; while CD43 ligation induced a short-lived and weaker TFII-I activation response relative to TCR ligation, simultaneous TCR and CD43, or TCR and CD28 crosslinking, resulted in a synergized activation response. Our data thus support the notion that TFII-I activation can be modulated in a trigger-specific manner to accommodate a cell’s environmental and functional requirements. The significance of stimulus-specific TFII-I activation remains to be determined; indeed, it will be interesting to explore which downstream signaling targets/genes are differentially induced following trigger-specific TFII-I activation. One might also investigate whether crosslinking of other co-stimulatory molecules such as CD2, and/or CTLA-4 augment TFII-I-dependent phosphorylation.
We also conclude that TFII-I activation follows a similar and rapid phosphorylation pattern in T and B cells (within 2’ of receptor crosslinking) (Fig. 1). These results are consistent with previous findings showing tyrosine phosphorylation of TFII-I in the Ramos B cell line as early as 2’ post-IgM crosslinking [8]. When examining the kinetics of TFII-I tyrosine phosphorylation, we noticed that the response in Jurkat cells was prolonged past 15’ post-stimulation, whereas in B cells it appeared curtailed (declining at 10’ post-stimulation). The precise functional implications of these findings remain to be elucidated.
As a step towards uncovering whether Itk and TFII-I pathways could be linked in T cells, here we demonstrate both ex vivo and in vitro that TFII-I physically interacts with Itk in a constitutive manner (Fig. 3, 4, and S1). This concurs with previous findings establishing that Btk-TFII-I interactions are also constitutive in murine and human B cells [9]. However, our data indicate that in contrast with findings where Btk-TFII-I protein complexes dissociated upon 10’ IgM ligation in B cells [9], Itk-TFII-I protein complexes are quite stable at different activation times in T cells (Fig. 3B and data not shown). These findings may represent a distinguishing feature between Btk-TFII-I and Itk-TFII-I signaling pathways in B and T cells. Interestingly, it has been demonstrated that while wild type Btk readily associates with TFII-I, a mutant version of Btk responsible for the Xid mutation, Btk-R28C, does not [9]. Contrary to this, we have observed that the interaction of TFII-I with the homologous mutant Itk-R29C (Xid) is not abrogated (Fig. 5 and S3). This could partly account for the intrinsic stability of Btk-TFII-I vs. Itk-TFII-I protein complexes. Future experiments are thus required to fully resolve these differences and their functional outcomes.
We further establish that splice variants of TFII-I, (Δ, β, and α isoforms) are all capable of associating with Itk, in agreement with previous data demonstrating that these same isoforms interact with Btk [11] (Fig. 4). Together, the data suggest that functional differences between Itk-TFII-I and Btk-TFII-I interactions are independent from the ability of Tec kinases to associate with distinct TFII-I isoforms.
Structural analysis of TFII-I and Itk interaction domains indicated that mutations in the proline rich, pleckstrin homology (Xid) or kinase domains of Itk did not abrogate TFII-I-Itk protein binding (Fig. 5). With regard to the TFII-I domains, we found that the first N-terminal 90 amino acids of TFII-I, although utilized, are not critical for Itk binding (Fig. 5). Regions required for Itk binding must reside beyond the first N-terminal 90 residues of TFII-I, and future work should target such regions. We have ensured in GST pull-down assays, comparable transfected protein levels of mutant and wild type TFII-I and Itk in whole cell extracts (Supporting Figure S3). However, it is still possible that experimental conditions used in our pull-down assays may not allow to fully distinguish subtle differences in Itk binding with either full length TFII-I, or ΔN90 and N90 mutants. Thus, the involvement of the first 90 amino acids of TFII-I in binding Itk needs to be detailed more precisely. The resolution of our assays does not provide information of changes in secondary structure that could affect TFII-I binding to Itk. Consequently, future work should aim to expand the current findings. Notwithstanding, the first 90 amino acids of TFII-I are required for Btk binding, and thus, our findings represent an additional distinguishing feature between TFII-I-Btk and TFII-I-Itk protein interactions, whose functional significance remains to be determined.
Interestingly, preliminary data obtained with partial Itk constructs demonstrated that combined point mutations in the SH3 and kinase domains could impair Itk-TFII-I interactions (data not shown). A more comprehensive structure-function analysis of the Itk interaction domains (including the role of SH3, SH2, kinase and Tec regions) is therefore warranted. Taken together, our data provide new insights into the putative interaction domains between Itk and TFII-I.
