Abstract
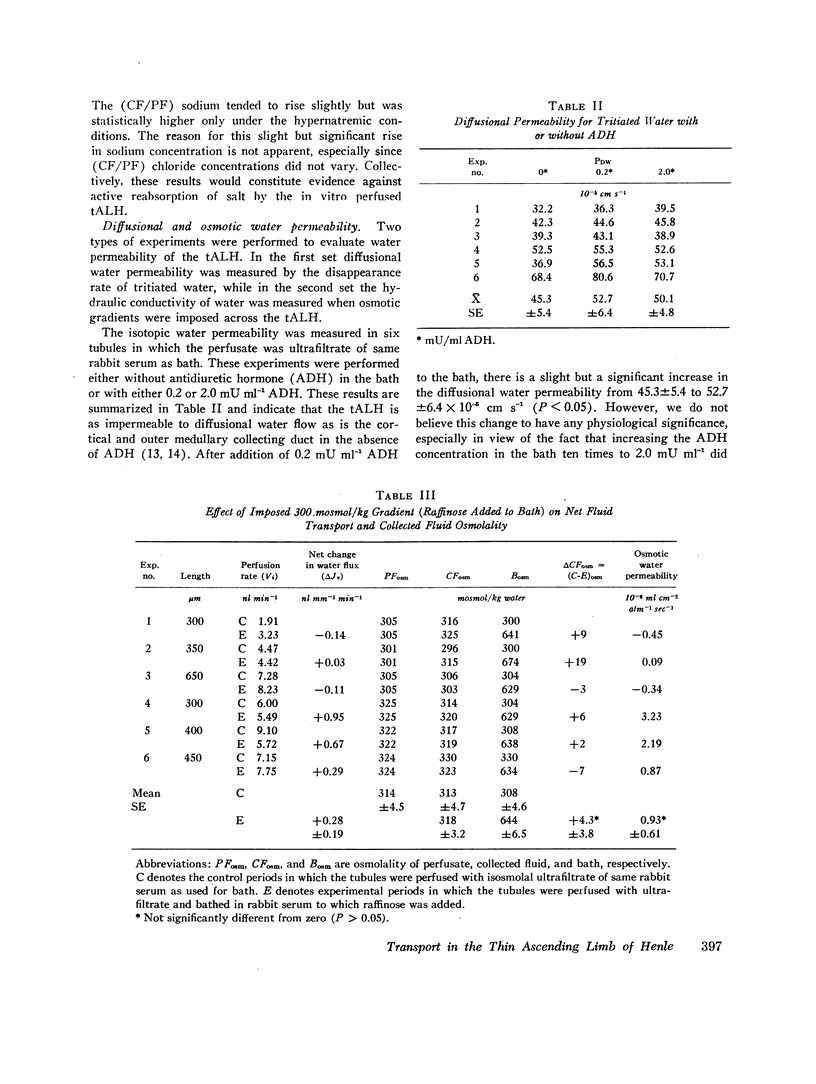
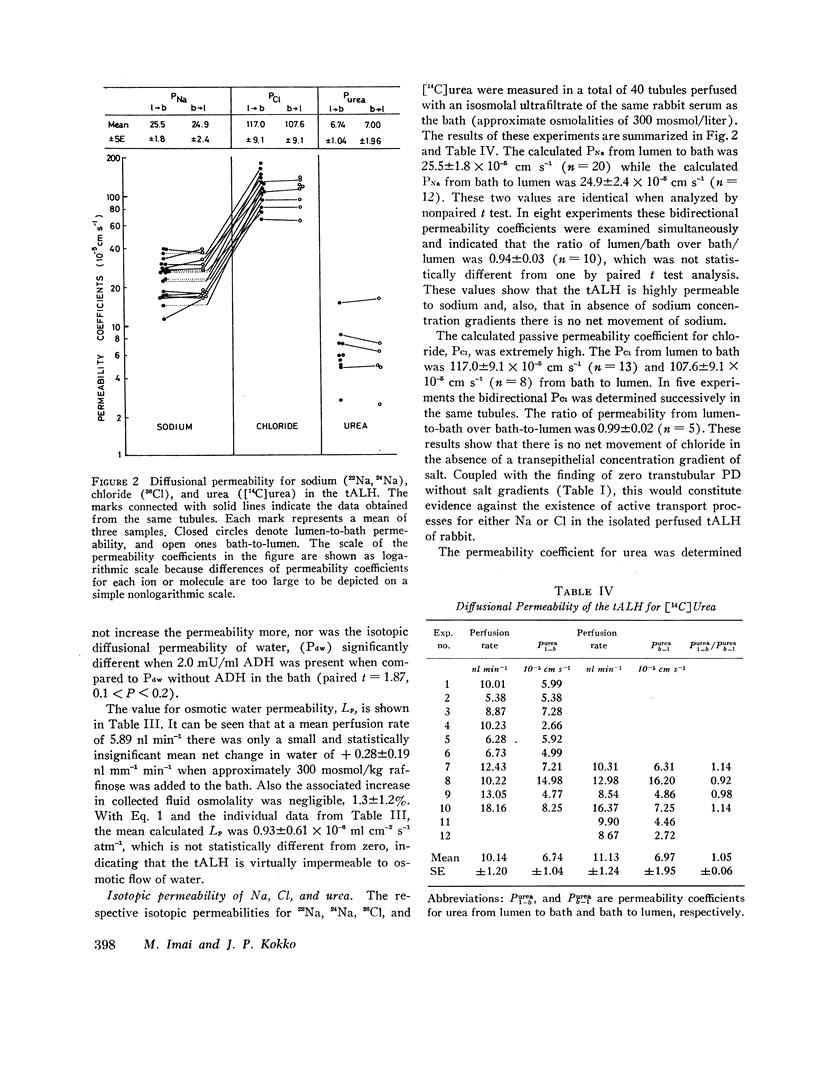
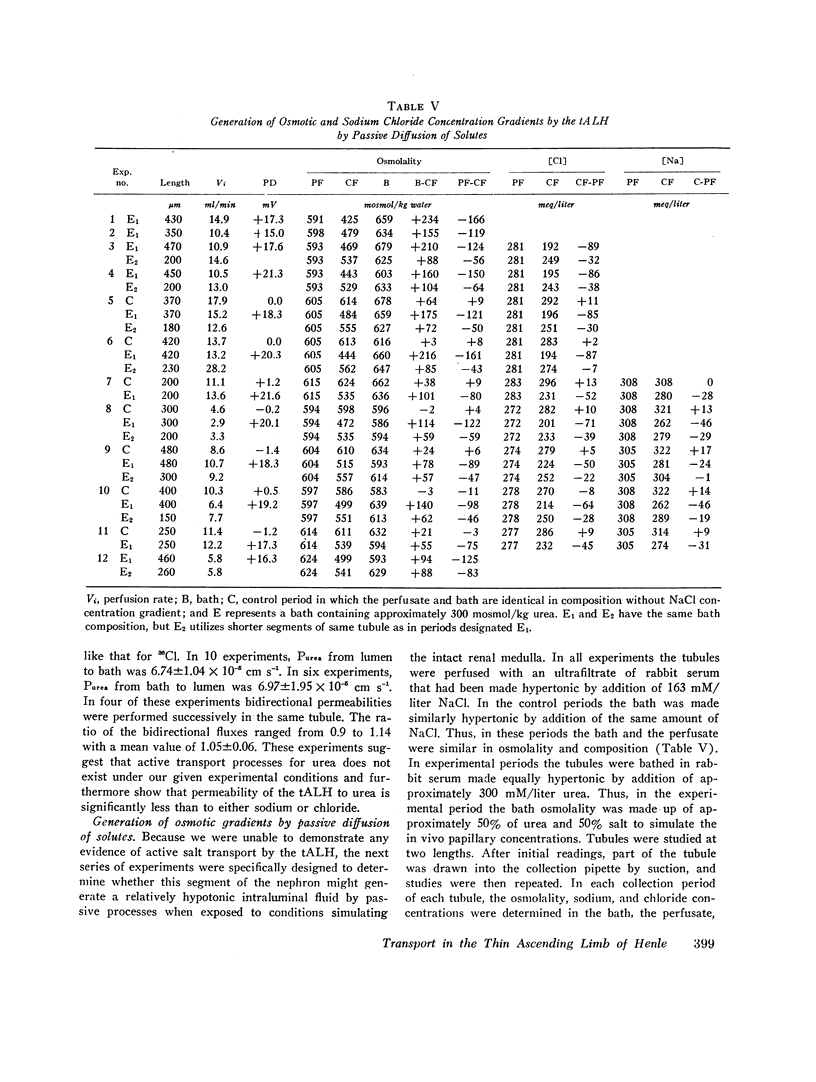
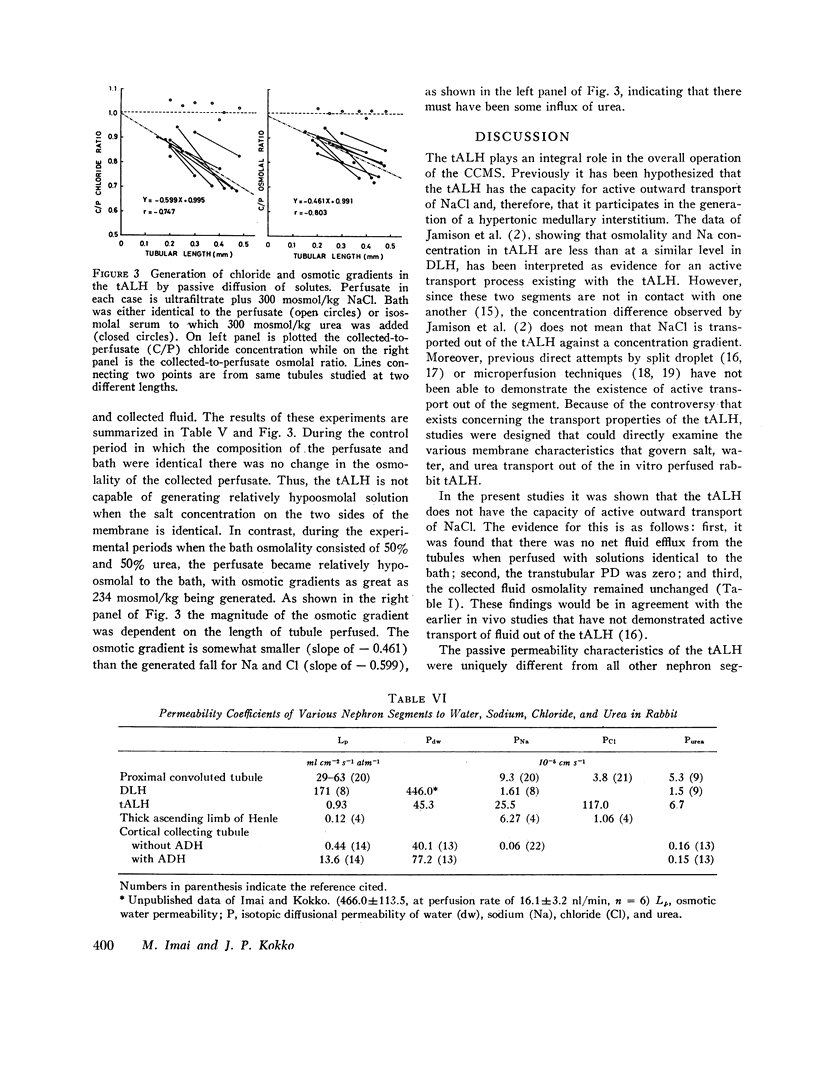
Studies were designed to examine whether the thin ascending limb of Henle (tALH) decreases its luminal solute concentration by an active or a passive transport process. In all experiments isolated segments of rabbit tALH were perfused in vitro. When tubules were perfused with solutions identical to the bath, active transport of NaCl was excluded by the following: (a) osmolality of the collected fluid remained unchanged and the same as the bath. (b) net water reabsorption could not be demonstrated, and (c) transtubular potential difference was zero. Isotopic permeability coefficients (x 10(-5) cm s-1) were calculated from the disappearance rate of the respective isotope added to the perfusate. These values indicate that tALH is moderately permeable to [14C]urea (6.97 +/- 1.95) while having a higher permeability to 22Na (25.5 +/- 1.8) and [not readable: see text]Cl (117 +/- 9.1) than any other segment similarly studied. The influx (bath-to-lumen) isotopic permeabilities were not statistically different from the above efflux permeabilities. Osmotic water permeability was immeasurably small. When tALH were perfused with a 600 mosmol/liter solution predominantly of NaCl against a 600 mosmol/liter bath in which 50% of osmolality was NaCl and 50% urea (to simulate in vivo papillary