Abstract
Six randomized clinical trials have been implemented to examine the efficacy of tenofovir disoproxil fumarate (TDF) and/or TDF/emtricitabine (TDF/FTC) as preexposure prophylaxis for HIV-1 infection (PrEP). Although largely complementary, the six trials have many similar features. As the earliest results become available, an urgent question may arise regarding whether changes should be made in the conduct of the other trials. To consider this in advance, a Consultation on the Implications of HIV Pre-Exposure Prophylaxis (PrEP) Trials Results sponsored by the Division of AIDS (DAIDS) of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), and the Bill and Melinda Gates Foundation (BMGF) was held on January 29, 2010, at the Natcher Conference Center, NIH, Bethesda, MD. Participants included basic scientists, clinical researchers (including investigators performing the current PrEP trials), and representatives from the U.S. Food and Drug Administration (FDA) and the agencies sponsoring the trials: the U.S. Centers for Disease Control and Prevention (CDC), the U.S. Agency for International Development (USAID), the BMGF, and the U.S. NIH. We report here a summary of the presentations and highlights of salient discussion topics from this workshop.
Introduction
Six phase IIB and III randomized clinical trials have been implemented to assess the efficacy of tenofovir disoproxil fumarate (TDF) and/or TDF/emtricitabine (TDF/FTC) as preexposure prophylaxis for HIV-1 infection (PrEP). The primary results of one trial were recently reported and those from two others are expected in late 2010 or early 2011. The results of the remaining three trials will follow in 2012 and 2013. These six efficacy trials are largely complementary. They are examining TDF/FTC in different high-risk populations and/or settings, comparing TDF (alone) versus TDF/FTC and/or comparing oral versus topical dosing. Nevertheless, as the earliest results become available, an urgent question may arise. If compelling efficacy is found in one of the trials, what, if any, changes should be made in the conduct of the other trials?
This question will be particularly urgent for the Data Safety and Monitoring Boards (DSMBs) and sponsors of the ongoing trials, but expert guidance on the extent to which the results can be generalized to other populations and settings will also be of value to clinicians. If any of the trials shows even partial efficacy, patients may ask their providers to prescribe these already-licensed agents. While some patients may have the same route of exposure and a similar level of risk compared to participants in the trial(s), others will not.
Current trials
The six PrEP efficacy trials are described in Table 1.1 When all of the trials are fully enrolled, nearly 20,000 HIV-negative persons will participate. In addition, safety, adherence, and other behavioral data are available or will be available for the more than 2000 persons who enrolled in the West Africa TDF Study (heterosexual women), the TDF Extended Safety Study [U.S. men who have sex with men (MSM)], and the TDF2 Study (TDF/FTC in heterosexual men and women in Botswana).2–4
Table 1.
| Trial (sponsors) | Sites | Population | Intervention | Status (date results expected) |
|---|---|---|---|---|
| Bangkok Tenofovir Study (CDC) | Thailand | 2400 injecting drug users | Daily oral TDF | Follow-up continuing (2011) |
| CAPRISA 004 (USAID, DST) | South Africa | 889 heterosexual women | Topical 1% TDF gel applied intravaginally ≤12 h prior to and as soon as possible after coitus (but within 12 h) | Completed (2010) TDF gel use was associated with a 39% overall reduction in HIV acquisition (95% CI = 6, 60; p = 0.017)16 |
| iPrEx (NIH, BMGF) | Peru, Ecuador, Brazil, South Africa, Thailand, United States | 2,500 men who have sex with men | Daily oral TDF/FTC | Follow up continuing (2010 or 2011) |
| Partners PrEP (BMGF) | Kenya, Uganda | 3900 serodiscordant heterosexual couples | Daily oral TDF or TDF/FTC | Enrolling (2012) |
| FEM-PrEP (USAID, BMGF) | Kenya, Malawi, South Africa, Tanzania, Zambia | 3900 heterosexual women | Daily oral TDF/FTC | Enrolling (2012) |
| VOICE (MTN 003) (NIH) | South Africa, Malawi, Uganda, Zimbabwe | 5000 heterosexual women | Daily oral TDF or TDF/FTC or daily topical TDF gel (or daily oral placebo or topical placebo gel) | Enrolling (2013) |
Modified from AVAC.1
BMGF, Bill and Melinda Gates Foundation; CAPRISA, Centre for the AIDS Programme of Research in South Africa; CDC, U.S. Centers for Disease Control and Prevention; DST, Department of Science and Technology, Republic of South Africa; MTN, Microbicide Trials Network; NIH, U.S. National Institutes of Health; USAID, United States Agency for International Development; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate.
The six trials are quite varied. The Bangkok TDF Study is examining daily oral TDF alone in injection drug users (IDUs). Directly observed dosing was encouraged and more than 85% of the participants selected this option. The Centre for the AIDS Program of Research in South Africa (CAPRISA) 004 trial examined topical 1% TDF gel in women at urban and rural sites in South Africa. The gel was applied intravaginally ≤ 12 h prior to and as soon as possible (but within 12 h) after coitus. The PrEP initiative (iPrEX) study is iPrEX is examining daily oral TDF/FTC in MSM at multiple sites in Peru, Ecuador, Brazil, South Africa, Thailand, and the United States. Partners PrEP is comparing daily oral TDF and daily oral TDF/FTC in serodiscordant couples in Kenya and Uganda. Randomization is 1:1:1 (TDF:TDF/FTC:placebo). Fem-PrEP is examining oral TDF/FTC in women at sites in Kenya, Malawi, South Africa, Tanzania, and Zambia. The Vaginal and Oral Interventions to Control the Epidemic (VOICE) trial [Microbicide Trials Network (MTN) 003] is a five-arm study comparing oral TDF, TDF/FTC, oral placebo, TDF gel, and placebo gel, all dosed daily, in Malawi, South Africa, Uganda, and Zimbabwe.
