Abstract
In the present study we extended errorless learning to a conditional temporal discrimination. Pigeons' responses to a left–red key after a 2-s sample and to a right–green key after a 10-s sample were reinforced. There were two groups: One learned the discrimination through trial and error and the other through an errorless learning procedure. Then, both groups were presented with three types of tests. First, they were exposed to intermediate durations between 2 s and 10 s, and given a choice between both keys (stimulus generalization test). Second, a delay from 1 s to 16 s was included between the offset of the sample and the onset of the choice keys (delay test). Finally, pigeons learned a new discrimination in which the stimuli were switched (reversal test). Results showed that pigeons from the Errorless group made significantly fewer errors than those in the Trial-and-Error group. Both groups performed similarly during the stimulus generalization test and the reversal test, but results of the delay test suggested that, on long stimulus trials, responding in the errorless training group was less disrupted by delays.
Keywords: errorless, fading, timing, conditional discrimination, key peck, pigeons
When experimental psychologists train discriminations, in addition to reinforcing correct responses they typically allow errors (that is, incorrect responses) to occur and be extinguished. Before the 1960s it was believed that discrimination learning required the selection of both correct and incorrect choices so that the consequences of each could be experienced (Hull, 1950; Spence, 1936). However, Terrace (1963a) developed a new method, later termed errorless learning, which allows learning to occur with few or no responses to the incorrect choice. In his first experiment, pigeons learned a simple successive discrimination between a red key (S+) and a green key (S−). Initially the red key was presented with full brightness and duration, whereas the green key was dark and presented only for a short duration. Gradually, the brightness and duration of the S− increased until the stimuli differed only in hue (fading). The main result showed that the group trained with the fading procedure gave few or no responses to the S−.
Terrace's subsequent studies with animals (Terrace, 1963b,c; 1964; 1966a; 1969; 1971; see also Terrace, 1966b, 1972) and humans (Terrace, 1974) confirmed and extended his original findings. He found that subjects exposed to errorless training performed differently in a variety of tests than subjects exposed to trial-and-error training. Specifically, subjects trained with the fading procedure did not show explicit “emotional” responses, such as wing flapping and striking the key, in the presence of the S−. Perhaps because these subjects did not experience nonreinforcement from responding to the S−, the S− did not become an aversive stimulus and therefore escape and antagonistic responses in its presence did not occur. Terrace also found that the generalization gradients for the errorless discrimination group did not show peaks shifted away from the S− (i.e., no peak shift) or with a minimum at the S− (i.e., no inhibitory stimulus control), did not increase response rate or decreased response latency to the S+ (i.e., absence of behavioral contrast), and did not show a disruption in their performance when sedatives (chlorpromazine and imipramine) were administered. Terrace (1972) classified these behavioral phenomena, which occurred only following a discrimination learned through trial and error, as by-products of discrimination learning.
Terrace's findings had a significant impact and generated a considerable number of follow-up studies, both basic and applied (e.g., basic: Arantes & Berg, 2009; Lyons, 1969a,b; Mackay & Brown, 1971; Moore & Goldiamond, 1964; Rilling & Caplan, 1975; Robinson, Foster & Bridges, 1976; Schusterman, 1966; Sidman & Rosenberger, 1967; Wessells, 1973; applied: Barlow & Agras, 1973; Birkan, McClannahan & Krantz, 2007; Corey & Shamow, 1972; Pace, Iwata, Cowdery, Andree & McIntyre, 1993; Sidman & Stoddard, 1967; Touchette & Howard, 1984). The strong interest shown by applied researchers for errorless learning has continued until the present because reducing errors and their associated costs is beneficial in a variety of settings (e.g., interventions with children and adults with developmental disabilities). However, interest among basic researchers declined after the 1970s, which may have been due to criticisms of Terrace's original studies raised by some researchers (e.g., Kodera & Rilling, 1976; Lyons, 1969a,b; Marsh & Johnson, 1968; Rilling, Kramer & Richards, 1973; Wessells, 1973). Specifically, these studies found that some of the by-products of discrimination learning have little relationship to the occurrence or nonoccurrence of errors during S− (see Rilling, 1977, for review).
One limitation of errorless learning procedures was that they applied only to a restricted range of discriminations, typically simple discriminations in the visual modality. Some researchers (e.g., Gollin & Savoy, 1968; Guralnick, 1975; Schilmoeller, Schilmoeller, Etzel & LeBlanc, 1979) unsuccessfully tried to teach conditional discriminations to children based on simple discriminations learned through fading procedures. Others were only partly successful: Schilmoeller et al. were able to teach an errorless conditional discrimination to participants after teaching them two simple discriminations, not through fading but stimulus shaping, a technique that involves manipulating the topographical configuration of visual stimuli. To illustrate, in one condition of Schilmoeller et al.'s study, the S+ was a triangle with a single stripe background and the S− was a circle with a single stripe background, both presented on a card. Initially, the S+ was manipulated to look like a tree on a hill, while the S− was a blank white card. Then, the shape of the S− was changed from “an apple with a worm” to the circle with a single stripe background while the S+ remained constant as the “tree on a hill”. Finally the S+ changed from the “tree on a hill” to the triangle with a single stripe background. Results showed that 12 out of the 16 subjects made none or very few responses (≤ 8% incorrect responses) during the learning of the conditional discrimination between the final S+ and S− (i.e., triangle vs. circle with a single stripe background).
Stubbs (1968) adapted a fading procedure to teach a conditional temporal discrimination in which responses to a red key were reinforced if the preceding sample was short (from 1 s to 5 s), whereas responses to a green key were reinforced if the preceding sample was long (from 6 s to 10 s). Group 1 (N = 3 pigeons) was exposed to all sample durations from the beginning of training. The S+ was a full red key and the S− was a dark green that gradually became brighter such that at the end of the training both keys varied only in terms of hue. Group 2 (N = 3 pigeons) was exposed initially only to the two extreme sample durations (i.e., 1 s and 10 s); the other durations were introduced after performance stabilized. Although Stubbs stated that both groups learned through fading procedures, Group 2 actually acquired the discriminations through trial-and-error in two different phases, first with the extreme sample durations and then with the other eight intermediate durations. Because Stubbs' goal was not to study errorless learning per se, but the psychophysical relationship between sample durations and choice responding, he did not report the number of errors made by the pigeons during training. However, it seems that performance after fading (Group 1) was not particularly good. First, after being exposed to the fading procedure for more than 45 sessions, the pigeons from Group 1 had to be given additional sessions (M = 89) until their performance stabilized. Second, his Figure 1 suggests that the percentage of correct responses to the 10-s sample (longest duration) was lower in Group 1 than in Group 2 (range from Group 1: 75% to 90%; range from Group 2: 85% to 93%).
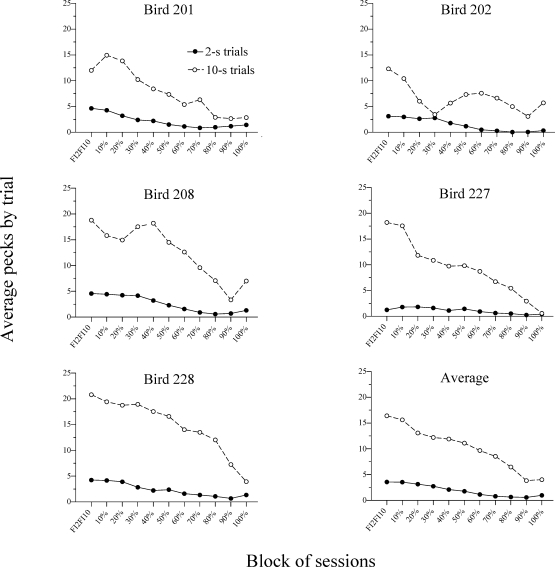
Fig 1.