Of relevance, our data show that TFII-I is tyrosine phosphorylated in the presence of wild type, but not kinase-dead Itk, suggesting that TFII-I is directly or indirectly, a downstream phosphorylation target of Itk (Fig. 5). It remains to be determined if Itk is capable of directly phosphorylating TFII-I. Importantly, mutations in both kinase and PH domains (Xid) of Itk severely impaired TFII-I tyrosine phosphorylation, suggesting that these regions are implicated in TFII-I tyrosine phosphorylation (Fig 5). Moreover, decreased tyrosine phosphorylation of TFII-I in the presence of kinase-dead or kinase-dead/Xid mutants was not due to decreased association of TFII-I-Itk protein complexes. Our data also showed that mutations in the PR domain of Itk did not affect TFII-I-Itk interactions, but caused a decrease in TFII-I phosphorylation, although to a lesser degree than other Itk mutations. Future experiments are required to fully discern these implications.
It will also be significant to address if CD43 ligation activates Itk, or co-stimulates its activation in conjunction with anti-CD3. In agreement with these results, our in vitro studies in Jurkat T cells, peripheral blood T lymphocytes and murine splenic T lymphocytes have consistently demonstrated TFII-I tyrosine phosphorylation upon extracellular stimulation. And, although the biochemical data suggest that Itk is capable of phosphorylating TFII-I, we do not exclude the possibility that TFII-I can be targeted by different kinases at distinct sites, simultaneously or not [11, 12]. Indeed, it has been documented that c-Src and Btk exert their effects on TFII-I through distinct and independent pathways, and moreover, that they target different tyrosine phosphorylation and interaction sites on TFII-I [11, 12]. Accordingly, other signaling pathways activating TFII-I might include Src-family kinases such as Lck and/or Fyn.
Presently, the functional consequences of TFII-I phosphorylation in T cells are not known. Given that TFII-I has multiple potential tyrosine phosphorylation sites, it is possible that these are utilized differentially. Future studies might focus on the determination of specific Itk-dependent TFII-I phosphorylation sites, and on the examination of putative phosphorylation differences in TFII-I in normal vs. Itk-deficient T cells. Experiments analyzing the phosphorylation status of TFII-I in T lymphocytes derived from Itk and/or Itk/Rlk knockout mice could shed some light into the role of Itk-dependent TFII-I activation.
As an additional approach to obtain a better assessment of the functional role of Itk-TFII-I signaling in T cells, we demonstrate in reporter assays that co-expression of Itk potentiates the TFII-I-driven transcriptional activity of the model c-fos promoter, under basal and stimulating conditions (Fig. 6). These results are in concordance with previous work demonstrating that Btk is a potent inducer of TFII-I-mediated transcription [11], suggesting a certain degree of similarity in the mechanisms governing TFII-I activation and gene regulation in various cell types. Because c-fos transcription was potentiated when both Itk and TFII-I were co-expressed in fibroblasts, our results further suggest additive cooperation between TFII-I and Itk proteins in transcriptional regulation, presumably through protein-protein interactions localizing to the c-fos promoter region. A comparison of Itk-TFII-I protein complexes in nuclear vs. cytoplasmic cell compartments might provide insight into the regulatory mechanisms of Itk-TFII-I interactions and gene regulation. While our results suggest that Itk regulates the transcriptional activity of TFII-I during T cell activation, the physiological target genes involved in this pathway remain to be elucidated.
To conclude, mechanistic and functional analyses from our current study demonstrate for the first time, activation of the multifunctional transcription factor TFII-I in response to receptor engagement in T cells and further implicate Itk in such activation. Our work thus provides an initial step in understanding the biological role of this pathway in T cell function and development. Future experiments should aim to expand the present findings, particularly in the context of T cell function, leading to a better understanding of the mechanisms underlying Itk-TFII-I protein interactions and their importance in T cell signaling and gene regulation.
