Abstract
Clopidogrel is one of the most commonly prescribed medications world-wide. Recent advisories from the US Food and Drug Administration (FDA) have drawn attention to the possibility of personalized decision-making for individuals who are candidates for clopidogrel. As is the case with antihypertensives, statins and warfarin, common genetic sequence variants can influence clopidogrel metabolism and its effect on platelet activity. These genetic variants have, in multiple studies, been associated with adverse clinical outcomes. Concurrent medication use also influences the body's handling of clopidogrel. Proton pump inhibitors, widely prescribed in conjunction with clopidogrel, may blunt its effectiveness. We address implications for bedside decision-making in light of accumulated data and current FDA advisories, and conclude that genetic testing for CYP2C19 genotype and limitation of PPI interactions do not yet appear to offer an opportunity to optimize treatment given the current state of knowledge.
Keywords: antiplatelet, genetics, stroke, review
Introduction
Clopidogrel, in combination with aspirin, is standard treatment for both medical and interventional management of acute coronary syndrome (ACS). Dual antiplatelet therapy reduces the risk of repeat ACS events and death compared with aspirin alone [1,2], and helps prevent stent thrombosis in patients undergoing percutaneous interventions (PCI) [2]. Patients presenting to neurovascular clinics are often on dual antiplatelet therapy because of coincident cardiac disease. Alternatively, clopidogrel monotherapy may be used as a first-line agent, or in the setting of an aspirin allergy or suspected aspirin ineffectiveness, in secondary prevention of ischemic stroke.
Concerns have been raised, however, regarding this agent's efficacy in specific subgroups. In November 2009, the Food and Drug Administration (FDA) published a post-market drug safety information announcement detailing drug-drug interactions between clopidogrel and omeprazole, urging against the concomitant use of these medications [TABLE 2]. In March 2010, the FDA announced that clopidogrel would require a new “black-box” warning regarding reduced effectiveness in individuals who are poor metabolizers of the medication, recommending testing for CYP2C19 genotype to aid clinical management [TABLE 3].
Table 2.
FDA Information for Healthcare Professionals: Update to the labeling of Clopidogrel Bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC)
|
FDA = Food and Drug Administration.
Table reprinted from the U.S. Food and Drug Administration.
Http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm (Accessed 8/1/2010).
Table 3.
FDA Black box warning on clopidogrel (Plavix)
|
FDA = Food and Drug Administration.
Table reprinted from the U.S. Food and Drug Administration.
http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020839s042lbl.pdf (Accessed 8/1/2010).
Both FDA announcements reflect increasing awareness of inter-individual differences in medication efficacy, and a growing desire to apply genetic discoveries to clinical medicine. These announcements place the clinician in the difficult position of weighing circumstantial and often contradictory information in pursuit of evidence-based practice. In this review, we discuss current evidence regarding the effect of CYP2C19 genotype and concurrent proton pump inhibitor (PPI) use on patients receiving clopidogrel for management of cardiovascular disease. We provide recommendations for clinical care and highlight areas of research that may clarify future practice.
Inter-Individual Variation in Clopidogrel Metabolism and Response
Common DNA sequence variants, called single nucleotide polymorphisms (SNPs), appear within genes, creating different alleles of that gene within the population. In the case of clopidogrel, variants have been identified in multiple genes which account for some, but not all, of the inter-individual variability in clopidogrel's antiplatelet effect. Several of these variants are fairly common, as represented by their allele frequency in the population, making them attractive targets for genetic screening. However, the effect size for these variants is small [3], with the consequence that the presence of any one accounts for only a small percentage of the inter-individual variation in clopidogrel response.
As with many medications, clopidogrel is dependent on multiple genetic and environmental factors to determine its circulating metabolite concentrations as well as its antiplatelet effect. As a result, there is wide inter-individual variability in response to clopidogrel [3–6]. A genetic test, if it is to be clinically useful, must explain enough of the inter-individual variation to provide sufficient predictive value to alter clinical decision making. Furthermore, there must be effective, presumably evidence-based, treatment alternatives for individuals who are tested.
