Abstract
Background:
Exhaled breath condensate (EBC) is composed of droplets of airway surface liquid (ASL) diluted by water vapor. The goal of this study was to determine if the composition of EBC is affected by changes in airway caliber, minute ventilation, or forceful exhalation, factors that may differ among subjects with asthma in cross-sectional studies.
Methods:
In a group of subjects with asthma, we measured the effects of the following: (1) a series of three deep-inspiration and forceful-exhalation maneuvers; (2) a doubling of minute ventilation; and (3) acute bronchoconstriction induced by methacholine on EBC volume, dilution of ASL, and concentration of cysteinyl leukotrienes (CysLTs).
Results:
With the exception of an increase in EBC volume with increased minute ventilation, there were no significant changes in the volume, dilution, or levels of CysLTs in EBC introduced by each of these factors. The CIs surrounding the differences introduced by each factor showed that the maximum systematic errors due to these factors were modest.
Conclusions:
These results indicate that changes in airway caliber, minute ventilation, or breathing pattern among subjects with asthma do not significantly alter the measurements of mediator concentrations in EBC.
Exhaled breath condensate (EBC) is often used to noninvasively evaluate the biologic characteristics of lung or airway diseases; however, levels of mediators such as cysteinyl leukotrienes (CysLTs) (eg, leukotrienes C4, D4, and E4) in EBC vary widely between different laboratories and patient populations.1-4 Significant within-subject variability in the volume and concentrations of lipid and protein mediators in EBC has been identified5-7 and may be explained by patient-related factors such as age, height, gender, and lung volumes.8,9 Differences in the characteristics of biologic assays used to detect mediators may also contribute to variations in mediator levels identified in different laboratories.10 The type of collection device5,11 and the minute ventilation also affect the volume of EBC.12,13 Factors that affect EBC composition are not fully understood.14,15
Because droplets of airway surface liquid (ASL) captured in EBC represent a small fraction of EBC fluid,16 patient-related factors that alter droplet formation in the airways may affect the composition of EBC. Turbulent flow introduced by airway narrowing or deep inhalation and/or forceful exhalation may affect the liberation of ASL droplets.15 Based on the ionic concentrations of Na+, K+, Cl−, lactate, and protein in EBC, Effros and colleagues16 demonstrated that ASL dilution can be accurately estimated and that this dilution can be estimated through a simple measurement of conductivity after lyophilization.17 It has been reported that the dilution factor varies by > 100 among normal subjects.16 Subsequent studies have revealed dilution factors ranging from 1:2,000 to about 1:20,000 in different patient groups and using various collection and analytical techniques.18-20 For example, the dilution factor was 1:4,248 in normal subjects and 1:2,259 in patients with cystic fibrosis.19 The concentration of Na+ in EBC was elevated in patients with asthma and cystic fibrosis relative to a group of subjects who did not have asthma,21 suggesting that the dilution factor is altered in these groups. Collectively, these studies suggest that dilution of EBC is variable and that this variability may be due to factors that alter droplet liberation and/or dilution by water vapor.
In this study, we tested the hypothesis that EBC composition is altered by acute changes in airway caliber, minute ventilation, and forceful exhalation. We measured the volume, dilution of ASL by water vapor, and the concentrations of CysLTs in EBC (1) before and after a series of three deep-inspiration and forceful-exhalation maneuvers, (2) before and during a doubling of minute ventilation for 10 min, and (3) before and during methacholine-induced bronchoconstriction in a group of adults with asthma. As the gold standard for dilution of EBC, sample conductivity was assessed after lyophilization.17 Levels of CysLTs were measured after solid-phase extraction, followed by fivefold to 7.5-fold concentration.10 The maximum amount of error that each of these factors could introduce in the measurement of a mediator in EBC was estimated. Some of these data were previously reported in abstract form.22
Materials and Methods
Study Subjects
The study was approved by the Institutional Review Board of the University of Washington, and informed consent was obtained from all participants. Adults with at least a 1-year history of physician-diagnosed asthma were recruited for this study. Subjects were excluded if they had an acute exacerbation or had taken systemic corticosteroids within 1 month of participation, had an upper or lower respiratory tract infection within 3 weeks of participation, or had a known electrolyte abnormality or renal disorder.