interstitium), the collected fluid osmolality was decreased significantly below that of the bath (300 mosmol/liter/mm of tubule). The decrease in osmolality was due to greater efflux of NaCl as compared to influx of urea. We conclude that active transport of salt by the tALH was not detected by the experimental protocol of the current studies, and that the unique membrane characteristics of tALH allows for generation of osmotic gradients (lumen less concentrated than adjacent surroundings) on purely passive mechanisms when perfused with isosmolal salt solutions in a bath with appropriate salt and urea concentrations. These findings are consistent with the passive counter-current model previously proposed from this laboratory.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burg M. B., Green N. Function of the thick ascending limb of Henle's loop. Am J Physiol. 1973 Mar;224(3):659–668. doi: 10.1152/ajplegacy.1973.224.3.659. [DOI] [PubMed] [Google Scholar]
- Frindt G., Burg M. B. Effect of vasopressin on sodium transport in renal cortical collecting tubules. Kidney Int. 1972 Apr;1(4):224–231. doi: 10.1038/ki.1972.32. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK C. W. RENAL TUBULAR FUNCTION: LESSONS FROM MICROPUNCTURE. Harvey Lect. 1963;58:99–124. [PubMed] [Google Scholar]
- Grantham J. J., Burg M. B. Effect of vasopressin and cyclic AMP on permeability of isolated collecting tubules. Am J Physiol. 1966 Jul;211(1):255–259. doi: 10.1152/ajplegacy.1966.211.1.255. [DOI] [PubMed] [Google Scholar]
