Abstract
Background:
Sarcoidosis is a systemic granulomatous disorder of unknown cause that occurs among men and women of all races. In the United States, black women are most frequently and most severely affected. There have been few epidemiologic studies of sarcoidosis focusing on black women.
Methods:
In this article, we present data on incidence, prevalence, and clinical characteristics of sarcoidosis among participants in the Black Women’s Health Study, a cohort study of 59,000 black women from across the United States. Data on incident disease and potential risk factors are obtained through biennial questionnaires. Follow-up has been > 80% through six completed cycles.
Results:
There were 685 prevalent cases of sarcoidosis at baseline in 1995 and 435 incident cases reported during 611,585 person-years of follow-up through 2007, for an average annual incidence rate of 71/100,000 and a current prevalence of 2.0%. The sarcoid diagnosis was confirmed in 96% of self-reported cases for whom medical records or physician checklists were obtained. The most frequently affected site was the lung. Most patients also had extrapulmonary involvement, with the most common sites being lymph nodes, skin, and eyes. Prednisone had the highest prevalence of use, followed by inhaled corticosteroids.
Conclusions:
This study confirms previous reports of high incidence and prevalence of sarcoidosis among black women, as well as the extent of extrapulmonary disease, frequent need for steroid therapy, and comorbid conditions in this population. The prospective identification of sarcoidosis cases from a defined population will enable a valid assessment of risk factors for incident disease as follow-up continues.
Sarcoidosis is a chronic, systemic disorder that causes a wide spectrum of symptoms and illnesses.1-3 The disease is characterized by its pathologic hallmark, the noncaseating granuloma, which is formed in response to an unknown antigen or stimulant.1-3 Clinical presentation of sarcoidosis is highly variable, with pulmonary disease being the major source of morbidity and mortality in affected patients.1-6 Nonpulmonary organ systems affected by sarcoidosis can result in serious cardiac, skeletal, neurologic, ocular, or cutaneous disease.1,3,6-9 Genetic, autoimmune, environmental, and social factors are believed to be related to the onset and development of sarcoidosis, but the cause and pathogenesis remain poorly understood.1-4,6,8,10
Sarcoidosis occurs worldwide and affects men and women of all ages and races.1,3,9-11 The disease shows a consistent predilection for adults < 40 years of age.1,3,4,9-11 Familial sarcoidosis has been well documented and reports of families with two or more affected members is common.12-14 In the United States, patterns of sarcoidosis differ between men and women and between whites and blacks.10,11,15 Population-based incidence data from Minnesota have shown higher age-adjusted rates for women,15 and health maintenance organization and case-control study data have demonstrated a threefold to fourfold higher risk of disease in blacks compared with whites, as well as a decade earlier age of onset among blacks.7,10 In addition, blacks are more likely to experience progressive or extrathoracic disease requiring corticosteroid therapy7,11 and to die of the disease.10,16
Black women experience the highest incidence of sarcoidosis in the United States.10,11 Previous research has estimated the lifetime risk of sarcoidosis to be 2.7% for black women compared with 1.0% for white women10,11 and the annual incidence to be 39.1 per 100,000 for black women compared with 12.1 per 100,000 for white women.10,11
Despite the high burden of morbidity and mortality, few epidemiologic studies of sarcoidosis have focused on disease etiology in black women. Rybicki et al11 conducted a retrospective cohort study of 254 incident cases of sarcoidosis occurring among members of a Detroit, Michigan health maintenance organization between 1990 and 1994; 104 of the cases were black women. In A Case Control Etiologic Study of Sarcoidosis (ACCESS), a multicenter study sponsored by the National Heart, Lung, and Blood Institute, 736 incident cases of sarcoidosis from 10 clinical centers were enrolled between November 1997 and May 1999.7,17 Of the 736 cases, 234 were black women.
The Black Women’s Health Study (BWHS), a national prospective study of black women in the United States, is following its 59,000 participants for the occurrence of sarcoidosis, among other outcomes. In the present report, we describe methods of follow-up and identification of sarcoidosis cases in the BWHS, validation of cases, and the frequency of occurrence and characteristics of incident and prevalent cases.
