Abstract
The process of aging is linked to oxidative stress, microglial activation, and proinflammatory factors, which are known to decrease cell proliferation and limit neuroplasticity. These factors may lead the transition from normal aging to more severe cognitive dysfunction associated with neurodegenerative diseases. We have shown that natural compounds such as polyphenols from blueberry and green tea and amino acids like carnosine are high in antioxidant and antiinflammatory activity that decreases the damaging effects of reactive oxygen species (ROS), in the blood, brain, and other tissues of the body. Furthermore, we have shown that the combination of these nutrients (called NT-020) creates a synergistic effect that promotes the proliferation of stem cells in vitro and in vivo. In the current study, we examined the effects of NT-020 on neurogenesis and performance on a Morris water maze (MWM). Aged (20-month-old) male Fischer 344 rats were treated with 135.0 mg/kg per day (n = 13) of NT-020. Young (3-month-old) (n = 10) and aged (20-month-old) (n = 13) control male Fischer 344 rats were treated with water by oral gavage. All groups were treated for a period of 4 weeks. Although there was no difference in performance in the MWM when comparing all aged rats, when the data for aged impaired rats were compared, there was a significant difference between groups on the last day of training with the treatment group performing better than controls. Using the cell cycle–regulating protein (Ki67), doublecortin (DCX), and OX6 antibody markers, cell proliferation, neurogenesis, and microglial activation were estimated in the dentate gyrus (DG) of young and aged animals. Cell proliferation was also examined in the subventricular zone (SVZ). A decreased number of OX6 MHC II–positive cells, increased neurogenesis, and increased number of proliferating cells were found in rats treated with NT-020 in comparison with aged control rats. In sum, NT-020 may promote health, proliferation, and maintenance of neurons in the age animals and exert antiinflammatory actions that promote function in the aged stem cell niche.
Introduction
The normal aging process is associated with changes in the physiology and neuroplasticity of the brain as well as cognitive impairments that vary in severity from mild to severe. Aging is also associated with an increase in oxidative stress, proinflammatory cytokines, and microglial activation. All of these events seen in normal aging make the process of aging one of the biggest risk factors in the majority of neurodegenerative diseases.1 However, researchers are investigating these neurophysiologic events and how they may lay the ground for the transition between normal cognitive decline and the risk for developing neurodegenerative diseases such as stroke, Parkinson disease, dementias and Alzheimer disease.2 For instance, increased inflammatory cytokines and complement proteins produced by endogenous glial cell, such as microglia and astrocytes, have been indentified in the Alzheimer pathology as well as other neurodegenerative diseases such as Parkinson disease. It is not known exactly how age-related cognitive declines correlate with the effects of increased oxidation and increased inflammation, yet, in the last decade, nutritional supplementation has been shown to decrease the age-related reactive oxygen species (ROS) production and to increase synaptic plasticity and learning and memory.3–8 Neurogenesis is one form of synaptic plasticity; it occurs throughout the life span and occurs primarily in stem cells niches. There are two main stem cell niches in the brain, namely the subventricular zone (SVZ) and the subgranular zone (SGZ) of the hippocampus. The de novo production of new neurons into the hippocampus has been shown to be important for some forms of learning.9 Although numerous studies have shown that neurogenesis is physiologically relevant for cognitive function, the relationship is complex (for review, see refs. 10 and 11). Nonetheless, neurogenesis is clearly linked to plasticity and repair mechanisms,12 and alterations in neurogenesis have been also been attributed to some affective disorders.13 Second, it has been increasingly suggested that aging may be a stem cell disease, because a major aspect of aging is a decline in the proliferation of stem cells niches throughout the body, including the brain.14,15
That cellular senescence occurs with age has been known since the 1960s,16 but the importance of the cellular senescence within the aged stem cell niche has only recently become an area of active interest. A clear example of the importance of the extrinsic or systemic influence on the stem cell niche was demonstrated in the stem cells that are found in the muscle, called satellite cells. Like neural stem cells, satellite cells in the muscle lose the potential to regenerate damaged tissue with age. In an elegant experiment, when aged rats were exposed to the systemic environment of a young rat by parabiosis, the satellite cells were rejuvenated in the aged rats, as demonstrated by an increase in the proliferation rate. Conversely, in young rats, the exposure to the circulation of the aged rats caused a decrease in the regenerative potential of the satellite cells,17 again supportive of an extrinsic/circulating factor that is influencing the proliferation of the stem cells in the aged animals. It is not clear whether the mechanism involved in the effect in the muscle would hold true in the brain, but the implication is that the aged environment is detrimental to stem cell function. When embryonic stem cells are transplanted into aged tissue, they are not able to repair damaged tissue as well as when transplanted into young tissue.14