- Jamison R. L., Bennett C. M., Berliner R. W. Countercurrent multiplication by the thin loops of Henle. Am J Physiol. 1967 Feb;212(2):357–366. doi: 10.1152/ajplegacy.1967.212.2.357. [DOI] [PubMed] [Google Scholar]
- Jamison R. L. Micropuncture study of segments of thin loop of Henle in the rat. Am J Physiol. 1968 Jul;215(1):236–242. doi: 10.1152/ajplegacy.1968.215.1.236. [DOI] [PubMed] [Google Scholar]
- Kokko J. P., Burg M. B., Orloff J. Characteristics of NaCl and water transport in the renal proximal tubule. J Clin Invest. 1971 Jan;50(1):69–76. doi: 10.1172/JCI106485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko J. P., Rector F. C., Jr Countercurrent multiplication system without active transport in inner medulla. Kidney Int. 1972 Oct;2(4):214–223. doi: 10.1038/ki.1972.97. [DOI] [PubMed] [Google Scholar]
- Kokko J. P. Sodium chloride and water transport in the descending limb of Henle. J Clin Invest. 1970 Oct;49(10):1838–1846. doi: 10.1172/JCI106401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko J. P. Urea transport in proximal tubule and the descending limb of Henle. J Clin Invest. 1972 Aug;51(8):1999–2008. doi: 10.1172/JCI107006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSH D. J., SOLOMON S. ANALYSIS OF ELECTROLYTE MOVEMENT IN THIN HENLE'S LOOPS OF HAMSTER PAPILLA. Am J Physiol. 1965 Jun;208:1119–1128. doi: 10.1152/ajplegacy.1965.208.6.1119. [DOI] [PubMed] [Google Scholar]