Average number of key pecks (to the correct key) emitted per trial throughout the sample duration over Phase I and Phase II.
Because there are not enough data to make conclusions about the effectiveness of the fading procedure in training a temporal discrimination, the present study aimed to answer this question. In a matching-to-sample task, pigeons learned to choose a red left key after a 2-s sample and a green right key after a 10-s sample. For one group of pigeons, both keys were lit simultaneously after the sample and no attempt was made to prevent errors (Trial-and-Error group). For another group of pigeons, a novel errorless learning procedure was used (Errorless group).
The errorless procedure described below is based on the concept of mediating behavior. Some theories of timing (e.g., the Behavioral theory of Timing, BeT; the Learning-to-Time model, LeT) hypothesize that temporal discrimination is affected by mediating behavioral states, and a number of studies have supported the hypothesis that, at least in some tasks, subjects use their own behavior to discriminate sample durations (e.g., Fetterman, Killeen & Hall, 1998; Machado & Arantes, 2006). For example, a pigeon exposed to a discrimination between 2-s and 10-s samples presented on a center key might start the trial facing the front panel, then approach the center key and peck it once or twice; if the signal persists because the sample is long, the pigeon may then peck the center key at a higher rate. Differences in the ongoing behavior at the moment of choice correlate with, and thus may mediate, choice performance.
In our experiment, we used a fading procedure to establish a pattern of behavior that should facilitate the acquisition of a temporal discrimination. If successful, the fading procedure should train the discrimination with few or no errors. Specifically, we trained one group of pigeons to face the intelligence panel at trial onset, stand for a few seconds in front of the left key (which later would be the correct comparison stimulus following the short sample), and then move to the right key (later the correct comparison stimulus following the long sample) and stand in front of that key until the end of the trial. Initially, the positive stimulus (left red or right green key, depending on the duration of the sample) also was lit during the sample. After the pigeons responded reliably, we introduced a delay between the illumination of the sample and the positive stimulus. When the delay reached 100%, the positive stimulus was lit only after the sample had elapsed (2 s or 10 s). Next, on an increasing percentage of trials the negative stimulus also was lit together with the positive stimulus. Finally, both comparison stimuli were always lit simultaneously after the sample. We expected that the pigeons would learn the temporal discrimination with few or no errors.
To determine if there were behavioral differences between subjects that learned the task via trial-and-error or errorless learning, we compared the performance of the two groups, henceforth named Errorless group and Trial-and-Error group, on three types of tests. First, the pigeons were exposed to sample durations ranging from 2 s to 10 s and given a choice between the two comparisons (stimulus generalization test). We were interested in determining not only whether the generalization gradients differed, but also whether the mediating behavior of the Errorless group, established in the pretraining, predicted the results from the generalization test.
Second, the pigeons were exposed to a delay test in which blackouts of 1 s to 16 s were interposed between the offset of the sample and the onset of the comparison stimuli. The goal was to determine whether responding acquired through trial-and-error or errorless learning would be differentially resistant to disruption.
Third and finally, the pigeons learned a new discrimination in which the comparison stimuli were switched, that is, a peck on the left red key now produced food after the 10-s samples, whereas a peck on the right green key produced food after the 2-s samples (reversal test). Marsh and Johnson (1968) examined reversal learning following the acquisition through a fading procedure of a successive simple discrimination between a red (S+) and a green key (S−). The results were mostly negative: 4 of their 5 pigeons made fewer than two responses to the new S+ (which was the S− during the previous discrimination) during five test sessions. Based on this result, Rilling (1977) concluded that “errorless learning is clearly not the best learning for an organism exposed to a changing environment”. However, other studies have shown appropriate reversal following errorless learning (e.g., Aronsohn, Castillo & Pinto-Hamuy, 1978; Robinson & Storm, 1978; Schusterman, 1965, 1966; Stettner & Matyniak, 1969).
In our experiment, both comparison stimuli were associated with reward and nonreward, albeit in different (sample) contexts. Therefore, we wanted to determine whether the Errorless and Trial-and-Error pigeons differed, when later exposed to the reversed contingencies, in their speed of detecting the new response–reinforcer relationship.
METHOD
Subjects
Twelve pigeons (Columba livia) were maintained at 85% of their free-feeding weights (± 15 g) through appropriate postsession feeding. Pigeons were housed individually in a vivarium with a 12h/12h light/dark cycle (lights on at 07:00 am). Eight pigeons were experimentally naïve and the remaining 4 were experienced with choice procedures (concurrent schedules), but had not been exposed to timing-related tasks. Water and grit were freely available in the home cages.
Apparatus
Eight standard three-key operant chambers, 35 cm deep by 36 cm wide by 35 cm high, were used. The keys were arranged in a row, 26 cm above the floor. The side keys could be illuminated from behind with red or green light. In each chamber there was a houselight located 7 cm above the center key and a grain magazine with a 6-cm by 5-cm aperture located 13 cm below the center key. The magazine was illuminated when wheat was made available. A force of approximately 0.10 N was necessary to operate each key, and effective responses produced an audible feedback click. Chambers were enclosed in a sound-attenuating box, and ventilation and white noise were provided by an attached fan. Event scheduling and data recording were controlled with a MEDSTATE® notation program and a MED-PC® system interfaced to an IBM®-compatible microcomputer that was located in an adjacent room.
Procedure
Sessions were conducted 7 days a week at approximately the same time of day. The pigeons were divided into two groups, an Errorless group (Pigeons 201, 202, 207, 208, 227 and 228), and a Trial-and-Error group (Pigeons 203, 204, 205, 206, 225 and 226). Four pigeons from each group were experimentally naïve (Pigeons 201–208), and thus learned first to peck the keys using an autoshaping procedure. The remaining 2 birds of each group (Pigeons 225–228) were exposed directly to the experimental procedure. Because of a programming error, during the fading of the multiple FI 2 s FI 10 s for Pigeon 207, the percentage delay between the illumination of the houselight (sample) and the response key changed abruptly from 10% to 80%. Therefore, results from Pigeon 207 were excluded from analysis.
Pretraining - Errorless Group
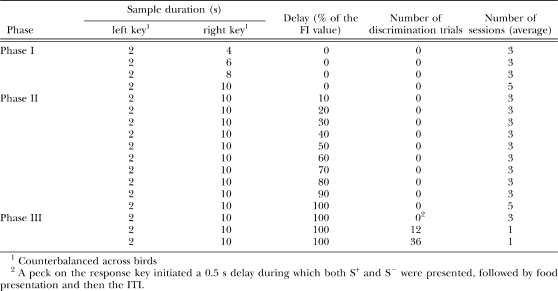
The pretraining, which pertains only to the Errorless group, was composed of three phases. Table 1 summarizes the main features and number of sessions in each phase. The main goal of Phases I and II was to train mediating behaviors that would facilitate acquisition of the temporal discrimination. Specifically, we wanted the pigeons to stand in front of the left key at the beginning of each trial, and switch to standing in front of the right key after several seconds had elapsed. To that end, during Phases I and II, only one key was illuminated on each trial (e.g., the left key on 2-s trials and the right key on 10-s trials). The duration of the houselight (2 s or 10 s) was the sample. The difference between both phases was that whereas in Phase I the response key and the houselight were illuminated simultaneously, in Phase II the response key was illuminated only after a delay that increased gradually such that, in the end, the key was illuminated only when the houselight was turned off. In Phase III, discrimination trials were introduced gradually, that is, on some trials the left and the right keys were turned on simultaneously after the sample, and a response to either key ended the trial with reinforcement. Finally, in the baseline procedure all trials were (the usual) choice trials: Following the sample stimulus, the two comparison stimuli were presented.