Materials and Methods
Establishment of the BWHS and Follow-up
The human subjects’ protocol for this study was approved by the Boston University Medical Center Institutional Review Board. The BWHS began in 1995 when 59,000 women aged 21 to 69 years enrolled through postal health questionnaires, which were sent to subscribers of Essence magazine, members of selected black women’s professional organizations, and friends and relatives of early respondents. Participants indicated their informed consent by completing the questionnaires. At baseline, subjects were 21 to 69 years of age (median, 38 years), and 97% had completed high school (Table 1). More than 80% were from California, Georgia, Illinois, Indiana, Louisiana, Maryland, Massachusetts, Michigan, New Jersey, New York, South Carolina, Virginia, and the District of Columbia. Participants are mailed biennial questionnaires to obtain updated information on disease outcomes and potential risk factors. They are asked to report on newly diagnosed disease and to list medications currently taken at least 3 days a week. Over six completed follow-up cycles, cohort retention has averaged > 80%.
Table 1.
—Baseline (1995) Characteristics of the Black Women’s Health Study Cohort
| BWHS Cohort (N = 59,027) |
||
| Characteristic | No. | % |
| Age, y | ||
| < 30 | 12,814 | 22 |
| 30-39 | 19,598 | 33 |
| 40-49 | 16,459 | 28 |
| 50-59 | 7,241 | 12 |
| ≥ 60 | 2,915 | 5 |
| Education, y | ||
| ≤ 12 | 11,411 | 19 |
| 13-15 | 21,206 | 36 |
| ≥ 16 | 26,294 | 45 |
| Geographic region | ||
| Northeast | 16,119 | 27 |
| South | 18,049 | 31 |
| Midwest | 13,800 | 23 |
| West | 10,994 | 19 |
BWHS = Black Women’s Health Study.
On the 1995 baseline questionnaire, BWHS participants provided demographic data and information on medical and reproductive history, smoking and alcohol use, physical activity currently and in the past, current weight, waist and hip circumference, adult height, use of selected medications including oral contraceptives and female hormone supplements, diet, and use of medical care. Many of these topics are included in the follow-up questionnaires. In particular, participants are asked to report on newly diagnosed disease and to list medications currently taken at least 3 days a week. Since 1995, the BWHS investigators have published > 70 peer-reviewed manuscripts, including etiologic analyses of breast cancer,18-24 hypertension,25,26 type 2 diabetes,27-31 and uterine fibroids.32-35
Ascertainment and Validation of the Diagnosis of Sarcoidosis
On the 1995 baseline questionnaire, BWHS participants were asked if a physician had ever told them that they had any of a list of medical conditions. The list of diagnoses provided did not specify sarcoidosis, but numerous women wrote it in under “other conditions.” The 1997 and all subsequent follow-up questionnaires asked specifically about sarcoidosis.
In 2005, we began validating all self-reports of sarcoidosis reported since 1995. All women who reported an incident case of sarcoidosis were asked for permission to contact their physicians for information on diagnosis and treatment. The physicians were asked to complete an assessment questionnaire (e-Appendix 1), which asked detailed, specific questions about the study participant’s diagnosis and treatment. We relied on the clinical and diagnostic judgment of the physician and accepted their diagnosis classification (definite, possible, or not sarcoidosis). Physicians who were unwilling to complete the questionnaire were asked for a copy of the patient’s medical records pertaining to sarcoidosis. Similarly, for those subjects for whom medical records were obtained, we classified the person as having sarcoidosis if the reporting physician had noted the diagnosis within the medical record. The diagnosis of sarcoidosis was confirmed for 95% (113 definite, 9 possible or probable) of the 129 cases for whom physician questionnaires or medical records were obtained. Among the cases for which a diagnosis of sarcoidosis was not confirmed, the reasons given were that the current physician did not make the diagnosis and was unable to find diagnostic evidence of sarcoidosis (n = 4), a different disease or condition was confirmed (asthma, keloids) (n = 2), or simply that the patient “does not have sarcoid” without further detail provided (n = 1).
We also asked women who reported either an incident or prevalent case of sarcoidosis to complete a supplemental survey (e-Appendix 2) regarding diagnosis and symptoms. To date, we have received 691 supplemental surveys, 234 from incident cases and 457 from prevalent cases. Based on the level of agreement between self-reports and physician data, women who report sarcoidosis on a BWHS questionnaire are included as cases of sarcoidosis unless the diagnosis has been disconfirmed by medical record or the woman has responded to our request for a supplemental questionnaire by notifying us that she did not have the diagnosis.