Materials and Methods
Cell cultures
Human T cells
Jurkat T cells, and peripheral blood lymphocyte - T cells (PBL-Ts) (see below) were cultured in RPMI 1640, supplemented with 2 mM L-glutamine, 10% FBS, 50 units/ml of penicillin and 50 µg/ml streptomycin (Gibco - Invitrogen), under reducing conditions (50 µM β-mercaptoethanol) (supplemented RPMI). PBL-Ts were isolated from adult healthy donors as previously described using Ficoll-Hypaque separation and nylon wool columns [27]. The acquisition and isolation of human PBLs has been routinely performed in Dr. Rosenstein’s laboratory and has been approved by institutional committees. Purified T cells were predominantly TCR+ (>90%) and CD43+ (>95%) as determined by flow cytometry. Cells were used in biochemical assays the following day of isolation.
Human B cells
The human B cell line, BJAB (ATCC, Rockville, MD), was cultured in supplemented RPMI.
COS-7 fibroblasts
COS-7 cells (from monkey kidney epithelium, ATCC), were cultured in DMEM supplemented with 10% FBS and antibiotics (as above).
Human T cell and B cell activation and immunoprecipitation assays
Purified T lymphocytes and Jurkat cells were serum-starved in RPMI 1640 for 3.5 h at 37 °C, 5% CO2 in a humidified incubator, subsequently resuspended in 0.5 ml of cold RPMI 1640 (2 × 107 cells) and activated as follows:
For anti-CD43, anti-CD28 and/or anti-CD3 stimulation, cells were incubated 10 min on ice with either L10 (anti-CD43) [48], anti-CD28 (clone CD8.2), and/or OKT3 (anti-CD3ε) mAbs (4 µg/ml). Subsequently, primary Abs were crosslinked with the secondary Ab, RaMIg (4 µg/ml) [27], and cells were activated in a 37°C water-bath for specific times, after which cells were lysed as previously described [11].
BJAB cells were serum-starved in RPMI 1640 for 2 h at 37 °C, 5% CO2 in a humidified incubator, subsequently resuspended in cold RPMI 1640 (2 × 107 cells) and activated with rabbit anti-human IgM F(ab’)2 (µ-chain-specific, 5.6 µg/ml, Dako) in a 37°C water-bath for specific times, after which cells were lysed, as previously described [11].
T cell and B cell lysates were pre-cleared of debris by centrifugation 15 min, 14,000 rpm, at 4°C. Total protein concentration was measured using the Bradford method (Bio-Rad). For immunoprecipitations (IP), 1000 µg to 2000 µg of whole cell lysates were incubated with appropriate Abs; TFII-I was immunoprecipitated with an anti-TFII-I Ab mix (equivalent amounts of Abs: mAb from BD Transduction Labs or Cell Signaling plus rabbit polyclonal anti-TFII-I [15, 44]); Itk was immunoprecipitated with an anti-Itk Ab mix (equivalent amounts of Abs: 2F12 plus 10B2 mouse mAbs [46]). RaMIg, an irrelevant control Ab for IPs was used at the same concentration as test Abs. Ab-lysate mixtures were incubated for 20 min at 4 °C (rotating motion), followed by incubation with a proportionate amount of protein A/G-sepharose (Pharmacia) beads (1:1 slurries) for 1h 40 min at 4 °C (rotating motion). As an additional control for the IP assays, A/G-sepharose beads were incubated with whole cell lysates alone, in the absence of Abs, revealing no protein band expression upon immunoblotting. Pre-clearing or non-pre-clearing cell lysates with protein A/G prior to addition of Abs did not result in major differences of experimental outcomes. Following incubation periods, samples were washed 4 times in lysis buffer. Whole cell extracts (WCE) (50 µg) and immunoprecipitated proteins were subsequently boiled in Laemmli buffer and resolved by SDS-PAGE/ Western blot analysis (see below).
Expression vectors
TFII-I plasmids
The GST fusion plasmids, pEBG vector, pEBG-II-I (TFII-I-Δ) wild type, pEBG-II-Iβ (TFII-I-β), pEBG-II-Iα (TFII-I-α) have been previously described [11, 43, 44]. Mutant pEBG-N90-II-I consists of the first 90 amino acids on the N-terminus of TFII-I-Δ, and pEBG-ΔN90-II-I consists of the entire TFII-I-Δ sequence, yet lacks the first 90 amino acids from the N-terminal end of TFII-I-Δ; these constructs have been previously described [11, 43, 44].
