Abstract
Aged dogs exhibit a spectrum of cognitive abilities including a syndrome similar to Alzheimer's disease. A major impediment to research so far has been the lack of a quick and accurate test of visuospatial memory appropriate for community-based animals. We therefore report on the development and validation of the Canine Sand Maze. A 4.5-m-diameter circular pool was filled with a sand and powdered food reward mix to a depth of 10 cm. Dogs were given 4 habituation and 16 learning trials which alternated a food reward being half (control trials) or fully-buried (acquisition trials) in a fixed location. After a 90-min break, a probe trial was conducted. Cognitively normal, aged (> 8 years, n = 11) and young (1–4 years, n = 11), breed-matched dogs were compared. After correction for differences in control trials, average probe times were 2.97 and 10.81 s for young and aged dogs, respectively. In the probe trial, both groups spent significantly more time in the target quadrant but there was a trend for young dogs to cross a 1 m2 annulus zone around the buried reward more frequently (2.6 times) than aged dogs (1.5 times). Test–retest reliability in a subset of young dogs (n = 5) was high. On the basis of these findings, the Canine Sand Maze is presented as a quick, sensitive and nonaversive tool for assessing spatial learning and reference memory in dogs.
Keywords: memory; cognition; aging; sand maze; canine, dog
Decline of cognitive function with age is a phenomenon that affects all mammals including humans (Berchtold & Cotman, 2009). Both developed and developing nations are experiencing demographic aging (Beck, 2009) and associated with this is an increasing prevalence of age-related cognitive disorders (Brookmeyer, Johnson, Ziegler-Graham, & Arrighi, 2007). Researchers are therefore exploring new animal models of brain aging, beyond the traditional rodent species. Research on canine brain aging has produced many interesting findings. Older dogs naturally exhibit varying degrees of cognitive decline, including successful aging, incipient cognitive impairment and overt cognitive dysfunction or dementia (Adams, Chan, Callahan, & Milgram, 2000a; Cummings, Head, Ruehl, Milgram, & Cotman, 1996). Along with this, they also show evidence of Alzheimer's pathology, including beta-amyloid plaques (Head, McCleary, Hahn, Milgram, & Cotman, 2000) and brain atrophy (Tapp, Head, Head, Milgram, Muggenburg, & Su, 2006). Furthermore, when testing new pharmacological treatments aimed at improving cognitive function, dogs show superior prediction of human responses compared with rodent models (Studzinski, Araujo, & Milgram, 2005).
In addition to the biological advantages of the dog as a model of brain aging, dogs are popular pets and there is hence the possibility of access to a large pool of aging animals which share the human environment and possible exposure to environmental risk factors. The use of community-based (pet) dogs in aging research presents some unique challenges. In particular, there is a requirement to develop noninvasive and easily administered tests that are compatible with the owner's lifestyle and ethical beliefs.
For community-based dogs to be used effectively as a model for brain aging, it is vital that appropriate measures of decline in the areas of episodic spatial reference memory and visuospatial working memory are available. Age-related memory decline in these specific domains occur in both dogs (Adams et al., 2000b) and humans (Trollor & Valenzuela, 2001), and are severely affected early in the course of Alzheimer's dementia. In colony-based dogs, the delayed-non-match-to-position task (DNMP) has been commonly used to assess visuospatial working memory (Adams et al, 2000b; Head et al., 1995). This task is an effective assessment tool, but requires up to 400 training trials over 40 days to reach learning criterion at any one delay. In addition, 18% of aged dogs fail to reach criterion for the task at a delay of 10 s (Adams et al., 2000b), and 39% fail at a delay of 30 s (Head et al., 1995). This level of difficulty, and the need for intensive training for DNMP, effectively rules out its use in community-based dogs.
In rodent studies of brain aging, some of the difficulties associated with intensive DNMP training are avoided by use of the classic Morris Water Maze (MWM) (D'Hooge & De Deyn, 2001; Morris, 1984). This task relies on an aversive stimulus (being trapped in a pool of water) to motivate the animal to learn and memorize the location of a hidden escape platform. The aversive nature of this task has been suggested to stimulate distinct brain structures compared to those involved when nonaversive stimuli are used (D'Hooge & De Deyn; Gotthard, 2006; McGaugh, 2000). Paradigms using aversive stimuli are also unsuitable for use with community-based dogs, as any testing must be acceptable to their owners.