Study Design
EBC was collected before and after performing spirometry in accordance with American Thoracic Society and European Respiratory Society guidelines.23 Subjects with a baseline FEV1 < 65% predicted were excluded from exercise and methacholine challenges. EBC was collected before and during exercise on a cycle ergometer calibrated to double resting minute ventilation based on real-time assessment of ventilation through a pneumotachometer. EBC was collected before and after methacholine-induced bronchoconstriction, defined as a ≥ 50% increase in airway resistance via plethysmography (VMAX; VIASYS Healthcare Inc; Yorba Linda, California).24 The studies were conducted on a single day, with a period of at least 20 min between each of the interventions to prevent any carryover effects.
EBC Collection
Each EBC collection was performed using the Ecoscreen (Jaeger; Hoechberg, Germany) during 10 min of tidal breathing. Subjects were asked not to eat solid food for 2 h prior to their study visit and wore a nose clip throughout each collection. The EBC volume collected during each session was recorded. Immediately following collection, the EBC sample was split into 2 aliquots: 0.2 mL for conductivity measurement and 1.0 to 1.5 mL for CysLT assay.
CysLT Measurement
All samples for CysLT analysis were overlaid with argon, protected from light exposure, and stored at −80°C until concentrated using a validated solid-phase extraction protocol.10 CysLT concentrations were measured by enzyme immunoassay with a detection limit of 13 pg/mL (Cayman Chemical; Ann Arbor, Michigan). The estimated CysLT level in the ASL was calculated from the dilution factor and EBC CysLT level.
Measurement of Conductivity and Estimation of ASL Dilution
EBC aliquots for conductivity measurement were lyophilized to dryness using a lyophilizer (Labconco; Kansas City, Missouri) at < −40°C and continuous vacuum of > 0.133 mBar. Following lyophilization, samples were reconstituted with 0.2 mL of deionized water, and conductivity was measured using a microflow cell digital conductivity meter (Amber Science; Eugene, Oregon). The deionized water used to reconstitute the samples had conductivity of ≤ 1 microsiemen/cm. The intrasubject coefficient of variation of conductivity measurements was < 5%. The dilution of ASL by water vapor was estimated according to the method of Effros et al17: dividing the total cation concentration in serum (150 mM) by the total concentration of cations estimated from the conductivity (in μmol/L of NaCl) in each lyophilized specimen.
Statistical Analysis
The sample size was selected to detect a 20% change in dilution with 95% power. The baseline lung function measures (FEV1 and FVC percent predicted), change in minute ventilation following exercise, and change in airway resistance following methacholine exposure were described by the mean and SD. Paired measurements of EBC volume, CysLT levels, and estimated ASL dilution were compared with a paired t test. CIs were calculated around the difference between premeasurements and postmeasurements of each of these factors based on a t test. Correlations between intraindividual changes in EBC volume, ASL dilution, and CysLT levels with minute ventilation were calculated using the Pearson correlation coefficient. Similar correlations with the changes mediated by acute bronchoconstriction were calculated after log transformation of the change in airway resistance. A P value < .05 was considered statistically significant.
Results
Study Population
Eighteen adults with stable asthma, including six men and 12 women, completed the study protocol (Table 1). EBC samples were obtained before and after spirometry in all 18 subjects, before and during exercise from 16 subjects, and before and after methacholine inhalation in 15 subjects. Two subjects were excluded from the exercise and methacholine interventions because their initial spirometry revealed an FEV1 < 65% predicted. A third subject did not complete the methacholine inhalation because of equipment malfunction.
Table 1.