Table 1.
Main features of Phases I, II and III of the Pretraining, which pertain only to the Errorless group.
Phase I: Multiple FI 2 s FI 10 s
Pigeons were initially exposed to a multiple FI 2 s FI 4 s schedule; then, across several sessions, the interval of the longer schedule was increased gradually to 10 s (i.e., multiple FI 2 s FI 10 s). Sessions lasted for 60 trials. Each trial was preceded by a 30-s ITI during which all the lights were off. After the ITI, the houselight was turned on and a side key was illuminated (red or green, depending on the sample duration). Although the assignment of the red and green colors to the two sample durations and to the left and right keys was counterbalanced across birds, for clarity we describe the procedure as if all birds were trained as follows: On half of the trials, the schedule in effect was the FI 2 s and the left key was illuminated red; on the remaining trials, the schedule in effect was the FI 10 s and the right key was illuminated green. When the FI duration elapsed, the houselight was turned off and the first response to the illuminated key was reinforced. During reinforcement, the keylight was turned off and the grain hopper was raised and illuminated for 3 s. If the pigeon did not respond within 5 s after the houselight was turned off, the trial was canceled. Training in this phase continued until response rates appeared approximately stable in both components for all pigeons, and it lasted 14 sessions on average.
Phase II: Fading of the multiple FI 2 s FI 10 s
The multiple FI 2-s FI 10-s schedule was identical to Phase I, with the following exception. Whereas in Phase I the response key was illuminated simultaneously with the houselight, in Phase II the response key was illuminated only after a delay. The delay was always a percentage of the FI schedule in effect and increased gradually from 10% to 100% in steps of 10% across blocks of sessions. Each fading value was maintained for at least three sessions, and until the response rates appeared stable. When the delay had reached 100% of the FI value, trials proceeded as follows: The houselight was illuminated for either 2 s or 10 s, and then it was turned off as the corresponding side key was illuminated with either red or green light. The first response to the illuminated key darkened the key and produced reinforcement. This phase lasted on average 32 sessions.
Phase III: Gradual introduction of the S−
This phase lasted five sessions and consisted of two steps. During the first three sessions, the first response to the sole lit key initiated a 0.5-s delay during which both S+ and S− were presented, followed by reinforcement. The goal of this step was to expose the pigeons to the S− in a context in which they would not be able to respond to it. In the second step, over the next two sessions, the pigeons were exposed to choice trials in which both S+ and S− were illuminated immediately after the houselight was turned off. In the first session, there were 12 choice trials (6 for each sample duration) randomly intermixed with 48 only S+ trials. In the second session, there were 36 choice trials (18 for each sample duration) randomly intermixed with 24 only S+ trials. During choice trials, after the sample duration had elapsed, the houselight was turned off and the left and the right side keys were illuminated red and green, respectively. A peck to either key turned all lights off. If the choice was correct (i.e., left–red key after a 2-s sample or right–green key after a 10-s sample), the hopper was activated and the ITI followed; if the choice was incorrect, the ITI began immediately. To reduce the number of potential errors (i.e., responses to S−) that pigeons could make during the gradual introduction of the choice trials, if pigeons made a response to the dark key that corresponded to S− after at least 80% of the sample duration had elapsed, the trial was cancelled by turning off the houselight and starting the ITI.
Baseline Training - Errorless Group
Sixty choice trials comprised each session and were identical to those of Phase III of pretraining for the Errorless group (see above). Baseline training continued until pigeons reached the criterion of 85% correct responses for each sample over three consecutive sessions.
Baseline Training – Trial-and-Error Group
The Trial-and-Error pigeons started the experiment in the Baseline condition. This condition was similar to that for the Errorless group, except that pecks on a dark key did not cancel the trial. Baseline training continued until the same criterion of 85% correct responses for each sample over three consecutive sessions was reached.
Testing
After birds had reached criterion, they were exposed to three different tests: stimulus generalization test, delay test and reversal test. Contingencies during all test sessions were the same for both groups.
Stimulus generalization test
Each session consisted of 30 test trials randomly intermixed with 30 choice trials identical to those used in baseline for the Trial-and-Error group. Sample durations on test trials were sampled from a set of three logarithmically-spaced values, in which the middle duration corresponded to the geometric mean of the training durations: 3.0 s, 4.5 s, and 6.7 s. Each of these test durations occurred 10 times per session. Because we wanted the Errorless group to experience as little extinction as possible throughout the experiment, responses on test trials were always nondifferentially reinforced for both groups. The stimulus generalization test lasted for three sessions.
Delay test
After the stimulus generalization test, pigeons were given three additional sessions of baseline training prior to the delay test. During the delay test, each session lasted for 60 trials. These were identical to choice trials from baseline training except that after the sample duration had elapsed and the houselight was extinguished, the side keys were lit simultaneously following a delay. Five different delays were arranged in each session: 1 s, 2 s, 4 s, 8 s, and 16 s. Each delay occurred 12 times per session, 6 for each of the two sample durations. During the delay, the chamber was dark. After the side keys were lit, a response to the correct key produced reinforcement. The delay test lasted 20 sessions.
Reversal test
After the delay test, pigeons returned to baseline for three sessions. During the reversal test the procedure was similar to baseline except that the contingencies were reversed. To receive food, pigeons needed to choose the right–green key after the 2-s samples and the left–red key after the 10-s samples. Training continued until the pigeons reached an accuracy criterion of at least 85% correct responses following each sample for three consecutive sessions.
RESULTS
Pretraining - Errorless Group
Phases I and II: Multiple FI 2 s FI 10 s and fading of the multiple FI 2 s FI 10 s
Our first question was whether the fading procedure established the desired mediating behavior for the Errorless group. Figures 1 and 2 address this issue. Figure 1 shows the average number of responses to the correct key (i.e., left–red key on 2-s trials and right–green key on 10-s trials) made on each trial while the sample was on. The data come from the last five sessions in Phase I (i.e., prior to the fading of the multiple FI 2 s FI 10 s), for each block of three sessions in Phase 2 (i.e., during the fading procedure), and for the last five sessions in Phase II (i.e., after the fading procedure). Responses were counted regardless of whether or not the key was lit. Figure 1 shows that, as the duration that the key was illuminated decreased across sessions, responses on both 2-s and 10-s trials also decreased. This result suggests that the onset of the keylights evoked responding. However, all birds continued to respond to the dark keys to some extent during the 10-s trials in the 100% fading sessions (i.e., when the two keys were turned off during the houselight and only the right–green key was illuminated afterwards).
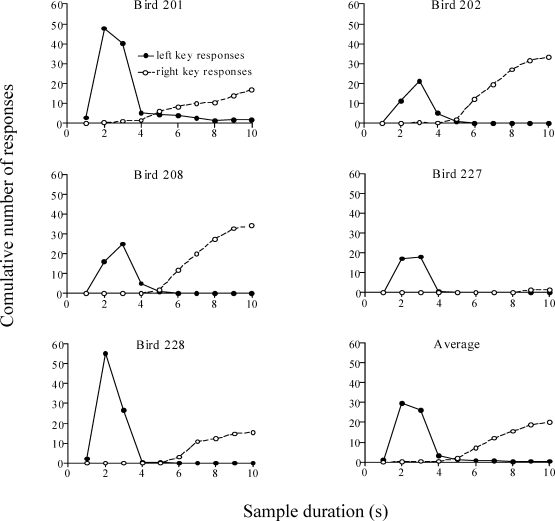
Fig 2.
Total number of key pecks emitted per session during the last sessions of Phase II over the 10-s trials. Solid and dotted lines correspond to the left and right keys, respectively.