Strength of evidence
The six trials differ regarding the statistical power they will have for making conclusions about efficacy. Some of the trials were intended as “proof of concept” only (i.e., to provide meaningful evidence for the hypothesis but not to be used, by themselves, to establish a new indication), but others—including iPrEX, Partners PrEP, Fem-PrEP, and VOICE—could have more powerful results. The latter trials were designed to have a 95% confidence interval lower bound of 25% or higher if the point estimate of efficacy is a 57%, 60%, or 70% reduction in HIV-1 incidence.
In general, in order to consider a product for a new treatment or prevention indication regulatory authorities such as the FDA require the results of two or more “adequate and well-controlled trials.”5,6 The results must corroborate one another, and the trials must be of adequate duration to provide convincing evidence for the durability of the effect and for safety. Depending on the study population(s) examined, a broad or more narrow, population-specific indication may be given.
In certain settings, one trial with “robust and compelling” evidence of efficacy may be considered sufficient to establish a new indication.5,6 This is more likely with conditions such as HIV infection in which the outcome can be death or serious disability. “Robust and compelling” evidence of efficacy may require an overall effect measure associated with a two-sided p-value < 0.001 (one-sided p-value < 0.0005). However, if there is considerable external evidence and the findings from the trial are consistent across the various subgroups, a two-sided p > 0.001 but < 0.01 may be considered sufficient. The final decision would, of course, require careful consideration of the risk–benefit ratio, effects on secondary endpoints, and the quality of the conduct of the trial.
Determining the safety of these agents in HIV-uninfected persons is a major aim of each of the trials. The larger PrEP trials will have several thousand person-years of follow-up. According to the “rule of three,” if 3000 persons are followed and no major adverse events occur, we can have 95% confidence that the risk of a major adverse event is < 1 in 1000.7 The duration of follow-up per participant is also important. If these agents are found to be effective in reducing HIV-1 incidence, then HIV-negative, healthy persons can be expected to take them for extended periods of time. In most of the six efficacy trials, individuals will be followed for at least 1–2 years.
Goals of the consultation
The central question addressed by the consultation was, “If we find efficacy in one PrEP trial, can the results be generalized to other populations and settings with the same or different route(s) of exposure?” More specifically, if there is “robust and compelling” evidence of efficacy in a study population with one predominant route of transmission (e.g., penile–rectal), do we know enough about the mechanism(s) of HIV-1 transmission to conclude that the intervention is highly likely to be effective in other populations with a different predominant route of exposure (e.g., penile–vaginal)? If so, can we say that this is so likely that it would be unethical to continue ongoing trial(s) in the other populations?
If “robust and compelling” evidence of efficacy is found in one trial, we must likewise ask whether we know enough about the pharmacokinetics (PK) and pharmacodynamics (PD) of TDF/FTC in the target tissues to conclude that it is highly likely to be effective in other populations with the same or different route(s) of exposure. Other questions discussed at this consultation included whether there were other factors (viral, hormonal, ethnic, behavioral, or other) that should also be considered in making this assessment. The potential implications that the CAPRISA 004 results might have for the daily PrEP trials were also discussed.
After an overview of the ongoing trials, invited experts led the participants in a review of the state-of-the-science regarding (1) the biology of HIV-1 transmission by route of exposure and (2) the PK/PD of FTC and TDF relevant to their potential efficacy as PrEP agents. The participants then met in break-out groups to discuss the questions outlined above. A final plenary discussion was held to determine whether there was a consensus regarding the central issues addressed by the consultation.
Workshop Summary
Mechanisms of HIV-1 transmission
Panel: Peter Anton, M.D. (Chair); Cecilia Cheng-Mayer, Ph.D.; Florian Hladik, M.D., Ph.D.; Thomas Hope, Ph.D.; Brandon Keele, Ph.D.; and Charles Wira, Ph.D.
The first panel presented what is currently known regarding the immunological and anatomical issues surrounding the transmission of HIV-1 in the female and male genital tracts and rectum. Although the main focus was on human studies, data from the nonhuman primate (NHP) repeated low-dose mucosal challenge model were also presented.
Dr. Charles Wira (Department of Physiology and Cell Biology, Dartmouth Medical School) discussed immune defenses in the human female reproductive tract (FRT) throughout the menstrual cycle. The FRT must protect against pathogens at the same time that it supports the allogeneic sperm and the immunologically distinct fetal-placental unit. Ten to twenty percent of all immune cells in the FRT are CD45+. The percentages of T cells (CD3+), neutrophils (CD66b+), monocytes (CD14+), and B cells (CD19+) vary throughout the menstrual cycle. Cyclical sex hormones play a critical role in modulating immunity in the FRT, alternately enhancing and suppressing specific immune responses at various stages of the cycle.
