Abstract
Obesity, the metabolic syndrome, and aging share several pathogenic features in both humans and non-human primates, including insulin resistance and inflammation. Since muscle and liver are considered key integrators of metabolism, we sought to determine in biopsies from lean and obese aging rhesus monkeys the nature of defects in insulin activation and, further, the potential for mitigation of such defects by an in vivo insulin sensitizer, rosiglitazone, and a thiazolidinedione activator of the peroxisome proliferator-activated receptor gamma. The peroxisome proliferator-activated receptor gamma agonist reduced hyperinsulinemia, improved insulin sensitivity, lowered plasma triglycerides and free fatty acids, and increased plasma adiponectin. In muscle of obese monkeys, previously shown to exhibit defective insulin signaling, the insulin sensitizer improved insulin activation of atypical protein kinase C (aPKC), the defective direct activation of aPKC by phosphatidylinositol (PI)-3,4,5-(PO4)3, and 5′-AMP-activated protein kinase and increased carnitine palmitoyltransferase-1 mRNA expression, but it did not improve insulin activation of insulin receptor substrate (IRS)-1-dependent PI 3-kinase (IRS-1/PI3K), protein kinase B, or glycogen synthase. We found that, although insulin signaling was impaired in muscle, insulin activation of IRS-1/PI3K, IRS-2/PI3K, protein kinase B, and aPKC was largely intact in liver and that rosiglitazone improved insulin signaling to aPKC in muscle by improving responsiveness to PI-3,4,5-(PO4)3. Antioxid. Redox Signal. 14, 207–219.
Introduction
The pathogenesis of metabolic syndrome clearly involves alterations in the intracellular signaling cascades that are thought to underlie insulin resistance and to be specifically involved in diseases of aging (22). These diseases of aging are very similar in humans and in rhesus monkeys; the middle-aged onset of metabolic syndrome includes obesity, dyslipidemia, and impaired glucose tolerance, often progressing to overt type 2 diabetes mellitus (8, 9, 21–23, 32, 33, 67). Rhesus monkeys and humans have highly similar metabolism (17, 42, 46, 68) and genomic features (20, 50). Diabetes and insulin resistance in monkeys and other non-human primates are likely to have the same mechanistic causes as underlie diabetes development in overweight middle-aged humans (11). Further, monkey insulin is identical to human insulin (51). Thus, this model is ideal for examining the nature of insulin signaling and insulin action, particularly in less accessible tissues that are difficult to obtain in humans under both basal and insulin-stimulated conditions, such as is true for liver. Insulin resistance seems to underlie the early stages in the development of the metabolic syndrome; and, thus, approaches to improving insulin action have been and remain key targets for potentially slowing or ultimately preventing type 2 diabetes (14, 18, 24).
Rhesus monkeys are also ideally suited to the examination of mechanisms of action of insulin-sensitizing agents, specifically where altered pathways may differ across different organs and at different stages of the disease progression. Such agents have been shown to have similar effects at the whole body level in humans and monkeys (16, 30, 52, 58). Prominent among the agents targeting insulin action are the peroxisome proliferator-activated receptor (PPAR) agonists and partial agonists including the thiazolidinediones (TZDs), such as rosiglitazone (RSGZ) and pioglitazone. Both are considered to be useful insulin-sensitizing agents for treating type 2 diabetes mellitus, and these, as well as other PPAR agonists, have been shown to be effective in humans and rhesus monkeys (10, 16, 30, 52, 56, 58, 86). The mechanisms by which they exert their insulin sensitizing effects are, however, only partially understood. The therapeutic effects of TZDs are thought to result from activation of PPARγ receptors primarily located in adipose tissue (26), and possibly to a lesser degree, but of still uncertain significance, in muscle and liver. Nevertheless, TZDs and other PPAR agonists improve whole body insulin sensitivity and insulin signaling, not only in adipocytes [e.g., (28, 66)] but also in muscle of rats (27), rhesus monkeys (58), and humans (6, 34). In conjunction with improved insulin signaling, TZDs and other partial and full PPAR agonists improve insulin-stimulated glucose transport in isolated adipocytes (28) and muscle preparations (90). Moreover, insulin-stimulated glucose disposal rates increase in subjects with type 2 diabetes after TZD treatment [e.g., (6, 34, 58)], among many), reflecting improved muscle uptake and metabolism of glucose.
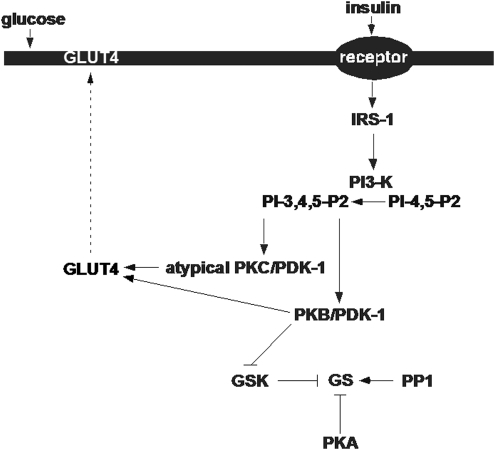
Most metabolic processes that are regulated by insulin in muscle, adipocytes, and liver appear to involve changes in the activity of protein phosphatases and of protein kinases, activities which may be altered by oxidative stress or intracellular redox imbalance. Insulin action at these organs involves the activation of insulin receptor substrate (IRS)-1-dependent and/or IRS-2-dependent phosphatidylinositol (PI) 3-kinase (PI3K), which, in turn, activate atypical protein kinase Cs (aPKCs) and protein kinase B ([PKB]/Akt). Both aPKC (2–5, 39, 69, 75) and PKB (1, 25, 38, 79, 85) appear to be required for insulin-stimulated glucose transport in muscle and adipocytes. Further, PKB, but not aPKC, is also required for insulin effects on glycogen synthesis in muscle, adipocytes, and liver and possibly other factors that limit hepatic glucose output, at least in rodents (12, 15, 65, 74) (Fig. 1). On the other hand, aPKCs appear to be important for mediating insulin effects on lipid synthesis in mouse liver (44, 78).
FIG. 1.
Model of insulin signaling in skeletal muscle. IRS-1, insulin receptor substrate-1; PI 3-K, phosphatidylinositol 3-kinase; PDK-1, 3-phosphoinositide dependent protein kinase-1; PKB, protein kinase B; GSK, glycogen synthase kinase; GS, glycogen synthase; PP1, protein phosphatase-1; PKA, protein kinase A; GLUT 4, glucose transporter 4.
From studies of the knockout of genes encoding IRS-1 or IRS-2 in mice, multiple tissue-specific differences in mechanisms used by insulin to activate aPKCs and PKB have become apparent between muscle, liver, and adipose tissue. In muscle, the activations of both aPKCs (71) and PKB (71, 81) are dependent on IRS-1-dependent PI3K rather than IRS-2-dependent PI3K; in liver, however, PKB activation is dependent on both IRS-1-dependent (71, 81) and IRS-2-dependent (82) PI3K, whereas aPKC activation is dependent on IRS-2-dependent (72, 82) but not on IRS-1-dependent (71) PI3K; in adipocytes, aPKC activation is dependent on both IRS-1-dependent and IRS-2-dependent PI3K, whereas PKB activation is not compromised by deficiency of either IRS-1 or IRS-2 (49, 71).
In rodent models, these insulin-induced activities have frequently been shown to differ between muscle and liver. For example, the activation of aPKC in muscle is consistently diminished in a variety of insulin-resistant conditions (6, 7, 27, 29, 35, 37, 76, 80), whereas aPKC activation in liver is maintained or increased (77). PKB activation in muscle is either maintained (6, 36, 76, 83) or diminished (40) and in liver either maintained or impaired (77) depending on the insulin-resistant state or animal model. Thus, there are divergent alterations in aPKC and PKB activation that seem to be, in part, due to alterations in IRS-1- and IRS-2-dependent PI3K. Accordingly, in high-fat-fed mice, an obesity-like model, insulin activation of IRS-1-dependent but not IRS-2-dependent PI3K is defective, thus explaining defects in aPKC and PKB activation in muscle; in contrast, in liver, insulin activation of both IRS-1- and IRS-2-dependent PI3K is intact, thus explaining the conserved activation of both aPKC and PKB (70, 71). In this high fat diet fed rodent model, there are defects in activation of aPKCs, with or without defects in PKB activation, in skeletal muscle, but apparently normal insulin signaling to IRS-1- and IRS-2-dependent PI3K, aPKCs, and PKB in liver (77). Further, in another insulin-resistant model, the GK-diabetic rat, insulin activation of IRS-1-dependent, but not IRS-2-dependent PI3K is defective in muscle, thus explaining the defect in aPKC activation (27, 72, 73, 77). Similarly, in liver of the GK rat, insulin activation of IRS-1-dependent PI3K is defective, thus explaining the defect in PKB activation, but, in contrast, insulin activation of IRS-2-dependent PI3K is intact, thus explaining conserved aPKC activation (73, 77). Of further note, in GK-diabetic rats, TZDs improve aPKC activation in muscle (27, 29, 34) but do not alter aPKC or PKB activation in liver (77).
In muscle and adipose tissue of type 2 diabetic rats (27–29) and humans (6, 27), the mechanism whereby TZDs improve insulin-stimulated aPKC activation in the absence of alterations in PI3K and PKB activation is enigmatic. However, the ability of aPKCs to directly respond to the lipid product of PI3K, namely, PI-3,4,5-(PO4)3 (PIP3), is impaired in muscles of obese and type 2 diabetic rhesus monkeys (76) and humans (6, 7) as well as in muscles of high-fat-fed rats and mice (27, 29) and diabetic mice (73). The possibility that TZDs may alter muscle aPKC responsiveness to PIP3, particularly in the absence of changes in IRS-1-dependent PI3K, has not been previously examined.