Results
Prevalence, Incidence, and Demographics Among BWHS and Sarcoidosis Cases
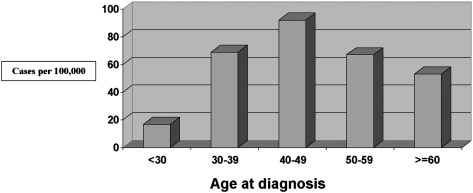
Among the 59,027 participants in the BWHS, 685 had sarcoidosis at the time they enrolled in the study, for a prevalence of 1,160/100,000 or 1.2%. In addition, 435 incident cases were reported during 611,585 person-years of follow-up from 1995 through 2007, for an average annual incidence rate of 71/100,000. Incidence was highest, 92/100,000, among women in their 40s (Fig 1). The median age at diagnosis was 32 years (range: 16-63 years) for prevalent cases and 44 years (range: 22-77 years) for incident cases. The current prevalence of sarcoidosis in the BWHS is 2.0%.
Figure 1.
Age-specific annual incidence rates of sarcoidosis in the Black Women’s Health Study Cohort (1995-2007).
As shown in Table 2, incident and prevalent cases of sarcoidosis were similar in terms of educational attainment, geographic area of current residence, and the type of area in which they grew up (eg, rural). Prevalent cases were more likely to be employed in white-collar occupations.
Table 2.
—BWHS Characteristics According to Sarcoidosis Status
| Characteristic | Incident Sarcoidosis, % (n = 435) | Prevalent Sarcoidosis, % (n = 685) | P Value |
| Education, y (1995) | .1757 | ||
| ≤ 12 | 20 | 18 | |
| 13-15 | 38 | 35 | |
| ≥ 16 | 42 | 47 | |
| Geographic region (1995) | .6589 | ||
| Northeast | 28 | 26 | |
| South | 31 | 32 | |
| Midwest | 28 | 27 | |
| West | 13 | 16 | |
| Occupation (1995) | .0302 | ||
| White collar | 54 | 61 | |
| Non-white collar | 42 | 34 | |
| Not employed/other | 2 | 3 | |
| Missing/unknown | 2 | 2 | |
| Where lived up to age 18 (1997) | .8680 | ||
| Urban setting | 42 | 40 | |
| Suburban setting | 13 | 15 | |
| Rural or small town | 19 | 21 | |
| Combination of settings | 14 | 14 | |
| Missing | 12 | 11 |
See Table 1 legend for expansion of abbreviation.
Clinical Characteristics of Sarcoidosis Cases Reported by Physicians
To date, physician assessment questionnaires or medical records have been received for 129 cases of sarcoidosis. Forty percent of reporting physicians were pulmonologists and 26% were primary care, internal medicine, or family medicine practitioners. Other specialties represented include rheumatology, ophthalmology, and dermatology. Characteristics of the 122 confirmed cases are presented in Table 3.
Table 3.
—Characteristics of Sarcoidosis Cases Based on Data From Physician Reports/Medical Records
| All With Checklist/Medical Records (N = 122) |
||
| Clinical Characteristic | No. | % |
| Organ involvement | ||
| Lung | 75 | 61 |
| Intrathoracic lymph nodes | 43 | 35 |
| Extrathoracic lymph nodes | 4 | 3 |
| Skin (including erythema nodosum) | 25 | 20 |
| Face (lupus pernio) | 4 | 3 |
| Eye | 20 | 16 |
| Liver | 9 | 7 |
| Joint | 8 | 6 |
| Othera | 11 | 9 |
| Diagnostic tests | ||
| Radiograph | 89 | 73 |
| Chest CT scan | 38 | 31 |
| Biopsy | 66 | 54 |
| Chest radiograph stageb | ||
| 0 | 10 | 13 |
| I | 22 | 27 |
| II | 26 | 33 |
| III or IV | 22 | 27 |
| Clinical coursec | ||
| Acute (with resolution) | 11 | 12 |
| Chronic progressive | 4 | 5 |
| Chronic stable | 45 | 51 |
| Never with symptoms | 7 | 8 |
| Information not provided | 22 | 24 |
| Comorbid illnessd | 68 | 56 |
| Medications | ||
| Prednisone | 63 | 52 |
| Methotrexate | 5 | 4 |
| Plaquenil | 12 | 10 |
| Inhaled corticosteroids | 20 | 16 |
| Nasal corticosteroids | 10 | 8 |
| Physician specialtye | ||
| Pulmonary | 52 | 40 |
| Primary care/internal/family medicine | 33 | 26 |
| Rheumatology | 5 | 4 |
| Ophthalmology | 4 | 3 |
| Dermatology | 2 | 2 |
| Otherf | 10 | 8 |
Other organs include: CNS, sinuses, throat, heart, kidney, and spleen.