It is important to consider immune defenses and conditions of vulnerability throughout the FRT, given that any agent introduced into the vagina may be capable of reaching the upper tract. Innate immune defenses such as SLPI, HBD2, and HNP1-3 decline in the cervicovaginal fluids during the ovulatory phase of the menstrual cycle. This may represent a “window of vulnerability” to HIV and other infectious agents.8 Cytotoxic T cell activity in the upper FRT (uterus) also varies significantly during the cycle. Similarly, antibody concentrations rise and fall in both the uterus and vagina.
It has been clearly shown that the innate and adaptive immune responses in the FRT differ significantly from those in the blood and gastrointestinal (GI) tract. Further research focusing on antiviral factors in the FRT at different stages of the cycle is needed. This information will assist us in designing effective prevention interventions.
Dr. Florian Hladik (Fred Hutchinson Cancer Research Center and University of Washington) discussed four scenarios in which topical microbicides and/or oral PrEP agents might fail to prevent HIV-1 infection after mucosal exposure. First, if the microbicide does not distribute evenly and fully penetrate the vaginal or rectal mucosa, some virions may be able to reach the submucosal compartment beyond the reach of the microbicide. HIV-1 has been shown to enter local Langerhans cells (LCs) within 2 h after mucosal exposure. Although these cells are not productively infected, they can transport virions to the submucosa where they can infect susceptible T cells. It has also been shown that HIV can productively infect vaginal intraepithelial CD4+ T cells within 2 h after initial exposure.
Second, if HIV-1 virions persist in the vaginal or rectal mucosa beyond the time of action of the microbicide or oral PrEP agent, they could potentially infect target cells in the submucosa at a subsequent time point. Third, exposure to HIV can have effects that are independent from the infection process. For instance, HIV has been shown to trigger long-lasting expression of thymic stromal lymphopoietin (TSLP) in vaginal epithelia. This could lead to increased expression of CCR5 on CD4+ T cells and a greater susceptibility of these cells to HIV infection. Fourth, an established sexually transmitted infection (STI) such as herpes simplex virus could foster a proinflammatory environment that favors HIV acquisition. Strategies could be developed to reduce the risk posed in each of these four scenarios, but they may prove to be extremely difficult to implement in real-world settings.
Dr. Hladik concluded that the key objective of any PrEP agent, independent of the route of exposure, is the prevention of productive infection in CD4+ target cells residing in the mucosa or submucosa. However, alternative mechanisms of HIV entry, indirect effects of exposure to HIV, and the influence of pathogenic cofactors on susceptibility to HIV infection will vary between anatomical sites, making the extrapolation of results from one site to another extremely difficult.
Dr. Peter Anton (David Geffen School of Medicine, University of California, Los Angeles) highlighted the unique characteristics of the rectal compartment and their influence on the risk of HIV acquisition in both men and women. The FRT and the colorectal compartment differ greatly both anatomically, immunologically, and functionally. The microbial environments are also distinctly different, as is the likelihood of trauma during sex and the physiological responses to injury and infection. Each of these factors may contribute to the 20-fold or greater risk of transmission per sexual act that is observed with unprotected receptive anal intercourse (RAI).
It is possible that RAI alone disrupts the columnar epithelium sufficiently for HIV to access vulnerable lamina propria target cells, without having to traverse intact epithelia. Further studies are needed to examine the extent to which epithelial sloughing occurs following RAI. Normative values in persons engaging in RAI must also be established to provide a baseline for evaluating the safety of candidate microbicides and other PrEP agents for rectal application.
Evidence of chronic inflammation has been found in the colorectal mucosa of men whether or not there is evidence of trauma, an STI, or a history of RAI.9 These findings have included a predominance of memory CD4+ T cells with high coreceptor expression and high levels of soluble cytokines. Similar baseline studies in women are also needed. In both men and women, further studies are required to more fully correlate these findings with a history of RAI and other specific practices.
Dr. Thomas Hope (Department of Cell and Molecular Biology, Northwestern University Medical School) further addressed the question, “How does HIV breach epithelial barriers to reach underlying target cells?” Figure 1 compares the squamous and columnar epithelia. The squamous epithelia (vagina, oral, penile shaft) are 30–100 μm thick and typically provide a robust multilayer barrier. The columnar epithelia (endocervix, rectum, gut) are only one cell layer thick and much more vulnerable.
FIG. 1.
Morphology of (a) columnar epithelium and (b) squamous epithelium. (Reprinted with permission of Dr. Robin Shattock, Saint George's Hospital Medical School, London, England.)
Recent studies have attempted to visualize events during the first 48 h after HIV exposure. Dr. Hope described his own studies using photoactivated green fluorescent protein viral particles. Penetration of both columnar and squamous (vaginal/ectocervix) epithelia by greater than 1 μm depth was observed. Only a small proportion of virions is able to penetrate the squamous epithelium, but most of those that do appear to move interstitially through the extracellular space between epithelial cells.
To ensure that these findings were not due to tissue degradation, two sets of supporting studies were performed: (1) Rhesus macaques were inoculated in vivo and virions were shown to penetrate the ectocervix and vagina. Approximately 50% of the virions appeared to move interstitially. (2) Using fluid phase markers in ex vivo human explants, virions were again seen to penetrate intact squamous (vaginal) epithelia, but penetration was 10-fold greater when the cell junctions were compromised.
In these studies, HIV was shown to penetrate the epithelium to depths of up to 50 μm, which would enable it to interact with LCs and other potential target cells. While HIV appears to move interstitially in the squamous epithelia, it likely penetrates the columnar epithelia by transcytosis, the process of virus translocation through the epithelium. Dr. Hope summarized that sexual transmission of HIV may vary significantly with different epithelia and body compartments.