—Subject Characteristics
| Subject Characteristics (N = 18) | Subject Data |
| Age | 33.6 ± 12.1 |
| Gender, male (female) | 6 (12) |
| Baseline FVC, % predicted | 89.8 ± 12.6 |
| Baseline FEV1, % predicted | 80.0 ± 11.5 |
| FVC/FEV1 ratio | 75.4 ± 8.7 |
| Methacholine PC50Raw, mg/dL | 0.3 ± 0.3 |
| Use of an inhaled corticosteroid, % | 33 |
| Use of a leukotriene receptor antagonist, % | 22 |
| Use of a long-acting β agonist, % | 22 |
| Use of an antihistamine, % | 11 |
| Increase in e with exercise | 173.6 ± 107.0 |
| Increase in Raw with methacholine | 130.5 ± 93.2 |
Data are presented as mean ± SD unless otherwise noted. PC50 Raw = concentration of methacholine needed to induce a 50% increase in airway resistance; Raw = airway resistance measured by plethysmography; e = minute ventilation.
Effects of Deep Inspiration and Forceful Exhalation on EBC Composition
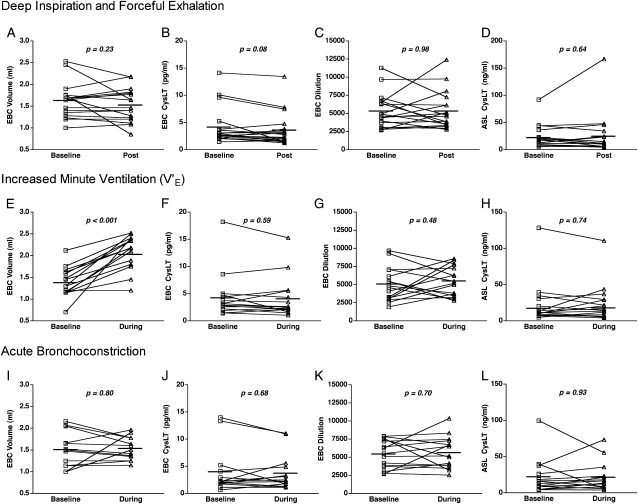
Deep inspiration followed by forced exhalation during three sequential spirometric maneuvers did not alter the EBC volume collected (mean, 1.63 mL prespirometry vs 1.53 mL postspirometry; P = .23) (Fig 1A) or CysLT levels (mean, 4.18 pg/mL prespirometry vs 3.60 pg/mL postspirometry; P = .08) (Fig 1B). The estimated ASL dilution factor was no different before and after spirometry (1:5,353 vs 1:5,337, P = .98) (Fig 1C). There was some intrasubject variability in ASL dilution of > 20% increase or decrease following spirometry in nine subjects. The estimated CysLT levels in ASL calculated from the dilution factor and EBC CysLT levels were no different before and after spirometry (mean, 22.30 ng/mL prespriometry vs 24.47 ng/mL postspirometry; P = .64) (Fig 1D). There was no correlation between the change in EBC ASL dilution induced by deep inspiration and forceful exhalation and either the change in EBC volume (r = 0.36, P = .15) or the change in CysLT levels (r = −0.18, P = .49).
Figure 1.
A-D, Effects of deep inspiration/forceful exhalation on the composition of EBC. (A, Volume. B, Level of CysLTs. C, ASL dilution. D, ASL CysLT concentrations.) No differences were introduced by a series of deep inspirations followed by forceful exhalation. E-H, Effects of minute ventilation on the composition of EBC. (E, Volume. F, Level of CysLTs. G, ASL dilution. H, ASL CysLT concentrations.) The volume of EBC was increased by a doubling of the minute ventilation. However, no differences were introduced by the increase in minute ventilation in the level of CysLTs, ASL dilution, or ASL CysLT concentrations. I-L, Effects of acute bronchoconstriction on the composition of EBC. (I, Volume. J, Level of CysLTs. K, ASL dilution. L, ASL CysLT concentrations.) No differences were introduced by acute bronchoconstriction. ASL = airway surface liquid; CysLT = cysteinyl leukotriene; EBC = exhaled breath condensate.