Itk plasmids
The Myc-tagged wild type pEF-ITK-Myc and mutant pEF-ITK-K390R-Myc (KR), pEF-ITK-R29C-Myc (Xid), pEF-ITK-R29C-K390R-Myc (Xid/KR), pEF-ITK- PR-Myc (P158A, P159A) have been previously described [42, 45, 46].
Reporter assay plasmids
The c-fos-luciferase reporter plasmid (pSVOAΔ5’, containing a 379 bp murine c-fos promoter upstream of a luciferase reporter gene), and the pRL-TK (thymidine kinase) (Renilla luciferase) plasmid (Promega) have both been previously described [43].
Transient transfection of COS-7 cells, GST pull-down assays and immunoprecipitations
COS-7 cells were transfected at 80% confluency using Polyfect lipofection reagent according to manufacturer’s instructions (Qiagen). pEBG-II-I (TFII-I-Δ) wild type, pEBG-II-I-β (TFII-I-β), pEBG-II-I-α (TFII-I-α), pEBG-N90-II-I, or pEBG-ΔN90-II-I cDNAs (8 µg) were transfected in the absence or presence of pEF-ITK-Myc wild type cDNA (10 µg). pEBG-II-I was also transfected in the absence or presence of pEF-ITK-K390R-Myc, pEF-ITK-R29C-Myc (Xid), pEF-ITK-R29C-K390R-Myc (Xid/KR), or pEF-ITK-PR-Myc (P158A, P159A) cDNAs (10 µg). WCE prepared as previously described [11]. WCE (500 – 1000 µg) were used for GST pull-down assays or immunoprecipitations with anti-TFII-I (see above). For GST pull-downs, WCE were incubated with proportionate amounts of glutathione-agarose beads (1:1 slurry) (Sigma) for 2 h at 4 °C (rotating motion). Following incubation periods, lysate-bead slurries were washed 4 times in lysis buffer and boiled in Laemmli buffer. WCE (10 – 50 µg) and pulled-down proteins were resolved by SDS-PAGE/Western blot analysis (see below).
Transient transfection of COS-7 cells and luciferase reporter assays
COS-7 cells (2 × 105) were transfected using supplemented DMEM with Polyfect lipofection reagent (Qiagen), as previously described [11]. Specifically, 600 ng of c-fos promoter-luciferase plasmid were combined with 35 ng of Renilla luciferase pRL-TK (internal control). One µg of pEBG-II-I wild type (TFII-I-Δ) was added to the mixture in the presence or absence of 1 µg pEF-ITK-Myc wild type. Total DNA concentration was normalized using empty pEBG vector. At 24 h post-transfection, cells were serum-starved for 20 h, and stimulated with human epidermal growth factor (EGF, 25 ng/ml, Sigma) for 4 h. Cells were subsequently harvested by using passive lysis (Promega). Relative luciferase activities of firefly and Renilla luminescence were measured from WCE using the Dual Luciferase Assay kit (Promega) and a TD luminometer. Firefly luciferase values were normalized to Renilla luciferase values representing relative luciferase activity units. To monitor protein expression, 50 µl of whole cell lysates from representative transfections were analyzed by SDS-PAGE/ Western blotting (see below).
Mice
C57BL/10 mice were used between 6 and 8 wk of age and were maintained at the University of Massachusetts Medical School specific pathogen-free animal facility after review and approval by the institutional animal care and use committee.
Murine T cell isolation and activation for immunoprecipitation assays
Murine T cells
Splenic T cells were purified from wild C57BL/10 mice by positive selection using anti-CD4 and anti-CD8 magnetic beads in the AutoMACS separation system according to manufacturer’s instructions (Miltenyi Biotec Inc.). Purity of T cells was > 95%, as determined by flow cytometry. Cells were purified in supplemented RPMI, serum-starved in RPMI 1640 for 3 hours at 37 °C, and used in biochemical assays the same day of isolation. Cells (1.5 × 107) were resuspended in 500 µl aliquots of cold RPMI 1640 and incubated with biotin-anti-CD3ε (1 µg/ml, clone 145-2C11) plus biotin-anti-CD28 (4 µg/ml, clone 37.51) Abs (BD Pharmigen), for 15 min on ice. Subsequently, cells were incubated with 20 µg/ml streptavidin (Pierce) and stimulated for 0, 2, and 15 min in a 37 °C water-bath. Cells were lysed on ice for 30 min in lysis buffer [11], additionally supplemented with PhosStop phosphatase inhibitor tablet cocktails (Roche Molecular Biochemicals). Lysates were pre-cleared of debris by centrifugation 10 min, 14,000 rpm, at 4°C, and total protein concentration measured. For IPs, 400 µg of whole cells lysates were incubated with anti-TFII-I Ab (10 µl, Cell Signaling), using the Catch and Release IP kit (Millipore) according to manufacturer’s instructions. Immunoprecipitated proteins were boiled in Laemmli buffer and resolved by SDS-PAGE/ Western blot analysis (see below). In preliminary assays, an irrelevant Ig Ab was incubated with whole cell lysates as a negative control for the IP, showing that no protein band was detected in this sample upon immunoblotting.