Epidemiological studies show that diets rich in colorful fruits and vegetables that are high in polyphenols or flavonoids may reduce the risk of developing neurodegenerative diseases, such as cognitive impairment, dementia, Parkinson disease, or Alzheimer disease.18,19 Previous studies have shown that nutraceuticals can have effects on adult stem cells. A nutraceutical combination of blueberry, green tea extract, carnosine, and vitamin D3 (a proprietary formulation known as NT-020) has been shown to promote migration of brain stem cells from the stem cell niche to the site of injury in an animal model of stroke.12 NT-020 was shown to stimulate the proliferation of human stem cells derived from bone marrow, bone marrow–derived CD34+, and progenitor cells from peripheral blood (CD133+) in vitro.20 NT-020 reduced the oxidative stress-induced apoptosis of microglia cells and neurons in vitro. Furthermore, cultured bone marrow cells removed from mice given NT-020 orally for 2 weeks exhibited a dose-related reduction of oxidative stress-induced cell death. This demonstrates that the action of this nutraceutical on stem cells is not dependent on the presence of the formulation, because the effect was observed when the cells were cultured in the absence of NT-020 for 3 days. In a further study by Yasuhara et al.,12 NT-020 supplementation protected male Sprague-Dawley rats against ischemic stroke. NT-020 was orally administered for 2 weeks prior to middle cerebral artery occlusion (MCAo). In the NT-020-treated animals, there was a 75% decrease in mean glial scarring at the infarction area in compared to the vehicle. More importantly, in this study it was demonstrated that NT-020 increased proliferation of stem cells in the SVZ and increased the migration of stem cells to the area of injury. In this model, the treatment was initiated 2 weeks prior to the injury and did not continue after the injury, and again demonstrates the long-lasting effect of this treatment because the increase in neurogenesis was observed 2 weeks following the injury.
The goal of this study was to examine if NT-020 has the potential to improve neural stem cell proliferation in aged rats and to see if there is a concurrent improvement in cognitive function. To carry out this goal, we treated 20-month-old old rats with NT-020 for 3 weeks prior to testing them in a Morris water maze (MWM). Following behavioral testing, the brains of the rats were examined for neural stem cell proliferation in the two stem cell niches of the brain (the SGZ and the SVZ), hippocampal neurogenesis, and activated micgroglia cells in SGZ.
Materials and Methods
Sources of NT-020
The ingredients of NT-020 are blueberry, green tea, vitamin D3, and carnosine. NT-020 is a patented proprietary formulation available from NaturaTherapeutics, Inc.
Subjects
Experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC). Fischer 344 rats, 3 months of age and 20 month of age, were obtained from Harlan to be used in scientific experiments. All animals were housed under normal conditions (20°C, 50% relative humidity, and a 12-h light/dark cycle) and provided a normal NIH-31 diet. All studies were performed by personnel blinded to the treatment condition.
Oral treatment of NT-020 in rats
Aged rats were treated with 135.0 mg/kg a day of NT-020 oral gavage. Young and aged control rats were treated with water by oral gavage. All groups were treated for a period of 4 weeks.
Behavioral testing
The MWM was used to evaluate the effects of natural compounds of NT-020 on spatial learning and memory by young and aged rats after 3 weeks of treatment. The tank used was 1.5 meters in diameter with a 10-cm-diameter platform submerged 1 cm below the surface of water, which was at 27°C. The performance in the MWM was measured over 5 days of training, with four trials per day. The platform was placed in any of the four positions north, south, east, or west. Every animal had an assigned platform position that was constant across days, yet the starting site (dropping zone) was changed per trial and the target quadrant varied between subjects. Once animals found their escape platform, they were allowed to remain on the platform for 30 sec between trials, then transferred to a warm resting cage for 30 sec before the next trial. If an animal did not find the platform, it was guided to the platform and allowed to rest on the platform for 30 sec. Probe trials were not assessed in this study.
Using a computer tracking software (Noldus), the cumulative search error was assessed. Cumulative search can be calculated using the average distance to the target platform in meters and multiplying it by the time to target. This type of measurement was chosen for our data because it mainly reveals the age-related cognitive impairments while restricting bias due to swimming capability. Cognitive impairment was defined as by Abrous22; to be conservative, we used a 40% cutoff rather than 30%.