Dr. Brandon Keele (AIDS and Cancer Virus Program, National Cancer Institute, Frederick) presented a phylogenetic model of HIV transmission and early virus evolution. This model was developed for the enumeration and identification of founder/early transmitted viruses, comparing different routes of exposure. Several studies in humans have examined the number of founder viruses in recently infected heterosexuals, MSM, and IDUs.10–14 Most of these studies have been performed in populations infected with clade B viruses, but similar results were obtained with those infected with clade C and clades A and C.
A single viral variant is responsible for productive infection in 80% of heterosexual HIV-1 infections (subtypes A, B, C). In most studies, the rate of multivariant transmission and the number of variants are significantly higher in MSM and IDUs than in heterosexuals (Fig. 2).
FIG. 2.
Frequency of transmitted number of variants depending on route of transmission. HSX, heterosexual; MSM, men having sex with men; IDU, injection drug users. (Reprinted with permission of Keele et al.10)
Very similar results were also obtained in a simian immunodeficiency virus (SIV)/macaque model using single genome amplification/sequencing with limiting dilution cDNA, which allows for the proportional representation of the viral sequences found within the plasma. The SIV-macaque model of mucosal and systemic infection recapitulates many features of HIV-1 infection including the degree of protection afforded by the mucosal barrier in reducing the number of transmitted variants (intravaginal > intrarectal > > intravenous).
Further studies are required to confirm and further elucidate these findings. Nevertheless, they suggest that it may be very important to assess the efficacy of PrEP and other prevention interventions separately in each of these populations.
Dr. Cecilia Cheng-Mayer (Aaron Diamond AIDS Research Center, Rockefeller University) described how the SIV/simian HIV(SHIV) NHP models of HIV-1infection can be useful for preclinical studies of HIV prevention modalities. There is clear evidence that the site of inoculation, dose and strain of virus administered, and monkey species all influence the efficiency of SIV/SHIV transmission. With the high dose of either SIV or R5 SHIV that is typically used, nearly 100% of macaques are infected with intravenous, oral, or intrarectal inoculation. Vaginal inoculation is associated with a somewhat lower but still very high transmission efficiency. By comparison, the per exposure risk of HIV infection in humans is estimated to be in the range of 0.01–10%. This difference may be explained by the high virus titer in the inoculums used in NHP studies compared to the range of viral titers found in semen or vaginal fluids of HIV-infected subjects.
To better mimic human exposure to the virus, various repeated low-dose challenge NHP models have been developed. In these models, the rate of transmission is generally higher with intrarectal than intravaginal inoculation. An atypical pattern of infection frequently accompanies low-dose mucosal inoculation, making it necessary to rigorously examine many tissues before concluding that infection has not occurred. Such occult infections may or may not be followed by systemic infection.
It is recognized that even the best NHP models imperfectly recapitulate HIV infection in humans. Limitations include the fact that the virus is usually administered cell-free, in the absence of semen, and in a totally atraumatic manner. In addition, there are important differences between humans and NHPs in microflora, innate immune mediators, and STIs.
Session summary
Dr. Anton summarized the transmission panel's presentations and the subsequent open discussion. It is clear that the likelihood of local trauma, the tissues and cell subsets involved in HIV transmission, and the risk of HIV-1 infection per act all differ for the vaginal, penile, rectal, and IDU routes of exposure. The role of various innate and adaptive immune responses in conferring protection against HIV infection at different mucosal sites remains to be elucidated.
The numbers of memory T cells and other subsets, local CD4+ and CD8+ T cell activation levels, the state of coreceptor expression on CD4+ target cells, the degree of immune cell migration, local humoral immunity, microbial-epithelial cell-lamina propria “crosstalk,” and other potentially important variables may vary substantially among individuals and by rectal, vaginal, or penile compartment. Further research is needed to clarify the clinical relevance of these differences. In the meantime, we must be very cautious in interpreting the significance of our findings for one population and body compartment.
Key unanswered questions include the following: (1) Which cells are first targeted by HIV and how does this interaction lead to productive infection and subsequent dissemination? and (2) What is the minimum number of infective particles (and time period) required to establish infection in various body compartments? While some findings suggest that one virion may be sufficient, it is clear that most exposures do not result in infection. Some critical variables clearly vary between compartments (e.g., rectal versus vaginal) and within compartments (e.g., upper versus lower FRT).
In summary, it must be recognized that our understanding of how transmission takes place in various compartments is insufficient for us to conclude that the results of one PrEP trial can be generalized to other populations and routes of exposure.
Pharmacokinetics and pharmacodynamics of tenofovir/emtricitabine
Panel: Ed Acosta, PharmD; Peter Anderson, PharmD; Courtney V. Fletcher, PharmD (Chair); Craig Hendrix, M.D.; and Angela Kashuba, PharmD.
The second panel addressed the PK and PD of TDF and FTC in the relevant target tissues. Of particular interest for PrEP is the length of time these drugs persist in the genital tract and rectal fluids, tissues, and specific target cells when administered using a given delivery system, dose, and dosing schedule.