Effects of Minute Ventilation on EBC Composition
During light exercise designed to double the minute ventilation, the mean increase in minute ventilation among subjects was 173% (range 91%-251%). The EBC volume collected while breathing at an increased minute ventilation was significantly greater than the volume collected immediately prior to exercise (mean, 1.38 mL before vs 2.03 mL during; P < .001) (Fig 1E). Despite this increase in EBC volume, the concentrations of CysLT in EBC were no different between samples collected at baseline and at increased minute ventilation (mean, 4.23 pg/mL before vs 4.04 pg/mL during; P = .59) (Fig 1F), and the ASL dilution factor was no different between samples collected at baseline and at increased minute ventilation (mean, 1:5,034 before vs 1:5,496 during; P = .48) (Fig 1G). Increased minute ventilation was associated with intrasubject changes of > 20% increase or decrease in ASL dilution in 11 of 16 subjects. The levels of CysLTs in ASL were no different during the period of increased minute ventilation (mean, 22.55 ng/mL before vs 23.54 ng/mL during; P = .74) (Fig 1H). There was no correlation between the percent change in minute ventilation and either the change in estimated ASL dilution (r = 0.11, P = .71) or the change in EBC volume collected (r = −0.06, P = .83). There was a trend between the change in EBC volume and the change in CysLT levels (r = −0.44, P = .09) but not between the change in EBC volume and the change in estimated dilution (r = −0.06, P = .81).
Effects of Acute Bronchoconstriction on EBC Composition
Methacholine inhalation at doses ranging from 0.06 to 1.00 mg/dL resulted in acute bronchoconstriction in all subjects, resulting in a mean increase in airway resistance of 131% (range 53%-346%). Acute bronchoconstriction did not significantly alter the volume of EBC (mean, 1.51 mL before vs 1.53 mL during; P = .80) (Fig 1I), CysLT concentration (mean, 3.89 pg/mL before vs 3.77 pg/mL during; P = .68) (Fig 1J), or ASL dilution factor (mean, 1:5,434 before vs 1:5,642 during; P = .70) (Fig 1K). However, intrasubject changes in EBC volume of > 20% occurred among six subjects, and intrasubject changes of > 20% in estimated ASL dilution occurred in four subjects following methacholine inhalation. Acute bronchoconstriction did not significantly alter the level of CysLTs in ASL (mean, 20.99 ng/mL before vs 21.44 ng/mL during; P = .93) (Fig 1L). There was no correlation between the increase in airway resistance and either the EBC volume (r = 0.22, P = .47) or the EBC CysLT concentration (r = 0.32, P = .26).
Estimation of the Magnitude of Variability in EBC Composition
We assessed the CIs surrounding the change in EBC volume, ASL dilution, and CysLT concentrations introduced by each of the physiologic factors to determine the maximum systematic alteration in EBC levels that could be introduced by each of these factors. The mean differences introduced by each factor and the 95% CIs around the differences are presented in Table 2. With the exception of the increase in volume with the increase in minute ventilation, the changes in the mean values for changes in EBC volume, ASL dilution, and CysLT levels were low, ranging from 0.3% to 13.9% of the baseline mean value. The CIs surrounding these estimates were also fairly low, ranging from 9.8% to 28.1% of the mean value for each measurement.
Table 2.
