Abstract
Adipose tissue inflammation in obesity is a major factor leading to cardiovascular disease and type 2 diabetes.12/15 lipoxygenases (ALOX) play an important role in the generation of inflammatory mediators, insulin resistance and downstream immune activation in animal models of obesity. However, the expression and roles of 12/15ALOX isoforms, and their cellular sources in human subcutaneous (sc) and omental (om) fat in obesity is unknown. The objective of this study was to examine the gene expression and localization of ALOX isoforms and relevant downstream cytokines in subcutaneous (sc) and omental (om) adipose tissue in obese humans. Paired biopsies of sc and om fat were obtained during bariatric surgeries from 24 morbidly obese patients. Gene and protein expression for ALOX15a, ALOX15b and ALOX 12 were measured by real-time PCR and western blotting in adipocytes and stromal vascular fractions (SVF) from om and sc adipose tissue along with the mRNA expression of the downstream cytokines IL-12a, IL-12b, IL-6, IFNγ and the chemokine CXCL10. In a paired analysis, all ALOX isoforms, IL-6, IL-12a and CXCL10 were significantly higher in om vs. sc fat. ALOX15a mRNA and protein expression was found exclusively in om fat. All of the ALOX isoforms were expressed solely in the SVF. Further fractionation of the SVF in CD34+ and CD34- cells indicated that ALOX15a is predominantly expressed in the CD34+ fraction including vascular and progenitor cells, while ALOX15B is mostly expressed in the CD34- cells containing various leucocytes and myeloid cells. This result was confirmed by immunohistochemistry showing exclusive localization of ALOX15a in the om fat and predominantly in the vasculature and non-adipocyte cells. Our finding is identifying selective expression of ALOX15a in human om but not sc fat. This is a study showing a major inflammatory gene exclusively expressed in visceral fat in humans.
Keywords: inflammation, 12/15 lipoxygenases, obesity, human, adipose tissue, vasculature, cytokines
Introduction
Adipose tissue inflammation plays a central role in obesity-related metabolic and cardiovascular complications. Obese individuals have at least six times the risk for having type 2 diabetes, leading to increases in cardiovascular morbidity and mortality. Multiple factors have been implicated, including inflammatory cytokines such as TNFα, IL-6, IL-12 and IFNγ [1]. In particular visceral (omental) fat in obesity is characterized by increased inflammation and correlates with elevated systemic inflammation in obesity and type 2 diabetes in humans [1–3]. Lipoxygenases constitute a family of enzymes that catalyze the oxygenation of cellular poly-unsaturated fatty acids into lipid mediators and are categorized according to their positional specificity. 12/15 ALOX enzymes regulate the expression of pro-inflammatory cytokines and chemokines in different tissues [4]. Important recent mouse studies demonstrated the role of 12/15 ALOX pathway in the development of insulin resistance, adipose tissue inflammation and atherosclerotic plaque formation [5–8]. In vivo rodent studies have demonstrated that deletion of 12/15 ALOX reduces inflammatory cytokine production and completely prevents insulin resistance in animals fed a western diet [6, 8]. In vitro studies show that direct addition of 12/15 ALOX lipid products( 12- and 15-HETEs) to adipocytes induces inflammatory cytokine expression and impairs insulin action [9]. In humans, ALOX12 was reported in endothelial and smooth muscle cells as well as in monocytes[10]. In macrophages, ALOX12 lipid products increase synthesis of the pro-inflammatory cytokines IL-12, TNFα, and IL-6, and also induce expression of inflammatory genes such as monocyte chemoattractant 1 (MCP-1) and Cox2[11, 12]. Two different human ALOX15 have been described: an ubiquitous ALOX15a and a more restrictively expressed ALOX15b [13, 14]. Interestingly, expression of ALOX15a is induced by cytokines [15, 16] and ALOX15b was reported to be expressed in human macrophages in response to hypoxia [17]. Also, macrophage ALOX15b overexpression stimulates the production of various chemokines and cytokines including IL-12a and increases T cell migration [18]. Importantly, ALOX15 variants in humans are associated with induced expression of IL-6, TNFα and IL-1b, indicating a broad role for the enzyme in systemic inflammation [19]. Existing data suggest an active interplay between the cytokine milieu and different lipoxygenase isoform expression which is tissue dependent and potentially highly pathogenic [4]. In this study we are showing the 12 and 15 ALOX expression, localization and downstream cytokine expression in sc and om adipose tissue in human obesity.