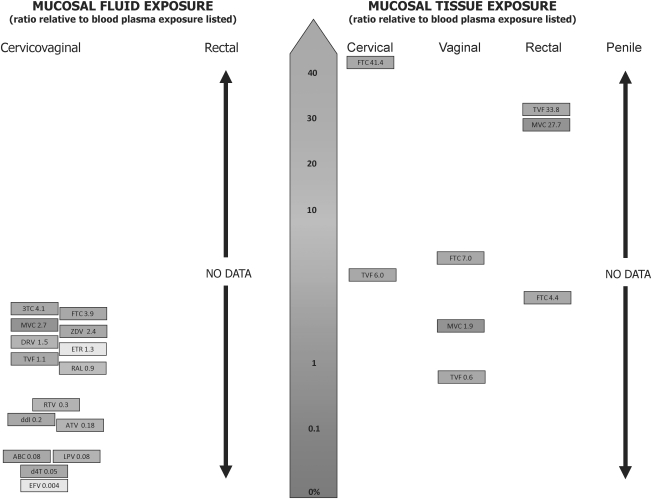
Dr. Angela Kashuba (School of Pharmacy, University of North Carolina at Chapel Hill) presented data demonstrating that oral administration of both tenofovir (TFV, a parent form of TDF) and FTC results in cervicovaginal fluids (CVF) concentrations that exceed those in blood plasma. Both drugs can be detected in the CVF within 2 h after a single dose and reach maximal levels in 4–6 h. For males, TFV concentrations in seminal plasma also exceed those of blood plasma. No data are presently available for the penetration of FTC into semen.
Figure 3 summarizes currently available data regarding the penetration of these and other antiretrovirals (ARVs) into the rectal and genital tract of men and women.15 After a single dose, both TDF and FTC have also been found to reach levels in the rectal tissues that exceed those in the plasma.
FIG. 3.
Penetration of antiretroviral drugs in mucosal fluids and tissues. (Reprinted with permission of Dr. Angela Kashuba, University of North Carolina at Chapel Hill. Adapted from Taylor et al.15)
Dr. Peter Anderson (Pharmaceutical Sciences, University of Colorado Denver) reviewed the cellular pharmacology relevant to the use of nucleoside reverse transcriptase inhibitors (NRTIs) for PrEP. To be pharmacologically active, NRTIs must be converted from the parent drug to the triphosphate anabolite, a process that takes place within the target cell. Once the drug is phosphorylated it becomes trapped within the cell. Thus, for all NRTIs, the half-life of the intracellular triphosphate is greater than the half-life of the parent drug in the plasma. Knowledge of the intracellular triphosphate levels of these agents is necessary to understanding their PK/PD.
Rational dosing strategies must be based on the NRTI's concentrations and intracellular half-life. Preliminary data indicate that intracellular levels can vary between men and women, by race, ethnicity, and other factors. They also differ by target cell type and activation status. For example, zidovudine-triphosphate (ZDV-TP) concentrations appear to be approximately 3-fold lower in CD4+ T cells than in paired CD4-depleted PBMCs, whereas lamivudine-TP (3TC-TP) concentrations are approximately equal in both subsets.16 Ongoing studies are elucidating these parameters for TDF and FTC in various tissues and CD4+ cell subsets in HIV-infected and uninfected individuals.
Dr. Craig Hendrix (Clinical Pharmacology, Johns Hopkins University School of Medicine) focused on the tissue pharmacology of the NRTIs used for PrEP. Understanding the PD of a given drug requires measuring the concentration of the pharmacologically active moiety at the site of action and relating it to the effects observed. Figure 4 shows the expected relationship between the intracellular triphosphate moiety of an NRTI and the risk of HIV acquisition.
FIG. 4.
Linkage of PK with protective outcome. Determining relationships among drug compartments may allow sampling in more accessible compartments (blood) to allow estimation of drug exposure in more distant compartments (tissue intracellular CD4 cells), which are expected to more closely correlate with seroconversion. (Reprinted with permission of Dr. Craig Hendrix, Johns Hopkins University.)
Dr. Hendrix discussed the considerable challenges involved in quantitating intracellular drug concentrations in genital tract and rectal tissues. Reproducible techniques are required for isolating the tissue of interest from superficial mucus and epithelial cells, processing the tissue to preserve the drug, and isolating the relevant cell subsets. The methods used to recover and measure TFV in tissue homogenates require further development, but current techniques appear to be capable of detecting intracellular TFV levels in cells extracted from tissue 1 week after a single dose of TFV. Epithelial cells predominate in vaginal and colon biopsies, but intracellular TDP levels are greater in CD4+ cells from these tissues.
Dr. Edward Acosta (Clinical Pharmacology, University of Alabama at Birmingham School of Medicine) discussed ways of modeling drug exposures to evaluate and optimize the use of potential PrEP agents. A number of critical questions must be considered:
How much drug is needed in a given compartment to prevent transmission?
Which drug(s) are optimal in this setting?
What is the most efficient way to deliver the drug to the critical compartment(s)?
Once these essential PK/PD questions are answered for TDF, FTC, and other candidate agents, appropriate models can be developed to identify the most effective drugs and predict the best dose and dosing interval.
Session summary
Dr. Courtney Fletcher (College of Pharmacy, University of Nebraska Medical Center) summarized the PK/PD discussion. As a class, the orally administered NRTIs have a wide distribution in the body, including rectal, vaginal, and cervical tissues, cervicovaginal secretions, semen, and the gut-associated lymphoid tissue (GALT). However, there is considerable drug-to-drug variability. Whereas stavudine (d4T) penetrates the female and male genital tracts very poorly (5% and 2%, respectively, as a percent of plasma concentrations), TDF and FTC penetrate extremely well (110–395% and 500–600%, respectively).
