Abstract
The current societal environment is marked by overabundant accessibility of food coupled with a strong trend of reduced physical activity, both leading to the development of a constellation of disorders, including central obesity, insulin resistance, dyslipidemia, and hypertension (metabolic syndrome). Prolonged calorie restriction (CR) has been shown to extend both the median and maximal lifespan in a variety of lower species such as yeast, worms, fish, rats, and mice. Mechanisms of this CR-mediated lifespan extension are not fully elucidated, but possibly involve significant alterations in energy metabolism, oxidative damage, insulin sensitivity, inflammation, and functional changes in both the neuroendocrine and sympathetic nervous systems. Here we review some of the major physiological, psychological, and behavioral changes after 6 months of CR in overweight otherwise healthy volunteers. Special emphasis is given to the first completed clinical studies that have investigated the effects of controlled, high-quality energy-restricted diets on both biomarkers of longevity and on the development of chronic diseases related to age in humans. With the incremental expansion of research endeavors in the area of energy or caloric restriction, data on the effects of CR in animal models and human subjects are becoming more accessible. Antioxid. Redox Signal. 14, 275–287.
Introduction
Antiaging research by modern scientists continues to echo the quest of the Spanish explorer Ponce de Leon, who searched for the “Fountain of Youth” on the shores of Florida in the early 1500s. Humans are no longer satisfied with simply living longer; they want increased quality of life (QOL) and prolonged health during their senior years. Basic and clinical research is therefore conducted to understand the physiological and molecular mechanisms of aging with the intent to postpone and possibly alleviate many of the illnesses associated with the aging process.
Ironically, as researchers aim to unravel the mysteries of delaying the biological aging process, the current societal environment is marked by overabundant accessibility of food coupled with a strong trend of reduced physical activity. As obesity rates have risen to over 30% among Americans (21), so has the prevalence of obesity-related chronic diseases such as diabetes mellitus, heart disease, and stroke. This alarming increase in obesity is further coupled with a lower age of onset for the emergence of obesity-related comorbidities. It is now understood that obesity may cause up to 300,000 deaths per year in the United States (1). Alarmingly, it now seems that babies born at the beginning of the 21st century will have shorter life expectancies than their parents (59).
Before the development of frank obesity, cardiovascular disease (CVD), and diabetes, individuals developed a constellation of disorders, including central obesity, insulin resistance, dyslipidemia, and hypertension, often termed the metabolic syndrome. Individuals with three or more of these key disorders have 2–3 times greater risk of dying or being struck by a heart attack or stroke and 3–5 times greater risk of developing diabetes mellitus (30, 46). It is estimated that worldwide one in three or four adults has the metabolic syndrome (57). The first line of treatment is to adopt a healthy lifestyle (46). However, the large individual variability in response to diet and exercise represents a huge challenge in clinical practice. Also, living in an obesogenic environment makes it often difficult for overweight or obese individuals to adhere to strict lifestyle modifications. A better understanding of the genetic and environmental influences in the physiopathology of the metabolic syndrome could ultimately deliver a customized treatment to those individuals who do not respond to intensive lifestyle changes and some medications.
Etiology of Obesity
Weight gain results from a sustained imbalance between energy intake and energy expenditure favoring positive energy balance. However, this simple statement belies the complex, multifactorial nature of obesity and the numerous biological and behavioral factors that can affect both sides of the energy balance equation.
Longitudinal studies in the same individuals over time have indicated that relative to body size, several factors can predict weight gain over time, including low metabolic rate, high respiratory quotient (impaired fat oxidation), insulin sensitivity, low sympathetic nervous system activity, and low plasma leptin concentrations (67). When an individual gains weight, the original abnormal metabolic state becomes normalized. This metabolic normalization with weight gain explains why cross-sectional studies are unable to identify metabolic risk factors for obesity. Weight gain thus causes an increase in metabolic rate, a decrease in respiratory quotient (better fat oxidation), a decrease in insulin sensitivity, an increase in sympathetic nervous activity, and an increase in plasma leptin concentrations, all of which serve to counteract further weight gain.
Aging and Obesity
Aging is associated with an increased risk for metabolic disorders, including overweight, obesity, insulin resistance, type 2 diabetes, atherosclerosis, and cancer. Cross-sectional and longitudinal studies suggest that overconsumption of energy-dense foods (20) and lack of physical activity (33) are the leading causes of weight gain, obesity, and their related health issues (10). Recently, researchers have learned that while increased adipose tissue per se is a health concern, the storage and distribution of fat within the body also has important implications for health. In particular, adipose tissues stored centrally in the visceral compartment of the abdomen and in nonadipose tissues, such as liver, heart, pancreas, and skeletal muscle, are considered to be metabolic abnormalities that precede the development of insulin resistance, impaired glucose tolerance, and hyperlipidemia. As individuals age, body weight, even if maintained, is composed of increased fat mass, decreased fat-free mass (109), and increased ectopic fat stores in the abdominal visceral compartment (83), the liver, and the skeletal muscle (15). These body composition changes are associated with increased incidence and prevalence of glucose intolerance and diabetes in older persons (23, 69). It therefore seems that the link between aging and chronic disease may be inevitable in our current obesogenic environment. Interventions that can attenuate the age-associated changes in body composition, therefore, could delay (even prevent) the onset of metabolic disturbances of aging and result in extended lifespan with better QOL.
Caloric Restriction
Calorie restriction (CR), a dietary intervention that is low in calories but maintains proper nutrition, is the only intervention known to date that consistently decreases the biological rate of aging and increases both average and maximal lifespan. Since the first report of prolonged lifespan in rodents more than 70 years ago (53), CR is gaining momentum as a nonpharmacological intervention with the potential to ward off age-associated diseases and delay death (Fig. 1). While the first observations were reported in rodents, similar observations have been reported across a wide range of species (Fig. 2), including yeast, worms, spiders, flies, fish, mice, and rats (32). While the effects of CR in longer-lived species remain unknown, results reported thus far from three nonhuman primate colonies suggest that CR might have a similar effect in longer-lived species. While lifespan data remain inconclusive (35), CR monkeys display a substantially reduced age-related morbidity (8, 41). In humans, data from controlled trials are lacking and of course no long-term prospective trials of CR have been conducted with survival being the primary end-point (31). There is, however, a lot that can be learned from a handful of epidemiological and cross-sectional observations in longer-lived humans, centenarians, and individuals who self-impose CR.
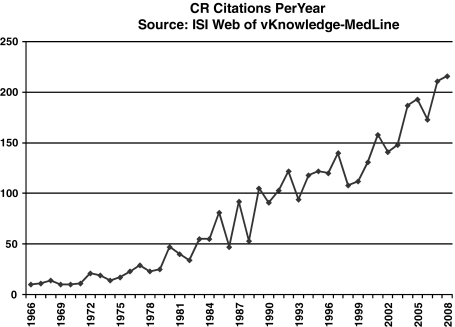
FIG. 1.
Caloric restriction literature. As shown by the ISI Web of Knowledge–MedLine® from 1966 until 2008, the numbers of published studies on caloric restriction have increased almost exponentially topping more than 200 citations per year for the last 2 years.
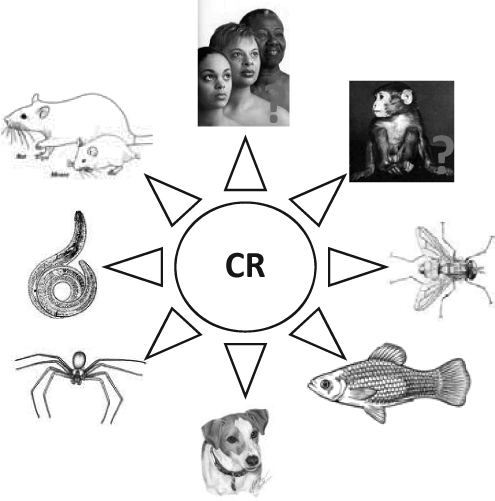
FIG. 2.
The effect of caloric restriction on lifespan. Prolonged lifespan in animals was first reported in rodents more than 70 years ago and since then similar observations are noted across a wide range of species, including yeast, worms, spiders, flies, fish, and dogs. Results reported thus far from three nonhuman primate colonies suggest that CR might have a similar effect in longer-lived species. Whether the benefits of CR extend to humans is not known. CR, calorie restriction.
Strong evidence shows that long-term energy restriction in lean and obese subjects improves insulin sensitivity, a mechanism by which CR may act to extend lifespan (97, 98). Additionally, prolonged CR reduces fasting glucose and insulin concentration, two factors believed to contribute to the aging process due to protein glycation (73) and mitogenic action (84), respectively. This compelling evidence suggests that weight loss due to CR may be the most effective means of improving insulin sensitivity, thereby decreasing the risk for the development of diabetes mellitus.