Materials and Methods
Human subjects
Twenty four morbidly obese subjects (3 males and 21 females) qualifying for bariatric surgery were included in this study. The average BMI was 42.13±5.94 kg/m2and the average age was 47.8±9.6 years (Table 1). Nine of the patients had type 2 diabetes, that was well controlled by established medications. Subjects were excluded for chronic auto-immune conditions, active tobacco use, type 1 diabetes, active malignancy or infection, or if they were on chronic immunosuppressive or anti-inflammatory medications. The protocol was approved by the Institutional Review Board of Eastern Virginia Medical School.
Table 1. Characteristics of obese subjects included in the study.
Twenty-four obese subjects qualifying for bariatric surgery were enrolled in this pilot study. Anthropomorphic measurements were obtained pre-operatively, including height and weight (to calculate body mass index, BMI). Hemoglobin A1c (A1c) was measured prior to surgery. Subjects were excluded if they had chronic autoimmune conditions, including type 1diabetes. Other exclusionary criteria included active tobacco use, active malignancy or infection, and the use of chronic immunosuppressive or anti-inflammatory medications. Values are expressed as mean ±SD.
| Mean ±SEM | |
|---|---|
| n | 24 |
| Gender (F/M) | 21/3 |
| Diabetic (% total) | 9 subjects (38%) |
| Age (years) | 46.3±2.1 |
| BMI (kg/m2) | 42.65±1.11 |
| A1C (%) | 6.58 ±0.26 |
Adipose tissue biopsies and preparation of adipocytes and stromal vascular fractions (SVF)
Paired samples of sc and om fat were obtained during each subject’s bariatric surgical procedure. Immediately after collection major blood clots and skin fragments were removed. Adipose tissue digestion was conducted as described by Fried et al [20] using KHR buffer supplemented with 1mg/mL collagenase type I (Sigma), 1% BSA and 50nM adenosine (Sigma) for ~1 hour at 37°C. After filtration, floating adipocytes were collected from the top of the tube while the infranatant was spun at 500 x g. The resulting pellet contained the SVF. Further fractionation of the SVF was performed for some of the samples by positive immuno-selection using anti-human CD34 coated magnetic beads, according to manufacturer’s instructions (StemCell Technologies Inc., Vancouver, Canada). The purity of the sorted fractions was verified by flow cytometry using PE-antiCD34 human antibody (BioLegend, San Diego, CA).
RNA isolation, reverse transcription and quantitative real-time PCR
These procedures were described in detail previously [12]. Briefly, total adipose tissue or isolated adipocytes and SVF were lysed in Trizol (Invitrogen, Carlsbad, CA) and RNA isolated using a RNeasy kit from Qiagen (Valencia, CA), according to manufacturer’s instructions. cDNA was prepared using a protocol, enzymes, and reagents provided by the Promega Reverse Transcription System (Promega Corporation, Madison, WI). Real-time PCR was performed using Taqman probes from Applied Biosystems (Carlsbad, CA). cDNA was amplified in an iCycler iQ Real-Time PCR Detection System (Bio-Rad laboratories, Hercules, CA). Results were normalized to 18s RNA and γ-actin, used as housekeeping genes. Data were calculated by the 2−ΔΔCT method and presented as fold change compared to a reference (defined as 1.0 fold).
Western blotting
Adipose tissue was powdered frozen and homogenized in RIPA buffer containing protease and phosphatase inhibitors, following by gel electrophoresis and electroblotting, as described in [21]. Incubation with primary antibodies (anti-human ALOX12, 1:500 dilution (Santa Cruz, Santa Cruz, CA) and anti-human ALOX15a (1:500 dilution, [22])) was performed overnight at 4°C. β-actin (Santa Cruz, 1:4,000 dilution) was used as a protein loading control. Secondary biotinylated antibodies and the SuperSignal West Pico Enhancer Solution were used for detection. Western blot quantifications were performed using Image J software (NIH, Bethesda, MD).
