Abstract
Chediak-Higashi syndrome (CHS) is a rare, autosomal recessive disorder characterized by oculocutaneous albinism, recurrent bacterial infections and progressive neurological dysfunction. We demonstrate novel heterogenous mutations of CHS1, the responsive gene of CHS, identified in five Japanese patients with CHS. Patients 1, 2, and 3 were siblings, and they had albinism of the skin and hair. They all had a heterogenous two-base deletion (c.5541-5542 del AA, p.Q1847fsX1850) in exon 18. Patient 4 had a heterogenous single-base insertion (c.3944-3945 ins C, p.T1315fsX1331) in exon 10. The patient exhibited severe early-onset phenotype and suffered from hemophagocytic lymphohistiocytosis. Patient 5 had two heterogenous nonsense mutations; c.7982C>G, p.S2661X in exon 30 and c.8281A>T, p.R2761X in exon 31. The patient suffered from infections in childhood and had visual disturbance and albinism of the skin and hair. The CHS1 mutations described here have not been reported previously.
1. Introduction
Chediak-Higashi syndrome (CHS; MIM 214500) is an autosomal recessive disorder characterized by oculocutaneous albinism, increased susceptibility to pyogenic infections, defective natural killer (NK) activity, delayed bactericidal activity of neutrophils, and the presence of giant lysosomes in many cell types [1–3]. We previously reported that abnormally downregulated protein kinase C activity is responsible for the impaired cellular functions of polymorphonuclear leukocytes, fibroblasts and NK cells of CHS mice and patients [4–9]. The manifestation of CHS may result from defective trafficking of proteins into late multivesicular endosomes [10]. Most CHS patients die young due to a lymphoproliferative histiocytosis called the accelerated phase unless they undergo bone marrow transplantation.
The genetic defect resulting in CHS was identified in 1996 [11, 12]. The human gene, CHS1, was also called LYST (Lysosomal Trafficking Regulator). A similar disorder has been identified in beige mice and many other mammalian species. Human CHS patients and beige mice have homologous disorders associated with the CHS1 mutation [11–13]. CHS1 consists of 51 coding exons with an open reading frame of 11,406 bp [12]. The CHS1 protein is cytosolic and is composed of 3801 amino acids with a molecular weight of 429 kDa. It is known that CHS1 has a pleckstrin homology domain, a BEACH domain, and WD-40 repeats in the C-terminal region [14]. While the exact function of the CHS1 protein has not been elucidated, the protein suggested to regulate lysosomal size or lysosomal fission and affect cellular events, such as those of nuclear phosphatidylinositol-4, 5-bisphosphate [15, 16].
Thus far, 31 mutations in the CHS1 gene have been reported, including frameshift, nonsense, and missense mutations [17–21]. Only five Japanese CHS patients have been examined to date. Two patients had a one-base substitution, and one patient had a deletion, whereas no mutation of the CHS1 gene was detected in the other two patients [17]. Thus, we attempted to examine the mutations in other Japanese CHS patients. Here, we report novel heterogenous mutations of the CHS1 gene identified in Japanese patients with CHS.
2. Patients and Methods
Informed consent for this study was obtained from the patients or their parents. The study protocol was approved by the Ethics Committee of the University of Yamanashi.
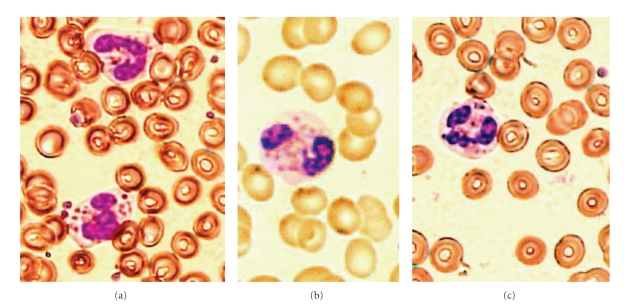
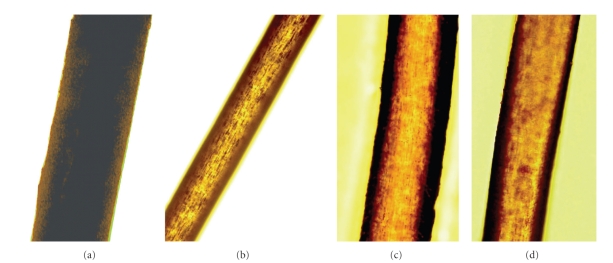
Patients 1, 2, and 3 (siblings) were 23-year-old male, 20-year-old female, and 17-year-old female, respectively. Giant granules were observed in polymorphonuclear cells from these three patients (Figure 1). They all had albinism of the skin and hair (Figure 2). However, there was no history of severe infection in any patient. Their parents were normal and healthy.