With regard to more naturally occurring forms of obesity, much less is known about alterations in insulin signaling in various tissues. Defective aPKC activation occurs in muscle of obese humans (7, 35) and monkeys (76) and in isolated adipocytes and myocytes of obese humans (70, 83). However, there is little information on insulin signaling in liver of spontaneously occurring forms of obesity, before the development of overt diabetes.
Here, we have examined insulin signaling in the liver of normal monkeys with or without insulin stimulation, for comparison to our previous study of insulin signaling in muscle. We also determined the effects of a TZD on mechanisms of insulin action in muscle of monkeys who spontaneously develop obesity while consuming standard chow ad libitum. Such obese monkeys have been previously shown to have defects in insulin action at skeletal muscle, specifically in the activation of IRS-1-dependent PI3K, PKB, aPKC (76), glycogen synthase (GS) (61), and protein phosphatase-1 (55) and in the inactivation of protein kinase A (53). We have previously found that in obese monkeys, insulin action on liver GS and glycogen phosphorylase is intact (57), although, as in muscle, liver triglyceride is higher in obese than in lean monkeys (64). It was, therefore, interesting to find that, whereas the insulin activation of IRS-1-dependent PI3K, PKB, aPKC and GS is impaired in muscle, the activation of all examined insulin signaling factors, namely, IRS-1-dependent PI3K, IRS-2-dependent PI3K, PKB, and aPKC, was not significantly compromised in liver. Due to the absence of demonstrably impaired signaling in liver, insulin signaling pathway alterations in response to whole body insulin sensitizing was pursued only in muscle. In muscle, the TZD, RSGZ, a PPARγ agonist, selectively improved aPKC activation without altering defects in the activation of IRS-1-dependent PI3K, PKB, or GS in muscle. Interestingly, the improvement in muscle aPKC activation was, at least, partly due to improved responsiveness of aPKCs to PIP3.
Research Design, Materials, and Methods
Subjects
Fifteen adult male rhesus monkeys (Macaca mulatta) were studied. All were bred in the United States and were of Indian origin. The monkeys were individually housed, and consistent care was provided according to the Guide for the Care and Use of Laboratory Animals of the ILAR/NAS 1996, including attention to environmental enrichment. Food intake was daily monitored, and body weight was weekly or biweekly monitored throughout the study. Blood and tissue samples were always obtained after a consistent 16 h overnight fast. Sedation for all blood draws and experimental procedures was accomplished with ketamine hydrochloride (10–15 mg/kg body weight) with supplemental ketamine, ∼5–10 mg/kg, given as needed at 20–30 min intervals during the procedures. All protocols were approved by the Institutional Animal Care and Use Committee.
Ten of these 15 monkeys were normal, studied to determine the normal action of insulin at the liver compared with muscle. This group consisted of mature adults (∼7 years old) that were lean (9.1 ± 0.5 kg body weight and 9.5 ± 1.5% body fat) and had normal fasting plasma glucose (FPG) (3.1 ± 0.1 mM) and normal insulin (300 ± 30 pM) levels. These monkeys also had normal chemistry and hematology values at the start of the study. Here we report the results of analysis of insulin signaling in the liver of these normal monkeys. We have previously reported the analysis of liver GS, glycogen phosphorylase (62), protein phosphatase-1, protein phosphatase-2C, protein kinase A, GS kinase-3, and PKC (54) regulation by insulin in these normal animals.
Five additional monkeys were both obese (body weights >12 kg) and insulin-resistant (glucose disposal rates under euglycemic clamp conditions of <8.0 mg/kg/FFM/min). These obese monkeys were prediabetic (FPG 3.3–7.0 mM) and were studied both before and at the end of treatment with the TZD, RSGZ (a PPARγ agonist), (kindly supplied by GlaxoSmithKline). RSGZ was administered orally at three doses (0.03, 0.1, 0.3 mg/kg body weight/day) for 4 weeks at each dose, a total of 12 weeks of treatment, preceded by vehicle and followed by washout periods. Blood samples were biweekly obtained, and tissue samples of muscle were obtained at the end of the 4-week period of administration of vehicle, and again at the end of the 12-week TZD treatment period. Blood and tissue samples were also obtained under maximally insulin-stimulated conditions during an euglycemic hyperinsulinemic clamp before and at the end of the treatment. Blood samples were analyzed for FPG, insulin, nonesterified fatty acids, adiponectin (human adiponectin RIA kit; Linco), triglycerides, very low-density lipoprotein (VLDL) and high-density lipoprotein lipid fractions, and routine clinical chemistry and hematology.
Procedures
Euglycemic hyperinsulinemic clamps to measure whole body insulin sensitivity were conducted after an overnight fast and included obtaining of skeletal muscle (vastus lateralis) and liver biopsies both basally and at maximal insulin stimulation at the end of the clamp period. Anesthesia was maintained with fentanyl citrate (0.01 mg/kg). Succinylcholine (1 mg/kg) was initially used followed by vecuronium bromide for muscle relaxation (0.1 mg/kg) and diazepam (2.5 mg/dose). The open biopsies of muscle and liver were obtained just before initiation of the insulin infusion and during steady-state insulin infusion (90–120 min after the onset of the insulin infusion), as previously described (61, 62). In normal monkeys, we determined the activities of multiple muscle and liver enzymes (see below) before (under basal conditions) and after maximal insulin stimulation and identified the differences in insulin signaling between these two target organs. In RSGZ-treated monkeys, the euglycemic hyperinsulinemic clamps were conducted just before the start of the initial dosing and at the end of the final highest dosing period after 12 weeks of RSGZ treatment. Maximal insulin-stimulated whole-body glucose disposal rate (M) was estimated using an insulin infusion rate of 400 mU/m2/min with maintenance of glucose at 4.7 pM using a variable rate infusion of 20% dextrose. M was corrected for fat-free mass determined by the tritiated water dilution method.
All tissues samples were rapidly frozen in situ (skeletal muscle) or immediately ex situ (liver) using aluminum clamps cooled in liquid nitrogen. Tissue samples were lyophilized and stored in liquid nitrogen until enzyme assays were performed.
Enzyme and substrate assays
Muscle and liver tissue samples were homogenized in appropriate buffers (76). Atypical PKC activity was measured as described (76). In brief, aPKCs, ζ, λ, and ι, were immunoprecipitated from cell lysates with a rabbit polyclonal antiserum (Santa Cruz Biotechnologies, Inc.) that recognizes the nearly identical C-termini of PKC-ζ and PKC-λ/ι [The individual aPKCs apparently function interchangeably during insulin-stimulated glucose transport (2–5, 39, 75)]. Precipitates were collected on Sepharose-AG beads and incubated for 8 min at 30°C in 100 μl buffer containing 50 mM Tris/HCl (pH 7.5), 100 μM Na3VO4, 100 μM Na4 P2O7, 1 mM NaF, 100 μM PMSF, 4 μg phosphatidylserine (Sigma), 50 μM (γ-32P) ATP (NEN Life Science Products), 5 mM MgCl2, and, as substrate, 40 μM serine analog of the PKC-ɛ pseudosubstrate (BioSource), a preferred substrate for aPKCs. In some assays, 10M PIP3 (Matreya), a maximally effective concentration, was added to activate aPKCs (6, 27, 29, 76). After incubation, 32P-labeled substrate was trapped on P-81 filter paper and counted.
PKB enzyme activity was measured using a kit obtained from Upstate Biotechnologies Inc. (UBI), as previously described (76). In brief, PKBα was immunoprecipitated with sheep polyclonal anti-PKBα antiserum (UBI), collected on Sepharose-AG beads, and assayed as per kit directions. PKB activation was also assessed by immunoblotting for phosphorylation of serine-473 (76, 77).
Immunoprecipitable IRS-1- and IRS-2-dependent PI3K activities were determined as previously described (76, 77) (rabbit polyclonal antisera for IRS-1 and IRS-2 were purchased from UBI). Autoradiographic results of chromatographically purified PI-3-32PO4, the lipid product of the PI3K assays, were quantified in a BioRad Phosphor-Imager, and finally expressed relative to the control samples developed on the same thin layer chromatography plate.
Immunoprecipitable 5′-AMP-activated protein kinase (AMPK) (combined 1 and 2) activity in muscle lysates was measured with the method of Wojtaszewski et al. (87) using rabbit polyclonal antiserum (Cell Signaling Technologies) and SAMS peptide (UBI) as substrate.
GS activity, glycogen, and glucose 6-phosphate (G6P) content were measured in lyophilized, micro dissected skeletal muscle as we have described (59, 63). We have previously reported these for liver.
Lipid was isolated from skeletal muscle using a modified method of Folch. Twenty milligram of lyophilized, micro dissected sample was homogenized in 4 ml chloroform:methanol (2:1) and rotated overnight in a glass vial. The following day, 2 ml of 0.6% NaCl was added to the vial, vortexed, and centrifuged for 10 min @ 2000 × g (4°C). The lower phase was removed and placed into a clean glass vial. After complete evaporation, 250 μL of 100% ethanol was added to the dried sample. Triglyceride content was determined by using an enzymatic kit (Glycerol; r-biopharm) in conjunction with the enzymes esterase and lipase (Sigma). Triglyceride content was expressed as nmol/mg dry tissue weight.
In all assays, comparisons between baseline and RSGZ-treated samples from the same monkey, or between samples from normal lean controls and obese monkeys, were made with samples that were simultaneously assayed.