Genetic Modifiers of Clopidogrel Response
Inter-individual variation in response to clopidogrel is driven by the pathways involved in the drug's pharmacokinetics and pharmacodynamics. The pharmacokinetics (absorption and metabolism) of clopidogrel is analyzed by measurement of the maximum blood concentration of clopidogrel or its metabolites (Cmax), or the area under the curve of clopidogrel's blood concentration over 24 hours (AUC0–24). The pharmacodynamics (end-organ effect) of clopidogrel are tested using techniques to examine the residual platelet function after dosing of the drug. The standard pharmacodynamic measurement is aggregometry, in which a blood sample is exposed to ADP, and the resulting platelet aggregation provides a measure of residual platelet reactivity [7]. Newer methods employ flow cytometry to measure vasodilator stimulated phosphoprotein (VASP), a direct marker of residual ADP-receptor activity [7]. Both pharmacodynamic assays detect residual platelet function in the presence of clopidogrel, although the differences in technique prevent the results from being directly comparable between studies.
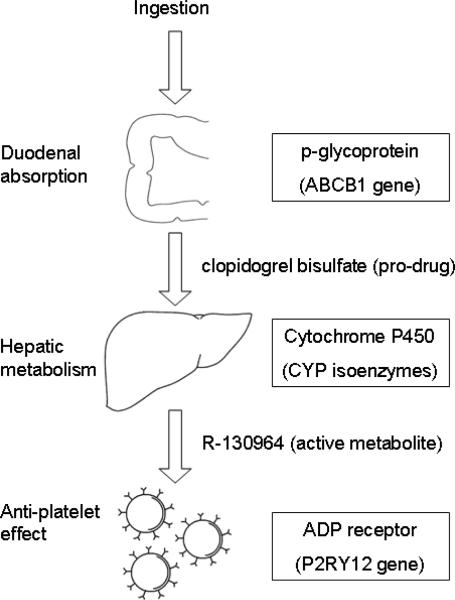
Clopidogrel is a pro-drug that must be absorbed and then metabolized into the active compound R-130964 in order to affect platelet function [FIGURE 3]. Absorbed chiefly in the duodenum, it passes into enterocytes and into the portal circulation [7–9]. On the luminal surface of the enterocyte, p-glyocoprotein (encoded by ABCB1) actively pumps clopidogrel back into the duodenum. Polymorphisms in ABCB1 influence the bioavailability of clopidogrel, and in some studies affect outcomes after ACS in patients on the drug [8,9].
Figure 3.
Clopidogrel metabolism
ADP = adenosine diphosphate. Proteins and genes listed in boxes play the dominant role in the pharmacokinetics and pharmacodynamics of clopidogrel.
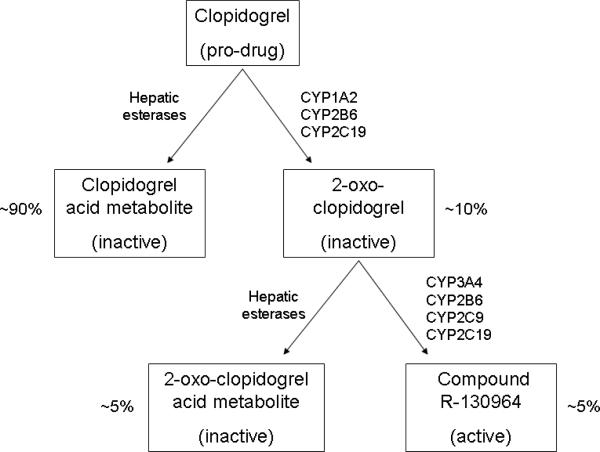
After absorption, clopidogrel passes into the liver. Hepatic metabolism of clopidogrel involves two steps, each catalyzed by members of the cytochrome P450 (CYP) system: oxidation to 2-oxo-clopidogrel and conversion to the active R-130964 [FIGURE 4]. Sequence variants in multiple CYP isoenzymes in the cytochrome P450 system have been implicated in inter-individual variation in the pharmacokinetics and pharmacodynamics of clopidogrel [4,10,11]. Hepatic esterases, which are active during both stages of metabolism, compete with the CYP enzymes to generate inactive metabolites. As a result, just 5–10% of ingested clopidogrel is ultimately converted to R-130964 [10].