Immunohistochemistry
Staining for the cell cycle–regulating protein Ki67, doublecortin (DCX), and and OX6 was carried out on every sixth sagittal section throughout the entire hippocampus or SVZ. Twenty-four free-floating sagittal sections (40 μm) were incubated in 0.3% hydrogen peroxide (H2O2) solution followed by 1-h of incubation in blocking solution (0.1 M phosphate-buffered saline (PBS) supplemented with 3% normal goat serum and 0.2% Triton X-100). Sections were then incubated overnight with Ki67 (1:400 Nocastra), DCX (1:200 Santa Cruz), and OX6 (major histocompatibility complex [MHC] class II; 1:750 BD) antibody markers in PBS supplemented with 3% normal goat serum and 0.1% Triton X-100. Sections were then washed and biotinylated secondary antibody (1:200; Vector Laboratories, Burlingame, CA) in PBS supplemented with 3% normal goat serum, and 0.1% Triton X-100 was applied for 1 h. Next, the sections were incubated for 60 min in avidin–biotin substrate (ABC kit, Vector Laboratories, Burlingame, CA). All sections were then incubated for 1 min in 3,3′-diaminobenzidine (DAB) solution (Vector Laboratories). Sections were then mounted onto glass slides and cover slipped with mounting medium.
Stereology
KI67-, DCX-, and OX6-positive cells were examined with a Nikon Eclipse 600 microscope and quantified using Stereo Investigator software, Version 8 MicroBrightField, Colchester, VT). Cells were counted within the granule cell layer using the optical fractionator method of unbiased stereological cell counting techniques.35 The sampling was optimized to count at least 200 cells per animal with error coefficients less than 0.07. Each counting frame (125 × 125 μm for OX6 and Ki67, and 175 × 125 μm for DCX) was placed at an intersection of the lines forming a virtual grid (125 ×125 μm), which was randomly generated and placed by the software within the outlined structure. Cell proliferation was also examined in the SVZ using Ki67. The sampling was optimized to count at least 200 cells per animal with error coefficients less than 0.07. Each counting frame was set at (75 × 75 μm) and a virtual grid size at (125 × 125 μm), generating random sampling of the SVZ.
Statistics
One-way analysis of variance (ANOVA) was used for multiple mean comparisons, followed by post hoc comparison using the Bonferonni method to compare all pairs of columns. Levels were set at α = 0.05 for all analyses.
Results
Cognitive function
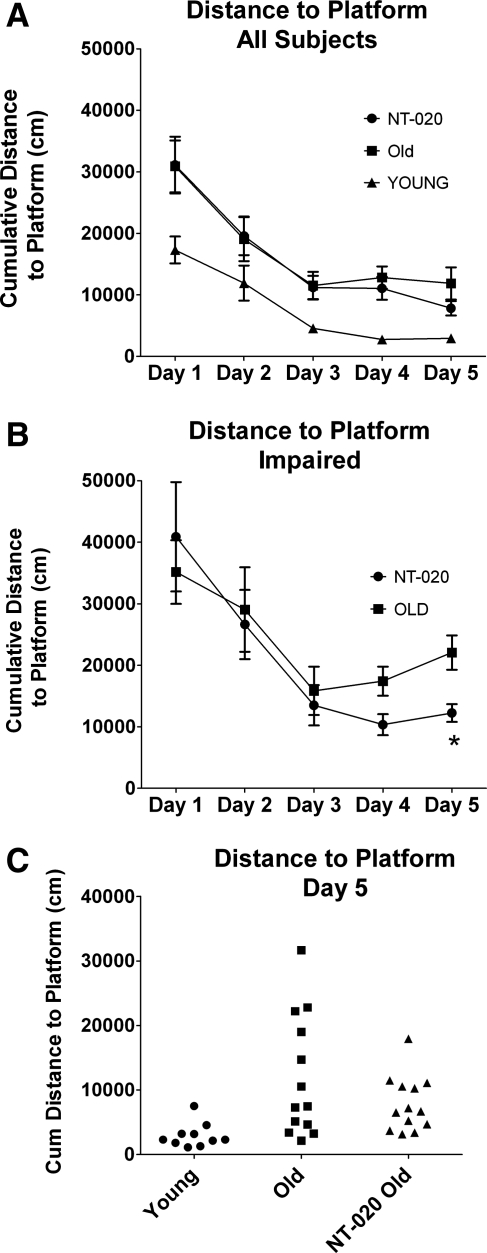
Rats were tested for spatial memory using a standard MWM design where the rats were trained for 5 consecutive days and 4 trials per day to find the hidden platform. To determine the effect of the treatment on the distribution of rats in a cognitively impaired group versus cognitively unimpaired groups, we examined performance on the fifth day of training. Figure 1A shows cumulative distance to platform from all subjects. Figure 1B shows a scatter plot of the individual rats performance on day 5 of training using cumulative distance to platform. The aged control group can be seen to have a high variance of performance. At 21 months of age, it is expected that only about 30% of the animals are considered aged impaired (AI), and this value has been used to define AI in other studies22; thus, the distribution of impaired in this group compares with other published data. To be conservative, AI for the control aged rats was determined as the lowest performing 40% of animals in this group. With the lowest score for the aged control group for cumulative distance as the cutoff for AI in the treatment group, there was only 1 rat in the treatment group that would be considered AI for the distance to platform value. To further compare between the two treatment groups, we compared the worst-performing rats in each group (old control and old NT-020) for all days for the acquisition phase on the MWM (Fig. 1C). The treatment group was significantly different from the aged control group in the cumulative distances to platform. Thus, there is a significantly lower distribution of rats as AI in the old NT-020 treatment group in compared with the old control and the means are significantly different.