Carnitine palmitoyltransferase-1 mRNA expression in skeletal muscle with and without insulin stimulation before and during TZD treatment
Total RNA was extracted from 20 mg lyophilized muscle obtained before and during the euglycemic hyperinsulinemic clamp during vehicle administration and at the end of the 12 weeks of RSGZ administration and processed as described (56). Muscle carnitine palmitoyltransferase-1 (CPT-1) mRNA expression was determined by real-time reverse transcription–polymerase chain reaction expressed relative to the housekeeping ribosomal gene 36B4 and based on concentrations from standard curves. Taqman probes (Assays on Demand) for this gene were purchased from Applied Biosystems (CPT-1 assay ID Hs00992651 g1) and used in the LightCycler (Roche Diagnostics) with the LightCycler FastStart DNA Master Hybridization Probe Kit. All samples were run in triplicate.
Western analyses
Lysate proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and immunoblotted as described (76, 77). Antibodies used for blotting included rabbit polyclonal anti-C-terminal PKC-ζ/λ/ι antiserum (Santa Cruz Biotechnologies, Inc.) (PKCs ζ and λ/ι have nearly identical C-termini that are recognized by this antiserum); sheep polyclonal anti-PKBα antiserum (UBI); rabbit polyclonal anti-IRS-1 and IRS-2 antisera (UBI); and rabbit polyclonal anti-phospho-ser-473-PKB antiserum (Cell Signaling). Immunoblots were quantified by measurement of chemiluminescence in a BioRad Molecular Analyst Chemiluminescence/Phosphorescence Imaging System or by lazer scanning densitometry.
Statistical methods
Data are expressed as mean ± standard error of the mean. Means were compared by Student's t-test for paired or unpaired samples. Pearson's correlation coefficient was used to test for significant linear relationships between variables.
Results
Assessment of regulation of insulin signaling in liver of lean and obese insulin-resistant monkeys
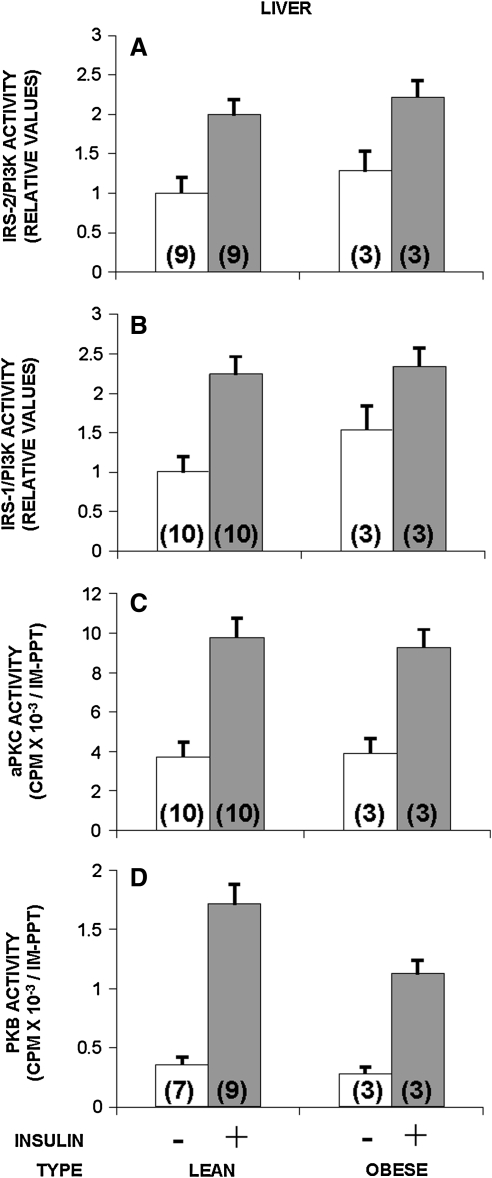
In the present study, we compared insulin signaling in the liver of obese monkeys with that of lean control monkeys and compared hepatic insulin signaling with our previous study of muscle insulin signaling in lean and obese monkeys (76). Unexpectedly, in view of the significant differences in insulin signaling at muscle in lean versus obese monkeys, in liver there were no obesity associated changes. Comparing basal activity with activity during the maximal hyperinsulinemic stimulation of a euglycemic clamp, the activities of several kinases involved in insulin signaling were equally increased in activity in lean and obese monkeys. As shown in Figure 2, there were no apparent differences in the activation by insulin of either IRS-2-dependent PI3K (Fig. 2A) or IRS-1-dependent PI3K (Fig. 2B). Thus, it appears that the initial steps of insulin signaling are not compromised in liver of obese monkeys compared with lean animals.
FIG. 2.
Comparison of insulin signaling to IRS-2-dependent PI3K (A), IRS-1-dependent PI3K (B), aPKCs (C), and PKB (D), and in liver of lean versus obese monkeys before (−) and during (+) the insulin stimulation of a euglycemic hyperinsulinemic clamp. Basal values are shown by clear bars, and insulin-stimulated values are shown by shaded bars. The number of determinations is shown in parentheses. In the liver, there were no significant differences between the effects of insulin in lean versus obese (insulin-resistant) monkeys (all p's > 0.05). aPKC, atypical protein kinase C.
As with IRS-2-dependent PI3K, which presumably functions upstream of aPKC in liver (71, 82), the activation of aPKC by insulin was essentially the same in liver of lean and obese monkeys (Fig. 2C).
The enzymatic activation of PKB by insulin, which appears to be dependent on both IRS-1- and IRS-2-dependent PI3K in liver (71, 81, 82), tended downward, but not significantly, in the liver of obese monkeys, relative to that seen in lean monkeys (Fig. 2D).
Effects of PPARγagonist RSGZ on in vivo metabolic parameters
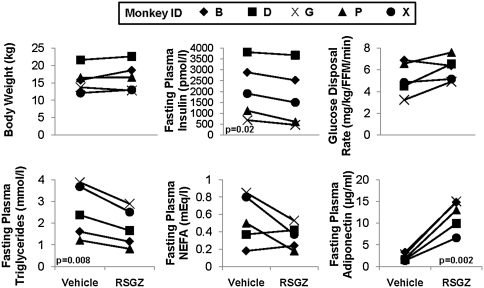
Treatment of obese insulin-resistant prediabetic monkeys with the TZD insulin sensitizer, RSGZ, over a 12 week escalating dose regimen reaching a maximal dose of 0.3 mg/kg in the final 4 weeks, resulted in an average body weight gain of 5%. Amount of weight gain widely ranged from 0% to 8% over 3 months (mean + 4.5%; p = n.s.; Fig. 3A), including a gain of 1% to 3% during 1 month at the highest dose (∼threefold human recommended dose levels; [p = n.s.]). Fasting plasma insulin levels were consistently reduced in all monkeys by an average of 16% (p = 0.02) in response to RSGZ treatment (Fig. 3B). Whole body insulin sensitivity increased by 17%, with four of five monkeys showing an improvement in insulin sensitivity under RSGZ treatment (Fig. 3C). The decline in fasting insulin was significantly negatively correlated with the improvement in insulin sensitivity. Plasma triglyceride levels significantly decreased (p = 0.008), with those having the highest initial triglyceride levels showing the greatest reduction in response to RSGZ (Fig. 3D); VLDL triglycerides declined at an average of 0.34 ± 0.12 mM (p < 0.05) (not shown). Fasting plasma nonesterified fatty acids decreased from elevated levels (>400 mEq/L) in three monkeys but were unchanged in the two monkeys in whom initial pretreatment levels were normal (Fig. 3E). Plasma adiponectin level increases were highly consistent across monkeys and were highly significant (p = 0.002, Fig. 3F). FPG levels were unchanged in the four monkeys that were normoglycemic at study initiation (64 vs. 70 mg/dl start to end of study) and slightly increased in the early diabetic monkey (not shown).
FIG. 3.
Alterations in body weight (upper left), fasting plasma levels of insulin (upper middle), and insulin-mediated glucose disposal rate (whole body insulin sensitivity) (upper right), and of fasting triglycerides (lower left), NEFA (lower middle), and adiponectin levels (lower right) after RSGZ treatment of obese insulin-resistant monkeys. RSGZ, rosiglitazone; NEFA, nonesterified fatty acid.