Figure 4.
Hepatic metabolism of clopidogrel
Hepatic enzymes catalyzing each reaction are shown adjacent to each arrow. Percentages represent the amount of each metabolite that his produced from each dose that reaches the liver [10].
From the liver, R-130964 passes into the general circulation, where it irreversibly binds the ADP-receptor on the platelet surface, preventing activation of glycoprotein IIb/IIIa [12]. The ADP receptor is encoded by the P2RY12 gene, and sequence variants within this gene have been associated with decreased antiplatelet effect in some studies [12], but not others [13].
In summary, the protein products of numerous genes [FIGURE 3, 4] are involved in the pharmacokinetics and pharmacodynamics of clopidogrel, with relevant sequence variants identified for several of them [7]. Of these genes, CYP2C19 has been the most thoroughly investigated.
CYP2C19*2 Genotype an Inter-individual differences in clopidogrel response
CYP2C19 is a critical enzyme in clopidogrel metabolism, instrumental in both the oxidation of clopidogrel to 2-oxo-clopidogrel, and conversion of this intermediate to R-130964 [10,14]. Presently, more than 33 distinct alleles of CYP2C19 gene have been identified [http://www.cypalleles.ki.se/cyp2c19.htm]; however, many of these alleles are rare in the general population. Each allele is defined by variations in DNA sequence, which can result in conformational and/or functional changes in the CYP2C19 enzyme. The CYP2C19*1 allele, the most common in individuals of European origin, enables extensive metabolism of clopidogrel to its active compound.
The CYP2C19*2 allele is a common alternative variant allele in Asian, Caucasian, and African American individuals, appearing in 30%, 15%, and 17% of these populations, respectively [7]. Healthy volunteer carriers of the CYP2C19*2 allele have reduced clopidogrel Cmax and AUC0–24 [11,14,15], both after initial loading doses and steady state dosing. Most studies have demonstrated an allelic dose-dependence of effect, with homozygous *1/*1 carriers showing higher blood concentrations of clopidogrel's active metabolite than *1/*2 carriers [15]. Pharmacodynamic studies on healthy volunteers have shown similar results, with significantly increased on-clopidogrel residual platelet activity (RPA, as determined by aggregometry), representing reduced drug effectiveness in carriers of the *2 allele [14,15]. Carriers of a reduced function CYP2C19 allele in one study had 9% higher residual platelet activity on clopidogrel, compared with CYP2C19*1/*1 individuals [15]. Furthermore, a genome-wide association study of platelet aggregation in volunteers taking clopidogrel revealed a strong association between high RPA and a variant genetically associated (i.e. in high linkage disequilibrium) with the marker defining the CYP2C19*2 allele [3]. Of note, this unbiased genetic survey showed that CYP2C19*2 status explained only 12% of the variability in clopidogrel response of individuals involved in the study.
Pharmacodynamic studies have also been performed in individuals presenting with ACS, receiving clopidogrel either prior to PCI or for medical management. These studies have shown similar results to those described above, with CYP2C19*2 carriers demonstrating significantly higher RPA on clopidogrel than *1/*1 individuals [3,16]. It should be noted, however, that although CYP2C19*2 status was associated with statistically significant differences in RPA, the very wide interquartile ranges within each genotype reported in these studies demonstrate substantial dramatic inter-individual variability beyond that explained by CYP2C19 genotype [3].