FIG. 1.
Cumulative distance to platform. (A) Morris Water maze learning acquisition performance of all subjects. When learning over days is graphed for all subjects, there is a clear age difference where young rats outperform the aged rats; then by days 4 and 5, a difference begins to emerge between the treatment group and the control aged group. (B) When the highest scoring 40% of rats are examined as the aged impaired (AI) groups for both the control and NT-020 treatment, there is a significant difference between groups on days 5 (one-way analysis of variance [ANOVA] followed by Bonferroni analysis; overall F = 16.55, degrees of freedom [df ] = 2, 12 post hoc for aged control vs. treatment p < 0.05; young vs. NT-020 is not significantly different) and distance to platform (ANOVA, F = 22.6 df = 2, 12, aged control vs. aged NT-020 p < 0.01). (C) Individual rat performance on day 5 of training is shown to demonstrate the wide variance of data within subjects and that there was lower variance in the NT-020-treated group.
Neural stem cell proliferation and neurogenesis in SGZ of the hippocampal dentate gyrus is increased in aged rats after 1 month of NT-020 dietary supplementation
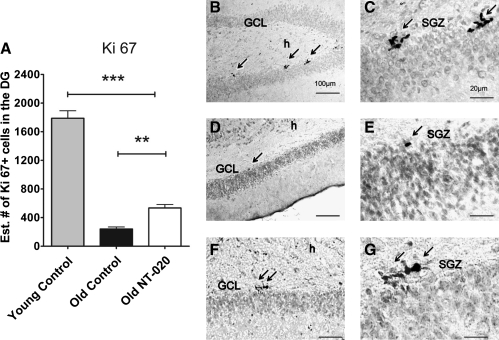
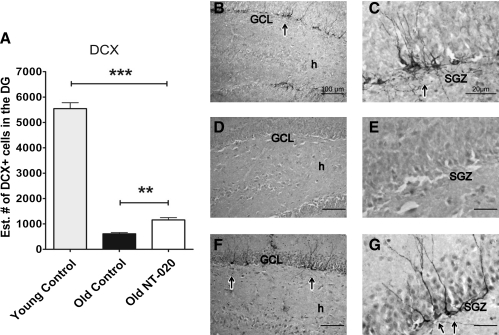
To test the hypothesis that NT-020 could impact the stem cell niche in aged animals, we examined the number of dividing cells in the SGZ of the hippocampus using the mitotic marker KI67. Treatment of 20-month-old rats with NT-020 for a period of 4 weeks was found to significantly increase the proliferative capacity of the cells present in the SGZ of the dentate gyrus in comparison with the aged control group, as seen by quantification of the mitotic marker KI67 (Fig. 2A). Quantification of the Ki67+ cells was done through unbiased stereology using the estimated number by optical fractionator. Also shown are representative sections of the dentate gyrus showing that in the aged rats there are fewer KI67 cells compared with young, but in the NT-020 treated rats there is a higher prevalence of labeled cells. To address the question of whether this increase in proliferation within the neurogenic niche translated into increased numbers of cells differentiating into the neuronal lineage, we quantified the staining for the neuronal lineage marker doublecortin. As can be seen in Fig. 3, neurogenesis as quantified by doublecortin-positive cells was significantly higher in the aged NT-020 group as compared with the control aged group. Representative micrographs are included to demonstrate this effect in the various groups.
FIG. 2.