Effects of RSGZ on insulin signaling and insulin action in skeletal muscle
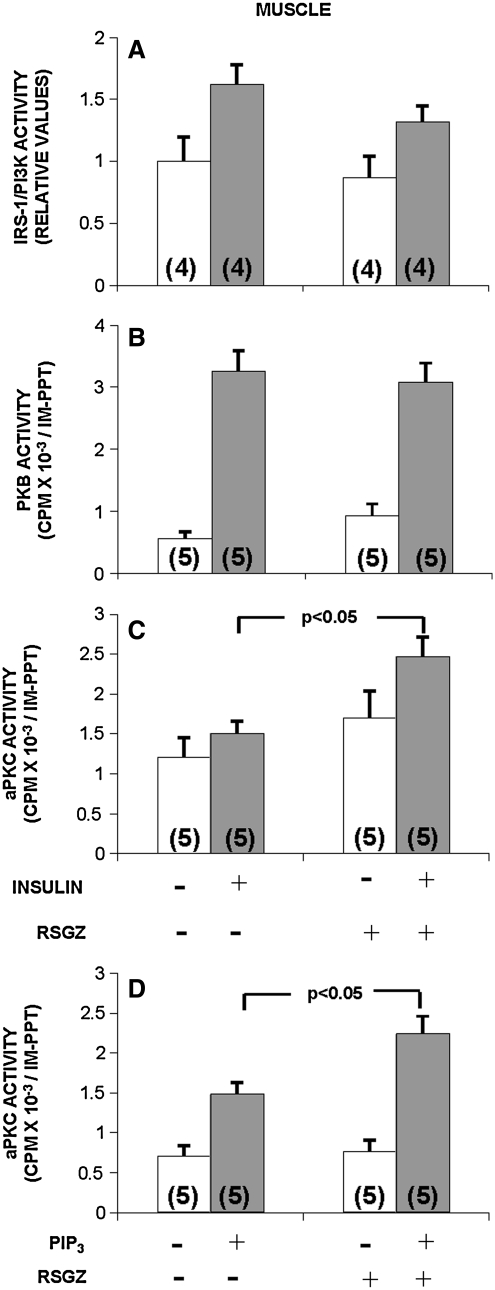
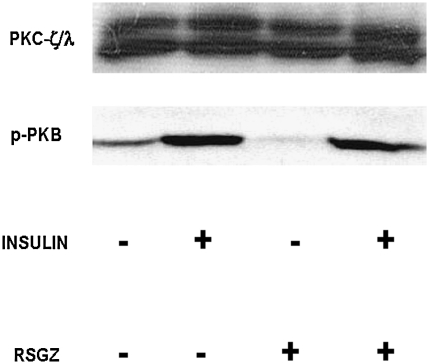
During euglycemic hyperinsulinemic clamp studies to examine whole body insulin sensitivity, we previously identified defects in the in vivo insulin activation in muscle of IRS-1-dependent PI3K, PKB, aPKC (76), and GS (61) (vastus lateralis) in obese monkeys. In the present study, we found that relative to pretreatment values, administration of RSGZ had no effect on the basal or insulin-stimulated activities of IRS-1/PI3K (Fig. 4A) in muscle. In keeping with the failure to observe alterations in IRS-1/PI3K, RSGZ did not affect the enzymatic activation of PKB by insulin in muscle of obese monkeys (Fig. 4B). In addition, RSGZ failed to alter the phosphorylation of serine-473 in PKB (Fig. 5).
FIG. 4.
Effects of RSGZ on basal and insulin-stimulated (A–C) or PIP3-stimulated (D) activation of IRS-1-dependent PI3K (A), PKB (B), and aPKCs (C and D) in muscle of obese monkeys. Euglycemic clamps with muscle biopsies were performed before and at the end of 3 months of RSGZ treatment. Basal values are shown by clear bars, and insulin-stimulated values are shown by shaded bars. Note that PIP3 was added only to assays of aPKCs immunoprecipitated from basal (noninsulin-treated) muscle. The number of determinations is shown in parentheses. RSGZ significantly increased aPKC activity (p < 0.05) and in basal (noninsulin-stimulated) samples RSGZ enhanced the response to PIP3 (p < 0.05). PIP3, PI-3,4,5-(PO4)3; RSGZ, rosiglitazone.
FIG. 5.
Failure of RSGZ to alter aPKC levels or insulin effects on serine-473 phosphorylation of PKB in muscle of obese monkeys. Shown here are representative blots from two monkeys.
By contrast, RSGZ treatment significantly improved the activation of aPKCs by insulin (p < 0.05, Fig. 4C), although, as seen in Figure 5, RSGZ did not alter protein expression of aPKCs in muscle.
In this study, we did not examine IRS-2-dependent PI3K activation, as other studies have suggested that in muscle the activation of aPKC and PKB are dependent on IRS-1 rather than on IRS-2 (71, 77) (also found in unpublished observations in IRS 2-knockout mice).
In view of the failure to observe an increase in insulin-stimulated IRS-1-dependent PI3K activation following RSGZ treatment, we examined the ability of muscle aPKC to respond to PIP3, the lipid product of PI3K. As seen in Figure 4D (shaded bars), aPKCs, immunoprecipitated from muscle of monkeys treated with RSGZ, responded significantly more effectively to PIP3 than aPKCs immunoprecipitated from muscle obtained prior to such treatment (p < 0.05). (Note that PIP3 was added only to aPKCs immunoprecipitated from muscle under basal conditions, not stimulated by insulin.) It, therefore, seems reasonable to suggest that this improvement in aPKC responsiveness to PIP3 importantly contributed to the increase in aPKC activation observed in response to insulin administration during the hyperinsulinemic clamp procedure (Fig. 4C).
In contrast to aPKC, but in keeping with a failure to see alterations in IRS-1-dependent PI3K and PKB, in these insulin-resistant monkeys, neither did RSGZ, compared with vehicle, increase the already impaired activation of GS by insulin (Maximal insulin-stimulated GS fractional activity during vehicle: 16.4% ± 3.7% vs. during RSGZ treatment: 17.5% ± 3.2%) nor did treatment affect GS independent activity or total GS activity. There was also no significant effect of RSGZ on muscle G6P or glycogen content. Muscle triglyceride content was slightly, but not significantly, lower during RSGZ treatment (vehicle; 73 ± 21 vs. RSGZ 50 ± 14 nmol/mg dry weight, n.s.). Nevertheless, the monkeys with the highest basal skeletal muscle triglyceride content before RSGZ treatment had the greatest decrease in basal triglyceride content after RSGZ treatment (r = −0.85, p < 0.05).
Effects of RSGZ on AMPK activity and CPT-1 gene expression in muscle
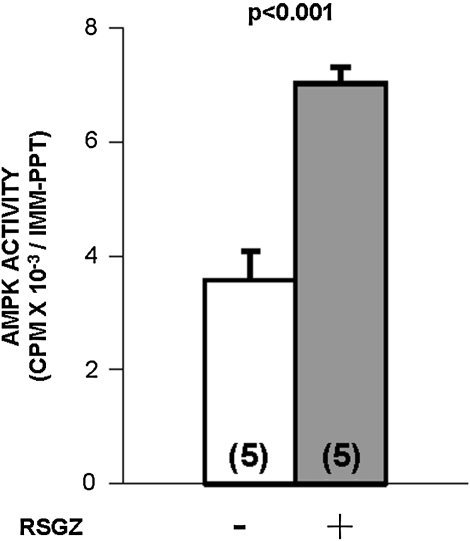
The failure to see an effect of RSGZ on IRS-1-dependent PI3K, and, on the other hand, the ability to see an effect of RSGZ on aPKC responsiveness to PIP3, prompted us to examine whether factors that may influence the intracellular metabolism of lipids (that conceivably may down-regulate aPKC activity in conditions of obesity) might be involved in RSGZ actions in muscle. One such factor is AMPK, which has been shown to be activated both by RSGZ itself (19) and by adiponectin (88). Similar to the robust effect of RSGZ treatment on plasma adiponectin, RSGZ treatment provoked significant increases in basal fasting AMPK activity in muscle of obese monkeys (Fig. 6).
FIG. 6.
Treatment of obese monkeys with RSGZ significantly increased AMPK activity in muscle (p < 0.001). The number of determinations is shown in parentheses. AMPK, 5′-AMP-activated protein kinase.
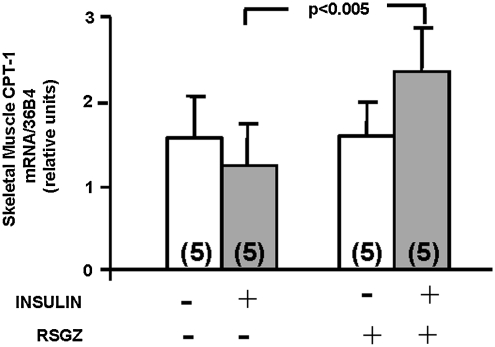
Another important factor that affects the intramyocellular metabolism of lipids, specifically long-chain acyl CoAs (LCA-CoAs), is CPT-1, the gene that controls fatty acid mitochondrial β-oxidation (48). Similar to the response of aPKC to in vivo insulin after RSGZ treatment (Fig. 4), CPT-1 mRNA expression in muscle during the maximal insulin stimulation of an euglycemic hyperinsulinemic clamp was significantly higher after RSGZ treatment compared with vehicle (Fig. 7).
FIG. 7.
Effect of RSGZ to increase CPT-1 mRNA gene expression during the euglycemic hyperinsulinemic clamp in muscle of obese monkeys. The number of determinations is shown in parentheses. CPT-1, carnitine palmitoyltransferase-1.
Discussion
Insulin resistance and obesity, accompanied by dyslipidemia, have been well documented in rhesus monkeys with metabolic syndrome (8, 9, 17, 22, 23, 32, 33, 41, 43, 67, 68, 84). Calorie restriction and calorie restriction mimetic agents have been demonstrated in rhesus monkeys to reverse or prevent these metabolic disorders (13, 21, 24, 42, 45, 46, 50, 60, 64). From previous findings in obese insulin-resistant monkeys (55, 57, 61, 76) and the present study, it seems clear that, whereas insulin signaling to IRS-1-dependent PI3K, PKB, aPKC, and GS is compromised in muscle of obese monkeys relative to lean healthy control monkeys (63, 76) and humans (7, 19, 35, 36, 40, 83, 87, 88), such signaling to each of these factors, and to IRS-2-dependent PI3K, is largely intact in liver of obese monkeys (57, 62). This pattern of compromised insulin signaling in muscle, coupled with conserved insulin signaling in liver, presently observed in obese insulin-resistant monkeys, is similar to the signaling pattern observed in muscle and liver of high-fat-fed mice (72, 77) These findings of differential apparent effects of insulin insensitivity in muscle and liver are in accord with our previous identification of insulin resistance (as measured by the euglycemic hyperinsulinemic clamp) as an early event in the progression from normal through sequential phases to overt diabetes (9), occurring in obese normoglycemic, normal glucose tolerant monkeys. Since glucose uptake rate under insulin stimulation is thought to principally reflect uptake by muscle, muscle insulin resistance is an early event, developing in the earliest phases of this diabetes trajectory (9, 53, 55, 61). By contrast, failure of insulin to suppress hepatic glucose production is a very late event in this progression, occurring in direct relationship to developing hyperglycemia (9, 62). Thus, this pattern would be in full accord with the absence of defects in insulin signaling at the liver in obese prediabetic monkeys (9). Thus, the development of impaired insulin action at the liver appears to be a late event in the progression to overt diabetes.