Data are from the women for whom staging of chest radiograph was reported (n = 80).
Data are restricted to the women with a date of diagnosis of 2003 or earlier (n = 89).
Comorbid illnesses include asthma, hypertension, type 2 diabetes, cancer, obesity, depression, scleroderma, and lupus.
Percentages are based on the 129 physicians who completed questionnaires or provided medical records.
Other physician specialties include gastroenterology, general surgery, gynecology, hepatology, infectious disease, neurology, and oncology.
As shown, the lung was the organ most commonly involved in the disease process, with 61% of women having lung involvement, followed by intrathoracic lymph nodes (35%); 96% had intrathoracic involvement. There was also substantial extrapulmonary disease; sites affected most often were the skin (including erythema nodosum) (20%), and eyes (16%). Cardiac sarcoidosis was reported for only one woman. Chest radiograph was reported as the method of diagnosis for 73% of women, followed by biopsy (54%), and chest CT scan (31%). Sixty percent of women with a diagnostic chest radiograph were classified by their physicians as stage II or higher. Comorbid illness was noted for 56% of cases: conditions reported by physicians included asthma, hypertension, type 2 diabetes, cancer, scleroderma, lupus, obesity, hypercholesterolemia, and depression. Of cases with a comorbid illness, 16% reported a single condition, 19% reported two conditions, and 60% reported three or more conditions.
Corticosteroids were the most commonly prescribed drugs for treatment of sarcoidosis, with prednisone prescribed for 52% of the women, followed by inhaled corticosteroids (16%) and nasal corticosteroids (8%) (Table 3). Methotrexate and hydroxychloroquine sulfate (Plaquenil) were prescribed for 4% and 10% of women, respectively. We restricted the analysis of clinical prognosis to cases whose date of diagnosis preceded the start of our validation by 2 or more years (n = 89). Fifty-one percent of cases were classified as chronic stable and only 5% were classified as chronic progressive; 24% of physicians did not provide information on the clinical course of disease.
Self-Reported Characteristics of Sarcoidosis Cases
Table 4 presents data collected on supplemental questionnaires completed by 691 cases. The most common presenting symptoms reported were shortness of breath (45%), fatigue (41%), and cough (40%). Other symptoms included muscle pain (29%), nasal/sinus congestion (25%), chest pain (22%), and palpitations (14%). Prednisone was the most commonly reported therapy (70%), followed by nasal (30%) and inhaled corticosteroids (29%). Nine percent reported use of methotrexate and 8% use of Plaquenil. Twenty-eight percent of women reported having a relative with a history of sarcoidosis, with half of those noting that the affected family member was a first-degree relative (mother, father, sister, or brother).
Table 4.
—Characteristics of Sarcoidosis Cases Based on Data From Participant Supplemental Surveys
| All With Supplemental Survey (N = 691) |
||
| Survey Item | No. | % |
| Symptoms at diagnosis | ||
| Shortness of breath | 309 | 45 |
| Cough | 275 | 40 |
| Nasal/sinus congestion | 173 | 25 |
| Chest pain | 152 | 22 |
| Numbness/tingling (arms, legs, face) | 99 | 14 |
| Muscle pain | 200 | 29 |
| Fatigue | 281 | 41 |
| Palpitations | 99 | 14 |
| Headaches | 114 | 17 |
| Othera | 87 | 13 |
| Medications | ||
| Prednisone | 485 | 70 |
| Methotrexate | 61 | 9 |
| Plaquenil | 54 | 8 |
| Inhaled corticosteroids | 202 | 29 |
| Nasal corticosteroids | 209 | 30 |
| Otherb | 108 | 16 |
| Family history of sarcoidosis | ||
| Primary relative | 93 | 14 |
| Any relative | 195 | 28 |
Other symptoms reported: skin rash (including erythema nodosum), unintentional weight loss, eye problems.
Other reported medications include topical corticosteroid creams, corticosteroid eye drops.
Discussion
Our study reports on the largest sample to date of sarcoidosis in black women in the United States. We estimated an annual incidence rate of sarcoidosis of 71/100,000 over a 12-year period based on 435 incident cases, with a peak age-specific rate of 92/100,000 at ages 40 to 49. The current prevalence is 2.0%. Rybicki et al11 observed an average annual incidence rate of 39/100,000 during a 5-year period of observation, and a peak age-specific incidence of 107/100,000 for black women aged 30 to 39 years, based on a sample of incident cases that included 105 black women. Our overall estimate, based on data from 12 years of observation, was higher. We also observed a later age-specific peak incidence, which may represent the age distribution of the participants in the BWHS. At enrollment in 1995, only 22% of BWHS participants were < 30 years of age. Indeed, the median age of diagnosis of prevalent cases in the BWHS was 32 years compared with 44 years for incident cases.