Western blot analysis
WCE, IP, or GST-pull-down samples were resolved by SDS-PAGE (7.5% – 10%) and transferred to nitrocellulose (Schleicher & Schuell) using a semi-dry transfer apparatus as described [43]. Blots were blocked in TBST with 5% nonfat milk or 5% BSA (Sigma) (the latter used for anti-phosphotyrosine immunoblotting) according to standard methods. The following primary Abs were used for blotting: anti-Myc (Santa Cruz) (1:2000 dilution), anti-TFII-I (rabbit polyclonal) (1:2500) [15, 44], anti-GST (GST-2 mouse mAb, Sigma) (1:3500), anti-phosphotyrosine (mouse mAb PY99, Santa Cruz Biotechnology or 4G10, Millipore) (1:2000), anti-Itk (2F12 plus 10B2 mouse mAb mix) (1:1000). Secondary HRP-conjugated goat anti-mouse and goat anti-rabbit Abs were used, and proteins visualized using enhanced chemiluminiscence according to standard methods. Data shown are representative of duplicate or triplicate independent experiments. Densitometry of scanned autoradiographs was performed using FluorChem 8800 software (Alpha Innotech) with appropriate auto-background correction. Fold-increases in tyrosine phosphorylation were calculated from normalized densitometric ratios of individual bands (anti-pTyr to anti-TFII-I ratio).
Supplementary Material
Acknowledgements
We’d like to thank Dr. José Moreno for the kind gift of the anti-IgM Ab, and Erika Melchy and Regina Whitehead for technical assistance. We’re also grateful to Dr. Gustavo Pedraza and Dr. David Schatz for critical reading of the manuscript and to Dr. Ana I. Sacristán for assistance with Graphic Converter X software. We’re greatly appreciative to the Blood Bank at the Hospital de Zona, Instituto Mexicano del Seguro Social, Cuernavaca, Morelos, for donating leukocyte concentrates from healthy donors. This work was partially supported by grants from the Consejo Nacional de Ciencia y Tecnología (U46505M to YR) and the Dirección General de Apoyo Académico, Universidad Nacional Autónoma de México (IN219307 to YR), Mexico, and by grants from the National Institutes of Health (AI37584 and AI66118 to LJB, and HD046034 to ALR).
Abbreviations
- Itk
Inducible Tyrosine Kinase
- Btk
Bruton’s tyrosine kinase
- TFII-I
Transcription Factor (RNA polymerase) II – binding the Initiator
- RaMIg
rabbit anti-mouse IgG
- PD
pull-down
- WCE
whole cell extracts
- IB
immunoblotting
- pTyr
phosphotyrosine
Footnotes
Disclosures
The authors have no financial or commercial conflicts of interest.