Figure 2 shows how the fading procedure affected the distribution of dark-key responses. The total number of pecks to the left and right keys when they were dark, emitted per session during the 10-s trials and averaged across the last five sessions of Phase II (i.e., 100% fading), is plotted as a function of elapsed duration. Temporal control over responding is evident. All pigeons responded more to the 2-s key at the start of the houselight, with responding peaking at approximately 2 to 3 s after houselight onset. Responding to the 10-s key then increased systematically for the remainder of the sample duration. This pattern was evident for all pigeons, with exception of Pigeon 227, which rarely pecked the 10-s key. These results suggest that the fading procedure successfully established the mediating behavior for the Errorless group that should facilitate the acquisition of the temporal discrimination.
The presence of a well-differentiated pattern during the sample also supported the following hypothesis: If the pattern remained stable in subsequent phases, then the moment the pigeons switched between the two dark keys (i.e., ceased to peck the left key and started to peck the right key) should predict the bisection point when they were exposed to the stimulus generalization test trials. We return to this issue below.
Phase III: Gradual introduction of the S−
During the two sessions in which the pigeons were exposed to choice trials for the first time (total of 48 trials), the Errorless group made an average of 46 correct responses (95.8%), 1 incorrect response (2.1%), 0.8 cancelled trials (1.7%), and virtually no missed trials (0.4%). Pigeons 202 and 227 did not make any errors, 1 pigeon (228) made only one error, and the remaining 2 pigeons (201 and 208) made three errors.
Baseline Training
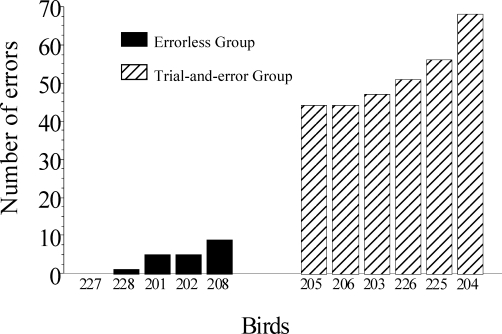
When exposed to sessions consisting of exclusively choice trials, the Errorless group made an average of 3 errors (Pigeons 201, 202, 208, 227 and 228 made 4, 5, 6, 0 and 0, respectively) and the Trial-and-Error group made an average of 52 errors (Pigeons 203, 204, 205, 206, 225 and 226 made 47, 68, 44, 44, 56 and 52 errors, respectively) until they reached the criterion of at least 85% correct responses over three consecutive sessions. Whereas pigeons in the Errorless group met the 85% criterion in all discrimination sessions, and therefore reached the criterion in the minimum number of 3 sessions (180 trials), the Trial-and-Error group met the criterion only after an average of 6.5 sessions (390 trials). An independent-means t test comparing the number of baseline sessions to acquire the discrimination showed that the Errorless group needed significantly fewer sessions to reached the criterion than the Trial-and-Error group, t(9) = −4.40, p < .01. These results show that the mediating behavior facilitated the acquisition of the temporal discrimination.
Next we compared performance of the two groups during all choice trials. For the Errorless group, this included the 48 trials presented in Phase III of pretraining and the 180 trials presented in baseline, for a total of 228 trials; for the Trial-and-Error group, this included 380 trials on average (range: 300 to 600 trials). Figure 3 shows the number of responses to the S− (errors) made by each pigeon. Whereas pigeons in the Errorless group made an average of 4 errors (98% correct responses; Pigeons 201, 202, 208, 227 and 228 made 5, 5, 9, 0 and 1 errors, respectively), those in the Trial-and-Error group made an average of 52 errors (86% correct responses; Pigeons 203, 204, 205, 206, 225 and 226 made 47, 68, 44, 44, 56 and 52 errors, respectively). It is important to note that the 2 experienced birds in the Errorless group (Pigeons 227 and 228) made 0 and 1 error, respectively. An ANOVA with sample duration and group as factors found a significant effect of group, F(1,9) = 117.78, p < .001, but no effect of sample duration, or of their interaction. Although the contingency for cancelling trials remained in place during baseline training for the Errorless group, virtually no trials were cancelled (0.4%). Specifically, with the exception of Pigeons 202 and 208, which throughout 180 choice trials had one and three cancelled trials, respectively, none of the other pigeons had cancelled trials.
Fig 3.
Number of responses to the S− made by each bird during all choice trials until they reached the learning criterion.
Performance during the last three sessions of baseline training did not differ appreciably between the two groups. The Errorless group averaged 98% correct responses (range: 96%–100%) and the Trial-and-Error group averaged 96% (range: 94%–98%). A repeated-measures ANOVA with sample duration and group as factors found no significant effect of group, F(1,9) = 4.40, sample duration, F(1,9) = 5.01, or their interaction, F(1,9) = 0.11 (all p's >.05, although the differences between groups almost reached significance, p = .06). In short, at the end of baseline training, the two groups were similarly accurate.
Stimulus Generalization Test
Figures 4 and 5 show the individual psychometric functions obtained during the stimulus generalization test for the Errorless and Trial-and-Error groups, respectively. Note that during the stimulus generalization test half of the trials were training trials (2-s and 10-s samples) and the other half were test trials (2.99-s, 4.47-s and 6.69-s samples). Data from both training and test trials are shown in Figures 4 and 5. For all birds, the probability of a “long” response, p(“long”), increased monotonically with sample duration. To describe the results concisely and estimate the bisection points, a three-parameter logistic function was fitted to the data from individual pigeons:
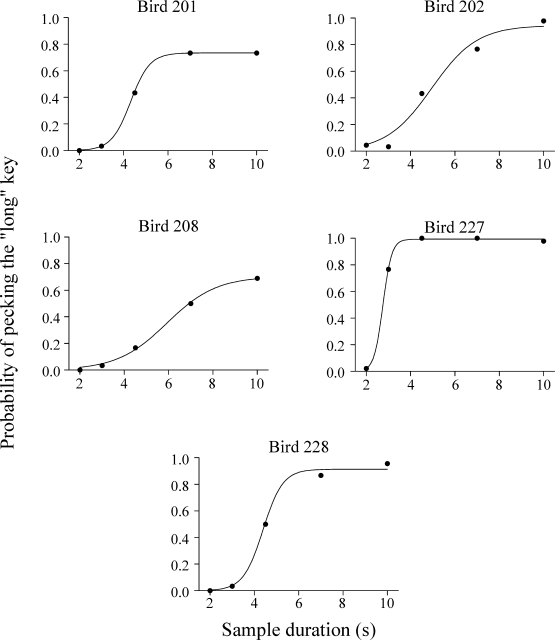
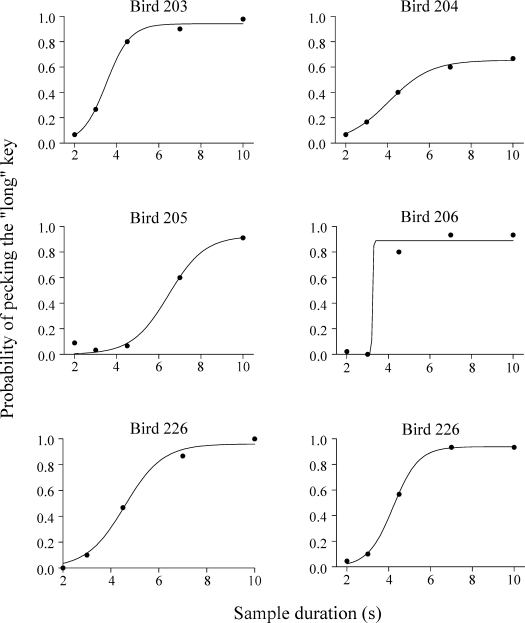
where t is the sample duration, μ is the mean of the function (an estimate of the bisection point), and σ is the standard deviation. Overall Equation 1 described the data well, accounting for 97.5% to 100% of the variance (VAC) in the individual data (M = 99.3%). Average values of the bisection point (μ) were 4.46 s (SEM = 0.52) for the Errorless group and 4.34 s (SEM = 0.51) for the Trial-and-Error group. In both cases, average bisection points were closer to the geometric mean (4.47) than the arithmetic mean (6) of the two sample durations. It should be noted that for 3 pigeons (2 from the Errorless group, 201 and 208, and 1 from the Trial-and-Error group, 204) the accuracy at the longest training stimuli was relatively low.