CYP2C19*2 and clinical outcome in Patients on Clopidogrel
The effect of CYP2C19*2 on clopidogrel's pharmacokinetics and pharmacodynamics appears to be sufficient to influence clinical outcomes. Data from the EXCELSIOR trial have shown an association between higher RPA on clopidogrel and adverse clinical outcomes [17]. Multiple studies have examined the association between CYP2C19 allele status and clinical outcomes such as recurrent cardiovascular events, in-stent thrombosis, and mortality in individuals on clopidogrel after ACS presentations [3,8,15,17,18]. A recent meta-analysis of all published studies on CYP2C19*2 and outcomes identified a risk OR = 1.96 (95% confidence interval (CI) 1.14 – 3.37) for recurrent cardiovascular events per CYP2C19*2 allele [19] and a risk OR = 3.82 (95% CI 2.23 – 6.54) per allele for stent thrombosis.
The mechanism for increased adverse outcomes associated with the CYP2C19*2 genotype is likely related to attenuated effect of clopidogrel on platelet aggregation, rather than another unmeasured cause. In one study which both performed platelet aggregometry and followed clinical outcomes in patients on clopidogrel after ACS events, carriers of the CYP2C19*2 allele had higher on-clopidogrel RPA as well as increased incidence of adverse outcomes [3]. This association between CYP2C19*2 and outcome was not observed after controlling for RPA in regression analysis. Hence, while unmeasured physiologic changes conferred by the CYP2C19*2 allele could theoretically impact outcome, the available evidence suggests that the increased risk of adverse outcomes is primarily mediated by impaired onclopidogrel platelet inhibition in carriers of this allele.
Proton Pump Inhibitors and Clopidogrel
Proton pump inhibitors are widely prescribed in conjunction with antiplatelet therapy. Current American Heart Association guidelines recommend that all patients on dual antiplatelet therapy be prescribed a PPI regardless of H. pylori status or gastrointestinal bleeding risk [20]. PPIs are inhibitors of CYP2C19 in vitro, with omeprazole showing more potent inhibition than newer generation PPIs such as pantoprazole [21]. Based on this evidence, several studies have assessed RPA in patients co-prescribed clopidogrel and a PPI. For omeprazole, there is a significant increase in RPA in individuals prescribed both clopidogrel and a PPI compared to clopidogrel alone [22]. The results for other PPIs, particularly pantoprazole, are less clear. One study showed that RPA in ACS patients on clopidogrel and pantoprazole was similar to those on clopidogrel alone [23], suggesting a compound-specific effect.
Despite the evidence that at least some PPIs affect residual platelet function in patients taking clopidogrel, the results of studies seeking to link this effect to adverse outcomes in ACS patients have been inconsistent. Multiple retrospective analyses, involving thousands of participants, have demonstrated a significant association between PPI/clopidogrel co-prescription and adverse cardiovascular outcomes and death [24,25]. These results might be confounded, however, by greater illness severity among individuals prescribed PPIs. Attempts to control for this confounding by indication using propensity matching indeed weaken the association between PPI use and adverse outcome [22,25]. The yet-to-be published COGENT trial, the only randomized, double-bind, placebo-controlled trial of ACS patients discharged on either clopidogrel alone or clopidogrel and PPI demonstrated no association between co-prescription and outcome [26]. Among the 3627 participants on clopidogrel after ACS events, the hazard ratio for combined endpoints of vascular events and death was 1.02 (95% CI 0.70–1.51). The survival curves for individuals taking and not taking PPIs were entirely superimposable. In a recent meta-analysis of all available outcome studies, there was a risk OR = 1.43 (95% CI 1.15 – 1.77) for adverse outcomes in patients co-prescribed clopidogrel and a PPI [25]. Meta-analysis restricted to only propensity-matched and randomized trials showed no association with outcome, with OR = 1.15 (95% CI 0.89 – 1.48) [25]. In contrast with the pharmacodynamic studies (which showed a compound-specific effect for RPA on clopidogrel), analyses of adverse outcomes have not demonstrated that pantoprazole and other “newer” PPIs have effects that differ from those of older PPIs such as omeprazole [24,27].