References
- 1.Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. Tec family kinases in T lymphocyte development and function. Annu. Rev. Immunol. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- 2.Berg LJ. Signalling through Tec kinases regulates conventional versus innate CD8+ T-cell development. Nature Rev. Immunol. 2007;7:479–485. doi: 10.1038/nri2091. [DOI] [PubMed] [Google Scholar]
- 3.Felices M, Berg LJ. The Tec kinases Itk and Rlk regulate NKT cell maturation, cytokine production, and survival. J. Immunol. 2008;180:3007–3018. doi: 10.4049/jimmunol.180.5.3007. [DOI] [PubMed] [Google Scholar]
- 4.Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, Sparkes RS, Kubagawa H, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- 5.Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, et al. The gene involved in X-linked agammaglobulinemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- 6.Rawlings DJ, Saffran DC, Tsukada S, Largaespada DA, Grimaldi JC, Cohen L, Mohr RN, Bazan JF, et al. Mutation of the unique region of Bruton's tyrosine kinase in immunodeficient XID mice. Science. 1993;261:358–361. doi: 10.1126/science.8332901. [DOI] [PubMed] [Google Scholar]
- 7.Thomas JD, Sideras P, Smith CIE, Vorechovsky I, Chapman V, Paul WE. Colocalization of X-linked agammaglobulinemia and X-linked immunodeficiency genes. Science. 1993;261:355–358. doi: 10.1126/science.8332900. [DOI] [PubMed] [Google Scholar]
- 8.Yang W, Desiderio S. BAP-135, a target for Bruton's tyrosine kinase in response to B cell receptor engagement. Proc. Natl. Acad. Sci. USA. 1997;94:604–609. doi: 10.1073/pnas.94.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novina CD, Kumar S, Bajpai U, Cheriyath V, Zhang K, Pillai S, Wortis HH, Roy AL. Regulation of nuclear localization and transcriptional activity of TFII-I by Bruton's tyrosine kinase. Mol. Cell. Biol. 1999;19:5014–5024. doi: 10.1128/mcb.19.7.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egloff AM, Desiderio S. Identification of phosphorylation sites for Bruton's tyrosine kinase within the transcriptional regulator BAP/TFII-I. J. Biol. Chem. 2001;276:27806–27815. doi: 10.1074/jbc.M103692200. [DOI] [PubMed] [Google Scholar]
- 11.Sacristán C, Tussié-Luna MI, Logan SM, Roy AL. Mechanism of Btk-mediated recruitment and regulation of TFII-I. J. Biol. Chem. 2004;279:7147–7158. doi: 10.1074/jbc.M303724200. [DOI] [PubMed] [Google Scholar]
- 12.Cheriyath V, Desgranges ZP, Roy AL. c-Src dependent transcriptional activation of TFII-I. J. Biol. Chem. 2002;277:22798–22805. doi: 10.1074/jbc.M202956200. [DOI] [PubMed] [Google Scholar]
- 13.Roy AL, Meisterernst M, Pognonec P, Roeder RG. Cooperative interaction of an initiator-binding transcription initiation factor and the helix-loop-helix activator USF. Nature. 1991;354:245–248. doi: 10.1038/354245a0. [DOI] [PubMed] [Google Scholar]
- 14.Roy AL, Carruthers C, Gutjahr T, Roeder RG. Direct role for Myc in transcription initiation mediated by interactions with TFII-I. Nature. 1993;365:359–361. doi: 10.1038/365359a0. [DOI] [PubMed] [Google Scholar]
- 15.Cheriyath V, Novina CD, Roy AL. TFII-I regulates Vbeta promoter activity through an initiator element. Mol. Cell. Biol. 1998;18:4444–4454. doi: 10.1128/mcb.18.8.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakre S, Tussie-Luna MI, Ashworth T, Novina CD, Settleman J, Sharp PA, Roy AL. Opposing functions of TFII-I spliced Isoforms in growth factor-induced gene expression. Mol. Cell. 2006;24:301–308. doi: 10.1016/j.molcel.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Caraveo G, van Rossum DB, Patterson RL, Snyder SH, Desiderio S. Action of TFII-I outside the nucleus as an inhibitor of agonist-induced calcium entry. Science. 2006;314:122–125. doi: 10.1126/science.1127815. [DOI] [PubMed] [Google Scholar]