The sand maze was developed for rats in response to these issues, and is a nonaversive, appetitive adaptation of the MWM paradigm (Gotthard, 2006; Hansen, 2003). This task involves the rodent learning and retaining the location of a food reward buried in sand and relies on the animal's motivation to forage for food. Direct comparison of rats' performances in the sand maze and MWM showed similar learning and retention response patterns, although the MWM produced shorter latencies and greater preferences shown for the correct region. Use of an N-methyl-D-aspartate (NMDA) antagonist (MK-801) in a sand maze reversal learning task also produced deficits in the learning of the new location compared with a placebo control, suggesting the sand maze is a valid and versatile spatial memory task (Gotthard, 2006).
Our aim was to adapt this approach to develop a sensitive measure of memory function that can reliably detect subtle longitudinal changes or group differences. The Canine Sand Maze (CSM) is a simple visuospatial memory paradigm that takes advantage of dogs' natural behavior to locate and dig-up buried food. Since no aversive stimuli are used, and the entire test can be conducted in a relatively short time-frame, it is potentially suitable for use in community-owned dogs.
METHOD
Subjects
This research was conducted with approval from the University of Sydney's animal ethics committee. Animals were community-based companion dogs (Canis lupis familiaris) categorized as young (1–4 years, n = 11) or aged (≥ 8 years, n = 11). They had no previous research experience. As our intention was to focus on the effects of normal aging on cognition, aged dogs were assessed for behaviors indicative of the canine equivalent of dementia using the Canine Cognitive Dysfunction Rating scale (CCDR) and excluded if they were above diagnostic threshold (Salvin, McGreevy, Sachdev, & Valenzuela, in press). All aged animals also underwent a full veterinary assessment to exclude noncognitive causes of poor performance on the CSM. This included a clinical history, physical examination, urinalysis, blood count, biochemistry, and thyroid function tests. Other reasons for exclusion were: body condition score over 4 (severely obese) (McGreevy, Thomson, Pride, Fawcett, Grassi, & Jones, 2005), separation-related distress, or severe visual impairment. Different gundog breeds were used due to their high food drive and medium size and the age groups were matched for breed. Test–retest reliability over a 6–9 month period was examined in a random subset of young dogs (n = 5).
Canine Sand Maze
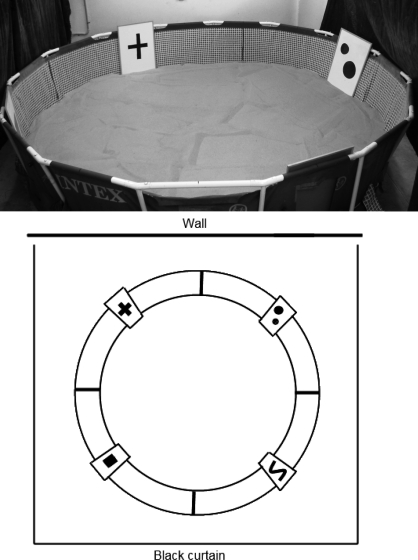
The CSM (Figure 1) consists of a 4.5-m-diameter round pool filled with 3 tons of washed river sand to a depth of 10 cm. Four doors were positioned equidistantly around the sides of the pool. The doors also served as visual cues, each one displaying a large black symbol: +, S, oO, □. Powdered dried liver (Show Em How Much You Love Em®, Somersby NSW, 10 kg/150 kg sand) was added to the top 5 cm of sand to mask any odor cues given by the reward, a dried liver drop (Show Em How Much You Love Em®, about 2 cm diameter). Owners were asked not to give their dogs any breakfast on the morning of the trial but no other food restrictions were applied. Black curtains were used to screen the pool from the rest of the room and lighting was provided from above by four fluorescent lights. The combination of large pool width and limited ceiling height in the test room required four CCTV cameras (one over each quadrant) to be used to monitor and record a dog's behavior during the CSM. Live video was streamed to a monitor outside the experimental area which allowed the experimenters to monitor a dog's behavior and performance on the task without being visible to the dog. All trial video recordings were saved and archived.
Fig 1.