Fig 4.
Preference for the right green key, the key associated with 10-s sample, for the Errorless group. Filled circles correspond to the data from the stimulus generalization test and the curves through the data points are the best-fitting logistic curves (see Equation 1). The psychometric functions combine data from training trials (2-s and 10-s samples) and test trials (2.99-s, 4.47-s and 6.69-s samples).
Fig 5.
Preference for the right green key, the key associated with 10-s sample, for the Trial-and-Error group. Filled circles correspond to the data from stimulus generalization test and the curves through the data points are the best-fitting logistic curves (see Equation 1). As in Figure 4, the psychometric functions combine data from training trials (2-s and 10-s samples) and test trials (2.99-s, 4.47-s and 6.69-s samples).
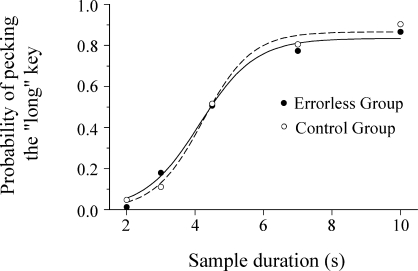
Figure 6 shows that the average psychometric functions did not differ between the two groups. An ANOVA with group and sample duration as factors found a significant effect of duration, F(4,36) = 71.62, p < .001, but the effect of group and the group x sample interaction was not significant. Furthermore, there were no significant differences between the groups in terms of the parameters of the logistic fits and VAC (for all t tests, p > .05).
Fig 6.
Average data from the stimulus generalization test (symbols) and average of the individual fitted curves (lines) for the Errorless and Trial-and-Error groups.
As mentioned before, we attempted to predict the bisection points for the Errorless group on the basis of the mediating behavioral pattern learned during the fading procedure. The distribution of the dark key pecks on the 10-s trials revealed a well-differentiated, highly stereotypical motion pattern for all birds: At trial onset, they pecked the left key (corresponding to the 2-s trials); after a few seconds, they switched to the right key (corresponding to the 10-s trials) and pecked this key until the end of the trial. The moment of switching should predict the bisection point.
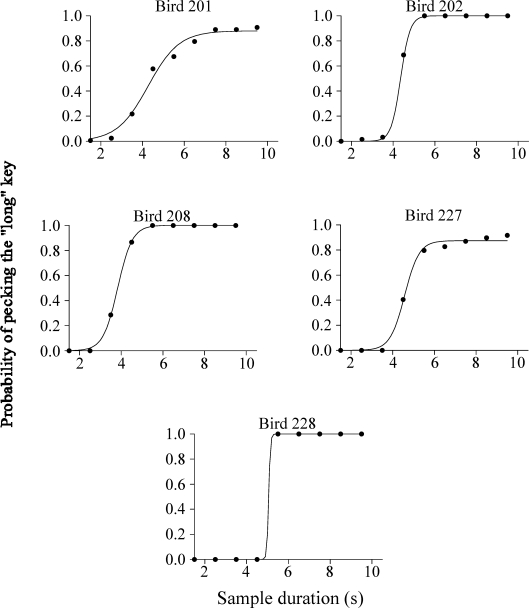
To determine whether this was the case, we calculated the proportion of pecks to the right key as a function of sample duration for all 10-s trials during the last five sessions of Phase II (100% fading). Equation 1 was fitted to those data and accounted for a very high percentage of the variance in the individual data (M = 99.7%; range: 99.6% to 100%). Figure 7 shows the individual and average data. The average value of the bisection point (μ) was 4.42 s (SEM = 0.20). A t test showed that there was no significant difference between these results and those obtained from the stimulus generalization test for the Errorless group (μ = 4.46 s), t(4) = −0.07, p = .95, suggesting that the hypothesis that the time when pigeons switched from pecking the left key to pecking the right key during the sample duration (when they were not illuminated) can predict the bisection points during the stimulus generalization test. In addition, we correlated for each pigeon the psychometric function obtained from the distribution of dark key pecks on the 10-s trials (Figure 7) and the psychometric function from the stimulus generalization test for the Errorless group (Figure 4). Results showed that correlations were highly positive for most pigeons (M = 0.88; Pigeons 201, 202, 208, 227 and 228: 1.00, 0.98, 0.85, 0.69 and 0.90, respectively), confirming our hypothesis.
Fig 7.
Proportion of pecks to the right key as a function of sample duration for all 10-s trials during the last five sessions of Phase II (100% fading), for the Errorless group.
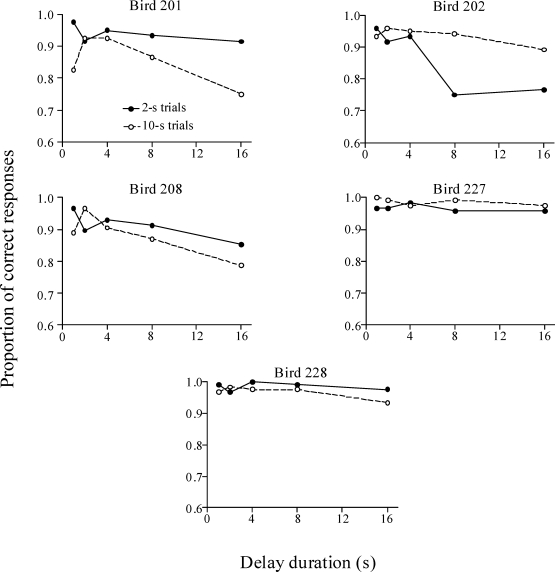
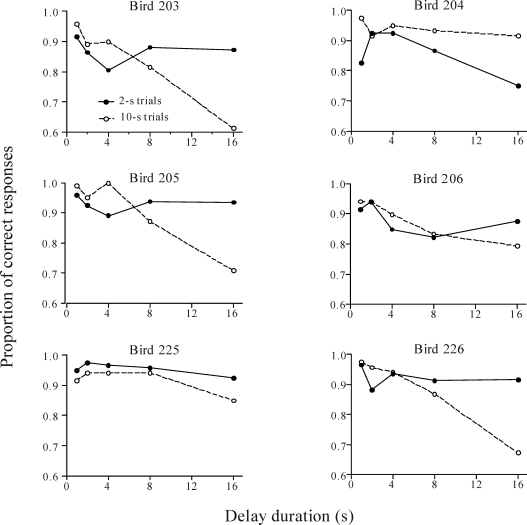
Delay Test
Figures 8 and 9 show the proportions of correct responses on 2-s and 10-s trials during the delay test. For all pigeons from the Trial-and-Error group, accuracy decreased with the delay interval. For 5 pigeons, accuracy was differentially degraded by delay on the 10-s sample trials, but for Pigeon 204 accuracy decreased more on 2-s than 10-s trials. Two subjects from the Errorless group (Pigeons 201 and 208) performed similarly to the majority of the subjects from the Trial-and-Error group, and 1 bird (Pigeon 202) performed the same as Pigeon 204. Interestingly, on both types of trials, Pigeons 225 and 228 from the Errorless group showed almost no reduction in accuracy with delay.
Fig 8.