Caloric Restriction: Effects on Lifespan and Healthspan
Caloric restriction (CR) has been shown since the 1930s by McCay et al. to retard the aging process (53), extending the median and maximal lifespan in various models and species (96). While the exact mechanisms through which CR is able to extend the lifespan have yet to be fully elucidated, CR reduces metabolic rate and oxidative damage, improves markers of age-related diseases such as insulin resistance for diabetes, and has been shown to alter neuroendocrine activities in animals (31). Results from studies in rhesus monkeys suggest that prolonged CR can also oppose many age-associated pathophysiological changes, including learning and behavior changes, plasma insulin concentrations, and resting energy expenditure (7, 8, 77). Since many changes associated with prolonged CR are important to the health and survival of humans, and excessive caloric intake is associated with morbidity and development of chronic diseases, it has become an important research objective to assess the feasibility, the safety, and the effects of prolonged CR in well-controlled human trials.
Centenarians from Okinawa
Probably the most intriguing epidemiological evidence supporting the role of CR in lifespan extension in humans comes from the Okinawans (37). Compared to most industrialized countries, Okinawa, Japan, has 4–5 times the average number of centenarians with an estimated 50 in every 100,000 people (36). Reports from the Japanese Ministry of Health, Labor, and Welfare show that both the average (50th percentile) and maximum (99th percentile) lifespan are increased in Okinawans (Fig. 3). From age 65, the expected lifespan in Okinawa is 24.1 years for women and 18.5 years for men compared to 19.3 years for women and 16.2 years for men in the United States (104). What is interesting about this population is that a low caloric intake was reported in school children on the island more than 40 years ago and later studies confirmed a 20% CR in adults residing on Okinawa compared to mainland Japan (87). A recent estimate of the energy balance of a cohort of Okinawa septuagenarians during youth to middle age suggested a 10%–15% energy deficit (102). This energy deficit can be attributed to laborious occupations and daily activities as farmers and a diet that was rich in nutrients yet low in energy density (102).
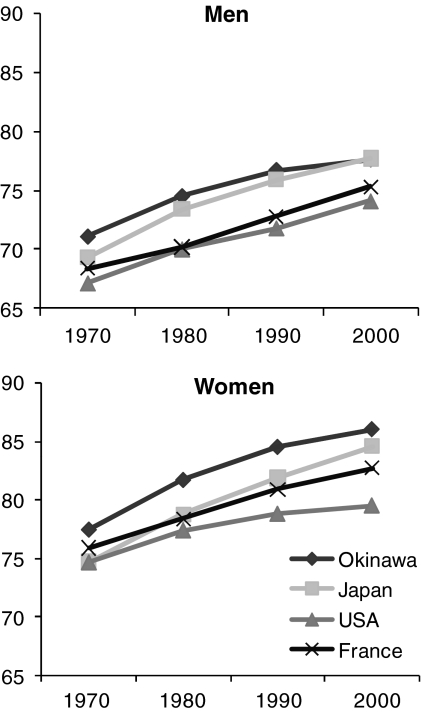
FIG. 3.
Life expectancy at birth in Okinawa, Japan. Okinawa is an isolated island off mainland Japan that has the lowest risk for age-related diseases and the longest life expectancy. The figure shows the average life expectancy at birth in Okinawa, Japan, United States, and France for men (top panel) and women (bottom panel) (103).
The Vallejo study
To our knowledge, there is only one study that was designed to test the effects of CR without malnutrition in nonobese humans (91). This was a study of alternate day feeding in 120 men whereby the 60 participants in the CR group received an average of 1500 kcal per day for 3 years, whereas the 60 others were ad libitum. This amounted to approximately 35% CR compared to the control group. While the initial report was brief, post hoc analyses conducted several years later (85) indicated that death rate tended to be lowered in the CR group and hospital admissions were reduced in these individuals by approximately 50% (123 days for CR vs. 219 days for Control).
Unexpected CR in biosphere 2
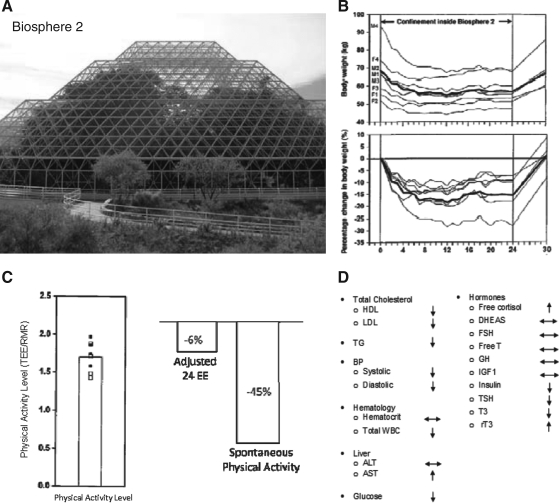
The unexpected low availability of food during the 2-year Biosphere 2 experiment provided a unique opportunity to observe the effects of CR in a group of nonobese humans (Fig. 4). Biosphere 2 was an enclosed 3.15-acre ecological laboratory that housed seven ecosystems or biomes resembling the earth: rainforest, savannah, ocean, marsh, desert, and agriculture and human/animal habitats (95). For 2 years, eight individuals, including Dr. Roy Walford, were completely isolated within this mini-world, where 100% of the air and water was recycled and all the food grown inside. Due to unforeseen problems with agriculture early on, food supply became quickly insufficient. Food intake for the eight individuals was projected at ∼2500 kcal/day and estimates from food records maintained by one of the biospherians suggested that diets were restricted by ∼750 kcal/day in each person during the first 6 months. The resulting ∼15% weight loss in the Biospherians was associated with many physiological, hematological, biochemical, and metabolic alterations (98, 101), consistent with caloric-restricted rodents and primate, including reductions in insulin, core temperature, and metabolic rate (41).
FIG. 4.
Caloric restriction in Biosphere 2. Biosphere 2 (A), a 3.15-acre ecological enclosure, provided an unexpectedly low availability of food for eight individuals who were housed inside for 2 years in the early 1990s. This study of nature of caloric restriction resulted in ∼15% weight loss (B), in changes in energy expenditure and physical activity (C), and many hematological, biochemical, and metabolic alterations (D) consistent with caloric-restricted rodents and primate, including reductions in insulin, core temperature, and metabolic rate (98, 101).
Randomized controlled trials of caloric restriction in nonobese humans
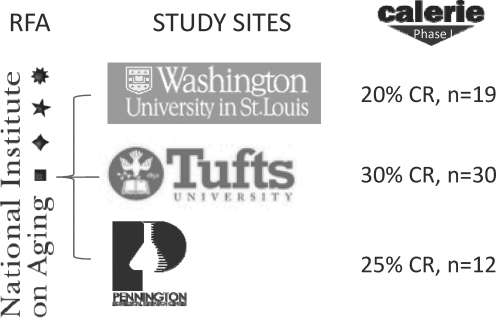
As for randomized controlled trials, results from a 2-year study of CR in humans are only a few years away. The National Institute on Aging is sponsoring a trial, comprehensive assessment of the long-term effect of reducing intake of energy (CALERIE), which is for the first time, scientifically testing the effects of 25% CR in ∼150 nonobese healthy men and women aged 25–45 years (Fig. 5). Three clinical sites are involved in the trial: Washington University in St. Louis, MO; Tufts University in Boston, MA; and the Pennington Biomedical Research Center in Baton Rouge, LA. The protocol and endpoints for this multicenter trial were developed from experience acquired in three independent phase 1 trials conducted at each clinical site (17, 31, 66).
FIG. 5.
The CALERIE Study. The first randomized trial of caloric restriction in humans is sponsored by the National Institute on Aging. In response to a request for applications (RFA), three clinical sites, Washington University, Tufts University, and Pennington Biomedical Research Center, were awarded funding. In the first phase of CALERIE, three independent studies were conducted and 61 individuals participated in a trial of 20%–30% caloric restriction for 6 or 12 months. In the second phase of CALERIE, 250 volunteers aged 21–45 years, with a body mass index between 23 and 27.9, were randomized 2/1 to a 2-year 25% caloric restriction or an ad libitum diet. CALERIE, comprehensive assessment of the long-term effect of reducing intake of energy.