The canine sand maze, a 4.5-m-diameter pool filled with sand to a depth of 10 cm. Four doors are positioned equidistantly around the pool; the doors also serve as visual cues. A black curtain screens the pool from the rest of the experimental area.
Testing Protocol
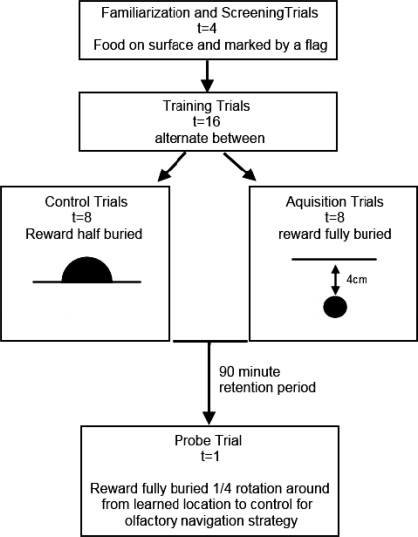
Each trial consisted of the dog being placed in the CSM without the experimenter present until the reward was located or for a maximum 90-s period. The dog entered through each door an equal number of times but the order of the doors used was randomized. Between each trial, the sand was raked thoroughly to disturb any visual or odor cues, and any sand contaminated with urine or feces was removed. The intertrial interval was 2 min. If the dog did not locate the reward within the 90-s period, it was shown the location by the experimenter, allowed to eat the reward and then removed from the CSM. An overview of the protocol is presented in Figure 2.
Fig 2.
The CSM trial protocol.
Familiarization and screening trials
Four screening trials were used to familiarize the dog to the testing environment as well as to screen for nonspecific factors that could confound task performance, such as motor and sensory dysfunction, or lack of motivation to find and consume visible food. A food reward was placed on the surface of the sand and, in successive trials, the dog entered the arena from each of the four different doors. The reward was placed below a marker (flag) to increase its visibility. Dogs that had to be directed to the location of the food reward on three or more of the screening trials were discontinued from testing. During all trials, the experimenter was screened from the dog's view to prevent it orientating to the experimenter or obtaining cues as to the location of the reward.
Training trials
Different training protocols were trialed during pilot testing and development on a separate cohort of dogs. The protocol which best maintained the dogs' attention and interest in the task, and reported here, involved alternating between trials in which the reward was half-buried but still visible (eight control trials) and trials in which the reward was fully buried (eight acquisition trials). Training therefore consisted of a total of 16 trials, in which control and acquisition trials were interleaved.
In the acquisition trials, four reward pieces were buried in a square configuration approximately 20 cm apart and at a depth of 4 cm. Multiple reward pieces were used to increase the likelihood of the dog locating the reward when digging in the correct location, but each dog was only allowed to retrieve one reward piece before being removed from the CSM. To remove the dogs, the experimenter called the dog or held the dog's collar from the outside of the pool and guided it to the closest door.
Delayed probe trial
After the last training trial, dogs were given a 90-min retention period in which they were toileted and then rested quietly in a separate room. At the end of the retention period, dogs were returned to the CSM for a final probe trial in which there was no food in the learned location; in addition, a dummy food reward was buried a ¼ rotation around the pool from the learned location. The dummy food reward was used to exclude the possibility that the dogs were using odor cues to locate the reward.
Outcome variables
Latency to reach the reward (start chewing) was recorded for all trials. For acquisition trials, the time taken to enter a 1 m2 annulus zone around the buried reward was also recorded. The percentage time spent in each quadrant was calculated for the probe trial, with the quadrant containing the learned location labeled as “target”, followed by quadrants A–C in a clockwise direction. The number of times a dog crossed the annulus in the probe trial was also recorded. An annulus-cross consisted of the dog placing at least its head in the annulus.
For three representative dogs, path diagrams are presented to demonstrate the variability in traveled distance and search patterns across animals with different levels of performance.