Immunohistochemical staining of adipose tissue
Tissue biopsies were fixed in 10% buffered formalin overnight then embedded in paraffin and following antigen retrieval were incubated for 2hrs with human anti-ALOX15 antibody (Abnova, 1:100 dilution). Detection was performed using the avidin-biotin -peroxidase method and slides were counterstained using Mayer’s hematoxylin. Sections incubated with goat IgG (Pierce) instead of the primary antibody were used as method controls. All pictures were captured with an Olympus microscope using 200x magnification.
Statistical analysis
Differences between gene and protein expression in the sc vs. om fat were determined using paired Student’s t-test. Unpaired analysis was performed to compare gene expression in different adipose tissue fractions. Data was represented as mean±SD. The null hypothesis was rejected for a p-value <0.05. Statistical analysis was performed using GraphPad InStat software (GraphPad Software Inc., La Jolla, CA).
Results
Expression of lipoxygenase isoforms in sc and om adipose tissue
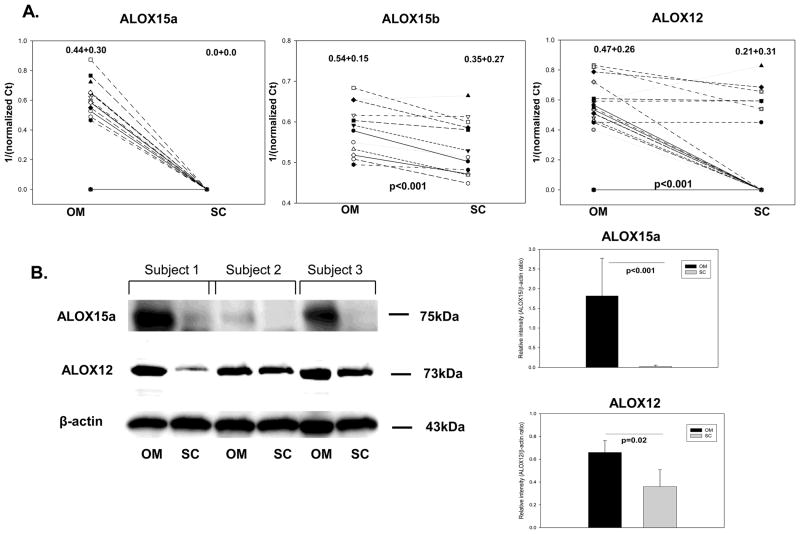
The relevant clinical parameters of the subjects that are included in the study are summarized in Table 1. Of great interest, we found mRNA expression of ALOX15a only in the om tissue, while ALOX15b and ALOX12 were expressed in both sc and om fat (Figure 1A). Using paired analysis we found significantly higher expression for all of the ALOX isoforms in the om compared to sc adipose tissue (Figure 1A). Protein expression of ALOX15a confirmed the real-time PCR results, showing selective expression in om adipose tissue (Figure 1B). Also, ALOX12 protein expression was significantly increased in the om vs. sc fat (Figure 1B). Importantly, ALOX15a and ALOX15b expression was not correlated with BMI or HbA1C values (data not shown) indicating that selective increased expression of both isoforms is an intrinsic property of the type of adipose depot in obese humans. Also, ALOX12 was independent of BMI and a weak direct correlation with HbA1c (r2 = 0.460) was found, suggesting a potential role of this isoform in type 2 diabetes.
Figure 1. mRNA and protein expression of ALOX isoforms in omental (OM) and subcutaneous (SC) adipose tissue.
Figure 1A: paired analysis of lipoxygenases in sc vs. om tissue from obese subjects (n=20) by real-time PCR. Data was expressed as 1/ΔCt (target gene Ct value normalized to housekeeping gene β-actin). Values on top of graphs represent mean±SD. The p-value was calculated using a paired two-tailed Student’s t-test. Figure 1B: representative western blots and semi-quantitative analysis of protein expression for ALOX15a and ALOX12 in paired samples of subcutaneous and omental adipose tissue. Data represent mean±SD and the p-value was calculated as in Figure 1A.