The remainder of this review will concentrate on the results from the phase 1 study conducted at the Pennington Center. For 6 months, 48 men and women were randomized to one of four treatment groups (4, 12, 43, 48, 50, 51, 71, 108). For the CR group, individuals were restricted to 75% (a 25% CR) of their weight maintenance energy requirements. The other groups were (a) CR plus exercise group for which the calorie deficit was also 25% from weight maintenance but half (12.5%) was achieved by CR and half (12.5%) by increasing energy expenditure with structured aerobic exercise, (b) a low calorie diet group in which participants consumed 890 kcal/day to achieve a 15% weight loss and thereafter followed a weight maintenance diet, and (c) a healthy diet control group, which followed a weight maintaining diet based on the American Heart Association Step 1 diet. The effects of the CR interventions were determined from changes in various physiological and psychological endpoints after 3 and 6 months.
Results from the CALERIE Study: Physiological Effects of Caloric Restriction
Aging is considered to be either primary, that is, the inevitable deterioration of cell and tissue structure and function that occurs independent of disease, lifestyle, and environmental causes, or secondary, where the decline in tissue structure and function occurs as a result of external influences, including diseases (34). Attenuation of primary aging therefore results in an increase in maximal lifespan, whereas delays in the progression of age-related disease or secondary aging increases mostly average lifespan. CR is the only known intervention that can slow primary aging and protect against secondary aging. Six months of CR produced favorable alterations in physiological and behavioral outcomes. Results from the first randomized study of caloric restriction (CALERIE) at the Pennington Center are discussed below:
Body composition
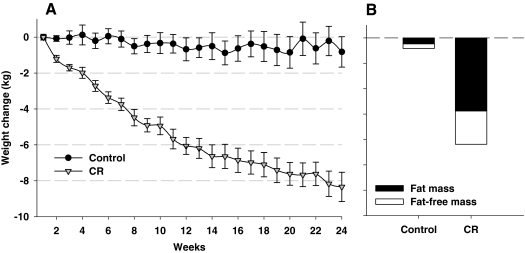
Throughout the 6-month intervention there was a progressive decline in body weight (Fig. 6) that reached ∼10% for the CR group at the completion of the study (71). Body composition analysis by dual X-ray absorptiometry (Fig. 6) and multislice computed tomography showed that the loss of tissue mass was attributable to significant reductions in both fat mass (CR: −24% ± 3%) and fat-free mass (CR: −4% ± 1%), and a 27% decrease in both visceral and subcutaneous fat depots. Abdominal adiposity, particularly abdominal visceral adiposity, is associated with increased risk for age-related diseases. It was interesting to note that the fat distribution within the abdomen was not altered by CR (71). The inability of caloric restriction to alter the distribution of fat suggests that individuals are genetically or epigenetically programmed for fat storage in a particular pattern and that this programming cannot be easily overcome by weight loss. In support of this contention, twin studies have shown that fat mass and regional fat distribution are largely determined by genetic factors and that genetic heritage can explain changes in body composition and fat distribution during negative energy balance (9, 47). We also observed a reduction in subcutaneous abdominal mean fat cell size by ∼20%, a lowering of hepatic lipid by ∼37% but no change in skeletal muscle lipid content (43).
FIG. 6.
CALERIE: Effect of 25% CR on body weight and body composition. Our 6-month study of 25% caloric restriction resulted in a progressive decline in body weight (A) that reached ∼10% the completion of the study (71). Body composition analysis by dual X-ray absorptiometry (B) showed that the loss of tissue mass was attributable to significant reductions in both fat mass (CR: −24% ± 3%) and fat-free mass (CR: −4% ± 1%).
Biomarkers of longevity
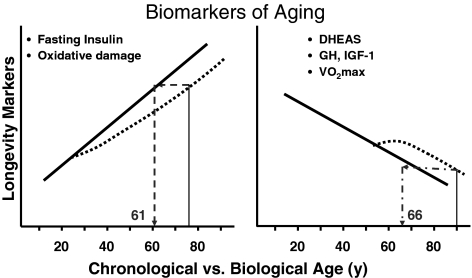
A “biomarker of aging or longevity” is considered to be any parameter that reflects physiological or functional age; it must undergo significant age-related changes, be slowed or reversed by treatments that increase longevity (e.g., caloric restriction), and must be reliably measured (Fig. 7). Biomarkers of aging are also presumed to be indicative of life expectancy. Numerous biomarkers have been identified in rodents and primates, including body temperature and hormones such as dehydroepiandrosterone-sulfate (DHEA-S) and insulin (75). In the CALERIE study, two out of the three biomarkers of longevity (75) were improved with 6 months of 25% CR (31). Significant reductions were observed in both fasting insulin concentrations (−29% ± 6%) and core body temperature (−0.20°C ± 0.05°C), whereas DHEA-S was unchanged by the intervention. These findings of course echo results previously reported in nonhuman primates and rodents on CR and long-lived men in The Baltimore Longitudinal Study of Aging (75).
FIG. 7.
Can caloric restriction improve biological age and extend chronological age? This figure summarizes some of the potential biomarkers of aging. It is hypothesized that caloric restriction will change the biological trajectory of these biomarkers and therefore improve biological age and extend chronological age. For example, the left panel shows an individual aged 75 years. With prolonged caloric restriction, it is hypothesized that fasting insulin and oxidative damage will be reduced in this individual. Therefore, an individual although 75 will have a biological age 14 years younger. Similarly, the individual on the right at 90 years with prolonged caloric restriction will be biologically similar to an individual aged 66 years. DHEA-S, dehydroepiandrosterone-sulfate; GH, growth hormone; IGF-1, insulin-like growth factor-1.
Cardiovascular and diabetes risk factors
With heart disease and stroke ranked numbers one and three in the causes of death in the United States (74), delaying the progression of atherosclerotic CVD may be one potential mechanism by which CR promotes longevity. Several risk factors for CVD, including blood lipids, blood pressure, hemostatic factors, inflammatory markers, and endothelial function, are worsened with age (11, 54). At least a portion of these age-related changes appear to be secondary to increases in adiposity and/or reductions in physical activity (19, 56) and, therefore, may be amenable to improvements through prolonged caloric restriction. Six months' CR significantly reduced triacylglycerol and factor VIIc by 18% and 11%, respectively (44). High-density lipoprotein cholesterol was increased, and fibrinogen, homocysteine, and endothelial function were not changed. According to total and high-density lipoprotein cholesterol (expressed as their ratio), systolic blood pressure, age, and gender, estimated 10-year CVD risk was 28% lower after only 6 months of CR.
Insulin resistance is an early metabolic abnormality that precedes the development of hyperglycemia, hyperlipidemia, and overt type 2 diabetes. Both insulin resistance and β-cell dysfunction are associated with obesity (27, 52, 89). Caloric restriction reduces fat mass and delays the development of age-associated diseases such as type 2 diabetes. While it is well established in obese humans that caloric restriction and weight loss improve insulin sensitivity (18, 58), the effects of caloric restriction on insulin sensitivity and therefore diabetes risk are not well understood in overweight and lean individuals. In our study of 6 months CR, we observed a 40% improvement in insulin sensitivity in the CR group, although this did not reach significance (p = 0.08) (43). The acute insulin response to glucose, however, was significantly decreased from baseline (CR: 29% ± 7%, p < 0.01), indicating an improvement in β-cell responsiveness to glucose.
Metabolic adaptation and oxidative stress
One of the most popular proposed theories by which CR promotes lifespan extension is the “rate of living theory” (78). It is hypothesized that a lowering of the metabolic rate (rate of living) reduces the flux of energy with a consequential lowering of reactive oxygen species and rate of oxidative damage to vital tissues (82). Indeed, CR is associated with a robust decrease in energy metabolism, including a lowering of resting metabolic rate (RMR or sleeping metabolic rate), lowering of the thermic effect of meals, and a decrease in the energy cost of physical activity. However, as mentioned earlier, whether total energy expenditure is reduced beyond the expected level (i.e., metabolic adaptation) for the reduction in the metabolizing mass (fat-free and fat mass) following CR is debated.
As expected, absolute 24-h energy expenditure and sleeping metabolic rate (both measured in a respiratory chamber) were significantly reduced from baseline with CR (p < 0.001). Importantly, however, both 24-h sedentary and sleeping energy expenditures were reduced ∼6% beyond what was expected for the loss of metabolic mass (i.e., fat-free and fat mass) (31). This metabolic adaptation was also observed for RMR measured by a ventilated hood indirect calorimeter (51). These physiological responses were associated with a reduced amount of oxidative stress as measured by DNA damage. DNA damage was reduced from baseline after 6 months in CR (p = 0.0005), but not in controls (31). In addition 8-oxo7,8-dihidro-2′deoxyguanosine was also significantly reduced from baseline in CR (p < 0.0001). These data confirm findings in animals that CR reduces energy metabolism and oxidative stress to DNA, both potentially attenuating the aging process.