(A) Ki 67+ cells (cell cycle marker) present in the subgranular zone (SGZ) of the dentate gyrus (DG) of young (4-month-old, n = 10) and aged (21-month-old, n = 13) rats quantified using stereology methods. Asterisks denote a significant age-related decrease in cell proliferation, demonstrating a significant increase of cell proliferation in the NT-020 oral gavage treatment group compared with age-matched controls. There is an overall age-related decrease in Ki67+ cells with age. (One-way analysis of variance [ANOVA] F = 157.6, degrees of freedom [df ] = 2, 32; followed by Bonferroni post hoc values [***] p = 0.005, [**] p < < 0.05). Photomicrographs depicting representative sections of Ki67 staining in the granular cell layer (GCL) and SGZ of the DG of the hippocampus (arrows) in young rats (B,C), in control aged rats (D,E), and in NT-020 aged, treated rats (F,G). Scale bars for B, D, F, 100 μm; C, E, G, 20 μm. h indicates the hilus of the dentate gyrus.
FIG. 3.
Doublecortin+ cells (DCX) present in granule cell layer of the dentate gyrus (DG) of young (4-month-old, n = 10) and aged rats (21-month-old, n = 13). Quantification of DCX shows a significant increase of DCX+ cells in the DG of aged rats treated with NT-020 for 1 month in comparison with aged rats treated with water oral gavage (one-way analysis of variance [ANOVA] F = 487.9, degrees of freedom [df ] = 2, 24; Bonferroni post hoc values [***] p < 0.005, [**] p < 0.05). Photomicrographs depicting representative sections of DCX staining in the granular cell layer (GCL) and subgranular zone (SGZ) of the DG of the hippocampus (arrows) in young rats (B,C), in control aged rats (D,E), and NT-020 aged, treated rats (F,G). Scale bars for B, D, F, 100 μm; C, E, G, 20 μm. h indicates the hilus of the dentate gyrus.
NT-020 treatment decreased activated microglia in the dentate gyrus of the hippocampus
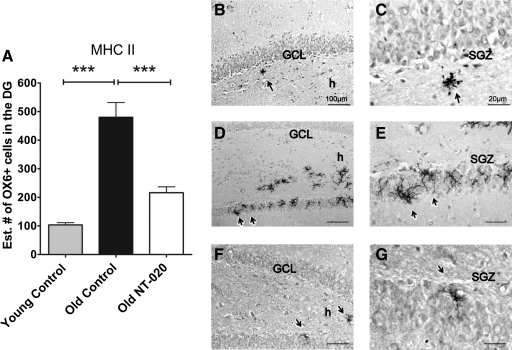
As discussed in the introduction, aging is associated with an increase in inflammation and oxidative stress, which are known to be deleterious to the stem cell niche. Thus, a second part of our hypothesis was that one aspect of the beneficial effects of NT-020 treatment would be a downregulation of inflammatory markers in the aged brain. To examine this, we estimated the total numbers of cells that express MHC class II receptors using OX6. Oral treatment of NT-020 was able to decrease the numbers of cells that express major (MCH class II) (Fig. 4A). In the NT-020-treated aged rats, the estimated numbers of OX6+ cells were significantly lower than the aged group fed with water oral gavages. Quantification of OX6+ cells was carried out using unbiased stereology. The estimated number of OX6+ cells was taken from the estimated number by optical fractionator.
FIG. 4.
(A) OX6+ cells (MHC class II) present in the granule cell layer of the dentate gyrus (DG) (arrows) of young (4-month-old, n = 10) and aged (21-month-old, n = 13) rats counted using stereology. Asterisks denote a significant age-related increase in MHC class II–expressing cells, demonstrating a significant decrease in MHC class II expression in the NT-020 oral gavage treatment group compared with age-matched controls. There is an overall age-related increase in OX6 expression in cells with age. (One-way analysis of variance [ANOVA] F = 31.63, degrees of freedom [df ] = 2, 32; followed by Bonferonni post hoc values [***] p < 0.005, [***] p < 0.05). Photomicrographs depicting representative sections of OX6 staining in the granular cell layer (GCL) and subgranular zone (SGZ) of the DG of the hippocampus (arrows) in young rats (B,C), in control aged rats (D,E), and NT-020 aged, treated rats (F,G). Scale bars, B, D, F, 100 μm; C, E, G, I 20 μm. h indicates the hilus of the dentate gyrus.