The study of the PD of these agents requires knowledge of the triphosphate concentration at the site of action. To date, the intracellular pharmacology of NRTIs, including TDF and FTC, has been largely based on studies in peripheral blood mononuclear cells (PBMCs). Accurate measurement of NRTI triphosphate concentrations in other compartments and cell types is essential, not only for evaluating the candidate agent's ability to prevent transmission but also for understanding potentially drug-limiting adverse reactions.
The formation of the intracellular triphosphate is known to be dependent on a variety of factors including the extracellular concentration, influx and efflux membrane transporters, the activation state of the target cell, intracellular regulatory control mechanisms, gender, and other genotypic factors. Combinations of NRTIs must be studied carefully before they are considered for coadministration. Whereas some combinations such as TDF and FTC have additive to synergistic HIV-inhibitory effects, others have been shown to be antagonistic.
In summary, our knowledge of the PK and PD of NRTIs for PrEP is not yet sufficient to determine whether the results of one ongoing trial can be generalized to other populations and routes of exposure. A number of key research questions should be addressed:
What are the mechanisms by which NRTIs penetrate the biological spaces and tissues relevant to HIV transmission?
Do in vitro measures of anti-HIV activity (IC50, IC95) correlate with the plasma or intracellular concentrations needed to block HIV infection?
Do the optimal combinations and concentrations of NRTIs for HIV prevention differ from those that are optimal for treatment (i.e., for long-term viral suppression and prevention of mutations)?
What are the PK/PD contributions to the heterogeneity in PrEP outcomes?
Summary of Break-Out Discussions
The break-out discussions proceeded in a similar manner in each group. There was a clear consensus that we do not yet know enough about either the biology of transmission or the PK/PD of the active agents in the relevant target tissues to generalize the results of any one of the current trials to other populations and settings. It was noted, however, that when the responsible DSMBs and other advisory groups address these questions in the future they will need to carefully consider new data as they become available.
A key question discussed by each group was whether there is a common pathway by which all HIV-1 sexual transmissions take place. If this is the case, the route of exposure to the virus would be less important than whether the active agents achieve protective levels in the relevant tissues. The route, dose, and frequency of administration required to achieve and maintain these levels for the required duration of time (before, at, and/or after exposure) would be paramount. Even if the virus can cross the epithelium by more than one mechanism and establish local infection in multiple cell types, there may still be a “final common pathway” by which systemic infection takes place.
On the other hand, if there are multiple distinct pathways by which sexual transmission occurs, different PrEP strategies may have substantially different levels of efficacy for different populations and routes of exposure. This might be particularly marked for daily versus coitally related dosing and/or for different formulations and delivery systems. These factors could have a critical impact on whether the agents reach and remain at protective levels in the target tissue(s) for the required period of time before, at, and/or after exposure.
The break-out groups agreed that there were also multiple other reasons for allowing each of the current trials to go to completion. These included gender, ethnic, behavioral, viral, and other differences across the various study populations. These factors might be associated with differences in drug absorption, distribution, and elimination; the frequency and route of HIV-1 exposure; relevant coinfections; substance use and other risk behaviors; product acceptability and adherence; and viral clade(s), activation phenotype, and level of primary antiretroviral drug resistance.
Participants from the FDA emphasized that when a product is intended for use by HIV-1-uninfected, healthy persons the evidence for safety must be particularly strong. Data from multiple populations and studies were preferable. It was also noted that different risk communities, national governments, and donor agencies [e.g., President's Emergency Plan for AIDS Relief (PEPFAR)] could come to different conclusions regarding what constitutes adequate evidence for safety and/or effectiveness for a given population and setting. It is also possible, for example, that what is considered an acceptable risk-benefit ratio may differ substantially for IDUs and high-risk adolescents.
The break-out groups did not expect the results of CAPRISA 004 to have a direct impact on the conduct of the oral PrEP trials, given that the product was dosed before and after coitus (not daily), and the study design was “proof of concept.” The CAPRISA study team trial has now reported that 1% tenofovir gel was associated with a 39% reduction overall in HIV-1 acquisition.17
Conclusions
In summary, there was a clear consensus that we do not yet know enough about the mechanism(s) of transmission, the PK/PD of the active agents in the relevant target cells and tissues, and other important factors to generalize the results of one PrEP trial to other populations and settings. Data from multiple populations and settings are necessary to make a comprehensive assessment of both safety and effectiveness. Nevertheless, this will need to be carefully reconsidered as new data become available from basic research, ongoing pharmacological studies, and the PrEP trials themselves.
Acknowledgments
The authors wish to thank the BMGF and DAIDS, NIAID, NIH [via its contract for conference planning and support services with B L Seamon (BLS) Corporation] for sponsoring this consultation. The authors also wish to extend their thanks to the organizing committee—Dr. Stephen Becker, BMGF (external member); and Drs. Paul Black, Roberta Black, David Burns, Diana Finzi, Jim Turpin, Fulvia Veronese, and Mrs. Sheryl Zwerski [internal (NIH/NIAID/DAIDS) members]—for their critical contributions in developing the workshop's objectives and agenda. Furthermore, we wish to thank the facilitators of the breakout sessions, Drs. Hoosen Coovadia (University of KwaZulu-Natal) and Veronica Miller (George Washington University), and the rapporteurs, Drs. Victor DeGruttola (Harvard School of Public Health) and Ian McGowan (Magee-Women's Research Institute/University of Pittsburgh School of Medicine) for their outstanding effort in highlighting important issues to consider for the field. Lastly, we wish to thank Ms. Jennifer Coulter and Mrs. Jean Morrow, Henry M. Jackson Foundation (HJF), for their assistance in all aspects of the workshop (in conjunction with BLS), and Mr. Lester Freeman (HJF) for his meticulous editorial assistance and contributions to the workshop. The authors are indeed grateful to all who participated in and supported this workshop in an effort to advance the field.