Endocrine Adaptations
Thyroid function
Thyroid hormone is an important determinant of energy expenditure and basal metabolic rate (16). Short-term studies of CR in humans have reported alterations in thyroid function. Four weeks of complete fasting resulted in a decrease in triiodothyronie (T3) and an increase in reverse T3, which was associated with a reduction in metabolic rate (90). The CRONIES (a self-selected group engaging on long-term CR) have significantly lower T3 but not thyroxine (T4) or thyroid-stimulating hormone (TSH) concentrations compared with age-, sex-, and weight-matched controls (24). In the CALERIE study, plasma T3 concentrations were reduced from baseline in the CR group after 3 (p < 0.01) and 6 months (p < 0.02) of intervention (31). Similar results were found for the change in plasma T4 in response to the treatment. When the data of the subjects in the three CR groups were combined into one intervention sample, we observed significant linear relationships between the change in plasma thyroid hormones and the degree of metabolic adaptation in 24-h sedentary energy expenditure after 3 months of intervention (T3; r = 0.40, p = 0.006 and T4; r = 0.29, p = 0.05) (31).
The somatotropic axis
Aging is marked by a reduction in both growth hormone (GH) and insulin-like growth factor-1 (IGF-1) concentrations in healthy adults resulting from a reduced amount of GH secreted at each burst without alterations of burst frequency or GH half life (92). Unlike rodents, weight loss via energy restriction in humans increases GH (81). After 6 months of CR, 11-h mean GH concentrations were not changed with CR, nor was the secretory dynamics in terms of the number of secretion events, secretion amplitude, and secretion mass (unpublished data). The fasting plasma concentration of ghrelin, a GH secretagogue, was significantly increased from baseline, but IGF-1 was unaffected. Despite a significant reduction in weight and visceral fat and an improvement in insulin sensitivity, mean GH concentrations were not altered by the 6-month intervention. In agreement with this observation was the finding that both GH and IGF-1 were not affected by the chronic food shortage experienced by the individuals in Biosphere 2 (98).
Dehydroepiandrosterone-sulfate
Given evidence from cross-sectional (60) and longitudinal studies (61) that DHEA-S declines with age, DHEA-S is considered to be a reliable endocrine marker of human aging and longevity (75). It was hypothesized that CR will delay or attenuate the age-associated decline in DHEA-S. In our 6-month study in young individuals (37 ± 2 years), we observed no alteration in DHEA-S (31). Similarly, DHEA-S was not changed with 2 years of energy restriction in the individuals within Biosphere 2 (98). To our knowledge, there has been no report of DHEA-S levels in those individuals from the Calorie Restriction Society (CRONIES) who are self-imposing CR. The lack of agreement between the human and nonhuman primate data is believed to be due to, first, the chronological age of the subjects at the onset of CR and, second, the duration of CR. Young adult monkeys undergoing CR for 3–6 years had an age-related decline in DHEA-S of 3% compared to 30% in monkeys fed ad libitum (42). In contrast, CR initiated in older animals (∼22 years) did not attenuate the age-associated decline in DHEA-S (88). These explanations remain to be tested in longer-term studies of CR in humans.
Physical activity
Daily energy expenditure has three major components: RMR, the thermic effect of food, and the energy cost of physical activity. Investigation of changes in physical activity are important in studies of CR not only because the contribution of physical activity to daily energy expenditure is variable, but also because it is not known if individuals volitionally or nonvolitionally decrease their level of physical activity in an attempt to conserve energy (40). In our study, we observed no change in spontaneous physical activity in a respiratory chamber (51), consistent with earlier reports of no alterations in spontaneous physical activity (68) or posture allocation in obese individuals after weight loss (45). If the hypothesis that spontaneous physical activity is inherited is true (45, 110), it is not surprising that we do not observe a change in spontaneous physical activity with CR.
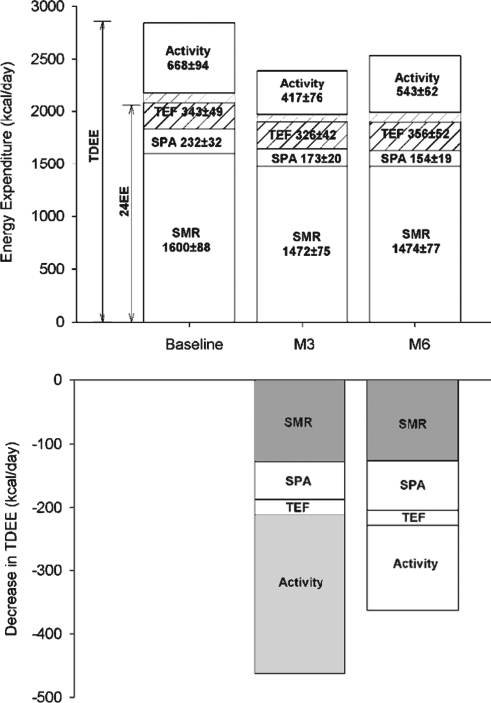
However, with a measure of energy metabolism in free-living conditions (total daily energy expenditure by doubly labeled water; Fig. 8), we found that a metabolic adaptation exists after 3 months (−386 ± 69 kcal/day) but not after 6 months of CR (72). This adaptation was evident even after total daily energy expenditure (TDEE) was adjusted for sedentary energy metabolism (24-h or sleeping energy expenditure), indicating that changes in other components of daily energy expenditure such as physical activity and diet-induced thermogenesis are also involved. In support of this, physical activity level calculated by either the ratios of TDEE to RMR or sleeping metabolic rate (51), or TDEE adjusted for sleeping metabolic rate, was significantly reduced at month 3 by 12% and returned toward baseline values after 6 months of intervention. Interestingly, despite lower physical activity levels, participants reported an improvement in physical functioning, a primary component of QOL. All the effects of caloric restriction on physiological outcomes are summarized in Table 1.
FIG. 8.
CALERIE: Effect of 25% CR on daily energy expenditure. The effect of caloric restriction on all components of daily energy expenditure (top panel). The changes in total daily energy expenditure after 3 and 6 months of CR (bottom panel) are shown and those representing a metabolic adaptation (larger than due to weight loss) are highlighted in gray (72). Combining two state-of-the-art methods (indirect calorimetry and doubly labeled water) for quantifying precisely the complete energy expenditure response to CR in nonobese individuals, we identified a reduction in sedentary energy expenditure that was 6% larger than what could be accounted for by the loss in metabolic size, that is, a metabolic adaptation (31) and a metabolic adaptation in the free-living situation as well. This adaptation comprised not only a reduction in cellular respiration (energy cost of maintaining cells, organs, and tissue alive) but also a decrease in free-living activity thermogenesis (behavioral adaptation). TDEE, total daily energy expenditure.
Table 1.
Summary of the Psychological and Behavioral Responses to 6 Months of Caloric Restriction in Humans
| Psychological/behavioral responses |
|---|
| Development of eating disorder symptoms |
| ↓ Disinhibition |
| ↓ Binge eating |
| ↓ Concern about body size and shape |
| ↔ Fear of fatness |
| ↔ Purgative behavior |
| Depressed mood |
| ↓ Multifactorial assessment of eating disorder symptoms depression scale |
| ↔ Beck depression inventory II |
| Subjective feelings of hunger |
| ↓ Eating inventory, perceived hunger scale |
| ↔ Subjective ratings of hunger, fullness, desire to eat |
| Quality of life |
| ↑ Physical functioning |
| ↔ Vitality |
| Cognitive performance |
| ↔ Verbal memory |
| ↔ Short-term memory and retention |
| ↔ Visual perception and memory |
| ↔ Attention/concentration |
Psychological and Behavioral Effects of Caloric Restriction
Caloric restriction in humans might prove to have positive effects on physical health and longevity, resulting in the practice of CR or the identification of CR mimetics. If people attempt to follow CR for health promotion, important questions must be answered about possible negative effects of CR on psychological well-being, cognitive functioning, mood, and subjective feelings of appetite. Determining the effect of CR on these parameters is critical to learn if adhering to a CR regimen is feasible and if CR has unintended negative consequences that would offset the potential of its health benefits. Phase I of CALERIE provided a unique opportunity to examine the effect of 6 months of CR on psychological and behavioral endpoints. We here summarize the effects of CR on the development of eating disorder symptoms, QOL, mood (symptoms of depression), subjective ratings of appetite, and cognitive function (Table 1).