Clinical presentation of sarcoidosis is highly variable.1,3,4,7 Although the lung is typically involved in > 90% of cases,4 the disease can affect any organ, and extrapulmonary disease is not uncommon.1,3,6,9 Our data are consistent with previous reports.7 Baughman et al7 reported involvement of the lungs in 95% of ACCESS participants followed by skin (excluding erythema nodosum) (16%), lymph nodes (15%), eye (12%), liver (12%), and bone/joint (0.5%). In the BWHS, the most common organs were the lungs (61%) followed by intrathoracic lymph nodes (35%), skin (including erythema nodosum) (20%), and eye (16%). We reported data on the lungs and intrathoracic lymph nodes separately, whereas the ACCESS investigators report intrathoracic lymph nodes as lung involvement.7 Were we to do the same, the proportion of lung involvement in our study would be 96%.
In the present study, 14% of sarcoidosis cases reported palpitations. A recent article by Mehta et al36 suggested that palpitations were a highly predictive symptom for cardiac involvement in sarcoidosis. In that study, 13 of 62 sarcoidosis patients had palpitations, and 11 of these 13 patients (86%) had evidence of cardiac sarcoidosis. We had only one physician report of cardiac involvement, possibly because of underrecognition of cardiac sarcoidosis. It is also possible the women with severe cardiac disease may have been less likely, or unable, to participate in the BWHS.
Comorbid illness is believed to occur in the majority of patients with sarcoidosis.37 Westney et al,37 in a retrospective cross-sectional study among predominantly African American women in Atlanta, observed that 56% of their study sample had one or more chronic conditions and that the most frequent chronic comorbid illnesses were hypertension (39%), diabetes mellitus (19%), anemia (19%), asthma (15%), gastroesophageal reflux disease (15%), depression (13%), and heart failure (10%). Among BWHS cases for which physician questionnaires or medical records were received, more than half were reported by their physicians to have comorbid illness. Self-reports of other medical conditions by the 1,120 sarcoidosis cases support this finding. Specifically, cases reported the following comorbid conditions: hypertension (57%), hypercholesterolemia (47%), arthritis (rheumatoid and/or osteoarthritis) (27%), asthma (26%), depression (24%), type 2 diabetes (21%), cardiovascular disease (20%), cancer (13%), and autoimmune diseases (lupus, scleroderma, or Sjögren) (4%). Sixty percent of women with a comorbid condition reported experiencing three or more comorbid conditions. As expected,3,4 corticosteroids were the most common treatment of symptomatic, systemic sarcoidosis, followed by noncorticosteroid therapies, such as methotrexate and Plaquenil.
Familial sarcoidosis has been commonly reported.10,12-14,38 Harrington et al12 observed a 13.5% prevalence of familial disease, defined as occurring among any related family member, among 673 patients diagnosed at the Henry Ford Health Sciences Center between 1965 and 1992. Other studies have reported a lower proportion of familial cases. Headings et al38 reported an estimated prevalence of sarcoidosis of 1.5% among first-degree relatives of 80 sarcoidosis cases in New York City compared with a population prevalence of 0.07%. Buck and McKusick39 reported a 2% prevalence of sarcoidosis among first-degree relatives of cases. In an analysis of 179 African American families of cases diagnosed within the Henry Ford Health System, Rybicki et al14 found that 3.7% of siblings and 6.8% of parents reported a history of sarcoidosis. In the present study, 14% of sarcoidosis cases who completed a supplemental survey reported a first-degree relative with sarcoidosis. The report of any relative (first or second degree) in this group was 28%. The variability in reports of familial sarcoidosis is not surprising. The diagnosis is not always clear and has become more common in recent years as physicians have become more aware of the disease.40 In addition, reports of familial disease depend on awareness by the index cases of the illness among their relatives and access to medical care among the relatives.38
It is not feasible in large observational studies to examine all participants for the presence of the disease of interest. Thus, in contrast to smaller studies that used clinical reports of sarcoidosis,11,15,17 the BWHS relies on self-report of sarcoidosis. Our validation effort in a subset of women showed a satisfactory degree of accuracy of self-report.