Participants
Participants in the Consultation on the Implications of PrEP Trial Results were Edward Acosta, Division of Clinical Pharmacology, University of Alabama at Birmingham; Peter Anderson, Pharmaceutical Sciences, University of Colorado Denver; Peter Anton, David Geffen School of Medicine, University of California, Los Angeles; Sally Blower, Center for Biomedical Modeling, University of California, Los Angeles; Connie Celum, Global Health and Medicine, University of Washington; Cecilia Cheng-Mayer, Aaron Diamond AIDS Research Center, Rockefeller University; Myron Cohen, Institute of Global Health and Infectious Diseases, University of North Carolina at Chapel Hill; Hoosen Coovadia, Reproductive Health and HIV Research Unit, University of the Witwatersrand; Jennifer Deese, Family Health International; Victor DeGruttola, Department of Biostatistics, Harvard School of Public Health; Courtney Fletcher, College of Pharmacy, University of Nebraska Medical Center; Lawrence Gostin, O'Neill Institute for National and Global Health Law, Georgetown University Law Center; Robert Grant, Gladstone Institute, University of California, San Francisco; Ronald Gray, Department of Population, Family, and Reproductive Health, Johns Hopkins Bloomberg School of Public Health; Yasmin Halima, Global Campaign for Microbicides; Catherine Hankins, Department of Evidence, Monitoring, and Policy, United Nations Joint Program on HIV/AIDS; Craig Hendrix, School of Medicine, Johns Hopkins University; Florian Hladik, Fred Hutchinson Cancer Research Center and University of Washington, Seattle; Thomas Hope, Department of Cell and Molecular Biology, Northwestern University Medical School; Angela Kashuba, School of Pharmacy, University of North Carolina at Chapel Hill; Brandon Keele, AIDS and Cancer Virus Program, National Cancer Institute, Frederick; Javier Lama, Investigaciones Medicas en Salud, Lima and School of Public Health, University of Washington; Jeanne Marrazzo, Division of Allergy and Infectious Diseases, University of Washington School of Medicine; Ian McGowan, Magee-Women's Research Institute, University of Pittsburgh School of Medicine; Veronica Miller, Forum for Collaborative HIV Research, George Washington University; Frances Priddy, International AIDS Vaccine Initiative; Walton Senterfitt, Community HIV/AIDS Mobilization Project; Jur Strobos, Forum for Collaborative HIV Research, George Washington University; Lut Van Damme, Family Health International; Mitchell Warren, AVAC, Global Advocacy for AIDS Prevention; Charles Wira, Department of Physiology and Cell Biology, Dartmouth Medical School; and Jennifer Coulter, Lester Freeman, and Jean Morrow, Henry M. Jackson Foundation; and Stephen Becker and Renee Ridzon, Global Health-HIV, Bill and Melinda Gates Foundation; Lee Claypool and Emmanuel Njeuhmeli, Office of Population and Reproductive Health Research, U.S. Agency for International Development; Lynn Paxton and Dawn Smith, Division of HIV/AIDS, U.S. Centers for Disease Control and Prevention; Debra Birnkrant, Linda Lewis, Jeffrey Murray, Jules O'Rear, and Mary Singer, Division of Antiviral Products, U.S. Food and Drug Administration; and Paul Black, Roberta Black, David Burns, Nandita Chopra, Vanessa Elharrar, Diana Finzi, Jeff Nadler, Jim Turpin, Fulvia Veronese, and Sheryl Zwerski, Division of AIDS, National Institute of Allergy and Infectious Diseases.