Development of eating disorder symptoms
One of the most pressing concerns about CR is the potential development of symptoms of eating disorders. This concern is based in part on the Keys (40) study, which found that 50% CR for 6 months among healthy men was associated with the development of eating disorder symptoms, for example, binge eating (28). Additionally, CR or the intent to restrict intake has been associated with the onset of eating disorders, including anorexia (105) and bulimia nervosa (65), and binge eating disorder (106). Hence, there is a need to examine both the benefit and potential harm of CR in humans, particularly for people who are not obese, and to answer important safety questions before CR is recommended (93, 94).
In our study, participants completed an assessment battery that included (a) the multifactorial assessment of eating disorder symptoms (MAEDS), which measures six symptom domains associated with eating disorders (binge eating, purgative behavior, depression, fear of fatness, avoidance of forbidden foods, and restrictive eating) (2); (b) the Eating Inventory, which measures dietary restraint, disinhibition, and perceived hunger (86); and (c) the body shape questionnaire (14), which measures concern about body size and shape.
As reported by Williamson et al. (107), the three dieting groups in CALERIE, including the CR group, reported higher dietary restraint scores in comparison to the control group at months 3 and 6, but measures of eating disorder symptoms did not increase and some decreased. All groups, except the control group, reported a significant reduction in disinhibition at month 6 and binge eating, measured by the MAEDS, decreased for all four groups at months 3 and 6. Concern about body size/shape decreased at 3 and 6 months among the three dieting groups but did not change in the control group. The Fear of Fatness and Purgative Behavior subscales of the MAEDS did not change during CR.
Subjective feelings of hunger
The ability of people to follow CR could be limited by feelings of increased hunger. We evaluated change in appetite ratings during CR using the perceived hunger scale of the Eating Inventory (86) and the Visual Analogue Scales (3), which have been found to be reliable and valid measures of appetite: hunger, fullness, desire to eat, satisfaction, and prospective food consumption (22). With CR, no changes were reported in with ratings of hunger, fullness, or prospective food consumption (3). After 6 months, CR participants reported an increased desire to eat and a decreased satisfaction of appetite. These appetite ratings measured by Visual Analogue Scales, however, were not different from those in the control group.
QOL and mood
The Minnesota Semi Starvation study (40) indicated that CR can negatively affect mood, and therefore the effect of CR on mood and QOL becomes an important factor when considering the feasibility of CR in humans. During CALERIE Phase I, the Medical Outcomes Study Short-Form 36 Health Survey (99, 100) was used to measure QOL, and the Beck Depression Inventory II (6) and depression scale of the MAEDS were used to measure mood. Our results indicate that depressed mood, measured by the BDI-II, did not change during the trial. Additionally, in the CR group, scores on the MAEDS depression subscale decreased at 3 and 6 months in comparison to baseline (107). Together, the results indicate that CR had no negative effect on mood during this trial, and, in fact, symptoms of depressed mood, measured with the MAEDS, decreased in the CR group.
The Medical Outcomes Study Short-Form 36 Health Survey was used to test the effects of CR on two components of QOL—physical functioning and vitality. All dieting groups, but not the control group, had improved physical functioning during the trial. For the CR group, physical functioning was significantly improved from baseline to month 3 and baseline to month 6. CR had no significant effect on vitality.
Cognitive function and performance
Self-reported dieting or caloric restriction has been associated with deficits in cognitive performance (e.g., memory and concentration deficits) (29, 39). Nevertheless, cognitive impairment is frequently mediated by preoccupation with food and body weight (38), suggesting that obsessive thoughts about food and weight, rather than CR, negatively affect cognitive performance. If CR has negative effects on cognitive performance, the feasibility of CR in humans would be in doubt.
In our trial, cognitive performance was evaluated empirically at baseline and months 3 and 6 with a comprehensive neuropsychological battery (49). Verbal memory was measured with the Rey Auditory and Verbal Learning Test (79), short-term memory and retention with the Auditory Consonant Trigram (63, 64), visual perception and memory with the Benton Visual Retention Test (80), and attention/concentration with the Conners' Continuous Performance Test-II (13). During CR, no pattern of memory or attention/concentration deficits emerged and effect sizes were small, indicating that no more than 7% of the variance in change in cognitive performance was due to treatment arm. The degree of daily energy deficit also was not correlated with change in cognitive performance; hence, these data indicate that CR did not have a negative effect on cognitive performance (49). All the effects of caloric restriction on psychological and behavioral outcomes are summarized in Table 1.
The psychological and behavioral findings from CALERIE provide important information about the feasibility and safety of CR in humans. Caloric restriction was not associated with the development of eating disorder symptoms, decreased QOL, depressed mood, or cognitive impairment. In fact, many of these endpoints improved, and changes in subjective ratings of appetite were similar in the CR group to those of the control group. These results suggest that CR might be feasible and have few unintended consequences, at least among overweight individuals. Additional research is needed to determine the feasibility and safety of CR in other samples.
Could CR Increase Longevity in Humans?
The wealth of CR literature in rodents, however, allows us to address some important questions relating to the practicality and feasibility of CR in humans. Relevant and practical questions are as follows: (a) How much CR do we need to improve age-related health and possibly longevity? (b) How long do we need to sustain CR to obtain these benefits? Analysis of 24 published studies of CR in mice or rats indicated a strong negative relationship between survival and energy intake (55), with more CR (up to 55%) associated with longer maximal lifespan.
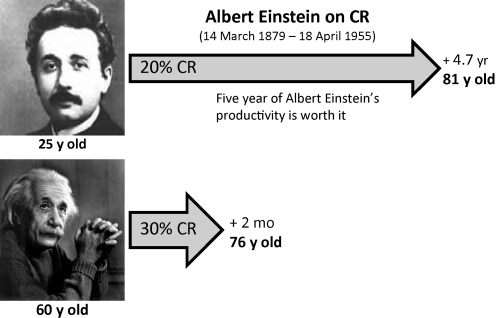
The rodent data indicate that CR has greater benefits when more extreme and sustained over a longer period of time. Using the prediction equations derived from the rodent data above (55), we and others estimated that a 5-year life extension could be induced by 20% CR starting at age 25 and sustained for 52 years, that is, the life expectancy from birth of a male in the United States. However, if a 30% CR was initiated at age 55 for the next 22 years, the gain would only be 2 months (Fig. 9).
FIG. 9.
Caloric restriction and life expectancy in humans. How can caloric restriction impact life expectancy in humans? By extrapolating the data from rodents to humans (55), one can predict the potential effect of caloric restriction in humans (70). As an example, if Albert Einstein started a 20% CR diet at 25 years of age, he could have increased his life expectancy by approximately 5 years. On the other hand, undertaking a 30% CR diet 45 years later (age 60) would have extended his life by only 2 months. Therefore, CR needs to be initiated early in adult life to significantly increase life expectancy.
Certainly, there are individuals who self-impose CR with the calorie restriction with optimal nutrition diet for health and longevity. A group of 18 CRONIES (only 3 women) have recently been studied after 3–15 years of CR (25, 26). Dietary analysis indicated an energy intake ∼50% less than age-matched controls. In terms of body composition, the mean body mass index of the males was 19.6 ± 1.9 kg/m2 with an extremely low percent body fat of ∼7%. Atherosclerosis risk factors including total cholesterol and low-density lipoprotein-c fell within the 10th percentile of values for people in their age group. This report provides further evidence that longer-term CR is highly effective in lowering the risk of developing coronary heart disease and other age-related comorbidities. It remains to be seen if the CRONIES live longer than their age- and sex-matched counterparts.
Concluding Remarks
While the rodent and primate data indicate that lifespan extension is possible with CR, collective analysis of the rodent data suggests that intensity and onset of CR required to induce these effects is probably not suitable for many individuals (70). Epidemiological studies certainly support the notion that a reduced energy intake that is nutritionally sound improves age-associated health. While results of the first randomized trials of CR, although short in duration, suggest a reduction in risk of age-related disease and improvements in some biomarkers of longevity, the ultimate effect of this intervention on lifespan in humans will probably never been determined in the scientific setting. In our short-term study, caloric restriction was not associated with the development of eating disorder symptoms, decreased QOL, depressed mood, or cognitive impairment, all probably indicating the feasibility and safety of CR in humans. However, it is a challenge for most individuals to practice caloric restriction in an obesogenic environment so conducive to overfeeding. Only a very few will be able to practice a lifestyle of caloric restriction and probably benefit from it. There is therefore a need for the search for organic or inorganic compounds that mimic the biological effects of CR. If such compounds, often called “CR mimetics” [such as resveratrol (5, 62)], prove viable in humans, individuals for the most part will opt to enjoy the effects of antiaging via a pill rather than CR.