Figure 1.
Wright stain of peripheral blood smear of CHS patients, showing polymorphonuclear leukocytes with giant granules (200×). (a) Patient 1, (b) patient 2, and (c) patient 3.
Figure 2.
Hair shafts under light microscopy (200×). (a) Hair sample of normal Japanese control, showing evenly distributed pigment. (b) Patient 1, (c) Patient 2, (d) Patient 3. Hair shafts of CHS patients showing atypical granular pigment pattern.
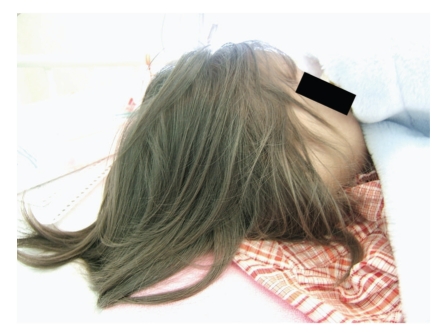
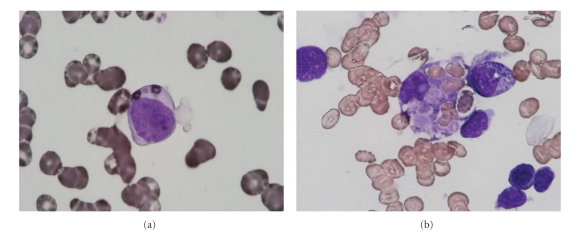
Patient 4 was a 6-year-old female with visual disturbance and hypopigmentation of the skin and hair (Figure 3). The diagnosis of CHS was determined by the presence of myeloperoxidase-positive giant granules in leukocytes (Figure 4(a)). At the age of 4, she suffered from hemophagocytic lymphohistiocytosis (Figure 4(b)), which is known as accelerated phase. At the time of the study, she had high fever, bleeding tendency, hepatosplenomegaly, and pancytopeny. She had low NK activity and decreased bactericidal activity of neutrophils. In addition, she had been treated with cyclosporine and steroids and then underwent bone marrow transplantation.
Figure 3.
Hair and skin of patient 4 at the age of 4, showing silvery hair and hypopigmentaion of skin.
Figure 4.
Bone marrow aspirate smear of patient 4, showing (a) myeloperoxidase-positive giant granules and (b) hemophagocytosis.
Patient 5 was a 21-year-old female who suffered from recurrent infections in childhood. At the time of the study, she had visual disturbance and neurological dysfunction including gait disturbance. Detailed information on this patient was not available.
Peripheral blood samples were obtained from five CHS patients who had not undergone bone marrow transplantation. Blood samples from parents of patients 1, 2, and 3 were also obtained. Genomic DNA was obtained from blood samples. Extraction of DNA was performed using the QIAamp DNA Blood kit (QIAGEN Inc., Valencia, CA, USA) according to the manufacturer's protocols. Polymerase chain reaction (PCR) primer pairs (25–35 bp) designed from intron sequences flanking each exon were used to amplify genomic DNA segments spanning each exon. For example, exons 9-10 were amplified using the F primer 5′-ATTTTTGCCACTAGATCTTCTAAATG-3′, and R primer was 5′-AGAAGCCATTATTATCAACTTTTCAC-3′. The F primer of exon 18 was 5′-TGCTACTGGCCACTAAGGTTGTGTGTC-3′, and R primer was 5′- GACTTTGATGACGAGATGAGTATCACTGC-3′. The F primer of exon 30 was 5′-CATTGTATCTATTACATCTAATACACCTGATACAC-3′, and R primer was 5′-ACGTATAATACAGTCAACATAAAACCTCTATTTCC-3′. PCR was performed using KOD-Plus-Ver. 2 according to the manufacturer's protocols (TOYOBO, Tokyo, Japan). The PCR products (220–3600 bp) were separated by electrophoresis on agarose gels. DNA was isolated from each band using the QIAquick Gel Extraction kit (QIAGEN Inc.). Direct sequencing using dye termination cycle sequencing was performed at FASMAC Co., Ltd. (Atsugi City, Kanagawa, Japan). The mutations were analyzed using Sequence Scanner Ver. 1 (Applied Biosystems, Tokyo, Japan) and were also checked at the cDNA level. For this purpose, total RNA was extracted from blood samples using Isogen-LS (Nippon Gene Co., Ltd., Tokyo, Japan) according to the manufacturer's protocols. cDNA was synthesized using PrimeScript reverse transcriptase (Takara Bio. Inc., Shiga, Japan), and PCR was performed as described above.