Analysis
SPSS v17 (SPSS Inc, Chicago) was used for all analyses and the significance threshold was set at p < .05. Correction for nonspecific interanimal differences such as motivational, motor and sensory differences was required to focus analysis on cognitive-dependent performance factors. This was accomplished by normalizing each individual dog's performance on acquisition trials or the probe trial by the square root of the average reward time on control trials. Square root transformation was used because of highly skewed raw control trial data. Both raw and corrected outcomes are presented. Difference in acquisition across trials (TRIAL) and between age groups (AGE) was assessed using a repeated measures factorial ANOVA. Probe performance differences between groups were assessed using independent samples t-test. Test–retest reliability was assessed using repeated measures ANOVA or Pearson correlation as appropriate. Test–retest accuracy of the path diagrams were analyzed by converting traced diagrams to a computer image, standardizing the sand maze arena dimensions and calculating the pairwise absolute difference and correlation between number of path pixels.
RESULTS
Subjects
After exclusion criteria had been applied, breeds were matched across age groups and included 11 dogs in each: Labrador retrievers (n = 4), golden retrievers (n = 4), English springer spaniels (n = 2) and a German short-haired pointer (n = 1). Average age was 2.8 years (SD = 1.17) for the young group and 9.7 years (SD = 1.42) for the aged group. The ratio of males to females was 4∶7 for the young group and 6∶5 for the aged group.
Dogs (n = 3) were excluded due to the inability to find a suitable breed match in the opposing age group. Additional dogs (n = 3) were excluded due to separation related distress not disclosed by their owners during recruitment.
Control Trials
On average, aged dogs took significantly longer to reach the reward during control trials with the half-buried reward, with aged and young dogs taking 13.14 s (SD = 6.89 s) and 6.18 s (SD = 3.46 s), respectively, F (1, 21) = 8.935, p = .007.
Acquisition Trials
There was no significant effect of TRIAL number on the raw time taken to reach the annulus, F(7, 140) = 2.111, p = .116. However, there was a nonsignificant trend for decreased time to the buried reward across trials, F(7, 140) = 2.321, p = .085. Further analysis showed that compared with Trial 1, Trials 3–8 showed a significant decrease in the raw time to the buried reward (p-values ranged from .002–.014). These results indicate that animals became generally faster at reaching the reward as they learned across the training trials.
There was no significant AGE effect for raw time taken to reach the buried reward, F(1, 20) = 0.033, p = .857, but AGE approached significance when analyzing for time to annulus, F(1, 20) = 2.283, p = .109. There was no significant TRIAL x AGE interaction for either time to annulus, F(1, 20) = 1.166, p = .384, or time to buried reward, F(1, 20) = 0.186, p = .984.
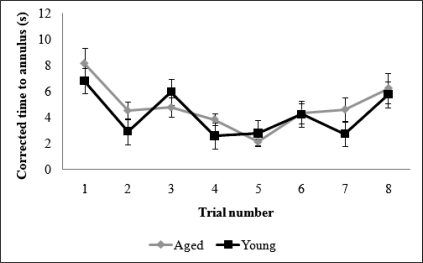
After normalizing for the noted group differences in control trial performance, there remained no significant effect of AGE (annulus time F(1, 20) = 0.685, p = .418; reward time F(1, 20) = 3.893, p = .062), TRIAL (annulus time F(7, 140) = 2.021, p = .130; reward time F(7, 140) = 1.947, p = .137), or TRIAL x AGE interaction (annulus time F(1, 20) = 0.773, p = .620; reward time F(1, 20) = 0.338, p = .923) (see Figure 3).
Fig 3.
Time taken by aged (n = 11) and young (n = 11) breed-matched dogs to reach a 1-m2 annulus around a buried food reward during eight acquisition trials. All times were corrected for nonspecific differences at an individual level. Standard error of the mean bars are given for each value.
Probe Retention Performance
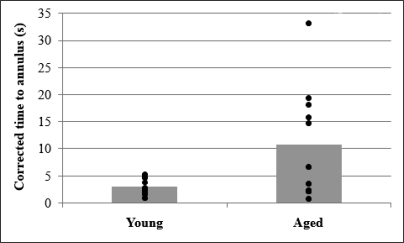
Aged dogs took significantly longer than young dogs, t(21) = −2.835; p = .017, to reach the annulus during the probe retention trial. After correcting for nonspecific factors, the difference in probe retention times remained significant, t(21) = −2.500; p = .031, with young dogs taking on average 2.97 s (SD = 1.45) compared with 10.81 s (SD = 10.30) for aged dogs. Given the skewed nature of the probe results, we tested these outcomes after natural log transformation. Results remained significant after transformation of either raw time to annulus, t(21) = 3.07, p = .006, or corrected time to annulus, t(21) = 2.14, p = 0.045. We also noted there was a higher level of variation in the probe times for aged compared with young dogs, as seen in Figure 4.