Abbreviations Used
- CALERIE
comprehensive assessment of reducing intake of energy study
- CR
calorie restriction
- CVD
cardiovascular disease
- DHEA-S
dehydroepiandrosterone-sulfate
- GH
growth hormone
- IGF-1
insulin-like growth factor-1
- MAEDS
multifactorial assessment of eating disorder symptoms
- QOL
quality of life
- RMR
resting metabolic rate
- T3
triiodothyronine
- T4
thyroxine
- TDEE
total daily energy expenditure
Acknowledgments
L.M.R. is supported by a Neil Hamilton-Fairley Training Fellowship awarded by the NHMRC of Australia (ID 349553). E.R. has initiated studies of caloric restriction in nonobese individuals supported by U01 AG20478.
References
- 1.Allison DB. Fontaine KR. Manson JE. Stevens J. VanItallie TB. Annual deaths attributable to obesity in the United States. JAMA. 1999;282:1530–1538. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DA. Williamson DA. Duchmann EG. Gleaves DH. Barbin JM. Development and validation of a multifactorial treatment outcome measure for eating disorders. Assessment. 1999;6:7–20. doi: 10.1177/107319119900600102. [DOI] [PubMed] [Google Scholar]
- 3.Anton SD. Han H. York E. Martin CK. Ravussin E. Williamson DA. Effect of calorie restriction on subjective ratings of appetite. J Hum Nutr Diet. 2009;22:141–147. doi: 10.1111/j.1365-277X.2008.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anton SD. Martin CK. Redman L. York-Crowe E. Heilbronn LK. Han H. Williamson DA. Ravussin E. Psychosocial and behavioral pre-treatment predictors of weight loss outcomes. Eat Weight Disord. 2008;13:30–37. doi: 10.1007/BF03327782. [DOI] [PubMed] [Google Scholar]
- 5.Baur JA. Pearson KJ. Price NL. Jamieson HA. Lerin C. Kalra A. Prabhu VV. Allard JS. Lopez-Lluch G. Lewis K. Pistell PJ. Poosala S. Becker KG. Boss O. Gwinn D. Wang M. Ramaswamy S. Fishbein KW. Spencer RG. Lakatta EG. Le Couteur D. Shaw RJ. Navas P. Puigserver P. Ingram DK. de Cabo R. Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck AT. Brown GK. Steer RA. Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 7.Blanc S. Schoeller D. Kemnitz J. Weindruch R. Colman R. Newton W. Wink K. Baum S. Ramsey J. Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J Clin Endocrinol Metab. 2003;88:16–23. doi: 10.1210/jc.2002-020405. [DOI] [PubMed] [Google Scholar]
- 8.Bodkin NL. Alexander TM. Ortmeyer HK. Johnson E. Hansen BC. Mortality and morbidity in laboratory-maintained Rhesus monkeys and effects of long-term dietary restriction. J Gerontol A Biol Sci Med Sci. 2003;58:212–219. doi: 10.1093/gerona/58.3.b212. [DOI] [PubMed] [Google Scholar]
- 9.Bouchard C. Tremblay A. Genetic influences on the response of body fat and fat distribution to positive and negative energy balances in human identical twins. J Nutr. 1997;127:943S–947S. doi: 10.1093/jn/127.5.943S. [DOI] [PubMed] [Google Scholar]
- 10.Calle EE. Teras LR. Thun MJ. Adiposity and physical activity as predictors of mortality. N Engl J Med. 2005;352:1381–1384. doi: 10.1056/NEJM200503313521322. author reply 1381–1384. [DOI] [PubMed] [Google Scholar]
- 11.Celermajer DS. Sorensen KE. Spiegelhalter DJ. Georgakopoulos D. Robinson J. Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 12.Civitarese AE. Carling S. Heilbronn LK. Hulver MH. Ukropcova B. Deutsch WA. Smith SR. Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conners CK. Conners' Continuous Performance Test (CPT II) Toronto: Multi-Health Systems, Inc.; 2000. [Google Scholar]
- 14.Cooper PJ. Taylor MJ. Cooper Z. Fairburn CG. The development and validation of the Body Shape Questionnaire. International Journal of Eating Disorders. 1987;6:485–494. [Google Scholar]
- 15.Cree MG. Newcomer BR. Katsanos CS. Sheffield-Moore M. Chinkes D. Aarsland A. Urban R. Wolfe RR. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89:3864–3871. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- 16.Danforth E., Jr. Burger A. The role of thyroid hormones in the control of energy expenditure. Clin Endocrinol Metab. 1984;13:581–595. doi: 10.1016/s0300-595x(84)80039-0. [DOI] [PubMed] [Google Scholar]
- 17.Das SK. Gilhooly CH. Golden JK. Pittas AG. Fuss PJ. Cheatham RA. Tyler S. Tsay M. McCrory MA. Lichtenstein AH. Dallal GE. Dutta C. Bhapkar MV. Delany JP. Saltzman E. Roberts SB. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85:1023–1030. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 18.Dengel DR. Pratley RE. Hagberg JM. Rogus EM. Goldberg AP. Distinct effects of aerobic exercise training and weight loss on glucose homeostasis in obese sedentary men. J Appl Physiol. 1996;81:318–325. doi: 10.1152/jappl.1996.81.1.318. [DOI] [PubMed] [Google Scholar]
- 19.DeSouza CA. Shapiro LF. Clevenger CM. Dinenno FA. Monahan KD. Tanaka H. Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 20.Drewnowski A. Energy density, palatability, and satiety: implications for weight control. Nutr Rev. 1998;56:347–353. doi: 10.1111/j.1753-4887.1998.tb01677.x. [DOI] [PubMed] [Google Scholar]
- 21.Flegal KM. Carroll MD. Ogden CL. Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 22.Flint A. Raben A. Blundell JE. Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 23.Folsom AR. Kushi LH. Anderson KE. Mink PJ. Olson JE. Hong CP. Sellers TA. Lazovich D. Prineas RJ. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women's Health Study. Arch Intern Med. 2000;160:2117–2128. doi: 10.1001/archinte.160.14.2117. [DOI] [PubMed] [Google Scholar]
- 24.Fontana L. Klein S. Holloszy JO. Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- 25.Fontana L. Meyer TE. Klein S. Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fontana L. Weiss EP. Villareal DT. Klein S. Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forsey RJ. Thompson JM. Ernerudh J. Hurst TL. Strindhall J. Johansson B. Nilsson BO. Wikby A. Plasma cytokine profiles in elderly humans. Mech Ageing Dev. 2003;124:487–493. doi: 10.1016/s0047-6374(03)00025-3. [DOI] [PubMed] [Google Scholar]
- 28.Garner DM. Psychoeducational principles in treatment. In: Garner DM, editor; Garfinkel PE, editor. Handbook of Treatment for Eating Disorders. New York: Guilford Press; 1997. pp. 145–177. [Google Scholar]
- 29.Green MW. Rogers PJ. Impairments in working memory associated with spontaneous dieting behaviour. Psychol Med. 1998;28:1063–1070. doi: 10.1017/s0033291798007016. [DOI] [PubMed] [Google Scholar]
- 30.Grundy SM. Cleeman JI. Daniels SR. Donato KA. Eckel RH. Franklin BA. Gordon DJ. Krauss RM. Savage PJ. Smith SC., Jr. Spertus JA. Costa F. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13:322–327. [PubMed] [Google Scholar]
- 31.Heilbronn LK. de Jonge L. Frisard MI. DeLany JP. Larson-Meyer DE. Rood J. Nguyen T. Martin CK. Volaufova J. Most MM. Greenway FL. Smith SR. Deutsch WA. Williamson DA. Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heilbronn LK. Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–369. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 33.Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev. 2006;27:750–761. doi: 10.1210/er.2006-0032. [DOI] [PubMed] [Google Scholar]
- 34.Holloszy JO. Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–712. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingram DK. Roth GS. Lane MA. Ottinger MA. Zou S. de Cabo R. Mattison JA. The potential for dietary restriction to increase longevity in humans: extrapolation from monkey studies. Biogerontology. 2006;7:143–148. doi: 10.1007/s10522-006-9013-2. [DOI] [PubMed] [Google Scholar]
- 36.Japan Ministry of Health LaW. Journal of Health and Welfare Statistics. Tokyo: Health and Welfare Statistics Association; 2005. [Google Scholar]
- 37.Kagawa Y. Impact of Westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians. Prev Med. 1978;7:205–217. doi: 10.1016/0091-7435(78)90246-3. [DOI] [PubMed] [Google Scholar]
- 38.Kemps E. Tiggemann M. Working memory performance and preoccupying thoughts in female dieters: evidence for a selective central executive impairment. Br J Clin Psychol. 2005;44:357–366. doi: 10.1348/014466505X35272. [DOI] [PubMed] [Google Scholar]