Taken together, the present and previous findings (9, 44, 57, 61, 62, 64, 71–73, 77, 78, 82) suggest that insulin signaling mechanisms in liver are initially largely intact in simple obesity and deteriorate only with the development of overt diabetes, selectively impairing IRS-1-dependent PI3K and PKB but sparing IRS-2-dependent PI3K and aPKCs (71, 77). The initial conservation of hepatic PKB activation contributes to the maintenance of relatively normal glucose tolerance in obesity, as PKB is important in regulating glycogen synthesis (65, 71), gluconeogenesis (15), and glucose release (74). The conservation of hepatic aPKC activation in both primate obesity (present results) and diabetic rodent models (72, 77), on the other hand, contributes to the maintenance of insulin-dependent synthesis and release of VLDL-triglycerides, as lipid synthesis effects of insulin in liver are thought to be largely mediated via aPKCs (44) and presumably IRS-2-dependent PI3K, which functions upstream of aPKCs in hepatocytes (82). Indeed, the development of hyperinsulinemia together with defects in insulin signaling in skeletal muscle, coupled with intact insulin action on IRS-2-dependent PI3K and aPKCs in liver, would be expected to lead to increased hepatic synthesis and release of VLDL-triglycerides, as seen in obese monkeys (17).
The reason insulin signaling is impaired in muscle, while normal in liver of both obese monkeys and high-fat-fed mice, is not entirely clear. Recently, we have found in high-fat-fed mice that basal activities of both conventional and novel diacylglycerol-sensitive PKCs are increased in muscle but not in liver, despite marked hyperlipidemia and hepatosteatosis (unpublished observations). Accordingly, the presence of insulin signaling defects in muscle and their absence in liver may be related to activities of these conventional/novel PKCs in muscle. Studies of conventional and novel PKC activities in monkey tissues across the entire progression from normal lean to overtly diabetic status are needed to test this hypothesis.
Effects of TZD treatment on insulin action
In the present study, we did not evaluate insulin signaling in liver before and after RSGZ treatment, as there were no significant defects in insulin signaling in liver of obese monkeys. Specifically, in previous studies of liver of GK diabetic rats, RSGZ did not improve defects in IRS-1-dependent PI3K or PKB activation (77); also, in a preliminary study involving liver biopsies in insulin-resistant obese monkeys during another TZD treatment (R-102380; Sankyo, Inc.), there was additional evidence of no alteration in hepatic insulin signaling to IRS-1-dependent PI3K, IRS-2-dependent PI3K, PKB, aPKC, and GS, despite significant whole body insulin resistance (unpublished observations).
The mechanism for improved aPKC activation in muscle after RSGZ treatment is uncertain. Surprisingly, we found no evidence for improvement in the activation of IRS-1-dependent PI3K or PKB. On the other hand, muscle aPKC responsiveness to PIP3 was increased after RSGZ treatment, and this most likely contributed to improved aPKC activation during insulin stimulation.
RSGZ did not increase GS activity, glycogen, or G6P content in muscle during the euglycemic hyperinsulinemic clamp assessing whole body insulin resistance in these obese monkeys. In contrast, a different TZD, R-102380, increased insulin activation of muscle GS and increased G6P content during a clamp in insulin-resistant monkeys (58). This difference may reflect the relative strength of the insulin sensitizing effects of RSGZ (mild) versus R-102380 (profound). Similarly, a PPARα agonist, K-111, increased GS activity and glycogen content during a clamp in obese insulin-resistant monkeys (56). Whether the PPAR agonists R-102380 or K-111 increase insulin activation of IRS-1-dependent PI3K, PKC and/or PKB in muscle of obese monkeys has not yet been determined.
Despite improvement in insulin sensitivity at the whole body level, RSGZ did not fully repair the defect in muscle aPKC activation by insulin in obese monkeys. The fold increase in insulin-stimulated aPKC activity was relatively small in RSGZ-treated muscle of obese monkeys (about 50%), as compared with that seen in normal muscle, that increased ∼two to fourfold (76). A more complete repair of the defect in aPKC activation by insulin would obviously require an improvement in IRS-1-dependent PI3K as well as an increase in responsiveness of aPKC to PIP3. Whether PIP3 could fully normalize aPKC activation in RSGZ-treated muscle was not directly tested.
The mechanism whereby aPKC responsiveness to PIP3 was improved by RSGZ treatment is uncertain, but several factors should be considered. First, in rats high-fat feeding impairs aPKC responsiveness to PIP3 (27, 29), and it is reasonable to propose that RSGZ may improve the lipid environment within muscle cells and thereby restore aPKC activity or activation by PIP3. In this regard, TZDs activate AMPK (19), which increases fatty acid oxidation and diminishes fatty acid synthesis. TZDs also increase adiponectin secretion from adipose tissue, and this factor similarly activates AMPK and increases fatty acid oxidation in muscle (88). Second, decreases in plasma levels of free fatty acids and triglycerides may improve the lipid environment within muscle cells. Third, TZD-induced increases in CPT-1 gene expression in muscle may increase ß-oxidation of fatty acids and thereby diminish lipids. In this regard, although intramyocellular triglycerides were not affected by RSGZ treatment in diabetic humans (47), or in the obese monkeys in the present study, pioglitazone decreased triglyceride and LCA-CoA levels in basal muscle samples and further decreased LCA-CoA content during hyperinsulinemic clamps in high-fat-fed rats (89). In the present study, mean triglyceride content for the group was not significantly affected by RSGZ treatment; however, the monkeys with the highest basal triglyceride before RSGZ treatment had the greatest improvement in triglyceride content after RSGZ treatment.
In conjunction with alterations in insulin signaling to aPKC in muscle, RSGZ improved circulating VLDL-triglyceride levels of these obese monkeys. Presumably, this plasma lipid-lowering effect of RSGZ reflected increased lipid synthesis in adipose or other tissues, due to improved glucose uptake (to provide glycerol-3-PO4) and activation of lipid-clearing enzymes, and/or decreased hepatic lipid synthesis. Given that insulin signaling to IRS-2-dependent PI3K and aPKCs, and thus lipid synthesis, in liver would be expected to be increased in insulin-resistant states, such as in obese hyperinsulinemic monkeys, any decrease in hepatic lipid synthesis would most likely be due to enhanced insulin action on glucose transport in muscle, leading to diminished insulin resistance in association with decreased ambient circulating insulin levels. Further studies are needed to test these hypotheses.
Abbreviations Used
- AMPK
5′-AMP-activated protein kinase
- aPKC
atypical protein kinase C
- CPT-1
carnitine palmitoyltransferase-1
- FPG
fasting plasma glucose
- G6P
glucose 6-phosphate
- GS
glycogen synthase
- GSK-3
glycogen synthase kinase-3
- IRS-1/PI3K
insulin receptor substrate-1-dependent phosphatidylinositol 3-kinase
- LCA-CoA
long-chain acyl CoA
- NEFA
nonesterified fatty acid
- PIP3
PI-3,4,5-(PO4)3
- PKA
protein kinase A
- PKB
protein kinase B
- PP1
protein phosphatase-1
- PPARγ
peroxisome proliferators-activated receptor gamma
- RSGZ
rosiglitazone
- TZD
thiazolidinedione
- VLDL
very low-density lipoprotein
Acknowledgments
The authors thank Drs. Adamandia D. Kriketos, Hiroyoshi Horikoshi, and Shinji Yoshioka for invaluable scientific support and the following people for excellent technical and data management support: Theresa Alexander, Karen Brockelhurst, Wallace Evans, Jr., LaKeisha Galloway, Georgielle Gerzanich, Joseph Haney, Carol St. Clair, Susannah Watson, Aaron Wimberly, and Jennifer Newcomb.
Funding
Support was provided by the National Institutes of Health, National Institute on Aging NO1-AG-0-2100 (B.C.H., N.L.B., H.K.O.), and NIH HHSN26320800022C (B.C.H.) and RO1-AG-1-9310 (A.S.R.); Department of Veterans Affairs and Veterans Affairs Medical Center Baltimore Geriatric Research, Education and Clinical Center (GRECC) (H.K.O. and A.S.R.); The National Institute on Aging (NIA) Claude D. Pepper Older Americans Independence Center P30-AG028747 (H.K.O. and A.S.R.); The NIDDK Mid-Atlantic Nutrition Obesity Research Center (NIH P30 DK072488) (H.K.O. and A.S.R.); Veterans Administration Merit Review Program (R.V.F., M.L.S.); NIH DK-38079-09A1 (R.V.F.); and the New England Regional Primate Research Center grant P51RR00168-40. Funding for the RSGZ portion of this study was provided by GlaxoSmithKline to B.C.H.
Author Disclosure Statement
None of the authors have any competing financial interest, as confirmed by all authors.