Distribution of different ALOX isoforms in sc and om adipose tissue
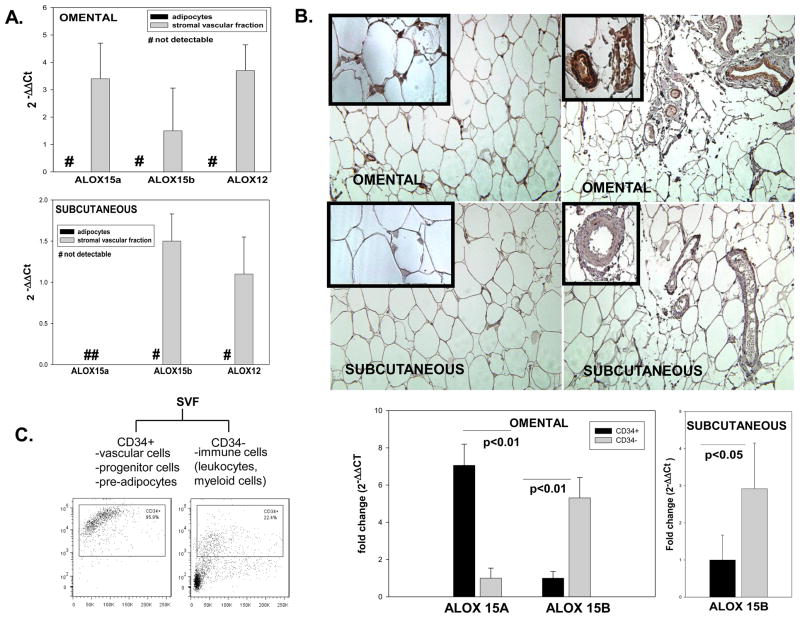
We also measured ALOX expression in adipocytes and SVF from sc and om fat and have found that all isoforms are solely present in the SVF (Figure 2A). Further, using immunohistochemistry we confirmed expression of ALOX15a exclusively in the om adipose tissue (Figure 2B). The strongest expression was detected in the adipose tissue vasculature (upper right panel) and to a lesser extent in areas possibly containing immune cells infiltrates (upper left panel). Next, we fractionated the SVF into CD34+ and CD34- sub-fractions. The CD34+ fraction contains vascular cells (endothelial cells, smooth muscle cells, pericytes) as well as progenitor cells and pre-adipocytes [23]; the CD34- fraction potentially contains leucocytes and cells of myeloid origin (macrophages, dendritic cells). Analysis of mRNA expression in the two SVF sub-fractions in the omental fat showed a 7.2-fold increase in mRNA expression of ALOX15a in the CD34+ fraction compared to the CD34- fraction (Figure 2C). This is consistent with previous reports showing ALOX15a expression in vascular cells. ALOX 15B mRNA expression in both omental and subcutaneous adipose tissue was ~5–6-fold increased in the CD34- fraction containing immune cells compared to the CD34+ cells (Figure 2C). Out of the five samples that were analyzed, none showed detectable expression for ALOX 12 in subcutaneous fat and 3 out of 4 samples showed borderline detectable expression in om fat.
Figure 2. Relative expression and adipose tissue distribution of 12/15 ALOX.
Figure 1A: relative gene expression of 12/15ALOX in adipocytes and SVF was expressed as 2−ΔΔCt using the expression in total adipose tissue as reference (a 2−ΔΔCt value of 1). Ct values of the target genes were normalized to 18 S rRNA expression. Values are expressed as group means±SD of n=5 samples for each of om and sc; # represents non-detectable values using a PCR threshold cycle of 50. Figure 1B. Representative immunohistochemistry photomicrographs illustrating expression of ALOX15a in paired omental and subcutaneous adipose tissue. Immunopositive staining was strongly and selectively present in the omental vasculature and likely associated with the immune infiltrate (arrows); Magnification 200x; insets represent selected magnified areas from each of the respective pictures. Figure 1C: mRNA expression of ALOX15a and ALOX15b in stromal vascular fraction (SFV) sub-fractions positively selected for CD34 (n=4–5); on left representative FACS plots illustrating % of the CD34+cells out of the total cell population in the CD34+ and CD34- fractions in omental fat; histograms represent fold change in gene expression and are shown as means±SD; two-tailed, unpaired Student’s t-test was used for data analysis.