Should genetic testing for CYP2C19 status be routinely performed?
There is good evidence that CYP2C19*2 is associated with increased residual platelet function on clopidogrel compared with CYP2C19*1. Furthermore, CYP2C19*2 genotype has been reliably associated with increased risk of adverse cardiovascular outcomes in ACS patients [3,8,19]. The mechanism for this association appears to be through reduction in clopidogrel's antiplatelet effect [3].
Presented with this data, the question arises whether testing for CYP2C19 allele status would be beneficial for patient care. A genetic test is clinically useful when it guides practice, through the reliable identification of individuals who are likely to benefit from a change in therapy. Because CYP2C19 genotype alone has a relatively small influence on an individual's response to clopidogrel, and there is dramatic inter-individual variation in residual platelet function on clopidogrel, knowledge of CYP2C19 status is insufficient to inform physicians of clopidogrel's effect on an individual patient. Numerous environmental, medical, and genetic factors play a role in clopidogrel pharmacology, including adherence to therapy, age, BMI, diabetes status, and drug and dietary inhibitors of hepatic metabolism [5,6]. CY2C19*1 carriers could have high residual platelet function for other reasons, and carriers of the *2 allele could have low residual platelet activity, limiting the predictive value of the test.
A further limitation on the clinical utility of CYP2C19 genetic testing is that there are no proven therapies that can supplant clopidogrel as part of a dual anti-platelet regimen for active cardiovascular disease. Clopidogrel dose escalation has been studied in a small case-series of CYP2C19*2 carriers, and has not been shown to significantly change residual platelet activity [28]. Additional studies of dose escalation in perceived clopidogrel resistance are needed, particularly given that alternative antiplatelet strategies such as prasugrel are as yet unproven. At this time, therefore, it is reasonable to conclude that there is currently no change in practice dictated by knowledge of CYP2C19*2 allele status.
Should patients routinely receive PPIs and clopidogrel?
Although co-prescription of clopidogrel and PPI medications appears to result in reduced antiplatelet effect, evidence linking co-prescription to outcome is far less clear. Multiple large retrospective studies have found such an association, but the fact that several propensity-matched studies and one randomized controlled trial did not validate this finding raises concern for confounding. Additionally, the utility of alternate forms of gastrointestinal prophylaxis is unclear. Although the FDA update [TABLE 2] points out that H2 blockers do not inhibit CYP2C19, their use in patients taking dual antiplatelets is not currently recommended by consensus guidelines [20]. In a recent case-control study of 2777 patients with history of upper-GI bleeding and 5532 controls, individuals on either non-steroidal anti-inflammatory drugs, aspirin, or clopidogrel were treated with either PPI, H2 blocker, or nitrates for GI prophylaxis. In those receiving clopidogrel therapy, only patients treated with PPIs had a significant reduction in GI bleeding risk [29]. In addition to this data, withdrawal of PPIs from individuals taking clopidogrel could have unintended gastrointestinal bleeding consequences, which have not yet been effectively weighed against the potential increase in adverse cardiovascular outcomes [27].
Recommendations
Based upon available evidence, systematic CYP2C19 genotype testing for individuals on clopidogrel appears to be of limited benefit to bedside decision-making [TABLE 1]. Further research into clinically useful platelet function analysis is likely to be of greater utility, as residual platelet function on clopidogrel represents the “final common pathway” of environmental and genetic factors affecting clopidogrel efficacy. Further insight into clopidogrel dose escalation strategies or other antiplatelet medications is also needed for CYP2C19 genetic testing to become a useful part of the vascular physician's armamentarium.
Table 1.