3. Molecular Analysis
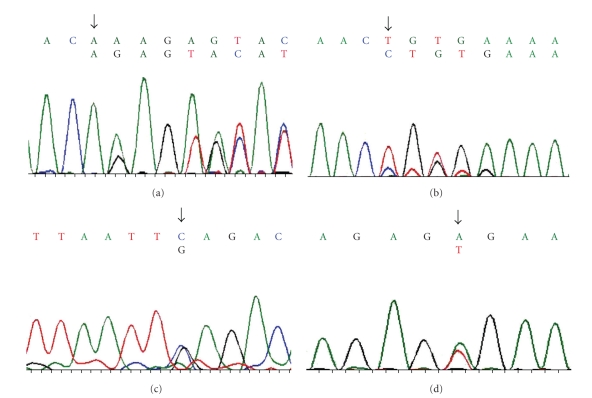
All 51 exons of the CHS1 gene of the five patients from three families were sequenced, and four patterns of novel heterogenous mutations were identified. In patients 1, 2, and 3, a two-base deletion (c.5541-5542 del AA) in exon 18 resulted in a frameshift mutation that eventually led to the formation of a stop codon (p.Q1847fsX1850) (Figure 5(a)). The second mutation was not found in the coding exons. The same heterogenous mutation was detected in their father. In their mother, no mutation was found in the CHS1 exons. Since their parents had no symptoms, it was possible that the second mutation lies in the intron sequence or splice mutation site. It was also possible that this mutation shows a mild phenotype associated with heterozygosity. Another possibility is the mutation in another gene, which affects the generation of lysosome-related organelles.
Figure 5.
The cDNA sequence patterns of CHS1 mutations. (a) Patients 1, 2, and 3: frameshift mutation in exon 18, c.5541-5542delAA, p. Q1847fsX1850. These three patients were siblings. The same sequence pattern of cDNA was observed in their father. (b) patient 4: Frameshift mutation in exon 10, c.3944-3945 ins C, p.T1315fsX1331. (c) patient 5: Nonsense mutation in exon 30, c.7982 C > G, p.S2661X. (d) patient 5: Nonesense mutation in exon 31, c.8281 A > T, p.R2761X.
In patient 4, we detected a heterogenous one-base (C) insertion (c.3944-3945 ins C) in exon 10, resulting in a frameshift mutation that led to the formation of a stop codon (p.T1315fsX1331) (Figure 5(b)). The second mutation was not found in any coding exon. The blood samples of her parents were not available.
In patient 5, two heterogenous mutations were identified; C-G substitution (c.7982 C > G) in exon 30 resulted in a nonsense mutation (p.S2661X) (Figure 5(c)), and A-T substitution (c.8281A > T) in exon 31 resulted in a nonsense mutation (p.R2761X) (Figure 5(d)). Blood samples of her parents were not available.