Writing Committee
The writing committee consisted of Fulvia Veronese, Peter Anton, Courtney V. Fletcher, Victor DeGruttola, Ian McGowan, Stephen Becker, Sheryl Zwerski, and David Burns.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.AVAC. Global Advocacy for HIV Prevention: Ongoing ARV-based prevention (oral PrEP and topical microbicide) trials (December 2009) http://www.avac.org/ht/a/GetImageAction/i/4474. [Feb 5;2010 ]. http://www.avac.org/ht/a/GetImageAction/i/4474
- 2.Peterson L. Taylor D. Roddy R. Belai G. Phillips P. Nanda K. Grant R. Clarke EEK. Doh AS. Ridzon R. Jaffe HS. Cates W. Tenofovir disoproxil fumarate for prevention of HIV infection in women: A phase 2, double-blind, randomized, placebo-controlled trial. [Feb 8;2010 ];PLoS Clin Trials. 2007 2:e27. doi: 10.1371/journal.pctr.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Extended safety study of tenofovir disoproxil fumarate (TDF) among HIV-1 negative men. http://clinicaltrials.gov/ct2/show/NCT00131677. [Feb 8;2010 ]. http://clinicaltrials.gov/ct2/show/NCT00131677
- 4.Centers for Disease Control and Prevention. Botswana TDF/FTC oral HIV prophylaxis trial. http://clinicaltrials.gov/ct2/show/NCT00448669?term = botswana&rank = 1. [Feb 8;2010 ]. http://clinicaltrials.gov/ct2/show/NCT00448669?term = botswana&rank = 1
- 5.Center for Biologics Evaluation and Research. Guidance for industry: providing clinical evidence of effectiveness for human drug and biological products. Food and Drug Administration; Rockville, MD: 1998. [Sep 30;2010 ]. [Google Scholar]
- 6.Fleming TR. Richardson BA. Some design issues in trials of microbicides for the prevention of HIV infection. J Infect Dis. 2004;190:666–674. doi: 10.1086/422603. [DOI] [PubMed] [Google Scholar]
- 7.Eypasch E. Lefering R. Kum CK. Troidl H. Probability of adverse events that have not yet occurred: A statistical reminder. BMJ. 1995;311:619–620. doi: 10.1136/bmj.311.7005.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wira CR. Fahey JV. A new strategy to understand how HIV infects women: Identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;1;22(15):1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGowan IM. Elliott J. Cortina G. Andrews K. Adler A. Cho D. Anton PA. HPTN-056: Characterization of baseline mucosal indices of injury and inflammation in men for use in rectal microbicide (RM) trials. J AIDS. 2007;46:417–425. doi: 10.1097/QAI.0b013e318156ef16. [DOI] [PubMed] [Google Scholar]
- 10.Keele BF. Giorgi EE. Salazar-Gonzalez JF. Decker JM. Pham KT. Salazar MG. Sun C. Grayson T. Wang S. Li H. Wei X. Jiang C. Kirchherr JL. Gao F. Anderson JA. Ping LH. Swanstrom R. Tomaras GD. Blattner WA. Goepfert PA. Kilby JM. Saag MS. Delwart EL. Busch MP. Cohen MS. Montefiori DC. Haynes BF. Gaschen B. Athreya GS. Lee HY. Wood N. Seoighe C. Perelson AS. Bhattacharya T. Korber BT. Hahn BH. Shaw GM. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. PMCID2387184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haaland RE. Hawkins PA. Salazar-Gonzalez J. Johnson A. Tichacek A. Karita E. Manigart O. Mulenga J. Keele BF. Shaw GM. Hahn BH. Allen SA. Derdeyn CA. Hunter E. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathogens. 2009;5(1):e1000274. doi: 10.1371/journal.ppat.1000274. PMID: 19165325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrahams M-R. Anderson JA. Giorgi EE. Seoighe C. Mlisana K. Ping L-H. Athreya GS. Treurnicht F. Keele BF. Wood N. Gonzalez-Salazar J. Passmore J. Roberts L. Bhattacharya T. Chu H. Hoffman I. Galvin S. Mapanje C. Kazembe P. Thebus R. Fiscus S. Hide W. Cohen MS. Abdool Karim S. Haynes BF. Shaw GM. Hahn BH. Korber BT. Swanstrom R. Williamson C for the CAPRISA Acute Infection Study Team and the Center for HIV-AIDS Vaccine Immunology Consortium. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-Poisson distribution of transmitted variants. J Virol. 2009;83:3556–3567. doi: 10.1128/JVI.02132-08. PMID: 19193811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H. Bar KJ. Wang S. Decker JM. Chen Y. Sun C. Salazar-Gonzalez JF. Salazar MG. Learn GH. Morgan CJ. Schumacher JE. Hraber P. Giorgi EE. Bhattacharya T. Korber BT. Perelson AS. Eron JJ. Cohen MS. Hicks CB. Haynes BF. Markowitz M. Keele BF. Hahn BH. Shaw GM. High multiplicity of infection by HIV-1 in men who have sex with men. http://www.ncbi.nlm.nih.gov/pubmed/20485520. PLoS Pathog. 2010;6(5):e1000890. doi: 10.1371/journal.ppat.1000890. Epub 2010 May 13. PMID: 20485520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bar KJ. Li H. Chamberland A. Tremblay C. Routy JP. Grayson T. Sun C. Wang S. Learn GH. Morgan CJ. Schumacher JE. Haynes BF. Keele BF. Hahn BH. Shaw GM. Wide variation in the multiplicity of HIV-1 infection among injection drug users. http://www.ncbi.nlm.nih.gov/pubmed/20375173. J Virol. 2010;84(12):6241–6247. doi: 10.1128/JVI.00077-10. Epub 2010 Apr 7. PMID: 20375173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor S. Jayasuriya A. Dufty N. Gilleran G. Berry A. Back D. Smit E the Birmingham Heartlands HIV Study Group. Darunavir (DRV) concentrations exceed the protein-corrected (PC) EC50 for wild type HIV in the semen of HIV-1 positive infected men. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. 2010. [Google Scholar]
- 16.Anderson PL. Zheng JH. King T. Bushman LR. Predhomme J. Meditz A. Gerber J. Fletcher CV. Concentrations of zidovudine- and lamivudine-triphosphate according to cell type in HIV-seronegative adults. AIDS. 2007;21:1849–1854. doi: 10.1097/QAD.0b013e3282741feb. [DOI] [PubMed] [Google Scholar]
- 17.Karim QA. Karim SS. Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010. Jul 19, http://www.sciencemag.org/cgi/content/abstract/science.1193748v1. [Jul 24;2010 ]. http://www.sciencemag.org/cgi/content/abstract/science.1193748v1 [Epub ahead of print] PubMed PMID: 20643915. [DOI] [PMC free article] [PubMed]