—Magnitude of the Changes in Exhaled Breath Condensate Composition Mediated by Deep Inspiration/Forced Exhalation, Increased V.e, and Acute Bronchoconstriction
| Difference Introduced by Physiologic Factors |
||||
| Physiologic Factors | Characteristics | Deep Inspiration/ Forceful Exhalation | Increased e (Exercise) | Increase in Raw (Methacholine) |
| EBC volume, mL | No. | 17 | 16 | 13 |
| Baseline, mean ± SD | 1.63 ± 0.40 | 1.38 ± 0.33 | 1.51 ± 0.40 | |
| Mean change ± SD | −0.10 ± 0.34 | 0.65 ± 0.43 | 0.03 ± 0.36 | |
| Mean change relative to baseline, % | −6.3 | 47.3 | 1.7 | |
| 95% CI for mean change relative to baseline, % | (−16, 4) | (32, 63) | (−11, 15) | |
| CysLT levels, pg/mL | No. | 18 | 16 | 14 |
| Baseline, mean ± SD | 4.18 ± 3.48 | 4.23 ± 4.15 | 3.89 ± 4.12 | |
| Mean change ± SD | −0.58 ± 1.30 | −0.19 ± 1.41 | −0.21 ± 1.84 | |
| Mean change relative to baseline, % | −13.9 | −4.5 | −5.4 | |
| 95% CI for mean change relative to baseline, % | (−28, 0) | (−21, 12) | (−30, 19) | |
| ASL dilution | No. | 18 | 16 | 15 |
| Baseline, mean ± SD | 5,353 ± 2,353 | 5,034 ± 2,327 | 5,434 ± 1,944 | |
| Mean change ± SD | −16 ± 2,383 | 462 ± 2,557 | 208 ± 2,109 | |
| Mean change relative to baseline, % | −0.3 | 9.2 | 3.8 | |
| 95% CI for mean change relative to baseline, % | (−21, 20) | (−16, 34) | (−16, 23) | |
| ASL CysLT levels, ng/mL | No. | 18 | 16 | 15 |
| Baseline, mean ± SD | 20.30 ± 21.19 | 22.55 ± 30.07 | 20.99 ± 24.44 | |
| Mean change ± SD | 2.17 ± 19.60 | 0.99 ± 11.92 | −0.46 ± 19.25 | |
| Mean change relative to baseline, % | 9.7 | 4.4 | −2.2 | |
| 95% CI for mean change relative to baseline, % | (−31, 50) | (−26, 30) | (−46, 50) | |
ASL = airway surface liquid; CysLT = cysteinyl leukotriene; EBC = exhaled breath condensate inspiration. See Table 1 for expansion of other abbreviations.
Discussion
In this study, we experimentally determined in a group of subjects with asthma if acute changes in breathing pattern, minute ventilation, and airway narrowing alter EBC composition, and found that with the exception of an increase in EBC volume with increased minute ventilation, these physiologic factors did not systematically change the composition EBC. The CIs surrounding the estimates for each of these parameters demonstrate that they introduce, at most, only modest bias in measurements of EBC. As in prior studies, there was substantial within-subject and between-subject variability in EBC dilution16-20 and mediator concentrations5-9 that was not explained by the physiologic factors studied in the present investigation.
Correction for the ASL dilution factor has been proposed to estimate the true airway level of a mediator in EBC.15,17 Because the ASL is essentially isotonic with serum,25 the dilution of ASL by water vapor can be estimated by assessing EBC ionic content. One validated approach is to lyophilize an aliquot of EBC to remove volatile constituents, including NH4+ and bicarbonate, then measure the sample conductivity to estimate EBC cation content.17,26 Using this approach, we found that clinically meaningful changes in airway resistance, minute ventilation, and breathing pattern did not systematically alter the estimated ASL dilution factor in EBC.
The use of deep inspiration and forceful expiration associated with spirometry was designed to test whether acute opening and closing of small airways and alveoli affect droplet liberation as reflected in the dilution factor and mediator concentrations.15,27-29 Although changes introduced by spirometry were not apparent in the dilution factor, there was a trend toward reduced CysLT levels following spirometry that could reflect increased droplet liberation during spirometry followed by a period of lower droplet formation following spirometry. Regardless, the mean change in CysLT levels introduced by spirometry was only 13.9%, suggesting that the effects of spirometry, cough, and other factors that induce deep inspiration and forceful exhalation are relatively small.