NT-020 treatment increases proliferation of neural progenitors in the SVZ
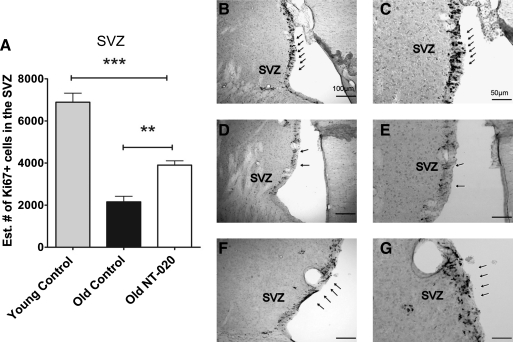
To determine if the effect that was observed in the SGZ was also observed in other neurogenic areas, we assessed the numbers of proliferating cells in the SVZ. As can be seen in Fig. 5, there is an age-related decrease in proliferation in the SVZ, and treatment with NT-020 for 1 month was able to increase the numbers of proliferating cells. Representative micrographs are included to demonstrate this effect in the various groups.
FIG. 5.
(A) Ki67+ cells (cell cycle marker) present in the subventricular zone (SVZ) of young (4-month-old, n = 10) and aged (21-month-old, n = 13) rats counted using stereology. Asterisks denote a significant age-related decline in Ki67-expressing cells, demonstrating a significant increase in number of cells expressing Ki67 staining in the NT-020 treatment group compared with age-matched controls. There is an overall age-related decrease in Ki67+ cells in the SVZ with age. (One-way analysis of variance [ANOVA] F = 52.82, degrees of freedom [df ] = 2, 24; followed by Bonferroni post hoc values [***] p < 0.005, [**] p < 0.05). Photomicrographs depicting representative sections of OX6 staining in the granular cell layer (GCL) and subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus (arrows) in young rats (B,C), in control aged rats, (D,E), and NT-020 aged, treated rats (F,G). Scale bars, B, D, F, 100 μm; scale bar, C, E, G, 50 μm. h indicates the hilus of the dentate gyrus.
Discussion
The present in vivo study has shown the effect of NT-020 to increase aspects of cognitive function and neural stem cell proliferation in the two main stem cell niches of the brain. In the SGL of the dentate gyrus, we also demonstrate that neurogenesis as indexed by DCX is increased by NT-020. Furthermore, one of the key changes that may relate to the changes assessed in neurogenesis is a decline in the numbers of MHC class II receptor expression on microglia. Our hypothesis is that the local environment of the aged animals has negative regulators on the proliferation of stem cells, some of which are being produced by activated microglia.
The notion of aging as a stem cell disease has been gaining popularity. Considerable evidence points to the fact that stem cells throughout the body have reduced regenerative capacity. For example, hematopoietic stem cells in the bone marrow of aged mice have a reduced repopulating potential when used for transplants to mice following irradiation.23 These authors also noted that epigenetic regulation of inflammation and stress response pathways was associated with the change in stem cell function. A further study identified p53 as a critical factor in that p53 gene dosage regulated this loss of stem cell function.24 Interestingly, this effect at the level of the bone marrow is also translated into the central nervous system (CNS) because it has been shown that mutant mice overexpressing p53 show early senescence, decreased stem cell proliferation in the SVZ and decreased learning on an olfactory cure learning task.25 Unfortunately, the positive effect of decreasing p53 gene expression on stem cell proliferation has the opposite effect on tumorogenesis and so there is an increased incidence of neoplasm.15
Many environmental influences on stem cell proliferation have also been examined. For example, high glucose26 has been shown to impair endothelial progenitor proliferation. Alcohol exposure can also reduce neurogenesis.27,28 Oxidative stress and inflammation are also both negative regulators of stem cell proliferation.29,30 It has clearly been demonstrated that circulating factors in the aged animal influence stem cell function. Studies using parabiosis where the circulation of 2 animals is connected have shown that the environment of an aged rat can reduce stem cell proliferation in young animals and, vice versa, that the circulation of a young rat can have a rejuvenating effect on the aged rats' stem cells.31 Thus, it is clear that negative regulators of stem cell genesis are present in aged animals. It is our hypothesis that one important negative regulator that occurs in the aged brain relates to increased proinflammatory cytokines and other factors coming from microglia. In this paper, we have demonstrated that one way to increase stem cell function in the aged animal is via dietary supplementation. NT-020 increases proliferation of neural progenitor cells in both the SVZ and the SGZ of the dentate gyrus. There is a concurrent change in the numbers of microglia expressing MHC class II, which supports our hypothesis that one aspect of the mechanism of action for this dietary manipulation is via the microglia.