References
- 1.Bae SS. Cho H. Mu J. Birnbaum MJ. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem. 2003;278:49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay G. Kanoh Y. Sajan MP. Standaert ML. Farese RV. Effects of adenoviral gene transfer of wild-type, constitutively active, and kinase-defective protein kinase C-lambda on insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 2000;141:4120–4127. doi: 10.1210/endo.141.11.7766. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay G. Sajan MP. Kanoh Y. Standaert ML. Quon MJ. Lea-Currie R. Sen A. Farese RV. PKC-zeta mediates insulin effects on glucose transport in cultured preadipocyte-derived human adipocytes. J Clin Endocrinol Metab. 2002;87:716–723. doi: 10.1210/jcem.87.2.8252. [DOI] [PubMed] [Google Scholar]
- 4.Bandyopadhyay G. Standaert ML. Galloway L. Moscat J. Farese RV. Evidence for involvement of protein kinase C (PKC)-zeta and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 1997;138:4721–4731. doi: 10.1210/endo.138.11.5473. [DOI] [PubMed] [Google Scholar]
- 5.Bandyopadhyay G. Standaert ML. Zhao L. Yu B. Avignon A. Galloway L. Karnam P. Moscat J. Farese RV. Activation of protein kinase C (alpha, beta, and zeta) by insulin in 3T3/L1 cells. Transfection studies suggest a role for PKC-zeta in glucose transport. J Biol Chem. 1997;272:2551–2558. doi: 10.1074/jbc.272.4.2551. [DOI] [PubMed] [Google Scholar]
- 6.Beeson M. Sajan MP. Dizon M. Grebenev D. Gomez-Daspet J. Miura A. Kanoh Y. Powe J. Bandyopadhyay G. Standaert ML. Farese RV. Activation of protein kinase C-zeta by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes. 2003;52:1926–1934. doi: 10.2337/diabetes.52.8.1926. [DOI] [PubMed] [Google Scholar]
- 7.Beeson M. Sajan MP. Gomez-Daspet J. Luna V. Dizon M. Grebenev D. Powe J. Lucudi S. Miura A. Kanoh Y. Bandyopadhyay G. Standaert ML. Yeko TR. Farese RV. Defective activation of protein kinase C-zeta in muscle by insulin and phosphatidylinositol-3,4,5-(PO4)3 in obesity and polycystic ovary syndrome. Metab Syndrome Relat Disord. 2004;2:49–56. doi: 10.1089/met.2004.2.49. [DOI] [PubMed] [Google Scholar]
- 8.Bodkin NL. Hannah JS. Ortmeyer HK. Hansen BC. Central obesity in rhesus monkeys: association with hyperinsulinemia, insulin resistance and hypertriglyceridemia? Int J Obes Relat Metab Disord. 1993;17:53–61. [PubMed] [Google Scholar]
- 9.Bodkin NL. Metzger BL. Hansen BC. Hepatic glucose production and insulin sensitivity preceding diabetes in monkeys. Am J Physiol. 1989;256:E676–E681. doi: 10.1152/ajpendo.1989.256.5.E676. [DOI] [PubMed] [Google Scholar]
- 10.Bodkin NL. Pill J. Meyer K. Hansen BC. The effects of K-111, a new insulin-sensitizer, on metabolic syndrome in obese prediabetic rhesus monkeys. Horm Metab Res. 2003;35:617–624. doi: 10.1055/s-2003-43510. [DOI] [PubMed] [Google Scholar]
- 11.Chavez AO. Lopez-Alvarenga JC. Tejero ME. Triplitt C. Bastarrachea RA. Sriwijitkamol A. Tantiwong P. Voruganti VS. Musi N. Comuzzie AG. DeFronzo RA. Folli F. Physiological and molecular determinants of insulin action in the baboon. Diabetes. 2008;57:899–908. doi: 10.2337/db07-0790. [DOI] [PubMed] [Google Scholar]
- 12.Cho H. Mu J. Kim JK. Thorvaldsen JL. Chu Q. Crenshaw EB., 3rd Kaestner KH. Bartolomei MS. Shulman GI. Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 13.Colman RJ. Anderson RM. Johnson SC. Kastman EK. Kosmatka KJ. Beasley TM. Allison DB. Cruzen C. Simmons HA. Kemnitz JW. Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cusi K. Maezono K. Osman A. Pendergrass M. Patti ME. Pratipanawatr T. DeFronzo RA. Kahn CR. Mandarino LJ. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105:311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daitoku H. Yamagata K. Matsuzaki H. Hatta M. Fukamizu A. Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes. 2003;52:642–649. doi: 10.2337/diabetes.52.3.642. [DOI] [PubMed] [Google Scholar]
- 16.Ding SY. Tigno XT. Braileanu GT. Ito K. Hansen BC. A novel peroxisome proliferator-activated receptor alpha/gamma dual agonist ameliorates dyslipidemia and insulin resistance in prediabetic rhesus monkeys. Metabolism. 2007;56:1334–1339. doi: 10.1016/j.metabol.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Ding SY. Tigno XT. Hansen BC. Nuclear magnetic resonance-determined lipoprotein abnormalities in nonhuman primates with the metabolic syndrome and type 2 diabetes mellitus. Metabolism. 2007;56:838–846. doi: 10.1016/j.metabol.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Folli F. Saad MJ. Backer JM. Kahn CR. Regulation of phosphatidylinositol 3-kinase activity in liver and muscle of animal models of insulin-resistant and insulin-deficient diabetes mellitus. J Clin Invest. 1993;92:1787–1794. doi: 10.1172/JCI116768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fryer LG. Parbu-Patel A. Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–25232. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs RA. Rogers J. Katze MG. Bumgarner R. Weinstock GM. Mardis ER. Remington KA. Strausberg RL. Venter JC. Wilson RK. Batzer MA. Bustamante CD. Eichler EE. Hahn MW. Hardison RC. Makova KD. Miller W. Milosavljevic A. Palermo RE. Siepel A. Sikela JM. Attaway T. Bell S. Bernard KE. Buhay CJ. Chandrabose MN. Dao M. Davis C. Delehaunty KD. Ding Y. Dinh HH. Dugan-Rocha S. Fulton LA. Gabisi RA. Garner TT. Godfrey J. Hawes AC. Hernandez J. Hines S. Holder M. Hume J. Jhangiani SN. Joshi V. Khan ZM. Kirkness EF. Cree A. Fowler RG. Lee S. Lewis LR. Li Z. Liu YS. Moore SM. Muzny D. Nazareth LV. Ngo DN. Okwuonu GO. Pai G. Parker D. Paul HA. Pfannkoch C. Pohl CS. Rogers YH. Ruiz SJ. Sabo A. Santibanez J. Schneider BW. Smith SM. Sodergren E. Svatek AF. Utterback TR. Vattathil S. Warren W. White CS. Chinwalla AT. Feng Y. Halpern AL. Hillier LW. Huang X. Minx P. Nelson JO. Pepin KH. Qin X. Sutton GG. Venter E. Walenz BP. Wallis JW. Worley KC. Yang SP. Jones SM. Marra MA. Rocchi M. Schein JE. Baertsch R. Clarke L. Csuros M. Glasscock J. Harris RA. Havlak P. Jackson AR. Jiang H, et al. (88 additional authors) Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 21.Gresl TA. Colman RJ. Havighurst TC. Byerley LO. Allison DB. Schoeller DA. Kemnitz JW. Insulin sensitivity and glucose effectiveness from three minimal models: effects of energy restriction and body fat in adult male rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1340–R1354. doi: 10.1152/ajpregu.00651.2002. [DOI] [PubMed] [Google Scholar]
- 22.Hansen BC. Chronomics of the metabolic syndrome. In: Hansen BC, editor; Bray GA, editor. Metabolic Syndrome: Its Epidemiology, Clinical Treatment, and Underlying Mechanisms. Philadelphia: Humana Press; 2007. pp. 373–386. [Google Scholar]
- 23.Hansen BC. Primate animal models of Type 2 diabetes. In: LeRoith D, editor; Olefsky JM, editor; Taylor S, editor. Diabetes mellitus: A Fundamental and Clinical Text. Philadelphia: Lippincott Williams and Wilkins; 2004. pp. 1060–1074. [Google Scholar]
- 24.Hansen BC. Bodkin NL. Primary prevention of diabetes mellitus by prevention of obesity in monkeys. Diabetes. 1993;42:1809–1814. doi: 10.2337/diab.42.12.1809. [DOI] [PubMed] [Google Scholar]
- 25.Hill MM. Clark SF. Tucker DF. Birnbaum MJ. James DE. Macaulay SL. A role for protein kinase Bbeta/Akt2 in insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol. 1999;19:7771–7781. doi: 10.1128/mcb.19.11.7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotta K. Gustafson TA. Yoshioka S. Ortmeyer HK. Bodkin NL. Hansen BC. Relationships of PPARgamma and PPARgamma2 mRNA levels to obesity, diabetes and hyperinsulinaemia in rhesus monkeys. Int J Obes Relat Metab Disord. 1998;22:1000–1010. doi: 10.1038/sj.ijo.0800718. [DOI] [PubMed] [Google Scholar]
- 27.Kanoh Y. Bandyopadhyay G. Sajan MP. Standaert ML. Farese RV. Rosiglitazone, insulin treatment, and fasting correct defective activation of protein kinase C-zeta/lambda by insulin in vastus lateralis muscles and adipocytes of diabetic rats. Endocrinology. 2001;142:1595–1605. doi: 10.1210/endo.142.4.8066. [DOI] [PubMed] [Google Scholar]
- 28.Kanoh Y. Bandyopadhyay G. Sajan MP. Standaert ML. Farese RV. Thiazolidinedione treatment enhances insulin effects on protein kinase C-zeta/lambda activation and glucose transport in adipocytes of nondiabetic and Goto-Kakizaki type II diabetic rats. J Biol Chem. 2000;275:16690–16696. doi: 10.1074/jbc.M000287200. [DOI] [PubMed] [Google Scholar]
- 29.Kanoh Y. Sajan MP. Bandyopadhyay G. Miura A. Standaert ML. Farese RV. Defective activation of atypical protein kinase C zeta and lambda by insulin and phosphatidylinositol-3,4,5-(PO4)(3) in skeletal muscle of rats following high-fat feeding and streptozotocin-induced diabetes. Endocrinology. 2003;144:947–954. doi: 10.1210/en.2002-221017. [DOI] [PubMed] [Google Scholar]
- 30.Kemnitz JW. Elson DF. Roecker EB. Baum ST. Bergman RN. Meglasson MD. Pioglitazone increases insulin sensitivity, reduces blood glucose, insulin, and lipid levels, and lowers blood pressure, in obese, insulin-resistant rhesus monkeys. Diabetes. 1994;43:204–211. doi: 10.2337/diab.43.2.204. [DOI] [PubMed] [Google Scholar]
- 31. This reference has been deleted.