- 18.Ashworth T, Roy AL. TFII-I controls B cell proliferation via regulating NF-kappaB. J. Immunol. 2007;178:2631–2635. doi: 10.4049/jimmunol.178.5.2631. [DOI] [PubMed] [Google Scholar]
- 19.Kim D-W, Cheriyath V, Roy AL, Cochran BH. TFII-I enhances activation of the c-fos promoter through interactions with upstream elements. Mol. Cell. Biol. 1998;18:3310–3320. doi: 10.1128/mcb.18.6.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D-W, Cochran BH. JAK2 activates TFII-I and regulates its interaction with extracellular signal-regulated kinase. Mol. Cell. Biol. 2001;21:3387–3397. doi: 10.1128/MCB.21.10.3387-3397.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crusselle-Davis VJ, Vieira KF, Zhou Z, Anantharaman A, Bungert J. Antagonistic regulation of beta-globin gene expression by helix-loop-helix proteins USF and TFII-I. Mol. Cell. Biol. 2006;26:6832–6843. doi: 10.1128/MCB.01770-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogura Y, Azuma M, Tsuboi Y, Kabe Y, Yamaguchi Y, Wada T, Watanabe H, Handa H. TFII-I down-regulates a subset of estrogen-responsive genes through its interaction with an initiator element and estrogen receptor alpha. Genes to Cells. 2006;11:373–381. doi: 10.1111/j.1365-2443.2006.00952.x. [DOI] [PubMed] [Google Scholar]
- 23.Wen YD, Cress WD, Roy AL, Seto E. Histone deacetylase 3 binds to and regulates the multifunctional transcription factor TFII-I. J. Biol. Chem. 2003;278:1841–1847. doi: 10.1074/jbc.M206528200. [DOI] [PubMed] [Google Scholar]
- 24.Novina CD, Cheriyath V, Roy AL. Regulation of TFII-I activity by phosphorylation. J. Biol. Chem. 1998;273:33443–33448. doi: 10.1074/jbc.273.50.33443. [DOI] [PubMed] [Google Scholar]
- 25.Cyster JG, Shotton DM, Williams AF. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO J. 1991;10:893–902. doi: 10.1002/j.1460-2075.1991.tb08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cruz-Munoz ME, Salas-Vidal E, Salaiza-Suazo N, Becker I, Pedraza-Alva G, Rosenstein Y. The CD43 coreceptor molecule recruits the zeta-chain as part of its signaling pathway. J. Immunol. 2003;171:1901–1908. doi: 10.4049/jimmunol.171.4.1901. [DOI] [PubMed] [Google Scholar]
- 27.Pedraza-Alva G, Merida LB, Burakoff SJ, Rosenstein Y. CD43-specific activation of T cells induces association of CD43 to Fyn kinase. J. Biol. Chem. 1996;271:27564–27568. doi: 10.1074/jbc.271.44.27564. [DOI] [PubMed] [Google Scholar]
- 28.Pedraza-Alva G, Merida LB, Burakoff SJ, Rosenstein Y. T cell activation through the CD43 molecule leads to Vav tyrosine phosphorylation and mitogen-activated protein kinase pathway activation. J. Biol. Chem. 1998;273:14218–14224. doi: 10.1074/jbc.273.23.14218. [DOI] [PubMed] [Google Scholar]
- 29.Pedraza-Alva G, Sawasdikosol S, Liu YC, Merida LB, Cruz-Munoz ME, Oceguera-Yanez F, Burakoff SJ, Rosenstein Y. Regulation of Cbl molecular interactions by the co-receptor molecule CD43 in human T cells. J. Biol. Chem. 2001;276:729–737. doi: 10.1074/jbc.M008494200. [DOI] [PubMed] [Google Scholar]
- 30.Santana MA, Pedraza-Alva G, Olivares-Zavaleta N, Madrid-Marina V, Horejsi V, Burakoff SJ, Rosenstein Y. CD43-mediated signals induce DNA binding activity of AP-1, NF-AT, and NFkappa B transcription factors in human T lymphocytes. J. Biol. Chem. 2000;275:31460–31468. doi: 10.1074/jbc.M005231200. [DOI] [PubMed] [Google Scholar]
- 31.Fierro NA, Pedraza-Alva G, Rosenstein Y. TCR-dependent cell response is modulated by the timing of CD43 engagement. J. Immunol. 2006;176:7346–7353. doi: 10.4049/jimmunol.176.12.7346. [DOI] [PubMed] [Google Scholar]