Fig 4.
Average time to annulus during the probe retention trial for aged (n = 11) and young (n = 11) breed-matched dogs and the distribution of individual scores within each group. All times were corrected for nonspecific differences at an individual level.
Search Strategy Analysis
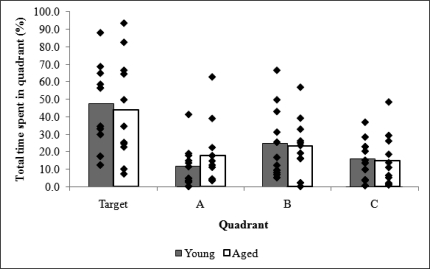
There was no significant difference between the percentage of time young and aged dogs spent in the target quadrant in the probe trial (T = 0.308, p = 0.761, df = 21). Both young and aged dogs (pooled data) spent significantly more time in the target quadrant than the other three quadrants (Target versus A: F(1, 20) = 14.859, p = .001; Target versus B: F(1, 20) = 6.273, p = .021; Target versus C: F(1, 20) = 17.886, p < .001, see Figure 5). There was a near-significant trend for young dogs to cross the annulus more frequently (2.6 times) in the probe retention trial than aged dogs (1.5 times), t(21) = 2.030, p = .056.
Fig 5.
Average percentage of time spent in each quadrant during the probe retention trial for aged (n = 11) and young (n = 11) breed-matched dogs and the distribution of individual scores. Quadrants were labeled: Target, the quadrant containing the learned location, and then A–C in a clockwise direction.
Test–Retest Stability
Of the 5 young dogs retested 6 months after their original testing, one could not complete testing due to separation-related distress. For the remaining 4 dogs, there was no significant difference between the two corrected probe times (mean difference = 42.9%, t(3) = −0.367, p = .738). The minimum and maximum within-dog difference in corrected probe times were 0.38 s and 7.31 s, respectively.
Individual Variation
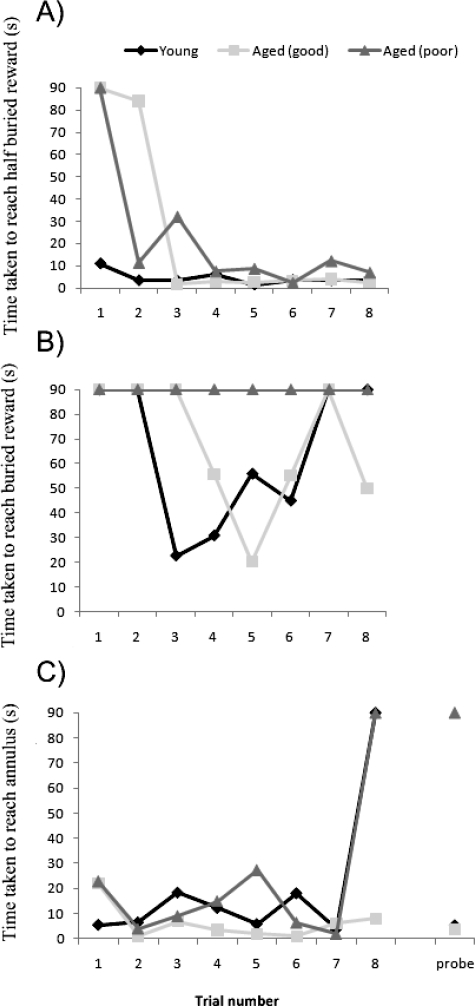
The uncorrected learning and retention data for an average-performing young dog (Labrador, 3 years), a high-performing (unimpaired) aged dog (Labrador, 10 yr) and a poor-performing (impaired) aged dog (Golden retriever, 10 yr) are provided to illustrate individual variation (Figure 6A–C).
Fig 6.
Individual acquisition and retention data for 3 example dogs, a young dog (Labrador, 3 years), an unimpaired aged dog (Labrador, 10 years) and an impaired aged dog (Golden retriever, 10 years). A) Time taken to reach the reward on control trials. B) Time taken to reach the buried reward on acquisition trials. C) Time to reach the 1-m2 annulus on acquisition trials and the probe retention trial.