- 32.Kemnitz JW. Francken GA. Characteristics of spontaneous obesity in male rhesus monkeys. Physiol Behav. 1986;38:477–483. doi: 10.1016/0031-9384(86)90414-2. [DOI] [PubMed] [Google Scholar]
- 33.Kemnitz JW. Goy RW. Flitsch TJ. Lohmiller JJ. Robinson JA. Obesity in male and female rhesus monkeys: fat distribution, glucoregulation, and serum androgen levels. J Clin Endocrinol Metab. 1989;69:287–293. doi: 10.1210/jcem-69-2-287. [DOI] [PubMed] [Google Scholar]
- 34.Kim YB. Ciaraldi TP. Kong A. Kim D. Chu N. Mohideen P. Mudaliar S. Henry RR. Kahn BB. Troglitazone but not metformin restores insulin-stimulated phosphoinositide 3-kinase activity and increases p110beta protein levels in skeletal muscle of type 2 diabetic subjects. Diabetes. 2002;51:443–448. doi: 10.2337/diabetes.51.2.443. [DOI] [PubMed] [Google Scholar]
- 35.Kim YB. Kotani K. Ciaraldi TP. Henry RR. Kahn BB. Insulin-stimulated protein kinase C lambda/zeta activity is reduced in skeletal muscle of humans with obesity and type 2 diabetes: reversal with weight reduction. Diabetes. 2003;52:1935–1942. doi: 10.2337/diabetes.52.8.1935. [DOI] [PubMed] [Google Scholar]
- 36.Kim YB. Nikoulina SE. Ciaraldi TP. Henry RR. Kahn BB. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J Clin Invest. 1999;104:733–741. doi: 10.1172/JCI6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim YB. Shulman GI. Kahn BB. Fatty acid infusion selectively impairs insulin action on Akt1 and protein kinase C lambda/zeta but not on glycogen synthase kinase-3. J Biol Chem. 2002;277:32915–32922. doi: 10.1074/jbc.M204710200. [DOI] [PubMed] [Google Scholar]
- 38.Kohn AD. Summers SA. Birnbaum MJ. Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 39.Kotani K. Ogawa W. Matsumoto M. Kitamura T. Sakaue H. Hino Y. Miyake K. Sano W. Akimoto K. Ohno S. Kasuga M. Requirement of atypical protein kinase clambda for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krook A. Roth RA. Jiang XJ. Zierath JR. Wallberg-Henriksson H. Insulin-stimulated Akt kinase activity is reduced in skeletal muscle from NIDDM subjects. Diabetes. 1998;47:1281–1286. doi: 10.2337/diab.47.8.1281. [DOI] [PubMed] [Google Scholar]
- 41.Lane MA. Nonhuman primate models in biogerontology. Exp Gerontol. 2000;35:533–541. doi: 10.1016/s0531-5565(00)00102-9. [DOI] [PubMed] [Google Scholar]
- 42.Lane MA. Ingram DK. Roth GS. Calorie restriction in nonhuman primates: effects on diabetes and cardiovascular disease risk. Toxicol Sci. 1999;52:41–48. doi: 10.1093/toxsci/52.2.41. [DOI] [PubMed] [Google Scholar]
- 43.Litwak KN. Cefalu WT. Wagner JD. Chronic hyperglycemia increases arterial low-density lipoprotein metabolism and atherosclerosis in cynomolgus monkeys. Metabolism. 1998;47:947–954. doi: 10.1016/s0026-0495(98)90349-3. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto M. Ogawa W. Akimoto K. Inoue H. Miyake K. Furukawa K. Hayashi Y. Iguchi H. Matsuki Y. Hiramatsu R. Shimano H. Yamada N. Ohno S. Kasuga M. Noda T. PKClambda in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest. 2003;112:935–944. doi: 10.1172/JCI18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattison JA. Lane MA. Roth GS. Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 46.Mattison JA. Roth GS. Lane MA. Ingram DK. Dietary restriction in aging nonhuman primates. Interdiscip Top Gerontol. 2007;35:137–158. doi: 10.1159/000096560. [DOI] [PubMed] [Google Scholar]
- 47.Mayerson AB. Hundal RS. Dufour S. Lebon V. Befroy D. Cline GW. Enocksson S. Inzucchi SE. Shulman GI. Petersen KF. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McGarry JD. Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 49.Miura A. Sajan MP. Standaert ML. Bandyopadhyay G. Kahn CR. Farese RV. Insulin substrates 1 and 2 are corequired for activation of atypical protein kinase C and Cbl-dependent phosphatidylinositol 3-kinase during insulin action in immortalized brown adipocytes. Biochemistry. 2004;43:15503–15509. doi: 10.1021/bi049221y. [DOI] [PubMed] [Google Scholar]
- 50.Moore CM. Dunn BG. McMahan CA. Lane MA. Roth GS. Ingram DK. Mattison JA. Effects of calorie restriction on chromosomal stability in rhesus monkeys (Macaca mulatta) Age (Dordr) 2007;29:15–28. doi: 10.1007/s11357-006-9016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Naithani VK. Steffens GJ. Tager HS. Buse G. Rubenstein AH. Steiner DF. Isolation and amino-acid sequence determination of monkey insulin and proinsulin. Hoppe Seylers Z Physiol Chem. 1984;365:571–575. doi: 10.1515/bchm2.1984.365.1.571. [DOI] [PubMed] [Google Scholar]
- 52.Oliver WR., Jr. Shenk JL. Snaith MR. Russell CS. Plunket KD. Bodkin NL. Lewis MC. Winegar DA. Sznaidman ML. Lambert MH. Xu HE. Sternbach DD. Kliewer SA. Hansen BC. Willson TM. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci USA. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ortmeyer HK. Insulin decreases skeletal muscle cAMP-dependent protein kinase (PKA) activity in normal monkeys and increases PKA activity in insulin-resistant rhesus monkeys. J Basic Clin Physiol Pharmacol. 1997;8:223–235. doi: 10.1515/jbcpp.1997.8.4.223. [DOI] [PubMed] [Google Scholar]
- 54.Ortmeyer HK. Insulin increases liver protein phosphatase-1 and protein phosphatase-2C activities in lean, young adult rhesus monkeys. Horm Metab Res. 1998;30:705–710. doi: 10.1055/s-2007-978963. [DOI] [PubMed] [Google Scholar]
- 55.Ortmeyer HK. Insulin resistance in skeletal muscle: a role for impaired insulin activation of glycogen synthase. In: Zierath J, editor; Walleberg-Henriksson H, editor. Muscle Metabolism. New York: Taylor and Francis; 2002. pp. 285–295. [Google Scholar]
- 56.Ortmeyer HK. Adall Y. Marciani KR. Katsiaras A. Ryan AS. Bodkin NL. Hansen BC. Skeletal muscle glycogen synthase subcellular localization: effects of insulin and PPAR-alpha agonist (K-111) administration in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1509–R1517. doi: 10.1152/ajpregu.00692.2004. [DOI] [PubMed] [Google Scholar]
- 57.Ortmeyer HK. Bodkin NL. Lack of defect in insulin action on hepatic glycogen synthase and phosphorylase in insulin-resistant monkeys. Am J Physiol. 1998;274:G1005–G1010. doi: 10.1152/ajpgi.1998.274.6.G1005. [DOI] [PubMed] [Google Scholar]
- 58.Ortmeyer HK. Bodkin NL. Haney J. Yoshioka S. Horikoshi H. Hansen BC. A thiazolidinedione improves in vivo insulin action on skeletal muscle glycogen synthase in insulin-resistant monkeys. Int J Exp Diabetes Res. 2000;1:195–202. doi: 10.1155/EDR.2000.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ortmeyer HK. Bodkin NL. Hansen BC. Adipose tissue glycogen synthase activation by in vivo insulin in spontaneously insulin-resistant and type 2 (non-insulin-dependent) diabetic rhesus monkeys. Diabetologia. 1993;36:200–206. doi: 10.1007/BF00399950. [DOI] [PubMed] [Google Scholar]
- 60.Ortmeyer HK. Bodkin NL. Hansen BC. Chronic calorie restriction alters glycogen metabolism in rhesus monkeys. Obes Res. 1994;2:549–555. doi: 10.1002/j.1550-8528.1994.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 61.Ortmeyer HK. Bodkin NL. Hansen BC. Insulin-mediated glycogen synthase activity in muscle of spontaneously insulin-resistant and diabetic rhesus monkeys. Am J Physiol. 1993;265:R552–R558. doi: 10.1152/ajpregu.1993.265.3.R552. [DOI] [PubMed] [Google Scholar]
- 62.Ortmeyer HK. Bodkin NL. Hansen BC. Insulin regulates liver glycogen synthase and glycogen phosphorylase activity reciprocally in rhesus monkeys. Am J Physiol. 1997;272:E133–E138. doi: 10.1152/ajpendo.1997.272.1.E133. [DOI] [PubMed] [Google Scholar]
- 63.Ortmeyer HK. Bodkin NL. Hansen BC. Relationship of skeletal muscle glucose 6-phosphate to glucose disposal rate and glycogen synthase activity in insulin-resistant and non-insulin-dependent diabetic rhesus monkeys. Diabetologia. 1994;37:127–133. doi: 10.1007/s001250050082. [DOI] [PubMed] [Google Scholar]