The goal of the present study within the BWHS is to accrue a large enough sample of incident cases for informative assessment of potential risk factors for the disease. Because of the higher incidence in women, hormone-related factors, such as pregnancy41 or menopausal status,42 will be assessed. Most studies of sarcoidosis in the United States have focused on patients from limited geographic regions.15,17 For example, the ACCESS study recruited patients exclusively from 10 clinical centers located on the East Coast and in the Midwest; no centers were located on the West Coast.17 Therefore, whether sarcoidosis incidence varies by geographic area is undetermined. Because the BWHS is a national cohort with participants from across the United States, this can be assessed. We will also explore the “rural hypothesis” of an increased risk of disease in the southeast and rural areas of the United States.43 Evidence that self-report of sarcoidosis is accurate, together with the prospective enrollment of sarcoidosis cases from a defined population, will enable the valid assessment of risk factors for incident disease.
The BWHS participants are not a random sample of black women in the United States. Participants need to be literate to fill out mailed questionnaires, and BWHS participants underrepresent the 15% of black women nationally of the same ages who did not graduate from high school.44 On the other hand, the participants represent the 85% of black women nationally who have completed 12 or more years of education, and the women represent all areas of the country.
In summary, the present study indicates accurate reporting of sarcoidosis in the BWHS. We also confirm previous reports of high incidence and prevalence of sarcoidosis among black women, as well as the extent of extrapulmonary disease, frequent need for steroid therapy, and comorbid conditions in this population. In the future, based on continued identification of incident cases in this large cohort, we will assess risk factors for the occurrence of the disease.
Supplementary Material
Acknowledgments
Author contributions: Dr Cozier: contributed to originating the idea for this study, supervising the data collection, designing the physician questionnaire and participant supplemental survey, analyzing and interpreting the data, and writing the article.
Dr Berman: contributed to originating the idea for this study, designing the physician questionnaire and participant supplemental survey, analyzing and interpreting the data, and writing the article.
Dr Palmer: contributed to originating the idea for this study, designing the Black Women’s Health Study, supervising the data collection, analyzing and interpreting the data, and writing the article.
Ms Boggs: contributed to analyzing and interpreting the data and writing the article.
Dr Serlin: contributed to designing the physician questionnaire and participant supplemental survey, analyzing and interpreting the data, and writing the article.
Dr Rosenberg: contributed to originating the idea for this study, designing the Black Women’s Health Study, supervising the data collection, analyzing and interpreting the data, and writing the article.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank the participants of the Black Women’s Health Study and the entire Black Women’s Health Study staff.
Additional information: The e-Appendixes can be found in the Online Supplement at http://chestjournal.chestpubs.org/content/139/1/144/suppl/DC1.
Abbreviations
- ACCESS
A Case Control Etiologic Study of Sarcoidosis
- BWHS
Black Women’s Health Study
Footnotes
Funding/Support: This work was supported by the National Cancer Institute [Grant CA58420]; and National Heart, Lung, and Blood Institute [Grant K01HL088709].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Bresnitz EA, Strom BL. Epidemiology of sarcoidosis. Epidemiol Rev. 1983;5:124–156. doi: 10.1093/oxfordjournals.epirev.a036255. [DOI] [PubMed] [Google Scholar]
- 2.Martin WJ, II, Iannuzzi MC, Gail DB, Peavy HH. Future directions in sarcoidosis research: summary of an NHLBI working group. Am J Respir Crit Care Med. 2004;170(5):567–571. doi: 10.1164/rccm.200308-1073WS. [DOI] [PubMed] [Google Scholar]
- 3.Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336(17):1224–1234. doi: 10.1056/NEJM199704243361706. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 5.Efferen LS. The challenge of sarcoidosis. Chest. 2001;120(3):697–699. doi: 10.1378/chest.120.3.697. [DOI] [PubMed] [Google Scholar]