The role of progenitor cells in the brain remains under debate. One factor that should be considered is that progenitor cells appear to have rejuvenating effects when used as a cell therapy in aged animals or in disease models. For example, when the mononuclear cell fraction of umbilical cord blood is used for treatment of stroke,32 amyotrophic lateral sclerosis (ALS),33 or normal aging,34 these cells have a neuroprotective function to reduce stroke damage, reduce motor symptoms of ALS, and increase neurogenesis in the aged brain. Treatments of these conditions with mature adult mononuclear fractions that contain a similar cell makeup have no such therapeutic properties, suggesting that there is something different with the immature progenitor cells. Thus, the decline in progenitor cells during aging may be an important factor underlying the reduced ability of the aged brain to respond to injury and may underlie the susceptibility to neurodegenerative diseases. Therapeutic approaches that improve progenitor cell function, such as the one described here, may have far-reaching effects for improving the health of the aging brain in more ways than by just increasing numbers of neurons in the dentate gyrus and olfactory bulb, known targets of the SGZ and SVZ. We have demonstrated that NT-020 increases proliferation of cells in these two stem cell niches and also results in a reduced number of aged impaired rats following 1 month of treatment. At 20 months of age, there is considerable spread of cognitive scores, with only about 30% of the rats being considered aged impaired for cognitive function.22 After treatment with NT-020, there was a significant reduction in the numbers of aged impaired rats and a significantly reduced spread in the cognitive scores. Although the results for cognition are preliminary in nature and suggest that the effect is in the aged impaired group only, these results should be examined further by prescreening rats and then testing the effects of treatment in the AI group so that the issue of dilution of the effect by the aged unimpaired rats can be resolved. This is of importance because humans show similar ranges of cognitive function with age, and this suggests that even those in the upper range of normal could achieve some cognitive benefit from these therapeutic approaches.
In summary, there is considerable accumulating evidence suggesting that aging is a stem cell disease and that one of the factors that influences stem cell function in aging is a change in the stem cell environment of the aged body, which has a strong negative influence on the function of stem cells. One change that occurs with aging is an increase in circulating factors such as cytokines and chemokines and local tissue conditions, which also increase proinflammatory factors that have a negative effect on the function of all cells, but most importantly on the progenitor cell pools. Furthermore, there is something that seems to be special about progenitor cells that has a rejuvenating effect on the aged environment. Thus, improving the proliferation and function of progenitor cells, as done here by treatment of aged rats with NT-020, has a positive influence on the stem cell niche and may have far-reaching effects on organ function beyond simple replacement of injured cells, as demonstrated by an improvement in cognitive function.
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants PO1AG04418 and MH–070430 (P.C.B.) and The U.S. Veterans Administration Medical Research Service (P.C.B/).
Author Disclosure Statement
P.C.B. and P.R.S. are the founders of NaturaTherapeutics, Inc.
References
- 1.Floyd RA. Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23:795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 2.Arnaiz E. Almkvist O. Neuropsychological features of mild cognitive impairment and preclinical Alzheimer's disease. Acta Neurol Scand Suppl. 2003;179:34–41. [PubMed] [Google Scholar]
- 3.Bickford PC. Gould T. Briederick L. Chadman K. Pollock A. Young D. Shukitt-Hale B. Joseph J. Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res. 2000;866:211–217. doi: 10.1016/s0006-8993(00)02280-0. [DOI] [PubMed] [Google Scholar]
- 4.Gemma C. Mesches MH. Sepesi B. Choo K. Holmes DB. Bickford PC. Diets enriched in foods with high antioxidant activity reverse age-induced decreases in cerebellar beta-adrenergic function and increases in proinflammatory cytokines. J Neurosci. 2002;22:6114–120. doi: 10.1523/JNEUROSCI.22-14-06114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu J. Atamna H. Kuratsune H. Ames BN. Delaying brain mitochondrial decay and aging with mitochondrial antioxidants and metabolites. Ann NY Acad Sci. 2002;959:133–166. doi: 10.1111/j.1749-6632.2002.tb02090.x. [DOI] [PubMed] [Google Scholar]