Expression of pro-inflammatory cytokines
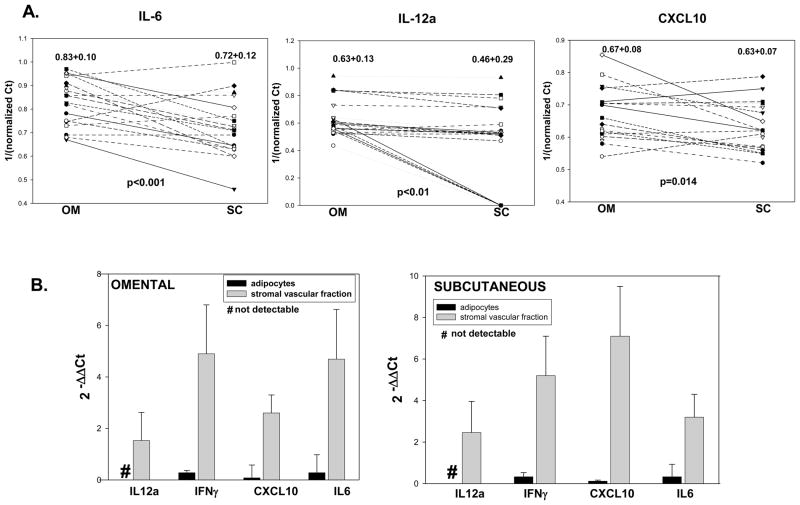
Inflammatory cytokines such as IL-12, IL-6, IFNγ and chemokines such as CXCL10 are downstream of 12/15 ALOX activation. Paired analysis of cytokine expression showed significantly higher levels in om vs. sc fat for IL-6, IL-12a and CXCL10 (Figure 3A). Interestingly, IL-12b expression was not detectable in either depot and no significant difference was measured for IFNγ expression between sc and om fat (data not shown). IL-6, IFNγ and CXCL10 were expressed in both adipocytes and in SVF, while IL-12a expression was detectable only in the SVF (Figure 3B). SVF expression for all of the cytokines tested was on average 2.5–7-fold higher compared to expression in adipocytes (Figure 3B). This distribution pattern closely follows the differential expression of the ALOX isoforms in the SVF in both the om and sc fat.
Figure 3. mRNA expression of downstream cytokines in omental (OM) and subcutaneous (SC) adipose tissue.
Figure 3A: paired analysis of cytokines in sc vs. om tissue from obese subjects (n=20) by real-time PCR. Data was expressed as 1/ΔCt (target gene Ct value normalized to housekeeping gene β-actin). Values on top of graphs represent mean±SD. The p-value was calculated using a paired two-tailed Student’s t-test. Figure 3B: relative gene expression of 12/15ALOX in adipocytes and SVF was expressed as 2−ΔΔCt using the expression in total adipose tissue as reference (a 2−ΔΔCt value of 1). Ct values of the target genes were normalized to 18 S rRNA expression. Values are expressed as group means±SD of n=5 samples for each of om and sc; # represents non-detectable values using a PCR threshold cycle of 50.
Discussion
Lipoxygenases (ALOX) constitute a family of enzymes that are categorized according to their positional specificity. ALOX 12 and 15 are implicated in the pathogenesis of insulin resistance, inflammation in fat, and atherosclerosis in mammals. In this study we evaluated the expression of the ALOX enzymes in om and sc adipose tissue in human obesity.