4. Discussion
The sequence pattern of CHS1 mutations described here has not been reported previously. All mutations were predicted to halt production of the complete CHS1 protein. Karim et al. [17] demonstrated that missense mutant alleles that likely encode CHS1 polypeptide with partial function were found in adolescent and adult forms of CHS, whereas functionally null mutant CHS1 alleles were found in case with severe childhood CHS. Westbroek et al. [19] also demonstrated that cellular defects in CHS correlate with the molecular genotype and clinical phenotype. In the present study, patient 4 and 5 exhibited early-onset CHS and were predicted to have a truncated CHS1 protein. Although the other three patients (patients 1, 2, and 3) were also predicted to have the truncated protein, they had clinically milder forms of CHS. In these patients, only single mutation in the CHS1 coding exons was detected. Since these patients were relatively healthy, it is possible that the mutant protein is acting as a dominant negative, resulting in a mild phenotype. In addition, we cannot exclude a possibility that the second mutation lies in the intron sequence or splice mutation site. However, the real reasons remain unknown, as we could not examine protein levels in these patients. In the previous report [17], no mutation in the CHS1 gene was found in 10 CHS patients, and only single mutations were found in 4 CHS patients. These findings suggest a possibility that the mutation lies in a gene other than CHS1, which affects the generation of lysosome-related organelles. In patient 5, two heterogenous nonsense mutations were identified. Recently, two heterogenous nonesense mutations in the CHS1 gene were reported in an African-American patient [20]. In that report, the patient exhibited severe childhood CHS. These findings support that the functionally null CHS1 mutant alleles are detected in severe childhood CHS.
In Japanese CHS patients, only three mutations have been identified so far, whereas no mutation in the CHS1 gene was found in two other patients [17]. We have described four patterns of novel mutations that are expected to result in a truncated CHS1 protein. However, the relationship between these mutations and the phenotype remains to be resolved. Examination of a large number of CHS patients will be required to clarify the genotype-phenotype correlation.
References
- 1.Ward DM, Shiflett SL, Kaplan J. Chediak-Higashi syndrome: a clinical and molecular view of a rare lysosomal storage disorder. Current Molecular Medicine. 2002;2(5):469–477. doi: 10.2174/1566524023362339. [DOI] [PubMed] [Google Scholar]
- 2.Shiflett SL, Kaplan J, Ward DM. Chediak-Higashi syndrome: a rare disorder of lysosomes and lysosome related organelles. Pigment Cell Research. 2002;15(4):251–257. doi: 10.1034/j.1600-0749.2002.02038.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan J, De Domenico I, Ward DM. Chediak-Higashi syndrome. Current Opinion in Hematology. 2008;15(1):22–29. doi: 10.1097/MOH.0b013e3282f2bcce. [DOI] [PubMed] [Google Scholar]
- 4.Ito M, Sato A, Tanabe F, Ishida E, Takami Y, Shigeta S. The thiol proteinase inhibitors improve the abnormal rapid down-regulation of protein kinase C and the impaired natural killer cell activity in (Chediak-Higashi syndrome) beige mouse. Biochemical and Biophysical Research Communications. 1989;160(2):433–440. doi: 10.1016/0006-291x(89)92451-0. [DOI] [PubMed] [Google Scholar]
- 5.Tanabe F, Cui SH, Ito M. Abnormal down-regulation of PKC is responsible for giant granule formation in fibroblasts from CHS (beige) mice—a thiol proteinase inhibitor, E-64-d, prevents giant granule formation in beige fibroblasts. Journal of Leukocyte Biology. 2000;67(5):749–755. doi: 10.1002/jlb.67.5.749. [DOI] [PubMed] [Google Scholar]
- 6.Sato A, Tanabe F, Ito M, Ishida E, Shigeta S. Thiol proteinase inhibitors reverse the increased protein kinase C down-regulation and concanavalin A cap formation in polymorphonuclear leukocytes from Chediak-Higashi syndrome (beige) mouse. Journal of Leukocyte Biology. 1990;48(5):377–381. doi: 10.1002/jlb.48.5.377. [DOI] [PubMed] [Google Scholar]