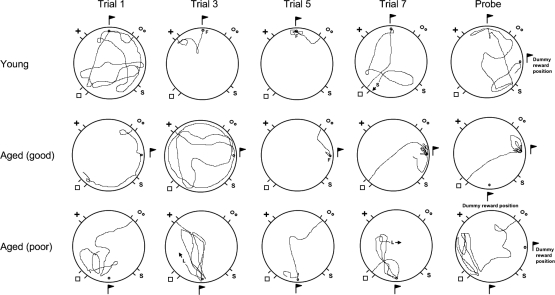
Figure 7 shows the distance travelled and the search strategy used by the 3 dogs for a selection of acquisition trials and the probe trial. It also shows variation in performance based on the door entered during the trial. Blinded test–retest analysis of these 15 pairs of path diagrams 3 weeks apart demonstrated high reliability: a 0.4% absolute mean difference in pixel numbers (SD = 8.3%) and a pairwise correlation of r(13) = 0.99 p < .0001.
Fig 7.
Path diagrams for 3 example dogs, a young dog (Labrador, 3 years), an unimpaired aged dog (Labrador, 10 years) and an impaired aged dog (Golden retriever, 10 years), for acquisition trials 1, 3, 5 and 7 and the probe retention trial. The flag indicates the learned location. F = found the reward, S = sitting facing the direction of the arrow, L = lying facing the direction of the arrow.
DISCUSSION
There has been increasing interest in the use of dogs as a model for brain aging (Adams et al., 2000a; Studzinski et al., 2005). To date, colony-based dogs have been favored for these studies, but there also exists a large population of aging pet dogs within the community that could potentially be utilized for research (Morell, 2009). Research in community-based dogs has so far been limited by the lack of a sensitive memory paradigm that is both nonaversive and practical over a short time-frame.
Our study has shown that the nonaversive sand maze test in rodents can be adapted to canines. The CSM is a visuospatial memory paradigm that can be completed in 3 hr, and relies on a dog's innate motivation for food that obviates the need for extensive training. While a number of different gundog breeds were used here, it is likely that any dog with a normal food motivation would be able to complete this task. To address whether dogs were using odor cues to spatially navigate, we employed a number of design strategies. Powdered food reward was mixed throughout the sand to help mask any reward-specific odors. Redistributing the sand by raking it in between trials also reduced the likelihood that dogs followed their own scent trail to the learned location. Furthermore, in the delayed probe trial, a dummy food reward was buried a quarter of a rotation around from the learned location. No dog successfully found this food, suggesting that our dogs were not primarily locating the food based on odor. Finally, we ensured that dogs entered the maze from different doors in the training phase, and hence we minimized their potential to develop a search strategy based on a predictable pattern such as “always turn left”.
With these design factors in mind, the CSM was found to be sensitive to subtle differences in spatial memory performance between young and cognitively unimpaired aged dogs. Importantly, older animals with evidence of a dementia-like syndrome were excluded from the current study, so our findings are relevant to normative age-related changes in canine memory. Given that both age groups exhibited evidence of a learning effect during acquisition trials in the form of decreasing latencies, and there were no group differences during this training phase, age-related divergence in delayed probe performance cannot be explained by different encoding of the training stimuli. In fact, both before and after correction for nonspecific factors at an individual level, aged dogs took, on average, over 3.5 times longer to reach the learned location after a 90-min retention period. Our findings therefore indicate that the CSM is sensitive to age-related difference in retention and recall of spatial reference memory.
We further investigated whether the increased time to reach the annulus in the delayed probe trial was the result of altered search strategies in the aged group, an effect sometimes observed in rodent MWM studies (Calhoun, 2009). In our CSM, despite the aged group taking longer to reach the 1 m2 annulus, both groups showed a significant spatial bias to the target quadrant. Since both groups of animals exhibited an appropriate spatial search strategy, our quantitative results are likely to be related to subtle age-related changes. This interpretation was further supported by a near-significant trend for older animals to cross the annulus less than young dogs (p = .056). Young dogs therefore showed more sustained interest in the learned location. An alternative explanation is that after not finding the food reward in the learned location, older dogs either lost motivation to continue searching in that area, or the spatial representation was more effectively extinguished than in younger animals. Further research is required to address these questions and the CSM is readily adaptable for this task.