Summary and recommendations
| CYP2C19*2 and clopidogrel pharmacokinetics/pharmacodynamics | |
|---|---|
| Summary | Clopidogrel's anti-platelet effect depends on multiple genetic and environmental factors, and there is substantial inter-individual variability in clopidogrel response. The CYP2C19*2 allele is associated with a decrease in both loading-dose and steady state concentrations of clopidogrel's active metabolite, as well as higher residual platelet function in patients on clopidogrel. However, CYP2C19 status appears to account for no more than 12% of the inter-individual variation in clopidogrel response. |
|
| |
| Recommendation | Genotyping CYP2C19 status alone is insufficient to inform clinicians about on-clopidogrel residual platelet function. |
| CYP2C19*2 and clopidogrel-associated clinical outcomes | |
|---|---|
| Summary | The CYP2C19*2 allele is associated with increased risk of adverse cardiovascular outcomes, stent thrombosis, and death in individuals placed on clopidogrel after acute coronary events. |
|
| |
| Recommendation | In the absence of clinical evidence for effective alternative therapies, the impact of CYP2C19*2 status on clinical management is unclear. Further research is needed into the utility and safety of clopidogrel dose escalation, as well as the potential utility of alternate antiplatelet agents less sensitive to CYP2C19 genotype. |
| Proton-pump inhibitor (PPI) use and clopidogrel-associated clinical outcomes | |
|---|---|
| Summary | Multiple retrospective studies have found an association between the dual prescription of clopidogrel and PPIs and increased risk of adverse cardiac outcomes and death in individuals placed on clopidogrel after acute coronary events. Other retrospective studies and one prospective randomized trial have not replicated this association. |
|
| |
| Recommendation | The association between PPI use in conjunction with clopidogrel and adverse outcomes is unclear. Potential unmeasured confounders may have influenced the results of retrospective analyses. Further randomized trials are needed. |
One situation where alternative therapies to clopidogrel have been established is secondary stroke prevention. Neurovascular patients on clopidogrel monotherapy could potentially benefit from knowledge of CYP2C19 genotype, as presence of the CYP2C19*2 allele could be used to justify re-trial of aspirin or a switch to an alternative antiplatelet agent. We note that the utility of this strategy has not yet been explored.
Finally, given conflicting evidence for association between co-prescription of clopidogrel and PPIs and adverse outcomes, it appears premature to recommend discontinuation of PPI therapy in individuals on clopidogrel as part of a dual antiplatelet regimen [TABLE 1]. Further research is needed to understand the ramifications of any increase in gastrointestinal bleeding events in clopidogrel users off PPIs. In management of neurovascular patients on clopidogrel monotherapy, testing for H. pylori and review of gastrointestinal risk factors could be entertained, with possible discontinuation of PPI therapy in low-risk patients to minimize any possible interactions.
Conclusion
Clopidogrel is a commonly-prescribed medication, and has been proven useful in the prevention of recurrent cardiovascular and cerebrovascular events. It is natural to want to guarantee that our therapy is having its desired effect in every patient. In the case of clopidogrel, CYP2C19 genetic testing and limitation of PPI interactions do not yet appear to offer an opportunity to optimize treatment given the current state of knowledge.
Acknowledgements
none
Funding: Bugher Foundation Centers for Stroke Prevention Research/American Stroke Association, National Institutes of Neurologic Disorders and Stroke R01 NS059727, Massachusetts General Hospital Deane Institute for the Integrative Study of Atrial Fibrillation and Stroke.