Proportion of correct responses during the delay test for the Errorless group. Solid and dotted lines correspond to the 2-s and 10-s trials, respectively.
Fig 9.
Proportion of correct responses during the delay test for the Trial-and-Error group. Solid and dotted lines correspond to the 2-s and 10-s trials, respectively.
An ANOVA with group, sample duration, and delay as factors found a significant effect of delay, F (4,36) = 22.62, p < .001, confirming that as the delay duration increased, the proportion of correct responses decreased for both groups. There was also a significant interaction between sample duration and delay, F (4,36) = 3.20, p < .05. This interaction indicates that correct responses decreased with delay more on the 10-s trials than the 2-s trials. There was also a significant three-way interaction, F (4,36) = 2.92, p < .05, because the just stated two-way interaction happened only in the Trial-and-Error group. That is, the Errorless group showed no significant difference between the performances on both trials.
These results suggest that the errorless training made pigeons' performance less sensitive to delay on 10-s sample trials. They are consistent with the predictions based on the behavioral pattern of the Errorless group: The pigeons were pretrained to peck at the left key at the onset of the sample and then, after a few seconds had elapsed, to switch and peck at the right key until reinforcement occured. Therefore, when a delay was included between the offset of the sample and the onset of the choice keys, we expected those pigeons to stand in front of the right key for longer than the pigeons from the Trial-and-Error group, which were not trained to emit this mediating behavior.
Reversal Test
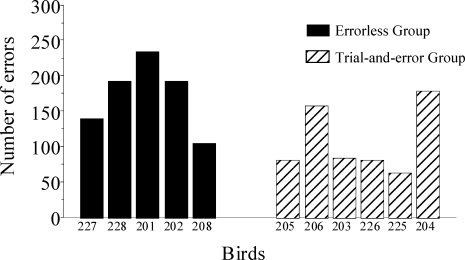
Figure 10 shows the number of errors made by each pigeon during the acquisition of the new discrimination. Whereas the Errorless group made an average of 173 errors before reaching the criterion (range: 104–234), the Trial-and-Error group made an average of 108 errors (range: 63–183). The individual data show that the pigeons for the Trial-and-Error group generally made fewer errors, but the difference between the group averages was not significant, t(9) = 2.13, p > .05.
Fig 10.
Number of responses to the S− made by each bird until they reached the learning criterion during the reversal test.
DISCUSSION
The main goal of the present study was to investigate whether a fading procedure could be used to teach a conditional discrimination with few or no errors. The fading procedure successfully established the intended mediating behavioral pattern: At the onset of the sample, the pigeons started to peck the 2-s key, with the response rate to this key attaining a maximum at 2 s or 3 s into the sample, and then they switched to the 10-s key, with the response rate to this key increasing for the remainder of the sample duration. Clare and Jones (2008) mentioned that the early animal experiments on errorless learning dealt only with very simple motor behaviors and that it would be difficult to imagine how complex behaviors could possibly be established using an errorless procedure. Our results showed that contrary to Clare and Jones' conclusion, it is possible to establish a complex behavioral pattern through an errorless learning procedure.
During the gradual introduction of the S−, the Errorless group averaged only one response to the S−, with 2 out of 5 pigeons making no errors at all. These results were similar to or better than those obtained in previous reports of successful errorless simple discriminations (e.g., Terrace, 1963a).
Comparisons of the Errorless group and the Trial-and-Error group on all discrimination trials before criterion was reached revealed a significant difference between the average number of errors made by each group (4 and 52, respectively). Notably, one pigeon from the Errorless group made 0 errors during all discrimination trials and another made only 1 error. These results show that the fading procedure was effective in teaching the errorless conditional discrimination, with all the pigeons learning the temporal discrimination with few or no errors. Ferster and Hammer (1966) speculated whether a fading procedure could be devised with complex stimuli to produce stimulus control without S− responding. Our study is the first to show that a fading procedure can be used to teach a complex, conditional discrimination with few or no responses to the S−. Because one of the reasons for the decreased interest in errorless learning by basic researchers was that it applied only to a restricted range of discrimination procedures, the extension of the fading procedure to a conditional discrimination is particularly noteworthy.
Rilling (1977) claimed that the early introduction of the S− during the discrimination training is the most powerful procedural variable in the errorless learning procedures. However, in our experiment the S− corresponding to each S+ was introduced only after an average of 46 S+ only sessions, showing that, contrary to Rilling's claim, the moment the S− is introduced may not be fundamental to learning a conditional discrimination without errors.
We hypothesized that if the mediating behavior established by the fading procedure remained stable, the time of switching from responding to the left key to responding to the right key should predict the bisection point of the stimulus generalization gradient. Results are consistent with this hypothesis, showing that (a) the average bisection point predicted from the pigeons' behavior and that obtained during the stimulus generalization test were similar (M's = 4.42 and 4.46), and (b) the behavioral pattern developed during pretraining and the pigeons' performance during the stimulus generalization test showed high positive correlation for all pigeons (M = .88). Machado and Keen (2003) have also compared the pigeons' behavioral patterns and their performance during a stimulus generalization test. In their experiment, they used a long chamber equipped with one key and feeder at each end, and with sensitive floor panels to record the subject's location. The pigeons had to choose one side key after a short sample and the other side key after a long sample. At the end of training, the pigeons started each trial by moving to the “short” side; they stayed there for a while, and then, if the trial continued, moved to the “long” side. For 4 out of 7 cases, the cumulative distribution of departure times (i.e., the time the pigeons left the “short” side) fitted well the psychometric function obtained from the stimulus generalization test. Thus, pigeons might display behavior during trials that suggests a kind of mediation of the temporal discrimination that could function similarly to the explicitly trained mediating behavior in the Errorless birds of the current study. This may account in part for the fact that, overall, results from the stimulus generalization test were similar for the Errorless and Trial-and-Error groups.
We also hypothesized that the performance of the Errorless group should be more resistant to disruption because their mediating behavior was explicitly trained. Because after a 10-s sample these pigeons should be standing in front of the right key, we predicted that when delays between the offset of the sample and the onset of the comparison stimuli were introduced (delay test), the accuracy levels should be higher than for the Trial-and-Error group. Although the Trial-and-Error birds may also have adopted, untrained by us, a kind of behavior that mediated their timing performance, explicit shaping and reinforcement in the Errorless birds established a robust and effective mediating performance that withstood the disruptive effects of the delays. Despite variability across subjects, results suggested that for the Errorless group, responding was overall less disrupted by the delay than responding for the Trial-and-Error group. In some sense, then, it may have been the greater resistance of the trained mediating behavior of the Errorless birds relative to the untrained mediating behavior of the Trial-and-Error birds that accounted for the difference in disruption during the delay tests.
Results from the reversal test showed that all pigeons learned the new discrimination, and the two groups made similar numbers of errors throughout its acquisition. Together with previous experiments in which researchers successfully reversed the contingences following a discrimination learned without errors (Aronsohn et al., 1978; Robinson & Storm, 1978; Schusterman, 1965, 1966; Stettner & Matyniak, 1969), our results challenge Rilling's claim that errorless learning may not be the “best learning for an organism exposed to a changing environment” (Rilling, 1977, p. 475).