- 6.James DG, Hosoda Y. Epidemiology. In: James DG, editor. Sarcoidosis and Other Granulomatous Disorders. New York, NY: Marcel Dekker; 1994. pp. 729–743. [Google Scholar]
- 7.Baughman RP, Teirstein AS, Judson MA, et al. Case Control Etiologic Study of Sarcoidosis (ACCESS) research group Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10 pt 1):1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 8.James DG. Sarcoidosis 2001. Postgrad Med J. 2001;77(905):177–180. doi: 10.1136/pmj.77.905.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynch JP, III, Sharma OP, Baughman RP. Extrapulmonary sarcoidosis. Semin Respir Infect. 1998;13(3):229–254. [PubMed] [Google Scholar]
- 10.Rybicki BA, Maliarik MJ, Major M, Popovich J, Jr, Iannuzzi MC. Epidemiology, demographics, and genetics of sarcoidosis. Semin Respir Infect. 1998;13(3):166–173. [PubMed] [Google Scholar]
- 11.Rybicki BA, Major M, Popovich J, Jr, Maliarik MJ, Iannuzzi MC. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145(3):234–241. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 12.Harrington DW, Major M, Rybicki B, Popovich J, Maliarik M, Iannuzzi MC. Familial sarcoidosis: analysis of 91 families. Sarcoidosis. 1994;11(suppl 1):240–243. [Google Scholar]
- 13.Rybicki BA, Kirkey KL, Major M, et al. Familial risk ratio of sarcoidosis in African-American sibs and parents. Am J Epidemiol. 2001;153(2):188–193. doi: 10.1093/aje/153.2.188. [DOI] [PubMed] [Google Scholar]
- 14.Rybicki BA, Iannuzzi MC, Frederick MM, et al. ACCESS Research Group Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS) Am J Respir Crit Care Med. 2001;164(11):2085–2091. doi: 10.1164/ajrccm.164.11.2106001. [DOI] [PubMed] [Google Scholar]
- 15.Henke CE, Henke G, Elveback LR, Beard CM, Ballard DJ, Kurland LT. The epidemiology of sarcoidosis in Rochester, Minnesota: a population-based study of incidence and survival. Am J Epidemiol. 1986;123(5):840–845. doi: 10.1093/oxfordjournals.aje.a114313. [DOI] [PubMed] [Google Scholar]
- 16.Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979-1991: an analysis of multiple-cause mortality data. Am J Med. 1996;100(4):423–427. doi: 10.1016/S0002-9343(97)89518-6. [DOI] [PubMed] [Google Scholar]
- 17.Access Research Group Design of a case control etiologic study of sarcoidosis (ACCESS) J Clin Epidemiol. 1999;52(12):1173–1186. doi: 10.1016/s0895-4356(99)00142-0. [DOI] [PubMed] [Google Scholar]
- 18.Palmer JR, Wise LA, Horton NJ, Adams-Campbell LL, Rosenberg L. Dual effect of parity on breast cancer risk in African-American women. J Natl Cancer Inst. 2003;95(6):478–483. doi: 10.1093/jnci/95.6.478. [DOI] [PubMed] [Google Scholar]
- 19.Palmer JR, Wise LA, Adams-Campbell LL, Rosenberg L. A prospective study of induced abortion and breast cancer in African-American women. Cancer Causes Control. 2004;15(2):105–111. doi: 10.1023/B:CACO.0000019484.29558.f7. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg L, Palmer JR, Wise LA, Adams-Campbell LL. A prospective study of female hormone use and breast cancer among black women. Arch Intern Med. 2006;166(7):760–765. doi: 10.1001/archinte.166.7.760. [DOI] [PubMed] [Google Scholar]
- 21.Taylor TR, Williams CD, Makambi KH, et al. Racial discrimination and breast cancer incidence in US Black women: the Black Women’s Health Study. Am J Epidemiol. 2007;166(1):46–54. doi: 10.1093/aje/kwm056. [DOI] [PubMed] [Google Scholar]
- 22.Palmer JR, Adams-Campbell LL, Boggs DA, Wise LA, Rosenberg L. A prospective study of body size and breast cancer in black women. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1795–1802. doi: 10.1158/1055-9965.EPI-07-0336. [DOI] [PubMed] [Google Scholar]