Our finding is identifying selective expression of ALOX15a in human om but not sc fat. Expression and function of ALOX15a (15-LO-1) has been studied in vascular cells and monocytes as well as atherosclerotic animal models, and has been shown to play active roles in vascular reactivity and remodeling as well as in atherosclerosis [7, 24]. Targeted deletion of the mouse form of ALOX15a completely prevented insulin resistance, glucose intolerance and inflammation in visceral adipose tissue in mice fed high fat diet [8]. Therefore, ALOX15a selective expression in om adipose tissue in humans may play a key pro-inflammatory role and may explain in part the mechanistic link between increased visceral fat and cardiometabolic risk in human obesity. ALOX15a may also play a role as modulator of angiogenesis in human om fat. A recent study demonstrated an anti-angiogenic role of ALOX15a in skeletal muscle [25]. Interestingly, a mechanistic role of 12/15 lipoxygenase in tumor angiogenesis and vessel maturation was recently reported in a rodent model [26]. Recent evidence indicate that visceral adipose tissue in obese humans is characterized by inflammation and rarefaction without the expected angiogenic response [27]. In this study we found the most abundant expression of ALOX15a in the CD34+ cells separated from SVF of the om fat, that include endothelial cells, smooth muscle cells and pericytes. The selective presence of ALOX15a in vascular cells of the visceral fat may contribute to the inadequate angiogenesis in response to the distinctive adipose tissue expansion of this fat depot in human obesity. Besides vascular cells, SVF contains pre-adipocytes and progenitor-type cells. 12/15 lipoxygenases are involved in 3T3L1 adipocyte differentiation [28, 29] but there is no evidence yet on role of the lipoxygenase pathway in human adipocyte differentiation. The presence of ALOX 15a and ALOX15b in the CD34+ fraction of human visceral fat raises the intriguing possibility that lipoxygenase pathway is also involved in adipocyte differentiation in humans. Additional studies will be needed to clarify the precise non-vascular source(s) of ALOX enzymes as well as their functional significance. In this study we also showed increased ALOX12 and ALOX15b expression in SVF of om vs. sc human fat. These ALOX isoforms have also been linked to diabetes complications and carotid atherosclerosis [30]. As opposed to ALOX15a, we found the ALOX15b isoform more abundantly expressed in the CD34- cells isolated from om and sc SVF. This fraction contains myeloid cells and leukocytes. Human macrophages in carotid plaques express ALOX15b and expression is increased by hypoxia via HIF-1α [17, 30]. Human adipose tissue in obesity is characterized by reduced oxygenation [27] and, enhanced macrophage content was reported in visceral compared to subcutaneous adipose tissue in obesity[31]. The more abundant expression of ALOX15b in the CD34- fraction containing macrophages as well as increased ALOX15b expression in om fat may reflect increased ALOX15b production by macrophages in response to hypoxia in human adipose tissue in obesity. Importantly, ALOX15b expression by macrophages induces chemokine production and T cell migration [18] and may therefore contribute to inflammation in om adipose tissue in human obesity.
Arachidonic acid metabolites generated from both the 15- and 12-ALOX are potent inducers of the pro-inflammatory cytokines leading to Th1 lineage differentiation of T cells [11]. We found significant increases in IL-12a, IL-6 and CXCL10 in om vs. sc fat. Consistent with our findings, a recent report showed increased CXCL10 expression in om vs. sc fat in obese humans [1]. Interestingly, IL-12b expression was not detectable suggesting a possible predominant expression of IL-35 vs IL-12 in om fat in obese humans. This may represent an adaptive response to limit inflammation in om fat in obesity. Our results suggest a complex interplay between the 12/15ALOX pathway active in SVF and the production of pro-inflammatory cytokines by both the adipocytes and cells of the SVF that act in concert to contribute to adipose tissue inflammation and insulin resistance. This pathway emerges therefore as a possible key link between obesity and associated cardiometabolic complications. Development of selective ALOX inhibitors could provide a novel approach to reduce the complications associated with increased visceral obesity and inflammation.
Research highlights.
ALOX15A is selectively expressed only in the omental adipose tissue in obese humans
Expression of all 12/15 ALOX isoforms is increased in omental vs. subcutaneous fat
ALOX isoforms are detectable only in the fraction containing immune, vascular and progenitor cells
Relevant downstream cytokines are also increased in omental vs. subcutaneous human fat
Acknowledgments
The authors wish to thank Dr. Michael Gonzales, Sentara Medical Group and our clinical study coordinators for their valuable assistance. This work was supported from NIH PO1 HL55798 to J.N. and A.D.