- 7.Cui SH, Tanabe F, Terunuma H, et al. A thiol proteinase inhibitor, E-64-d, corrects the abnormalities in concanavalin A cap formation and the lysosomal enzyme activity in leucocytes from patients with Chediak-Higashi syndrome by reversing the down-regulated protein kinase C activity. Clinical and Experimental Immunology. 2001;125(2):283–290. doi: 10.1046/j.1365-2249.2001.01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morimoto M, Tanabe F, Kasai H, Ito M. Effect of a thiol proteinase inhibitor, E-64-d, on susceptibility to infection with Staphylococcus aureus in Chediak-Higashi syndrome (beige) mice. International Immunopharmacology. 2007;7(7):973–980. doi: 10.1016/j.intimp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe F, Kasai H, He L, et al. Improvement of deficient natural killer activity and delayed bactericidal activity by a thiol proteinase inhibitor, E-64-d, in leukocytes from Chediak-Higashi syndrome patients in vitro. International Immunopharmacology. 2009;9(3):366–370. doi: 10.1016/j.intimp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Faigle W, Raposo G, Tenza D, et al. Deficient peptide loading and MHC class II endosomal sorting in a human genetic immunodeficiency diseasethe Chediak-Higashi syndrome. Journal of Cell Biology. 1998;141(5):1121–1134. doi: 10.1083/jcb.141.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbosa MDFS, Nguyen QA, Tchernev VT, et al. Identification of the homologous beige and Chediak-Higashi syndrome genes. Nature. 1996;382(6588):262–265. doi: 10.1038/382262a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagle DL, Karim MA, Woolf EA, et al. Identification and mutation analysis of the complete gene for Chediak-Higashi syndrome. Nature Genetics. 1996;14(3):307–311. doi: 10.1038/ng1196-307. [DOI] [PubMed] [Google Scholar]
- 13.Barbosa MDFS, Barrat FJ, Tchernev VT, et al. Identification of mutations in two major mRNA isoforms of the Chediak-Higashi syndrome gene in human and mouse. Human Molecular Genetics. 1997;6(7):1091–1098. doi: 10.1093/hmg/6.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jogl G, Shen Y, Gebauer D, et al. Crystal structure of the BEACH domain reveals an unusual fold and extensive association with a novel PH domain. EMBO Journal. 2002;21(18):4785–4795. doi: 10.1093/emboj/cdf502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perou CM, Leslie JD, Green W, Liangtao Li H, Ward DM, Kaplan J. The Beige/Chediak-Higashi syndrome gene encodes a widely expressed cytosolic protein. Journal of Biological Chemistry. 1997;272(47):29790–29794. doi: 10.1074/jbc.272.47.29790. [DOI] [PubMed] [Google Scholar]
- 16.Ward DM, Shiflett SL, Huynh D, Vaughn MB, Prestwich G, Kaplan J. Use of expression constructs to dissect the functional domains of the CHS/beige protein: identification of multiple phenotypes. Traffic. 2003;4(6):403–415. doi: 10.1034/j.1600-0854.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 17.Karim MA, Suzuki K, Fukai et al. F. Apparent genotype-phenotype correlation in childhood, adolescent, and adult Chediak-Higashi syndrome. American Journal of Medical Genetics. 2002;108(1):16–22. [PubMed] [Google Scholar]
- 18.Zarzour W, Kleta R, Frangoul H, et al. Two novel CHS1 (LYST) mutations: clinical correlations in an infant with Chediak-Higashi syndrome. Molecular Genetics and Metabolism. 2005;85(2):125–132. doi: 10.1016/j.ymgme.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Westbroek W, Adams D, Huizing M, et al. Cellular defects in Chediak-Higashi syndrome correlate with the molecular genotype and clinical phenotype. Journal of Investigative Dermatology. 2007;127(11):2674–2677. doi: 10.1038/sj.jid.5700899. [DOI] [PubMed] [Google Scholar]
- 20.Morrone K, Wang Y, Huizing M, et al. Two novel mutations identified in an African-American child with Chediak-Higashi syndrome. Case Reports in Medicine. 2010;2010:4 pages. doi: 10.1155/2010/967535. Article ID 967535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manoli I, Golas G, Westbroek W, et al. Chediak-Higashi syndrome with early developmental delay resulting from paternal heterodisomy of chromosome. American Journal of Medical Genetics A. 2010;152(6):1474–1483. doi: 10.1002/ajmg.a.33389. [DOI] [PMC free article] [PubMed] [Google Scholar]