- 23.Min C, Yu Z, Kirsch KH, et al. A loss-of-function polymorphism in the propeptide domain of the LOX gene and breast cancer. Cancer Res. 2009;69(16):6685–6693. doi: 10.1158/0008-5472.CAN-08-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer JR, Boggs DA, Adams-Campbell LL, Rosenberg L. Family history of cancer and risk of breast cancer in the Black Women’s Health Study. Cancer Causes Control. 2009;20(9):1733–1737. doi: 10.1007/s10552-009-9425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cozier YC, Palmer JR, Horton NJ, Fredman L, Wise LA, Rosenberg L. Racial discrimination and the incidence of hypertension in U.S. black women. Ann Epidemiol. 2006;16(9):681–687. doi: 10.1016/j.annepidem.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Cozier YC, Palmer JR, Horton NJ, Fredman L, Wise LA, Rosenberg L. Relation between neighborhood median housing value and hypertension risk among black women in the United States. Am J Public Health. 2007;97(4):718–724. doi: 10.2105/AJPH.2005.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan S, Rosenberg L, Djoussé L, Cupples LA, Palmer JR. Overall and central obesity and risk of type 2 diabetes in U.S. black women. Obesity (Silver Spring) 2007;15(7):1860–1866. doi: 10.1038/oby.2007.220. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan S, Rosenberg L, Singer M, et al. Glycemic index, glycemic load, and cereal fiber intake and risk of type 2 diabetes in US black women. Arch Intern Med. 2007;167(21):2304–2309. doi: 10.1001/archinte.167.21.2304. [DOI] [PubMed] [Google Scholar]
- 29.Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008;168(14):1487–1492. doi: 10.1001/archinte.168.14.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan S, Coogan PF, Boggs DA, Rosenberg L, Palmer JR. Consumption of restaurant foods and incidence of type 2 diabetes in African American women. Am J Clin Nutr. 2010;91(2):465–471. doi: 10.3945/ajcn.2009.28682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishnan S, Cozier YC, Rosenberg L, Palmer JR. Socioeconomic status and incidence of type 2 diabetes: results from the Black Women’s Health Study. Am J Epidemiol. 2010;171(5):564–570. doi: 10.1093/aje/kwp443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wise LA, Palmer JR, Harlow BL, et al. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol. 2004;159(2):113–123. doi: 10.1093/aje/kwh016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wise LA, Palmer JR, Harlow BL, et al. Risk of uterine leiomyomata in relation to tobacco, alcohol and caffeine consumption in the Black Women’s Health Study. Hum Reprod. 2004;19(8):1746–1754. doi: 10.1093/humrep/deh309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wise LA, Palmer JR, Spiegelman D, et al. Influence of body size and body fat distribution on risk of uterine leiomyomata in U.S. black women. Epidemiology. 2005;16(3):346–354. doi: 10.1097/01.ede.0000158742.11877.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wise LA, Palmer JR, Stewart EA, Rosenberg L. Polycystic ovary syndrome and risk of uterine leiomyomata. Fertil Steril. 2007;87(5):1108–1115. doi: 10.1016/j.fertnstert.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest. 2008;133(6):1426–1435. doi: 10.1378/chest.07-2784. [DOI] [PubMed] [Google Scholar]
- 37.Westney GE, Habib S, Quarshie A. Comorbid illnesses and chest radiographic severity in African-American sarcoidosis patients. Lung. 2007;185(3):131–137. doi: 10.1007/s00408-007-9008-z. [DOI] [PubMed] [Google Scholar]
- 38.Headings VE, Weston D, Young RCJ, Jr, Hackney RL., Jr Familial sarcoidosis with multiple occurrences in eleven families: a possible mechanism of inheritance. Ann N Y Acad Sci. 1976;278:377–385. doi: 10.1111/j.1749-6632.1976.tb47049.x. [DOI] [PubMed] [Google Scholar]
- 39.Buck AA, McKusick VA. Epidemiologic investigations of sarcoidosis. III. Serum proteins; syphilis; association with tuberculosis: familial aggregation. Am J Hyg. 1961;74:174–188. doi: 10.1093/oxfordjournals.aje.a120209. [DOI] [PubMed] [Google Scholar]
- 40.Aladesanmi OA. Sarcoidosis: an update for the primary care physician. MedGenMed. 2004;6(1):7. [PMC free article] [PubMed] [Google Scholar]
- 41.Selroos O. Sarcoidosis and pregnancy: a review with results of a retrospective survey. J Intern Med. 1990;227(4):221–224. doi: 10.1111/j.1365-2796.1990.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 42.Shirai M, Sato A, Chida K. The influence of ovarian hormones on the granulomatous inflammatory process in the rat lung. Eur Respir J. 1995;8(2):272–277. doi: 10.1183/09031936.95.08020272. [DOI] [PubMed] [Google Scholar]
- 43.Gentry JT, Nitowsky HM, Michael M., Jr Studies on the epidemiology of sarcoidosis in the United States: the relationship to soil areas and to urban-rural residence. J Clin Invest. 1955;34(12):1839–1856. doi: 10.1172/JCI103240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.U.S. Department of Commerce . Educational Attainment in the United States. Washington, DC: Department of Commerce; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.