The results of our study may have implications for theories of timing, theories of learning and applied psychology. We consider each in turn. Some theories of timing do not assume that errors (i.e., nonreinforced responses) are important for discrimination learning, as is the case of the popular Scalar Expectancy Theory (SET; Gibbon, 1977). However, other accounts such as the Learning-to-Time model (LeT; Machado, 1997) postulate that both reinforced and nonreinforced responses contribute to the acquisition of a discrimination. Previous research using a temporal double bisection task (e.g., Arantes, 2008; Arantes & Machado, 2008; Machado & Arantes, 2006), in which subjects are trained on two temporal conditional discriminations and later given a transfer test in which stimuli from the two discriminations are paired together, have provided some evidence in favor of LeT's assumption that responses to both S+ and S− are important. Future research should further investigate the role of the errors by using a fading procedure similar to that used in the present study to reduce or eliminate the errors made in a temporal double bisection task. If the errors are important for the discrimination learning, then these animals should perform differently than the animals that learn with a large number of errors. However, if the errors are not important, the animals' performance should be the same regardless of the number of errors.
Our results are important also for theories of learning. For example, they challenge the classical generalization theory (e.g., Spence, 1936, 1937; Hull, 1943). Specifically, because the S+ and S− differed only with respect to wavelength, were presented simultaneously, and the response to S+ had a long history of reinforcement before the S− was introduced (M = 46 sessions), the classical generalization theory would predict a generalization from the S+ to the S− and therefore that the pigeons would initially make some responses to the S− during choice trials. However, as we mentioned before, the pigeons did not generalize from the S+ to the S−, giving few or no responses to the latter.
One of the reasons why errorless learning attracted so much attention was because the S− did not seem to work as a conditioned inhibitor, but rather as a neutral stimulus (Terrace 1966b, 1967, 1971). A conditioned inhibitor is “a stimulus that develops during conditioning the capacity to decrease response strength below the level occurring when that stimulus is absent'” (Hearst, Besley & Farthing, 1970). Proposed by Pavlov (1927), the concept of conditioned inhibition has attracted the interest of several researchers (e.g., Hearst et al., 1970; Jenkins, 1965; Rescorla; 1969; Terrace, 1964, 1966a, 1972) and played an important role in models of discrimination learning (Hull, 1943, 1952; Spence, 1936, 1937). Several researchers have criticized Terrace's conclusion that the S− did not function as an inhibitor (e.g., Wessels, 1973; Wilkie & Ramer, 1974; see Rilling, 1977, for a review).
Our experiment sheds some light on this issue. In previous experiments on errorless learning the S+ (e.g., red key) was always associated with reinforcement while the S− (e.g., green key) was always associated with nonreinforcement. In our conditional discrimination study, the red key and the green keys were associated with reinforcement and nonreinforcement (depending on the preceding sample duration), and both keys were presented simultaneously during the discrimination trials. During approximately the first 46 sessions, the pigeons were presented exclusively with S+ trials, responding to the keys with a probability close to 100%. When the corresponding S− stimuli were introduced, the probability with which a response occurred did not decrease, that is, the presence of the S− did not seem to reduce the response strength to the S+. These results are consistent with Terrace's (1966b, 1967, 1971) assumptions that the errorless S− is not an inhibitor.
In summary, we showed that the errorless training is effective in reducing and even eliminating completely errors during the acquisition of a conditional temporal discrimination. Also, when exposed to stimulus generalization, delay, and reversal tests, the results from the Errorless group were similar to those from the Trial-and-Error group, although there was some indication that the Errorless group was less disrupted by delays. These results challenge some of the previous criticisms of errorless learning and suggest that the abandonment of this topic by basic researchers may have been premature. In fact, when first discovered, errorless learning attracted much attention because it was considered anomalous. Contrary to the prevailing view on discrimination, which assumed that associations with both reward and nonreward are necessary, in a simple errorless training procedure, one stimulus is associated with reward but the other remains neutral, for it is rarely or never chosen. In our task, both stimuli are associated with reward, albeit in different contexts, and yet the discrimination is learned without errors. The processes underlying errorless conditional discrimination learning remain to be elucidated.
Acknowledgments
This research was presented in part at the 33rd Annual Convention of the Association of Behavioral Analysis (ABA) in San Diego, California. The authors were supported by the Portuguese Foundation for Science and Technology (FCT). The authors thank Herbert S. Terrace for his valuable thoughts at the 31st Annual Meeting of the Society for the Quantitative Analyses of Behavior (SQAB) about this research.
REFERENCES
- Arantes J. Comparison of Scalar Expectancy Theory (SET) and the Learning-to-Time (LeT) model in a successive temporal bisection task. Behavioural Processes. 2008;78((2)):269–278. doi: 10.1016/j.beproc.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Arantes J, Berg M.E. Intermodal transfer from a visual to an auditory discrimination using an errorless learning procedure. Behavioral Processes. 2009;81:303–308. doi: 10.1016/j.beproc.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Arantes J, Machado A. Context effects in a temporal discrimination task: Further tests of the Scalar Expectancy Theory and Learning-to-Time models. Journal of the Experimental Analysis of Behavior. 2008;90:33–51. doi: 10.1901/jeab.2008.90-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronsohn S, Castillo O, Pinto-Hamuy T. Fading procedure effects on a visual pattern discrimination reversal in the albino rat. Animal Learning & Behavior. 1978;6:72–76. [Google Scholar]
- Barlow D.H, Agras S. Fading to increase heterosexual responsiveness in homosexuals. Journal of Applied Behavior Analysis. 1973;6:355–366. doi: 10.1901/jaba.1973.6-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkan B, McClannahan L.E, Krantz P.J. Effects of superimposition and background fading on the sight-word reading of a boy with autism. Research in Autism Spectrum Disorders. 2007;1:117–125. [Google Scholar]
- Clare L, Jones R.S.P. Errorless learning in the rehabilitation of memory impairment: A critical review. Neuropsychology Review. 2008;18:1–23. doi: 10.1007/s11065-008-9051-4. [DOI] [PubMed] [Google Scholar]
- Corey J.R, Shamow J. The effects of fading on the acquisition and retention of oral reading. Journal of Applied Behavior Analysis. 1972;5:311–315. doi: 10.1901/jaba.1972.5-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster C.B, Hammer C.E., Jr . Synthesizing the components of arithmetic behavior. In: Honig W.K, editor. Operant behavior: areas of research and application. New York: Appleton-Century-Crofts; 1966. (Ed.) [Google Scholar]
- Fetterman J.G, Killeen P, Hall S. Watching the clock. Behavioral Processes. 1998;44:211–224. doi: 10.1016/S0376-6357(98)00050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J. Scalar expectancy theory and Weber's law in animal timing. Psychological Review. 1977;84:279–325. [Google Scholar]
- Gollin E.S, Savoy P. Fading procedures and conditional discrimination in children. Journal of the Experimental Analysis of Behavior. 1968;11:443–451. doi: 10.1901/jeab.1968.11-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnick M.J. Effects of distinctive-feature training and instructional technique on letter and form discrimination. American Journal of Mental Deficiency. 1975;80:202–207. [PubMed] [Google Scholar]
- Hearst E, Besley S, Farthing G.W. Inhibition and the stimulus control of operant behavior. Journal of the Experimental Analysis of Behavior. 1970;14:373–409. doi: 10.1901/jeab.1970.14-s373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C.L. Principles of Behavior: An Introduction to Behavior Theory. New York: Appleton-Century-Crofts; 1943. [Google Scholar]