Footnotes
Disclosures. The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Rourke RW, Metcalf MD, White AE, Madala A, Winters BR, Maizlin, Jobe BA, Roberts CT, Slifka MK, Jr, Marks DL. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 2009;33:978–990. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 3.Sam S, Haffner S, Davidson MH, D’Agostino RB, Sr, Feinstein S, Kondos G, Perez A, Mazzone T. Relation of abdominal fat depots to systemic markers of inflammation in type 2 diabetes. Diabetes Care. 2009;32:932–937. doi: 10.2337/dc08-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobrian AD, Lieb DC, Cole BK, Taylor-Fishwick DA, Chakrabarti SK, Nadler JL. Functional and pathological roles of the 12- and 15-lipoxygenases. Prog Lipid Res. 2010 doi: 10.1016/j.plipres.2010.10.005. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Law IK, Xu A, Lam KS, Berger T, Mak TW, Vanhoutte PM, Liu JT, Sweeney G, Zhou M, Yang B, Wang Y. Lipocalin-2 deficiency attenuates insulin resistance associated with ageing and obesity. Diabetes. 2010;59:872–882. doi: 10.2337/db09-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nunemaker CS, Chen M, Pei H, Kimble SD, Keller SR, Carter JD, Yang Z, Smith KM, Wu R, Bevard MH, Garmey JC, Nadler JL. 12-Lipoxygenase-knockout mice are resistant to inflammatory effects of obesity induced by Western diet. Am J Physiol Endocrinol Metab. 2008;295:E1065–1075. doi: 10.1152/ajpendo.90371.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poeckel D, Zemski Berry KA, Murphy RC, Funk CD. Dual 12/15- and 5-lipoxygenase deficiency in macrophages alters arachidonic acid metabolism and attenuates peritonitis and atherosclerosis in ApoE knock-out mice. J Biol Chem. 2009;284:21077–21089. doi: 10.1074/jbc.M109.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sears DD, Miles PD, Chapman J, Ofrecio JM, Almazan F, Thapar D, Miller YI. 12/15-lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS One. 2009;4:e7250. doi: 10.1371/journal.pone.0007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakrabarti SK, Cole BK, Wen Y, Keller SR, Nadler JL. 12/15-Lipoxygenase Products Induce Inflammation and Impair Insulin Signaling in 3T3-L1 Adipocytes. Obesity (Silver Spring) 2009 doi: 10.1038/oby.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JA, Gu JL, Natarajan R, Berliner JA, Nadler JL. A leukocyte type of 12-lipoxygenase is expressed in human vascular and mononuclear cells. Evidence for upregulation by angiotensin II. Arterioscler Thromb Vasc Biol. 1995;15:942–948. doi: 10.1161/01.atv.15.7.942. [DOI] [PubMed] [Google Scholar]
- 11.Wen Y, Gu J, Chakrabarti SK, Aylor K, Marshall J, Takahashi Y, Yoshimoto T, Nadler JL. The role of 12/15-lipoxygenase in the expression of interleukin-6 and tumor necrosis factor-alpha in macrophages. Endocrinology. 2007;148:1313–1322. doi: 10.1210/en.2006-0665. [DOI] [PubMed] [Google Scholar]
- 12.Wen Y, Gu J, Vandenhoff GE, Liu X, Nadler JL. Role of 12/15-lipoxygenase in the expression of MCP-1 in mouse macrophages. Am J Physiol Heart Circ Physiol. 2008;294:H1933–1938. doi: 10.1152/ajpheart.00260.2007. [DOI] [PubMed] [Google Scholar]
- 13.Brash AR, Boeglin WE, Chang MS. Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci U S A. 1997;94:6148–6152. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadel JA, Conrad DJ, Ueki IF, Schuster A, Sigal E. Immunocytochemical localization of arachidonate 15-lipoxygenase in erythrocytes, leukocytes, and airway cells. J Clin Invest. 1991;87:1139–1145. doi: 10.1172/JCI115110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrad DJ, Kuhn H, Mulkins M, Highland E, Sigal E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc Natl Acad Sci U S A. 1992;89:217–221. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nassar GM, Morrow JD, Roberts LJ, 2nd, Lakkis FG, Badr KF. Induction of 15-lipoxygenase by interleukin-13 in human blood monocytes. J Biol Chem. 1994;269:27631–27634. [PubMed] [Google Scholar]