Footnotes
Disclosures: The authors have no pertinent financial disclosures/conflicts of interest
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 2.Steinhubl SR, Berger PB, Mann JT, 3rd, Fry ET, DeLago A, Wilmer C, Topol EJ. CREDO Investigators. Clopidogrel for the Reduction of Events During Observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 3.Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farid NA, Payne CD, Small DS, Winters KJ, Ernest CS, 2nd, Brandt JT, Darstein C, Jakubowski JA, Salazar DE. Cytochrome P450 3A inhibition by ketoconazole affects prasugrel and clopidogrel pharmacokinetics and pharmacodynamics differently. Clin Pharmacol Ther. 2007;81:735–741. doi: 10.1038/sj.clpt.6100139. [DOI] [PubMed] [Google Scholar]
- 5.Steinhubl SR. Genotyping, clopidogrel metabolism, and the search for the therapeutic window of thienopyridines. Circulation. 2010;121:481–483. doi: 10.1161/CIR.0b013e3181d1e0e1. [DOI] [PubMed] [Google Scholar]
- 6.Aleil B, Léon C, Cazenave JP, Gachet C. CYP2C19*2 polymorphism is not the sole determinant of the response to clopidogrel: implications for its monitoring. J Thromb Haemost. 2009;7:1747–1749. doi: 10.1111/j.1538-7836.2009.03554.x. [DOI] [PubMed] [Google Scholar]
- 7.Ellis KJ, Stouffer GA, McLeod HL, Lee CR. Clopidogrel pharmacogenomics and risk of inadequate platelet inhibition: US FDA recommendations. Pharmacogenomics. 2009;10:1799–1817. doi: 10.2217/pgs.09.143. [DOI] [PubMed] [Google Scholar]
- 8.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, Steg PG, Ferrières J, Danchin N, Becquemont L. French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 9.Taubert D, von Beckerath N, Grimberg G, Lazar A, Jung N, Goeser T, Kastrati A, Schömig A, Schömig E. Impact of P-glycoprotein on clopidogrel absorption. Clin Pharmacol Ther. 2006;80:486–501. doi: 10.1016/j.clpt.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol. 2010;50:126–142. doi: 10.1177/0091270009343005. [DOI] [PubMed] [Google Scholar]
- 11.Brandt JT, Close SL, Iturria SJ, Payne CD, Farid NA, Ernest CS, 2nd, Lachno DR, Salazar D, Winters KJ. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5:2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 12.Fontana P, Dupont A, Gandrille S, Bachelot-Loza C, Reny JL, Aiach M, Gaussem P. Adenosine diphosphate-induced platelet aggregation is associated with P2Y12 gene sequence variations in healthy subjects. Circulation. 2003;108:989–995. doi: 10.1161/01.CIR.0000085073.69189.88. [DOI] [PubMed] [Google Scholar]
- 13.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramírez C, Cavallari U, Trabetti E, Sabaté M, Jimenez-Quevedo P, Hernández R, Moreno R, Escaned J, Alfonso F, Bañuelos C, Costa MA, Bass TA, Pignatti PF, Macaya C. Lack of association between the P2Y12 receptor gene polymorphism and platelet response to clopidogrel in patients with coronary artery disease. Thromb Res. 2005;116:491–497. doi: 10.1016/j.thromres.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Hulot JS, Bura A, Villard E, Azizi M, Remones V, Goyenvalle C, Aiach M, Lechat P, Gaussem P. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244–2247. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 15.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, Sabatine MS. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 16.Giusti B, Gori AM, Marcucci R, Saracini C, Sestini I, Paniccia R, Valente S, Antoniucci D, Abbate R, Gensini GF. Cytochrome P450 2C19 loss-of-function polymorphism, but not CYP3A4 IVS10 + 12G/A and P2Y12 T744C polymorphisms, is associated with response variability to dual antiplatelet treatment in high-risk vascular patients. Pharmacogenet Genomics. 2007;17:1057–1064. doi: 10.1097/FPC.0b013e3282f1b2be. [DOI] [PubMed] [Google Scholar]