- 39.Kemps E. Tiggemann M. Marshall K. Relationship between dieting to lose weight and the functioning of the central executive. Appetite. 2005;45:287–294. doi: 10.1016/j.appet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Keys A. Brozek J. Henschel A. Mickelson O. Taylor H. The Biology of Human Starvation. Minneapolis: University of Minnesota Press; 1950. [Google Scholar]
- 41.Lane MA. Baer DJ. Tilmont EM. Rumpler WV. Ingram DK. Roth GS. Cutler RG. Energy balance in rhesus monkeys (Macaca mulatta) subjected to long-term dietary restriction. J Gerontol A Biol Sci Med Sci. 1995;50:B295–B302. doi: 10.1093/gerona/50a.5.b295. [DOI] [PubMed] [Google Scholar]
- 42.Lane MA. Ingram DK. Ball SS. Roth GS. Dehydroepiandrosterone sulfate: a biomarker of primate aging slowed by calorie restriction. J Clin Endocrinol Metab. 1997;82:2093–2096. doi: 10.1210/jcem.82.7.4038. [DOI] [PubMed] [Google Scholar]
- 43.Larson-Meyer DE. Heilbronn LK. Redman LM. Newcomer BR. Frisard MI. Anton S. Smith SR. Alfonso A. Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefevre M. Redman LM. Heilbronn LK. Smith JV. Martin CK. Rood JC. Greenway FL. Williamson DA. Smith SR. Ravussin E. Caloric restriction alone and with exercise improves CVD risk in healthy non-obese individuals. Atherosclerosis. 2009;203:206–213. doi: 10.1016/j.atherosclerosis.2008.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levine JA. Lanningham-Foster LM. McCrady SK. Krizan AC. Olson LR. Kane PH. Jensen MD. Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 46.Lorenzo C. Williams K. Hunt KJ. Haffner SM. The National Cholesterol Education Program—Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30:8–13. doi: 10.2337/dc06-1414. [DOI] [PubMed] [Google Scholar]
- 47.Malis C. Rasmussen EL. Poulsen P. Petersen I. Christensen K. Beck-Nielsen H. Astrup A. Vaag AA. Total and regional fat distribution is strongly influenced by genetic factors in young and elderly twins. Obes Res. 2005;13:2139–2145. doi: 10.1038/oby.2005.265. [DOI] [PubMed] [Google Scholar]
- 48.Martin CK. Anton SD. Han H. York-Crowe E. Redman LM. Ravussin E. Williamson DA. Examination of cognitive function during six months of calorie restriction: results of a randomized controlled trial. Rejuvenation Res. 2007;10:179–190. doi: 10.1089/rej.2006.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin CK. Anton SD. Han H. York-Crowe E. Redman LM. Ravussin E. Williamson DA for the Pennington CALERIE Team. Examination of cognitive function during six-months of calorie restriction: results of a randomized controlled trial. Rejuvenation Res. 2007;10:179–189. doi: 10.1089/rej.2006.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin CK. Anton SD. York-Crowe E. Heilbronn LK. VanSkiver C. Redman LM. Greenway FL. Ravussin E. Williamson DA. Empirical evaluation of the ability to learn a calorie counting system and estimate portion size and food intake. Br J Nutr. 2007;98:439–444. doi: 10.1017/S0007114507708802. [DOI] [PubMed] [Google Scholar]
- 51.Martin CK. Heilbronn LK. de Jonge L. Delany JP. Volaufova J. Anton SD. Redman LM. Smith SR. Ravussin E. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity. 2007;15:2964–2973. doi: 10.1038/oby.2007.354. [DOI] [PubMed] [Google Scholar]
- 52.Matsumoto K. Sera Y. Abe Y. Ueki Y. Tominaga T. Miyake S. Inflammation and insulin resistance are independently related to all-cause of death and cardiovascular events in Japanese patients with type 2 diabetes mellitus. Atherosclerosis. 2003;169:317–321. doi: 10.1016/s0021-9150(03)00198-9. [DOI] [PubMed] [Google Scholar]
- 53.McCay CM. Crowel MF. Maynard LA. The effect of retarded growth upon the length of the life span and upon th eultimate body size. J Nutr. 1935:63–79. [PubMed] [Google Scholar]
- 54.Mendall MA. Patel P. Ballam L. Strachan D. Northfield TC. C reactive protein and its relation to cardiovascular risk factors: a population based cross sectional study. BMJ. 1996;312:1061–1065. doi: 10.1136/bmj.312.7038.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merry BJ. Calorie restriction and age-related oxidative stress. Ann NY Acad Sci. 2000;908:180–198. doi: 10.1111/j.1749-6632.2000.tb06646.x. [DOI] [PubMed] [Google Scholar]
- 56.Mora S. Lee IM. Buring JE. Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295:1412–1419. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 57.Nieman DC. Brock DW. Butterworth D. Utter AC. Nieman CC. Reducing diet and/or exercise training decreases the lipid and lipoprotein risk factors of moderately obese women. J Am Coll Nutr. 2002;21:344–350. doi: 10.1080/07315724.2002.10719233. [DOI] [PubMed] [Google Scholar]
- 58.Niskanen L. Uusitupa M. Sarlund H. Siitonen O. Paljarvi L. Laakso M. The effects of weight loss on insulin sensitivity, skeletal muscle composition and capillary density in obese non-diabetic subjects. Int J Obes Relat Metab Disord. 1996;20:154–160. [PubMed] [Google Scholar]
- 59.Olshansky SJ. Passaro DJ. Hershow RC. Layden J. Carnes BA. Brody J. Hayflick L. Butler RN. Allison DB. Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 60.Orentreich N. Brind JL. Rizer RL. Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 61.Orentreich N. Brind JL. Vogelman JH. Andres R. Baldwin H. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab. 1992;75:1002–1004. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- 62.Pearson KJ. Baur JA. Lewis KN. Peshkin L. Price NL. Labinskyy N. Swindell WR. Kamara D. Minor RK. Perez E. Jamieson HA. Zhang Y. Dunn SR. Sharma K. Pleshko N. Woollett LA. Csiszar A. Ikeno Y. Le Couteur D. Elliott PJ. Becker KG. Navas P. Ingram DK. Wolf NS. Ungvari Z. Sinclair DA. de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peterson LR. Short-term memory. Sci Am. 1966;215:90–95. doi: 10.1038/scientificamerican0766-90. [DOI] [PubMed] [Google Scholar]
- 64.Peterson LR. Peterson MJ. Short-term retention of individual verbal items. J Exp Psychol. 1959;58:193–198. doi: 10.1037/h0049234. [DOI] [PubMed] [Google Scholar]
- 65.Polivy J. Herman CP. Dieting and binging. A causal analysis. Am Psychol. 1985;40:193–201. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- 66.Racette SB. Weiss EP. Villareal DT. Arif H. Steger-May K. Schechtman KB. Fontana L. Klein S. Holloszy JO. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61:943–950. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ravussin E. Bogardus C. A brief overview of human energy metabolism and its relationship to essential obesity. Am J Clin Nutr. 1992;55(1 Suppl):242S–245S. doi: 10.1093/ajcn/55.1.242s. [DOI] [PubMed] [Google Scholar]
- 68.Ravussin E. Burnand B. Schutz Y. Jequier E. Energy expenditure before and during energy restriction in obese patients. Am J Clin Nutr. 1985;41:753–759. doi: 10.1093/ajcn/41.4.753. [DOI] [PubMed] [Google Scholar]
- 69.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 70.Redman LM. Ravussin E. Could calorie restriction increase longevity in humans? Aging Health. 2007;3:1–4. [Google Scholar]
- 71.Redman LM. Heilbronn LK. Martin CK. Alfonso A. Smith SR. Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92:865–872. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Redman LM. Heilbronn LK. Martin CK. De Jonge L. Williamson DA. DeLany JP. Ravussin E. Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009;4:e4377. doi: 10.1371/journal.pone.0004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 74.Rosamond W. Flegal K. Friday G. Furie K. Go A. Greenlund K. Haase N. Ho M. Howard V. Kissela B. Kittner S. Lloyd-Jones D. McDermott M. Meigs J. Moy C. Nichol G. O'Donnell CJ. Roger V. Rumsfeld J. Sorlie P. Steinberger J. Thom T. Wasserthiel-Smoller S. Hong Y. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 75.Roth GS. Lane MA. Ingram DK. Mattison JA. Elahi D. Tobin JD. Muller D. Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- 76. This reference has been deleted.