- 17.Rydberg EK, Krettek A, Ullstrom C, Ekstrom K, Svensson PA, Carlsson LM, Jonsson-Rylander AC, Hansson GI, McPheat W, Wiklund O, Ohlsson BG, Hulten LM. Hypoxia increases LDL oxidation and expression of 15-lipoxygenase-2 in human macrophages. Arterioscler Thromb Vasc Biol. 2004;24:2040–2045. doi: 10.1161/01.ATV.0000144951.08072.0b. [DOI] [PubMed] [Google Scholar]
- 18.Danielsson KN, Rydberg EK, Ingelsten M, Akyurek LM, Jirholt P, Ullstrom C, Forsberg GB, Boren J, Wiklund O, Hulten LM. 15-Lipoxygenase-2 expression in human macrophages induces chemokine secretion and T cell migration. Atherosclerosis. 2008;199:34–40. doi: 10.1016/j.atherosclerosis.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Fairfax BP, Vannberg FO, Radhakrishnan J, Hakonarson H, Keating BJ, Hill AV, Knight JC. An integrated expression phenotype mapping approach defines common variants in LEP, ALOX15 and CAPNS1 associated with induction of IL-6. Hum Mol Genet. 2010;19:720–730. doi: 10.1093/hmg/ddp530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 21.Dobrian AD, Schriver SD, Khraibi AA, Prewitt RL. Pioglitazone prevents hypertension and reduces oxidative stress in diet-induced obesity. Hypertension. 2004;43:48–56. doi: 10.1161/01.HYP.0000103629.01745.59. [DOI] [PubMed] [Google Scholar]
- 22.Ma K, Nunemaker CS, Wu R, Chakrabarti SK, Taylor-Fishwick DA, Nadler JL. 12-Lipoxygenase Products Reduce Insulin Secretion and {beta}-Cell Viability in Human Islets. J Clin Endocrinol Metab. 2010;95:887–893. doi: 10.1210/jc.2009-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curat CA, Miranville A, Sengenes C, Diehl M, Tonus C, Busse R, Bouloumie A. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 24.Kuhn H, Chaitidis P, Roffeis J, Walther M. Arachidonic Acid metabolites in the cardiovascular system: the role of lipoxygenase isoforms in atherogenesis with particular emphasis on vascular remodeling. J Cardiovasc Pharmacol. 2007;50:609–620. doi: 10.1097/FJC.0b013e318159f177. [DOI] [PubMed] [Google Scholar]
- 25.Viita H, Markkanen J, Eriksson E, Nurminen M, Kinnunen K, Babu M, Heikura T, Turpeinen S, Laidinen S, Takalo T, Yla-Herttuala S. 15-lipoxygenase-1 prevents vascular endothelial growth factor A- and placental growth factor-induced angiogenic effects in rabbit skeletal muscles via reduction in growth factor mRNA levels, NO bioactivity, and downregulation of VEGF receptor 2 expression. Circ Res. 2008;102:177–184. doi: 10.1161/CIRCRESAHA.107.155556. [DOI] [PubMed] [Google Scholar]
- 26.Schneider M, Wortmann M, Mandal PK, Arpornchayanon W, Jannasch K, Alves F, Strieth S, Conrad M, Beck H. Absence of glutathione peroxidase 4 affects tumor angiogenesis through increased 12/15-lipoxygenase activity. Neoplasia. 2010;12:254–263. doi: 10.1593/neo.91782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madsen L, Petersen RK, Sorensen MB, Jorgensen C, Hallenborg P, Pridal L, Fleckner J, Amri EZ, Krieg P, Furstenberger G, Berge RK, Kristiansen K. Adipocyte differentiation of 3T3-L1 preadipocytes is dependent on lipoxygenase activity during the initial stages of the differentiation process. Biochem J. 2003;375:539–549. doi: 10.1042/bj20030503. [DOI] [PubMed] [Google Scholar]
- 29.Hallenborg P, Jorgensen C, Petersen RK, Feddersen S, Araujo P, Markt P, Langer T, Furstenberger G, Krieg P, Koppen A, Kalkhoven E, Madsen L, Kristiansen K. Epidermis-type lipoxygenase 3 regulates adipocyte differentiation and peroxisome proliferator-activated receptor gamma activity. Mol Cell Biol. 2010;30:4077–4091. doi: 10.1128/MCB.01806-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hulten LM, Olson FJ, Aberg H, Carlsson J, Karlstrom L, Boren J, Fagerberg B, Wiklund O. 15-Lipoxygenase-2 is expressed in macrophages in human carotid plaques and regulated by hypoxia-inducible factor-1alpha. Eur J Clin Invest. 2009 doi: 10.1111/j.1365-2362.2009.02223.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee MJ, Wu Y, Fried SK. Adipose tissue remodeling in pathophysiology of obesity. Curr Opin Clin Nutr Metab Care. 2010;13:371–376. doi: 10.1097/MCO.0b013e32833aabef. [DOI] [PMC free article] [PubMed] [Google Scholar]