- Hull C.L. Simple qualitative discrimination learning. Psychological Review. 1950;57:303–313. doi: 10.1037/h0062099. [DOI] [PubMed] [Google Scholar]
- Hull C.L. A Behaviour System. New Haven, CT: Yale University Press; 1952. [Google Scholar]
- Jenkins H.M. Generalization gradients and the concept of inhibition. In: Mostofsky D.I, editor. Stimulus generalization. Stanford: Stanford University Press; 1965. pp. 55–61. (Ed.) [Google Scholar]
- Kodera T.L, Rilling M. Procedural antecedents of behavioral contrast: a re-examination of errorless learning. Journal of the Experimental Analysis of Behavior. 1976;25:27–42. doi: 10.1901/jeab.1976.25-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons J. Stimulus generalization as a function of discrimination learning with and without errors. Science. 1969a;163:490–491. doi: 10.1126/science.163.3866.490. [DOI] [PubMed] [Google Scholar]
- Lyons J. Stimulus generalization along dimension of S+ as a function of discrimination learning with and without errors. Journal of Experimental Psychology. 1969b;81:95–100. [Google Scholar]
- Machado A. Learning the temporal dynamics of behavior. Psychological Review. 1997;104:241–265. doi: 10.1037/0033-295x.104.2.241. [DOI] [PubMed] [Google Scholar]
- Machado A, Arantes J. Further tests of the Scalar Expectancy Theory (SET) and the Learning-to-Time (LeT) model in a temporal bisection task. Behavioural Processes. 2006;72:195–206. doi: 10.1016/j.beproc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Machado A, Keen R. Temporal discrimination in a long operant chamber. Behavioural Processes. 2003;62:157–182. doi: 10.1016/s0376-6357(03)00023-8. [DOI] [PubMed] [Google Scholar]
- Marsh G, Johnson R. Discrimination reversal following learning without “errors.”. Psychonomic Science. 1968;10:261–262. [Google Scholar]
- Mackay H.A, Brown S.M. Teaching serial position sequences to monkeys with a delayed matching-to-sample procedure. Journal of the Experimental Analysis of Behavior. 1971;15:335–345. doi: 10.1901/jeab.1971.15-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R, Goldiamond I. Errorless establishment of visual discrimination using fading procedures. Journal of the Experimental Analysis of Behavior. 1964;7:269–272. doi: 10.1901/jeab.1964.7-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace G.M, Iwata B.A, Cowdery G.E, Andree P.J, McIntyre T. Stimulus (instructional) fading during extinction of self-injurious escape behavior. Journal of Applied Behavior Analysis. 1993;26:205–212. doi: 10.1901/jaba.1993.26-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov I.P. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. Translated and edited by G. V. Anrep. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla R.A. Pavlovian conditioned inhibition. Psychological Bulletin. 1969;72:77–94. [Google Scholar]
- Rilling M. Stimulus control and inhibitory processes. In: Honig W.K, Staddon J.E.R, editors. Handbook of operant behavior. Englewood Cliffs, NJ: Prentice-Hall; 1977. pp. 432–480. (Eds.) [Google Scholar]
- Rilling M, Caplan H.J. Frequency of reinforcement as a determinant of extinction-induced aggression during errorless discrimination learning. Journal of the Experimental Analysis of Behavior. 1975;23:121–129. doi: 10.1901/jeab.1975.23-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling M, Kramer T.J, Richards R.W. Aversive properties of the negative stimulus during learning with and without errors. Learning and Motivation. 1973;4:1–10. [Google Scholar]
- Robinson P.W, Foster D.F, Bridges C.V. Errorless learning in newborn chicks. Animal Learning & Behavior. 1976;4:266–268. [Google Scholar]
- Robinson P.W, Storm R.H. Effects of error and errorless discrimination acquisition on reversal learning. Journal of the Experimental Analysis of Behavior. 1978;29:517–525. doi: 10.1901/jeab.1978.29-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilmoeller G.L, Schilmoeller K.J, Etzel B.C, LeBlanc J.M. Conditional discrimination after errorless and trial-and-error training. Journal of the Experimental Analysis of Behavior. 1979;31:405–420. doi: 10.1901/jeab.1979.31-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schusterman R.J. Errorless discrimination-reversal learning in the California sea lion. Proceedings of the American Psychological Association. 1965;1:141–142. [Google Scholar]
- Schusterman R.J. Serial discrimination-reversal learning with and without errors by the California sea lion. Journal of the Experimental Analysis of Behavior. 1966;9:593–600. doi: 10.1901/jeab.1966.9-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Rosenberger P.B. Several methods for teaching serial position sequences to monkeys. Journal of the Experimental Analysis of Behavior. 1967;10:467–478. doi: 10.1901/jeab.1967.10-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Stoddard L.T. The effectiveness of fading in programming a simultaneous form discrimination for retarded children. Journal of the Experimental Analysis of Behavior. 1967;10:3–15. doi: 10.1901/jeab.1967.10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence K.W. The nature of discrimination learning in animals. Psychological Review. 1936;43:427–449. doi: 10.1037/h0063067. [DOI] [PubMed] [Google Scholar]
- Spence K.W. The differential response in animals to stimuli varying within a single dimension. Psychological Review. 1937;44:430–444. [Google Scholar]
- Stettner L.J, Matyniak K.A. The use of stimulus fading in assessing behavioral deficits produced by brain damage. Physiology and Behavior. 1969;4:859–861. [Google Scholar]
- Stubbs D.A. The discrimination of stimulus duration by pigeons. Journal of the Experimental Analysis of Behavior. 1968;11:223–238. doi: 10.1901/jeab.1968.11-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrace H.S. Discrimination learning with and without “errors”. Journal of the Experimental Analysis of Behavior. 1963a;6:1–27. doi: 10.1901/jeab.1963.6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrace H.S. Errorless transfer of a discrimination across two continua. Journal of the Experimental Analysis of Behavior. 1963b;6:223–232. doi: 10.1901/jeab.1963.6-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrace H.S. Errorless discrimination learning in the pigeon: effects of chlorpromazine and imipramine. Science. 1963c;140:318–319. doi: 10.1126/science.140.3564.318. [DOI] [PubMed] [Google Scholar]
- Terrace H.S. Wavelength generalization after discrimination learning with and without errors. Science. 1964;144:78–80. doi: 10.1126/science.144.3614.78. [DOI] [PubMed] [Google Scholar]
- Terrace H.S. Discrimination learning and inhibition. Science. 1966a;154:1677–1680. doi: 10.1126/science.154.3757.1677. [DOI] [PubMed] [Google Scholar]
- Terrace H.S. Stimulus control. In: Honig W.K, editor. Operant behavior: Areas of research and application. New York: Appleton-Century-Crofts; 1966b. pp. 271–344. (Ed.) [Google Scholar]
- Terrace H.S. Discrimination learning and inhibition. Science. 1967;156:988–989. doi: 10.1126/science.156.3777.988. [DOI] [PubMed] [Google Scholar]
- Terrace H.S. Extinction of a discriminative operant following discrimination learning with and without errors. Journal of the Experimental Analysis of Behavior. 1969;12:571–582. doi: 10.1901/jeab.1969.12-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrace H.S. Escape from S−. Learning and Motivation. 1971;2:148–163. [Google Scholar]
- Terrace H.S. By-products of discrimination learning. In: Bower G.H, editor. The psychology of learning and motivation, vol. 5. New York: Academic Press; 1972. pp. 195–265. (Ed.) [Google Scholar]
- Terrace H.S. On the nature of non-responding in discrimination learning with and without errors. Journal of the Experimental Analysis of Behavior. 1974;22:151–159. doi: 10.1901/jeab.1974.22-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchette P.E, Howard J.S. Errorless learning: reinforcement contingencies and stimulus control transfer in delayed prompting. Journal of Applied Behavior Analysis. 1984;17:175–188. doi: 10.1901/jaba.1984.17-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells M.G. Errorless discrimination, autoshaping and conditioned inhibition. Science. 1973;182:941–943. doi: 10.1126/science.182.4115.941. [DOI] [PubMed] [Google Scholar]
- Wilkie D.M, Ramer D.G. Errorless discrimination established by differential autoshaping. Journal of the Experimental Analysis of Behavior. 1974;22:333–340. doi: 10.1901/jeab.1974.22-333. [DOI] [PMC free article] [PubMed] [Google Scholar]