- Marsh D. J. Solute and water flows in thin limbs of Henle's loop in the hamster kidney. Am J Physiol. 1970 Mar;218(3):824–831. doi: 10.1152/ajplegacy.1970.218.3.824. [DOI] [PubMed] [Google Scholar]
- Morgan T., Berliner R. W. Permeability of the loop of Henle, vasa recta, and collecting duct to water, urea, and sodium. Am J Physiol. 1968 Jul;215(1):108–115. doi: 10.1152/ajplegacy.1968.215.1.108. [DOI] [PubMed] [Google Scholar]
- Rocha A. S., Kokko J. P. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest. 1973 Mar;52(3):612–623. doi: 10.1172/JCI107223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai F., Tadokoro M., Yamaguchi I. Osmolality of loop of Henle fluid in golden hamster. Jpn J Pharmacol. 1966 Dec;16(4):492–492. doi: 10.1254/jjp.16.492. [DOI] [PubMed] [Google Scholar]
- Schafer J. A., Andreoli T. E. Cellular constraints to diffusion. The effect of antidiuretic hormone on water flows in isolated mammalian collecting tubules. J Clin Invest. 1972 May;51(5):1264–1278. doi: 10.1172/JCI106921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. L. Concentration of urine in a central core model of the renal counterflow system. Kidney Int. 1972 Aug;2(2):85–94. doi: 10.1038/ki.1972.75. [DOI] [PubMed] [Google Scholar]
- WINDHAGER E. E. ELECTROPHYSIOLOGICAL STUDY OF RENAL PAPILLA OF GOLDEN HAMSTERS. Am J Physiol. 1964 Apr;206:694–700. doi: 10.1152/ajplegacy.1964.206.4.694. [DOI] [PubMed] [Google Scholar]