- 32.Ji HB, Gupta A, Okamoto S, Blum MD, Tan L, Goldring MB, Lacy E, Roy AL, et al. T cell-specific expression of the murine CD3delta promoter. J. Biol. Chem. 2002;277:47898–47906. doi: 10.1074/jbc.M201025200. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Malcolm T, Estable MC, Roeder RG, Sadowski I. TFII-I regulates induction of chromosomally integrated human immunodeficiency virus type 1 long terminal repeat in cooperation with USF. J. Virol. 2005;79:4396–4406. doi: 10.1128/JVI.79.7.4396-4406.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadowski I, Mitchell DA. TFII-I and USF (RBF-2) regulate Ras/MAPK-responsive HIV-1 transcription in T cells. Eur. J. Cancer. 2005;41:2528–2536. doi: 10.1016/j.ejca.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Malcolm T, Chen J, Chang C, Sadowski I. Induction of chromosomally integrated HIV-1 LTR requires RBF-2 (USF/TFII-I) and Ras/MAPK signaling. Virus Genes. 2007;35:215–223. doi: 10.1007/s11262-007-0109-9. [DOI] [PubMed] [Google Scholar]
- 36.Park JK, Rosenstein YJ, Remold-O'Donnell E, Bierer BE, Rosen FS, Burakoff SJ. Enhancement of T-cell activation by the CD43 molecule whose expression is defective in Wiskott-Aldrich syndrome. Nature. 1991;350:706–709. doi: 10.1038/350706a0. [DOI] [PubMed] [Google Scholar]
- 37.Bagriacik EU, Tang M, Wang HC, Klein JR. CD43 potentiates CD3-induced proliferation of murine intestinal intraepithelial lymphocytes. Immunol. Cell. Biol. 2001;79:303–307. doi: 10.1046/j.1440-1711.2001.01007.x. [DOI] [PubMed] [Google Scholar]
- 38.Mao C, Zhou M, Uckun FM. Crystal structure of Bruton's tyrosine kinase domain suggests a novel pathway for activation and provides insights into the molecular basis of X-linked agammaglobulinemia. J. Biol. Chem. 2001;276:41435–41443. doi: 10.1074/jbc.M104828200. [DOI] [PubMed] [Google Scholar]
- 39.Brown K, Long JM, Vial SC, Dedi N, Dunster NJ, Renwick SB, Tanner AJ, Frantz JD, et al. Crystal structures of interleukin-2 tyrosine kinase and their implications for the design of selective inhibitors. J. Biol. Chem. 2004;279:18727–18732. doi: 10.1074/jbc.M400031200. [DOI] [PubMed] [Google Scholar]
- 40.Pérez-Jurado LA, Wang Y-K, Peoples R, Coloma A, Cruces J, Franke U. A duplicated gene in the breakpoint regions of the 7q11.23 Williams-Beuren syndrome deletion encodes the initiator binding protein TFII-I and BAP-135, a phosphorylation target of Btk. Hum. Mol. Genet. 1998;7:325–334. doi: 10.1093/hmg/7.3.325. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y-K, Pérez-Jurado LA, Francke U. A mouse single-copy gene, Gtf2i, the homolog of human GTF2I, that is duplicated in the Williams-Beuren Syndrome deletion region. Genomics. 1998;48:163–170. doi: 10.1006/geno.1997.5182. [DOI] [PubMed] [Google Scholar]
- 42.Bunnell SC, Diehn M, Yaffe MB, Findell PR, Cantley LC, Berg LJ. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J. Biol. Chem. 2000;275:2219–2230. doi: 10.1074/jbc.275.3.2219. [DOI] [PubMed] [Google Scholar]
- 43.Cheriyath V, Roy AL. Alternatively spliced isoforms of TFII-I. J. Biol. Chem. 2000;275:26300–26308. doi: 10.1074/jbc.M002980200. [DOI] [PubMed] [Google Scholar]
- 44.Cheriyath V, Roy AL. Structure-function analysis of TFII-I. Roles of the N-terminal end, basic region, and I-repeats. J. Biol. Chem. 2001;276:8377–8383. doi: 10.1074/jbc.M008411200. [DOI] [PubMed] [Google Scholar]
- 45.Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heyeck SD, Wilcox HM, Bunnell SC, Berg LJ. Lck phosphorylates the activation loop tyrosine of the Itk kinase domain and activates Itk kinase activity. J. Biol. Chem. 1997;272:25401–25408. doi: 10.1074/jbc.272.40.25401. [DOI] [PubMed] [Google Scholar]
- 47.Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nat. Rev. Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 48.Remold-O’Donnell E, Davis AE, III, Kenney D, Bhaskar KR, Rosen FS. Purification and chemical composition of gpL115, the human lymphocyte surface sialoglycoprotein that is defective in Wiskott-Aldrich syndrome. J. Biol. Chem. 1986;261:7526–7530. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.