Increased minute ventilation during light exercise was used to assess the effect of increased water transfer to exhaled breath on EBC composition. EBC volume is increased with increased minute ventilation as the air is equilibrated with the lower airway conditions, and is decreased by inspiration of colder and drier air.13 Subjects with asthma also have increased respiratory water loss relative to subjects who do not have asthma.30 Remarkably, in the present investigation, this increase in volume did not systematically result in dilution of EBC measured by conductivity or a reduction in the level of CysLTs in EBC. Similarly, Rosias and colleagues5 found that the volume of EBC markedly differed in different collection systems, but mediator concentrations were similar despite the differences in volume. These results indicate that droplet formation is proportionate to minute ventilation and that the effect of any change related to minute ventilation on dilution is small.
The effects of acute bronchoconstriction with resulting increase in turbulent airflow on EBC composition were assessed by methacholine-induced bronchoconstriction. We conducted the methacholine challenges using plethysmography to examine the effects of bronchoconstriction in isolation of the effects of deep inspiration.31 Methacholine challenge was used because it does not result in the release of mediators in the airways.32 We were unable to demonstrate any significant effect of acute bronchoconstriction on the measurement of CysLT levels in EBC, EBC volume, or ASL dilution. It is possible that the degree of bronchoconstriction introduced in this study was not of sufficient to alter the turbulent flow enough to change droplet formation.
In conclusion, we tested the hypothesis that deep inspiration/forceful exhalation, increased minute ventilation, and acute bronchoconstriction alter EBC solute and mediator composition in a group of adults with stable asthma. We used a validated method to estimate the dilution of ASL in EBC and measured one mediator that can be detected at very low levels in EBC to address this hypothesis. With the exception of the volume of EBC that increased with increasing minute ventilation, these physiologic factors did not systematically change the concentration of a mediator in the lower airways or the estimated dilution of ASL in EBC, and, at maximum, these factors only appear to introduce a modest level of bias into the measurement of a biomarker in EBC.
Acknowledgments
Author contributions: Dr Debley: contributed to the planning, data analysis, and writing of the manuscript.
Ms Ohanian: contributed to the laboratory analysis of the study.
Dr Spiekerman: contributed to the statistical analysis of the data.
Dr Aitken: contributed to the planning, data analysis, and writing of the manuscript.
Dr Hallstrand: contributed to the planning, data analysis, and writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Debley has received grant support over the past three years from the National Institutes of Health and the American Lung Association, and has investigator-initiated grants from AstraZeneca and Merck & Co to perform research assessing biomarkers in the exhaled breath from children with asthma. Dr Aitken has received grant monies to the University of Washington from the Cystic Fibrosis Foundation, Pharmaxis Inc, Transave Inc, Vertex Pharmaceuticals, and PTC Therapeutics to perform clinical and translational studies in patients with cystic fibrosis, and has received travel expenses from the Cystic Fibrosis Foundation for continuing medical education courses at the North American Cystic Fibrosis Conference and travel money from Pharmaxis Inc and Vertex Pharmaceuticals to attend investigators meetings. Dr Hallstrand has received grant support over the past three years from the National Institutes of Health and the American Lung Association, has an investigator-initiated grant pending from Novartis on the pathogenesis of asthma, and has served on the speakers bureau of Merck & Co and Schering Plough. Ms Ohanian and Dr Spiekerman have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- ASL