- 77.Roth GS. Mattison JA. Ottinger MA. Chachich ME. Lane MA. Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004;305:1423–1426. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- 78.Sacher GA. Life table modifications and life prolongation. In: Finch CE, editor; Hayflick L, editor. Handbook of the Biology of Aging. New York: van Nostrand Reinold; 1977. pp. 582–638. [Google Scholar]
- 79.Schmidt M. Rey Auditory and Verbal Learning Test: A Handbook. Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- 80.Sivan AB. Benton Visual Retention Test. Fifth. San Antonio: The Psychological Corporation, Harcourt Brace & Company; 1992. [Google Scholar]
- 81.Smith SR. The endocrinology of obesity. In: Bray G, editor. Endocrinology and Metabolism Clinics of North America. W.B. Saunders; 1996. pp. 921–942. [DOI] [PubMed] [Google Scholar]
- 82.Sohal RS. Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stanforth PR. Jackson AS. Green JS. Gagnon J. Rankinen T. Despres JP. Bouchard C. Leon AS. Rao DC. Skinner JS. Wilmore JH. Generalized abdominal visceral fat prediction models for black and white adults aged 17–65 y: the HERITAGE Family Study. Int J Obes Relat Metab Disord. 2004;28:925–932. doi: 10.1038/sj.ijo.0802563. [DOI] [PubMed] [Google Scholar]
- 84.Stenkula KG. Said L. Karlsson M. Thorn H. Kjolhede P. Gustavsson J. Soderstrom M. Stralfors P. Nystrom FH. Expression of a mutant IRS inhibits metabolic and mitogenic signalling of insulin in human adipocytes. Mol Cell Endocrinol. 2004;221:1–8. doi: 10.1016/j.mce.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 85.Stunkard AJ. Nutrition, longevity and obesity. In: Rockstein M, editor; Sussman ML, editor. Nutrition, Aging and Obesity. New York: Academic Press; 1976. pp. 253–284. [Google Scholar]
- 86.Stunkard AJ. Messick S. Eating Inventory Manual (The Psychological Corporation) San Antonio, TX: Harcourt Brace & Company; 1988. [Google Scholar]
- 87.Suzuki M. Wilcox BJ. Wilcox CD. Implications from and for food cultures for cardiovascular disease: longevity. Asia Pac J Clin Nutr. 2001;10:165–171. doi: 10.1111/j.1440-6047.2001.00219.x. [DOI] [PubMed] [Google Scholar]
- 88.Urbanski HF. Downs JL. Garyfallou VT. Mattison JA. Lane MA. Roth GS. Ingram DK. Effect of caloric restriction on the 24-hour plasma DHEAS and cortisol profiles of young and old male rhesus macaques. Ann NY Acad Sci. 2004;1019:443–447. doi: 10.1196/annals.1297.081. [DOI] [PubMed] [Google Scholar]
- 89.Utzschneider KM. Carr DB. Hull RL. Kodama K. Shofer JB. Retzlaff BM. Knopp RH. Kahn SE. Impact of intra-abdominal fat and age on insulin sensitivity and beta-cell function. Diabetes. 2004;53:2867–2872. doi: 10.2337/diabetes.53.11.2867. [DOI] [PubMed] [Google Scholar]
- 90.Vagenakis AG. Burger A. Portnary GI. Rudolph M. O'Brian JR. Azizi F. Arky RA. Nicod P. Ingbar SH. Braverman LE. Diversion of peripheral thyroxine metabolism from activating to inactivating pathways during complete fasting. J Clin Endocrinol Metab. 1975;41:191–194. doi: 10.1210/jcem-41-1-191. [DOI] [PubMed] [Google Scholar]
- 91.Vallejo EA. Hunger diet on alternate days in the nutrition of the aged. Prensa Med Argent. 1957;44:119–120. (In Spanish). [PubMed] [Google Scholar]
- 92.Veldhuis JD. Erickson D. Iranmanesh A. Miles JM. Bowers CY. Sex-steroid control of the aging somatotropic axis. Endocrinol Metab Clin North Am. 2005;34:877–893. doi: 10.1016/j.ecl.2005.07.006. viii. [DOI] [PubMed] [Google Scholar]
- 93.Vitousek KM. Gray JA. Grubbs KM. Caloric restriction for longevity: I. Paradigm, protocols and physiological findings in animal research. Eur Eating Disord Rev. 2004;12:279–299. [Google Scholar]
- 94.Vitousek KM. Manke FP. Gray JA. Vitousek MN. Calorie restriction for longevity: II. The systematic neglect of behavioural and psychological outcomes in animal research. Eur Eating Disord Rev. 2004;12:338–360. [Google Scholar]
- 95.Walford RL. Harris SB. Gunion MW. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proc Natl Acad Sci USA. 1992:11533–11537. doi: 10.1073/pnas.89.23.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walford RL. Harris SB. Weindruch R. Dietary restriction and aging: historical phases, mechanisms and current directions. J Nutr. 1987;117:1650–1654. doi: 10.1093/jn/117.10.1650. [DOI] [PubMed] [Google Scholar]
- 97.Walford RL. Mock D. MacCallum T. Laseter JL. Physiologic changes in humans subjected to severe, selective calorie restriction for two years in biosphere 2: health, aging, and toxicological perspectives. Toxicol Sci. 1999;52(2 Suppl):61–65. [PubMed] [Google Scholar]
- 98.Walford RL. Mock D. Verdery R. MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci. 2002;57:B211–B224. doi: 10.1093/gerona/57.6.b211. [DOI] [PubMed] [Google Scholar]
- 99.Ware JE., Jr. Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 100.Ware JE., Jr. Kosinski M. Gandek B. SF-36 Health Survey: Manual & Interpretation Guide. Lincoln, RI: QualityMetric, Inc.; 1993, 2002. [Google Scholar]
- 101.Weyer C. Walford RL. Harper IT. Milner M. MacCallum T. Tataranni PA. Ravussin E. Energy metabolism after 2 y of energy restriction: the biosphere 2 experiment. Am J Clin Nutr. 2000;72:946–953. doi: 10.1093/ajcn/72.4.946. [DOI] [PubMed] [Google Scholar]
- 102.Willcox BJ. Willcox DC. Todoriki H. Yano K. Curb D. Suzuki M. Caloric restriction, enerhy balance and healthy aging in Okinawans and Americans: biomarker differences in Septuagenarians. Okinawan J Am Stud. 2007;4:62–74. [PMC free article] [PubMed] [Google Scholar]
- 103.Willcox DC. Willcox BJ. He Q. Wang NC. Suzuki M. They really are that old: a validation study of centenarian prevalence in Okinawa. J Gerontol A Biol Sci Med Sci. 2008;63:338–349. doi: 10.1093/gerona/63.4.338. [DOI] [PubMed] [Google Scholar]
- 104.Willcox DC. Willcox BJ. Todoriki H. Curb JD. Suzuki M. Caloric restriction and human longevity: what can we learn from the Okinawans? Biogerontology. 2006;7:173–177. doi: 10.1007/s10522-006-9008-z. [DOI] [PubMed] [Google Scholar]
- 105.Williamson DA. Assessment of Eating Disorders: Obesity, Anorexia, and Bulimia Nervosa. Elmsford, NY: Pergamon Press; 1990. [Google Scholar]
- 106.Williamson DA. Martin CK. Binge eating disorder: a review of the literature after publication of DSM-IV. Eat Weight Disord. 1999;4:103–114. doi: 10.1007/BF03339725. [DOI] [PubMed] [Google Scholar]
- 107.Williamson DA. Martin CK. Anton SD. York-Crowe E. Han H. Redman L. Ravussin E. Is caloric restriction associated with development of eating-disorder symptoms? Results from the CALERIE trial. Health Psychol. 2008;27:S32–S42. doi: 10.1037/0278-6133.27.1.S32. [DOI] [PubMed] [Google Scholar]
- 108.Williamson DA. Martin CK. York-Crowe E. Anton SD. Redman LM. Han H. Ravussin E. Measurement of dietary restraint: validity tests of four questionnaires. Appetite. 2007;48:183–192. doi: 10.1016/j.appet.2006.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zamboni M. Zoico E. Scartezzini T. Mazzali G. Tosoni P. Zivelonghi A. Gallagher D. De Pergola G. Di Francesco V. Bosello O. Body composition changes in stable-weight elderly subjects: the effect of sex. Aging Clin Exp Res. 2003;15:321–327. doi: 10.1007/BF03324517. [DOI] [PubMed] [Google Scholar]
- 110.Zurlo F. Ferraro RT. Fontvielle AM. Rising R. Bogardus C. Ravussin E. Spontaneous physical activity and obesity: cross-sectional and longitudinal studies in Pima Indians. Am J Physiol. 1992;263:E296–E300. doi: 10.1152/ajpendo.1992.263.2.E296. [DOI] [PubMed] [Google Scholar]