Our CSM results are therefore comparable to those seen in both the MWM and the rodent sand maze. In all instances, a decrease in latencies to the learned location was noted during training, and an increased preference for the target quadrant was seen in probe retention trials (D'Hooge & De Deyn, 2001; Gotthard, 2006). Dogs in the CSM spent approximately 45% of their time in the target quadrant in probe retention trials, slightly lower than the 50% or more commonly seen in the MWM (D'Hooge & De Deyn). A similar reduced level of quadrant preferences as in our CSM was seen in the rodent sand maze (Gotthard), and this may be due to the difference between foraging versus aversive stimuli as motivating factors.
There has been a large body of research using the MWM to study age-related effects (Brandeis, Brandys, & Yehuda, 1989; Calhoun, 2009; Gallagher, 1997). In general, age increases the overall latency and decreases accuracy of delayed probe performance as well as increasing interanimal variance. In fact, an age-related increase in memory performance variance is a canonical feature of both rodent and human studies (Rapp & Amaral, 1992). Our CSM findings of increased latency and variance in the delayed retention part of the paradigm are therefore consistent with memory studies in other animal models. We did not, however, find differences between age groups in the acquisition trials, an effect which has been found in MWM studies (Brandeis et al., 1989; D'Hooge & De Deyn, 2001). Further investigation on the effects of age in MWM studies has found that the performance of aged animals can be segregated into the unimpaired, whose memory function is indistinguishable to that of younger animals, and impaired animals that perform significantly worse (Gallagher, 1997). Since we excluded animals with a dementia-like syndrome, this may explain why there was no age effect seen in the acquisition trials. Further research using dogs with a wider spectrum of cognitive decline may clarify this issue.
The appetitive and naturalistic nature of the CSM has the benefit of potentially producing a more direct measure of memory function. MWM findings have been recently criticized because the aversive aspects of this protocol mean that age-related performance effects may represent an interaction between negative emotional states and cognitive substrates, rather than purely cognitive outcomes (Calhoun, 2009). Generally, this issue does not apply to the CSM due to its use of nonaversive stimuli, although this may lead to reduced motivation in some individuals. The paradigm can be completed within 3 hr, making it practical for owners to drop their pet at the testing centre for a day and not require animal feeding or overnight housing. Performance on the paradigm was also highly stable in young dogs over a 6–9 month period, with scores between the two time points differing by an average of only 2.8 s. The CSM may therefore be useful in the context of intervention trials which require pre- and posttesting or in longitudinal studies.
The development of the CSM will facilitate use of community-based dogs in memory research, allowing access to a large pool of aging dogs that exhibit the full spectrum of cognitive functioning. Despite this, some drawbacks to the use of community-based dogs are acknowledged. Research is limited to tests which are acceptable to the owner's ethical or moral beliefs. For example, studies that involve lesions to induce cognitive impairment are unlikely to be acceptable to owners. Likewise compliance rates in treatment trials cannot be fully controlled as in rodent studies, but are more likely to reflect patterns seen in human clinical trials. A role clearly remains for colony-based animals, but the CSM now gives researchers greater options in the development of the dog as a model for age-related cognitive decline.
Acknowledgments
This work was funded by NHMRC Program grant 350833. We thank Dr Bill Milgram for his expert comments on this work. Thanks to Tell Em How Much You Love Em® for the supply of all the dried liver reward product.
REFERENCES
- Adams B, Chan A, Callahan H, Milgram N.W. The canine as a model of human cognitive aging: Recent developments. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2000a;24:675–692. doi: 10.1016/s0278-5846(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Adams B, Chan A, Callahan H, Siwak C, Tapp D, Ikeda-Douglas C.J, …, Milgram N.W. Use of delayed non-matching to position task to model age-dependant cognitive decline in the dog. Behavioral Brain Research. 2000b;108:47–56. doi: 10.1016/s0166-4328(99)00132-1. [DOI] [PubMed] [Google Scholar]
- Beck B.A slow burning fuse: A special report on aging populations. Economist. 2009. June 25.