- 64.Ortmeyer HK. Collins CA. Tison A. Goldberg AP. Hansen BC. Chronic calorie restriction prevents obesity-associated increases in liver triglyceride (TG), but not age-related increases in muscle TG. FASEB J. 2004;18:A95. [Google Scholar]
- 65.Peak M. Rochford JJ. Borthwick AC. Yeaman SJ. Agius L. Signalling pathways involved in the stimulation of glycogen synthesis by insulin in rat hepatocytes. Diabetologia. 1998;41:16–25. doi: 10.1007/s001250050861. [DOI] [PubMed] [Google Scholar]
- 66.Peraldi P. Xu M. Spiegelman BM. Thiazolidinediones block tumor necrosis factor-alpha-induced inhibition of insulin signaling. J Clin Invest. 1997;100:1863–1869. doi: 10.1172/JCI119715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raman A. Colman RJ. Cheng Y. Kemnitz JW. Baum ST. Weindruch R. Schoeller DA. Reference body composition in adult rhesus monkeys: glucoregulatory and anthropometric indices. J Gerontol A Biol Sci Med Sci. 2005;60:1518–1524. doi: 10.1093/gerona/60.12.1518. [DOI] [PubMed] [Google Scholar]
- 68.Roth GS. Mattison JA. Ottinger MA. Chachich ME. Lane MA. Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004;305:1423–1426. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- 69.Sajan MP. Rivas J. Li P. Standaert ML. Farese RV. Repletion of atypical protein kinase C following RNA interference-mediated depletion restores insulin-stimulated glucose transport. J Biol Chem. 2006;281:17466–17473. doi: 10.1074/jbc.M510803200. [DOI] [PubMed] [Google Scholar]
- 70.Sajan MP. Standaert ML. Miura A. Bandyopadhyay G. Vollenweider P. Franklin DM. Lea-Currie R. Farese RV. Impaired activation of protein kinase C-zeta by insulin and phosphatidylinositol-3,4,5-(PO4)3 in cultured preadipocyte-derived adipocytes and myotubes of obese subjects. J Clin Endocrinol Metab. 2004;89:3994–3998. doi: 10.1210/jc.2004-0106. [DOI] [PubMed] [Google Scholar]
- 71.Sajan MP. Standaert ML. Miura A. Kahn CR. Farese RV. Tissue-specific differences in activation of atypical protein kinase C and protein kinase B in muscle, liver, and adipocytes of insulin receptor substrate-1 knockout mice. Mol Endocrinol. 2004;18:2513–2521. doi: 10.1210/me.2004-0045. [DOI] [PubMed] [Google Scholar]
- 72.Sajan MP. Standaert ML. Nimal S. Varanasi U. Pastoor T. Mastorides S. Braun U. Leitges M. Farese RV. The critical role of atypical protein kinase C in activating hepatic SREBP-1c and NFkappaB in obesity. J Lipid Res. 2009;50:1133–1145. doi: 10.1194/jlr.M800520-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sajan MP. Standaert ML. Rivas J. Miura A. Kanoh Y. Soto J. Taniguchi CM. Kahn CR. Farese RV. Role of atypical protein kinase C in activation of sterol regulatory element binding protein-1c and nuclear factor kappa B (NFkappaB) in liver of rodents used as a model of diabetes, and relationships to hyperlipidaemia and insulin resistance. Diabetologia. 2009;52:1197–1207. doi: 10.1007/s00125-009-1336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmoll D. Walker KS. Alessi DR. Grempler R. Burchell A. Guo S. Walther R. Unterman TG. Regulation of glucose-6-phosphatase gene expression by protein kinase Balpha and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. J Biol Chem. 2000;275:36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- 75.Standaert ML. Galloway L. Karnam P. Bandyopadhyay G. Moscat J. Farese RV. Protein kinase C-zeta as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J Biol Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 76.Standaert ML. Ortmeyer HK. Sajan MP. Kanoh Y. Bandyopadhyay G. Hansen BC. Farese RV. Skeletal muscle insulin resistance in obesity-associated type 2 diabetes in monkeys is linked to a defect in insulin activation of protein kinase C-zeta/lambda/iota. Diabetes. 2002;51:2936–2943. doi: 10.2337/diabetes.51.10.2936. [DOI] [PubMed] [Google Scholar]
- 77.Standaert ML. Sajan MP. Miura A. Kanoh Y. Chen HC. Farese RV., Jr. Farese RV. Insulin-induced activation of atypical protein kinase C, but not protein kinase B, is maintained in diabetic (ob/ob and Goto-Kakazaki) liver. Contrasting insulin signaling patterns in liver versus muscle define phenotypes of type 2 diabetic and high fat-induced insulin-resistant states. J Biol Chem. 2004;279:24929–24934. doi: 10.1074/jbc.M402440200. [DOI] [PubMed] [Google Scholar]
- 78.Taniguchi CM. Kondo T. Sajan M. Luo J. Bronson R. Asano T. Farese R. Cantley LC. Kahn CR. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 79.Tanti JF. Grillo S. Gremeaux T. Coffer PJ. Van Obberghen E. Le Marchand-Brustel Y. Potential role of protein kinase B in glucose transporter 4 translocation in adipocytes. Endocrinology. 1997;138:2005–2010. doi: 10.1210/endo.138.5.5136. [DOI] [PubMed] [Google Scholar]
- 80.Tremblay F. Lavigne C. Jacques H. Marette A. Defective insulin-induced GLUT4 translocation in skeletal muscle of high fat-fed rats is associated with alterations in both Akt/protein kinase B and atypical protein kinase C (zeta/lambda) activities. Diabetes. 2001;50:1901–1910. doi: 10.2337/diabetes.50.8.1901. [DOI] [PubMed] [Google Scholar]
- 81.Ueki K. Yamauchi T. Tamemoto H. Tobe K. Yamamoto-Honda R. Kaburagi Y. Akanuma Y. Yazaki Y. Aizawa S. Nagai R. Kadowaki T. Restored insulin-sensitivity in IRS-1-deficient mice treated by adenovirus-mediated gene therapy. J Clin Invest. 2000;105:1437–1445. doi: 10.1172/JCI7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valverde AM. Burks DJ. Fabregat I. Fisher TL. Carretero J. White MF. Benito M. Molecular mechanisms of insulin resistance in IRS-2-deficient hepatocytes. Diabetes. 2003;52:2239–2248. doi: 10.2337/diabetes.52.9.2239. [DOI] [PubMed] [Google Scholar]
- 83.Vollenweider P. Menard B. Nicod P. Insulin resistance, defective insulin receptor substrate 2-associated phosphatidylinositol-3′ kinase activation, and impaired atypical protein kinase C (zeta/lambda) activation in myotubes from obese patients with impaired glucose tolerance. Diabetes. 2002;51:1052–1059. doi: 10.2337/diabetes.51.4.1052. [DOI] [PubMed] [Google Scholar]
- 84.Wagner JD. Cline JM. Shadoan MK. Bullock BC. Rankin SE. Cefalu WT. Naturally occurring and experimental diabetes in cynomolgus monkeys: a comparison of carbohydrate and lipid metabolism and islet pathology. Toxicol Pathol. 2001;29:142–148. doi: 10.1080/019262301301418955. [DOI] [PubMed] [Google Scholar]
- 85.Wang Q. Somwar R. Bilan PJ. Liu Z. Jin J. Woodgett JR. Klip A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Winegar DA. Brown PJ. Wilkison WO. Lewis MC. Ott RJ. Tong WQ. Brown HR. Lehmann JM. Kliewer SA. Plunket KD. Way JM. Bodkin NL. Hansen BC. Effects of fenofibrate on lipid parameters in obese rhesus monkeys. J Lipid Res. 2001;42:1543–1551. [PubMed] [Google Scholar]
- 87.Wojtaszewski JF. Nielsen P. Hansen BF. Richter EA. Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528(Pt 1):221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamauchi T. Kamon J. Minokoshi Y. Ito Y. Waki H. Uchida S. Yamashita S. Noda M. Kita S. Ueki K. Eto K. Akanuma Y. Froguel P. Foufelle F. Ferre P. Carling D. Kimura S. Nagai R. Kahn BB. Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 89.Ye JM. Doyle PJ. Iglesias MA. Watson DG. Cooney GJ. Kraegen EW. Peroxisome proliferator-activated receptor (PPAR)-alpha activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-gamma activation. Diabetes. 2001;50:411–417. doi: 10.2337/diabetes.50.2.411. [DOI] [PubMed] [Google Scholar]
- 90.Zierath JR. Ryder JW. Doebber T. Woods J. Wu M. Ventre J. Li Z. McCrary C. Berger J. Zhang B. Moller DE. Role of skeletal muscle in thiazolidinedione insulin sensitizer (PPARgamma agonist) action. Endocrinology. 1998;139:5034–5041. doi: 10.1210/endo.139.12.6364. [DOI] [PubMed] [Google Scholar]