airway surface liquid
- CysLT
cysteinyl leukotriene
- EBC
exhaled breath condensate
Footnotes
For editorial comment see page 5
Funding/Support: This work was supported by the National Institutes of Health [Grants K23HL077626 and UL1RR025014], and by grants from the Firland Foundation and the World Bike for Breath Foundation.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Baraldi E, Carraro S, Alinovi R, et al. Cysteinyl leukotrienes and 8-isoprostane in exhaled breath condensate of children with asthma exacerbations. Thorax. 2003;58(6):505–509. doi: 10.1136/thorax.58.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Csoma Z, Kharitonov SA, Balint B, Bush A, Wilson NM, Barnes PJ. Increased leukotrienes in exhaled breath condensate in childhood asthma. Am J Respir Crit Care Med. 2002;166(10):1345–1349. doi: 10.1164/rccm.200203-233OC. [DOI] [PubMed] [Google Scholar]
- 3.Zanconato S, Carraro S, Corradi M, et al. Leukotrienes and 8-isoprostane in exhaled breath condensate of children with stable and unstable asthma. J Allergy Clin Immunol. 2004;113(2):257–263. doi: 10.1016/j.jaci.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 4.Samitas K, Chorianopoulos D, Vittorakis S, et al. Exhaled cysteinyl-leukotrienes and 8-isoprostane in patients with asthma and their relation to clinical severity. Respir Med. 2009;103(5):750–756. doi: 10.1016/j.rmed.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Rosias PP, Robroeks CM, Kester A, et al. Biomarker reproducibility in exhaled breath condensate collected with different condensers. Eur Respir J. 2008;31(5):934–942. doi: 10.1183/09031936.00073207. [DOI] [PubMed] [Google Scholar]
- 6.Chow S, Yates DH, Thomas PS. Reproducibility of exhaled breath condensate markers. Eur Respir J. 2008;32(4):1124–1126. doi: 10.1183/09031936.00085408. [DOI] [PubMed] [Google Scholar]
- 7.Borrill ZL, Starkey RC, Singh SD. Variability of exhaled breath condensate leukotriene B4 and 8-isoprostane in COPD patients. Int J Chron Obstruct Pulmon Dis. 2007;2(1):71–76. doi: 10.2147/copd.2007.2.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloemen K, Lissens G, Desager K, Schoeters G. Determinants of variability of protein content, volume and pH of exhaled breath condensate. Respir Med. 2007;101(6):1331–1337. doi: 10.1016/j.rmed.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Leung TF, Li CY, Yung E, Liu EK, Lam CW, Wong GW. Clinical and technical factors affecting pH and other biomarkers in exhaled breath condensate. Pediatr Pulmonol. 2006;41(1):87–94. doi: 10.1002/ppul.20296. [DOI] [PubMed] [Google Scholar]
- 10.Debley JS, Hallstrand TS, Monge T, Ohanian A, Redding GJ, Zimmerman J. Methods to improve measurement of cysteinyl leukotrienes in exhaled breath condensate from subjects with asthma and healthy controls. J Allergy Clin Immunol. 2007;120(5):1216–1217. doi: 10.1016/j.jaci.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 11.Czebe K, Barta I, Antus B, Valyon M, Horváth I, Kullmann T. Influence of condensing equipment and temperature on exhaled breath condensate pH, total protein and leukotriene concentrations. Respir Med. 2008;102(5):720–725. doi: 10.1016/j.rmed.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Thomas PS. Relationship between exhaled breath condensate volume and measurements of lung volumes. Respiration. 2007;74(2):142–145. doi: 10.1159/000094238. [DOI] [PubMed] [Google Scholar]
- 13.McCafferty JB, Bradshaw TA, Tate S, Greening AP, Innes JA. Effects of breathing pattern and inspired air conditions on breath condensate volume, pH, nitrite, and protein concentrations. Thorax. 2004;59(8):694–698. doi: 10.1136/thx.2003.016949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kostikas K, Koutsokera A, Papiris S, Gourgoulianis KI, Loukides S. Exhaled breath condensate in patients with asthma: implications for application in clinical practice. Clin Exp Allergy. 2008;38(4):557–565. doi: 10.1111/j.1365-2222.2008.02940.x. [DOI] [PubMed] [Google Scholar]