- 17.Trenk D, Hochholzer W, Fromm MF, Chialda LE, Pahl A, Valina CM, Stratz C, Schmiebusch P, Bestehorn HP, Büttner HJ, Neumann FJ. Cytochrome P450 2C19 681G>A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J Am Coll Cardiol. 2008;51:1925–1934. doi: 10.1016/j.jacc.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 18.Giusti B, Gori AM, Marcucci R, Saracini C, Sestini I, Paniccia R, Buonamici P, Antoniucci D, Abbate R, Gensini GF. Relation of cytochrome P450 2C19 loss-of-function polymorphism to occurrence of drug-eluting coronary stent thrombosis. Am J Cardiol. 2009;103:806–811. doi: 10.1016/j.amjcard.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 19.Sofi F, Giusti B, Marcucci R, Gori AM, Abbate R, Gensini GF. Cytochrome P450 2C19(*)2 polymorphism and cardiovascular recurrences in patients taking clopidogrel: a meta-analysis. Pharmacogenomics J. 2010 Mar 30; doi: 10.1038/tpj.2010.21. epub. [DOI] [PubMed] [Google Scholar]
- 20.Bhatt DL, Scheiman J, Abraham NS, Antman EM, Chan FK, Furberg CD, Johnson DA, Mahaffey KW, Quigley EM, Harrington RA, Bates ER, Bridges CR, Eisenberg MJ, Ferrari VA, Hlatky MA, Kaul S, Lindner JR, Moliterno DJ, Mukherjee D, Schofield RS, Rosenson RS, Stein JH, Weitz HH, Wesley DJ. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502–1517. doi: 10.1016/j.jacc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Li XQ, Andersson TB, Ahlström M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004;32:821–827. doi: 10.1124/dmd.32.8.821. [DOI] [PubMed] [Google Scholar]
- 22.O'Donoghue ML, Braunwald E, Antman EM, Murphy SA, Bates ER, Rozenman Y, Michelson AD, Hautvast RW, Ver Lee PN, Close SL, Shen L, Mega JL, Sabatine MS, Wiviott SD. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989–997. doi: 10.1016/S0140-6736(09)61525-7. [DOI] [PubMed] [Google Scholar]
- 23.Neubauer H, Engelhardt A, Krüger JC, Lask S, Börgel J, Mügge A, Endres HG. Pantoprazole Does Not Influence the Antiplatelet Effect of Clopidogrel - A Whole Blood Aggregometry Study Following Coronary Stenting. J Cardiovasc Pharmacol. 2010;56:91–7. doi: 10.1097/FJC.0b013e3181e19739. [DOI] [PubMed] [Google Scholar]
- 24.Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, Rumsfeld JS. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937–944. doi: 10.1001/jama.2009.261. [DOI] [PubMed] [Google Scholar]
- 25.Kwok CS, Loke YK. Meta-analysis: the effects of proton pump inhibitors on cardiovascular events and mortality in patients receiving clopidogrel. Aliment Pharmacol Ther. 2010;31:810–823. doi: 10.1111/j.1365-2036.2010.04247.x. [DOI] [PubMed] [Google Scholar]
- 26.Depta JP, Bhatt DL. Omeprazole and clopidogrel: Should clinicians be worried? Cleve Clin J Med. 2010;77:113–116. doi: 10.3949/ccjm.77a.09173. [DOI] [PubMed] [Google Scholar]
- 27.Laine L, Hennekens C. Proton pump inhibitor and clopidogrel interaction: fact or fiction? Am J Gastroenterol. 2010;105:34–41. doi: 10.1038/ajg.2009.638. [DOI] [PubMed] [Google Scholar]
- 28.Pena A, Collet JP, Hulot JS, Silvain J, Barthélémy O, Beygui F, Funck-Brentano C, Montalescot G. Can we override clopidogrel resistance? Circulation. 2009;119:2854–2857. doi: 10.1161/CIRCULATIONAHA.108.857722. [DOI] [PubMed] [Google Scholar]
- 29.Lanas A, García-Rodríguez LA, Arroyo MT, Bujanda L, Gomollón F, Forné M, Aleman S, Nicolas D, Feu F, González-Pérez A, Borda A, Castro M, Poveda MJ, Arenas J. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507–515. doi: 10.1111/j.1572-0241.2006.01062.x. [DOI] [PubMed] [Google Scholar]