- 6.Maguire EA. Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126(Pt 7):1511–1523. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- 7.Coultrap SJ. Bickford PC. Browning MD. Blueberry-enriched diet ameliorates age-related declines in NMDA receptor-dependent LTP. Age (Dordr) 2008;30:263–272. doi: 10.1007/s11357-008-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berglof E. Small BJ. Bickford PC. Stromberg I. Beneficial effects of antioxidant-enriched diet for tyrosine hydroxylase-positive neurons in ventral mesencephalic tissue in oculo grafts. J Comp Neurol. 2009;515:72–82. doi: 10.1002/cne.22002. [DOI] [PubMed] [Google Scholar]
- 9.Clelland CD. Choi M. Romberg C. Clemenson GD., Jr Fragniere A. Tyers P. Jessberger S. Saksida LM. Barker RA. Gage FH. Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drapeau E. Nora Abrous D. Stem cell review series: Role of neurogenesis in age-related memory disorders. Aging Cell. 2008;7:569–589. doi: 10.1111/j.1474-9726.2008.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leuner B. Gould E. Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 12.Yasuhara T. Hara K. Maki M. Masuda T. Sanberg CD. Sanberg PR. Bickford PC. Borlongan CV. Dietary supplementation exerts neuroprotective effects in ischemic stroke model. Rejuvenation Res. 2008;11:201–214. doi: 10.1089/rej.2007.0608. [DOI] [PubMed] [Google Scholar]
- 13.Sahay A. Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 14.Carlson ME. Conboy IM. Loss of stem cell regenerative capacity within aged niches. Aging Cell. 2007;6:371–382. doi: 10.1111/j.1474-9726.2007.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers SM. Shaw CA. Gatza C. Fisk CJ. Donehower LA. Goodell MA. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5:e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayflick L. Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 17.Conboy IM. Conboy MJ. Wagers AJ. Girma ER. Weissman IL. Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 18.Joseph JA. Shukitt-Hale B. Casadesus G. Reversing the deleterious effects of aging on neuronal communication and behavior: Beneficial properties of fruit polyphenolic compounds. Am J Clin Nutr. 2005;81(1 Suppl):313S–316S. doi: 10.1093/ajcn/81.1.313S. [DOI] [PubMed] [Google Scholar]
- 19.Arts IC. Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81(1 Suppl):317S–25S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 20.Bickford PC. Tan J. Shytle RD. Sanberg CD. El-Badri N. Sanberg PR. Nutraceuticals synergistically promote proliferation of human stem cells. Stem Cells Dev. 2006;15:118–123. doi: 10.1089/scd.2006.15.118. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher M. Nicolle MM. Animal models of normal aging: Relationship between cognitive decline and markers in hippocampal circuitry. Behav Brain Res. 1993;57:155–162. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- 22.Drapeau E. Mayo W. Aurousseau C. Le Moal M. Piazza PV. Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambers SM. Goodell MA. Hematopoietic stem cell aging: Wrinkles in stem cell potential. Stem Cell Rev. 2007;3:201–211. doi: 10.1007/s12015-007-0027-1. [DOI] [PubMed] [Google Scholar]
- 24.Dumble M. Moore L. Chambers SM. Geiger H. Van Zant G. Goodell MA. Donehower LA. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood. 2007;109:1736–1742. doi: 10.1182/blood-2006-03-010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medrano S. Burns-Cusato M. Atienza MB. Rahimi D. Scrable H. Regenerative capacity of neural precursors in the adult mammalian brain is under the control of p53. Neurobiol Aging. 2009;30:483–497. doi: 10.1016/j.neurobiolaging.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen YH. Lin SJ. Lin FY. Wu TC. Tsai CR. Huang PH. Liu PL. Chen YL. Chen JW. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007;56:1559–1568. doi: 10.2337/db06-1103. [DOI] [PubMed] [Google Scholar]
- 27.Crews FT. Mdzinarishvili A. Kim D. He J. Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- 28.Ieraci A. Herrera DG. Single alcohol exposure in early life damages hippocampal stem/progenitor cells and reduces adult neurogenesis. Neurobiol Dis. 2007;26:597–605. doi: 10.1016/j.nbd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Ito K. Hirao A. Arai F, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 30.Ekdahl CT. Claasen JH. Bonde S. Kokaia Z. Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brack AS. Conboy MJ. Roy S. Lee M. Kuo CJ. Keller C. Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 32.Vendrame M. Cassady J. Newcomb J. Butler T. Pennypacker KR. Zigova T. Sanberg CD. Sanberg PR. Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- 33.Garbuzova-Davis S. Sanberg CD. Kuzmin-Nichols N. Willing AE. Gemma C. Bickford PC. Miller C. Rossi R. Sanberg PR. Human umbilical cord blood treatment in a mouse model of ALS: optimization of cell dose. PLoS One. 2008;3(6):e2494. doi: 10.1371/journal.pone.0002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachstetter AD. Pabon MM. Cole MJ. Hudson CE. Sanberg PR. Willing AE. Bickford PC. Gemma C. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci. 2008;9:22. doi: 10.1186/1471-2202-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West MJ. Slomianka L. Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. The Anatomical Record. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]