- Berchtold N.C, Cotman C.W. Normal and pathological aging: From animals to humans. In: Bizon J.L, Woods A.G, editors. Animal Models of Human Cognitive Aging. New York: Humana Press; 2009. (Eds.) [Google Scholar]
- Brandeis R, Brandys Y, Yehuda S. The use of the Morris water maze in the study of learning and memory. International Journal of Neuroscience. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi H.M. Forecasting the global burden of Alzheimer's disease. Alzheimer's & Dementia. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- Calhoun M.E. Mouse models of cognitive aging: Behavioral tasks and neural substrates. In: Bizon J.L, Woods A.G, editors. Animal Models of Human Cognitive Aging. New York: Humana Press; 2009. (Eds.) [Google Scholar]
- Cummings B.J, Head E, Ruehl W, Milgram N.W, Cotman C.W. The canine as an animal model of human aging and dementia. Neurobiology of Aging. 1996;17:259–268. doi: 10.1016/0197-4580(95)02060-8. [DOI] [PubMed] [Google Scholar]
- D'Hooge R, De Deyn P.P. Applications of the Morris water maze in the study of learning and memory. Brain Research Reviews. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Gallagher M. Animal models of memory impairment. Philosophical Transactions of the Royal Society B: Biological Sciences. 1997;352:1711–1717. doi: 10.1098/rstb.1997.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotthard G.H. The sand maze. In: Anderson M.J, editor. Tasks and techniques: A sampling of the methodologies for the investigation of animal learning, behavior and cognition. New York: Nova Science Publishers; 2006. pp. 87–95. (Ed.) [Google Scholar]
- Hanson G.R. The sand maze: An appetitive alternative to the Morris water maze. Dissertation Abstracts International: Section B: The Sciences and Engineering. 2003;63:4958. [Google Scholar]
- Head E, Mehta R, Hartley J, Kameka M, Cummings B.J, Cotman C.W, …, Milgram N.W. Spatial learning and memory as a function of age in the dog. Behavioral Neuroscience. 1995;109:851–858. doi: 10.1037//0735-7044.109.5.851. [DOI] [PubMed] [Google Scholar]
- Head E, McCleary R, Hahn F, Milgram N, Cotman C.W. Region-specific age at onset of b-amyloid in dogs. Neurobiology of Aging. 2000;21:96. doi: 10.1016/s0197-4580(00)00093-2. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. Memory – A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGreevy P.D, Thomson P.C, Pride C, Fawcett A, Grassi T, Jones B. Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Veterinary Record. 2005;156:695–702. doi: 10.1136/vr.156.22.695. [DOI] [PubMed] [Google Scholar]
- Morell V. Going to the dogs. Science. 2009;325:1062–1065. doi: 10.1126/science.325_1062. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of Neuroscience Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Rapp P.R, Amaral D.G. Individual differences in the cognitive and neurobiological consequences of normal aging. Trends in Neurosciences. 1992;15:340–345. doi: 10.1016/0166-2236(92)90051-9. [DOI] [PubMed] [Google Scholar]
- Salvin H.E, McGreevy P.D, Sachdev P.S, Valenzuela M.J.The canine cognitive dysfunction rating scale (CCDR): A data-driven and ecologically relevant assessment tool. The Veterinary Journal. in press. (available online 2010), doi:10.1016/j.tvjl.2010.05.014. [DOI] [PubMed]
- Studzinski C.M, Araujo J.A, Milgram N.W. The canine model of human cognitive aging and dementia: Pharmacological validity of the model for assesment of human cognitive-enhancing drugs. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2005;29:489–498. doi: 10.1016/j.pnpbp.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Tapp P.D, Head K, Head E, Milgram N.W, Muggenburg B.A, Su M.-Y. Application of an automated voxel-based morphometry technique to assess regional gray and white matter brain atrophy in a canine model of aging. NeuroImage. 2006;29:234–244. doi: 10.1016/j.neuroimage.2005.07.043. [DOI] [PubMed] [Google Scholar]
- Trollor J.N, Valenzuela M. Brain aging in the new millennium. Australian and New Zealand Journal of Psychiatry. 2001;35:788–805. doi: 10.1046/j.1440-1614.2001.00969.x. [DOI] [PubMed] [Google Scholar]