- 15.Horváth I, Hunt J, Barnes PJ, et al. ATS/ERS Task Force on Exhaled Breath Condensate Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26(3):523–548. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 16.Effros RM, Hoagland KW, Bosbous M, et al. Dilution of respiratory solutes in exhaled condensates. Am J Respir Crit Care Med. 2002;165(5):663–669. doi: 10.1164/ajrccm.165.5.2101018. [DOI] [PubMed] [Google Scholar]
- 17.Effros RM, Biller J, Foss B, et al. A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am J Respir Crit Care Med. 2003;168(12):1500–1505. doi: 10.1164/rccm.200307-920OC. [DOI] [PubMed] [Google Scholar]
- 18.Esther CR, Jr, Boysen G, Olsen BM, et al. Mass spectrometric analysis of biomarkers and dilution markers in exhaled breath condensate reveals elevated purines in asthma and cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2009;296(6):L987–L993. doi: 10.1152/ajplung.90512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker EH, Clark N, Brennan AL, et al. Hyperglycemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. J Appl Physiol. 2007;102(5):1969–1975. doi: 10.1152/japplphysiol.01425.2006. [DOI] [PubMed] [Google Scholar]
- 20.Dwyer TM. Sampling airway surface liquid: non-volatiles in the exhaled breath condensate. Lung. 2004;182(4):241–250. doi: 10.1007/s00408-004-2506-3. [DOI] [PubMed] [Google Scholar]
- 21.Zacharasiewicz A, Wilson N, Lex C, et al. Repeatability of sodium and chloride in exhaled breath condensates. Pediatr Pulmonol. 2004;37(3):273–275. doi: 10.1002/ppul.10431. [DOI] [PubMed] [Google Scholar]
- 22.Debley JS, Ohanian A, Aitken ML, et al. Airway effects on exhaled breath condensate (EBC) composition [abstract] Am J Respir Crit Care Med. 2007;175:A318. [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 24.Crapo RO, Casaburi R, Coates AL, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161(1):309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 25.Tarran R. Regulation of airway surface liquid volume and mucus transport by active ion transport. Proc Am Thorac Soc. 2004;1(1):42–46. doi: 10.1513/pats.2306014. [DOI] [PubMed] [Google Scholar]
- 26.Effros RM. Saving the breath condensate approach. Am J Respir Crit Care Med. 2003;168(9):1129–1132. doi: 10.1164/ajrccm.168.9.951. [DOI] [PubMed] [Google Scholar]
- 27.Fairchild CI, Stampfer JF. Particle concentration in exhaled breath. Am Ind Hyg Assoc J. 1987;48(11):948–949. doi: 10.1080/15298668791385868. [DOI] [PubMed] [Google Scholar]
- 28.Papineni RS, Rosenthal FS. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 1997;10(2):105–116. doi: 10.1089/jam.1997.10.105. [DOI] [PubMed] [Google Scholar]
- 29.Hunt J. Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease. J Allergy Clin Immunol. 2002;110(1):28–34. doi: 10.1067/mai.2002.124966. [DOI] [PubMed] [Google Scholar]
- 30.Noble DD, McCafferty JB, Greening AP, Innes JA. Respiratory heat and moisture loss is associated with eosinophilic inflammation in asthma. Eur Respir J. 2007;29(4):676–681. doi: 10.1183/09031936.00071106. [DOI] [PubMed] [Google Scholar]
- 31.Scichilone N, Permutt S, Togias A. The lack of the bronchoprotective and not the bronchodilatory ability of deep inspiration is associated with airway hyperresponsiveness. Am J Respir Crit Care Med. 2001;163(2):413–419. doi: 10.1164/ajrccm.163.2.2003119. [DOI] [PubMed] [Google Scholar]
- 32.Kraft M, Bettinger CM, Wenzel SE, Irvin CG, Ackerman SJ, Martin RJ. Methacholine challenge does not affect bronchoalveolar fluid cell number and many indices of cell function in asthma. Eur Respir J. 1995;8(11):1966–1971. doi: 10.1183/09031936.95.08111966. [DOI] [PubMed] [Google Scholar]