Abstract
Rationale
The parasympathetic reduction in heart rate (HR) involves the sequential activation of m2 muscarinic cholinergic receptors (m2R), pertussis toxin-sensitive (Gi/o) heterotrimeric G proteins, and the atrial potassium channel IKACh. Molecular mechanisms regulating this critical signal transduction pathway are not fully understood.
Objective
To determine whether the G protein signaling regulator Rgs6/Gβ5 modulates m2R-IKACh signaling and cardiac physiology.
Methods and Results
Cardiac expression of Rgs6, and its interaction with Gβ5, was demonstrated by immunoblotting and immunoprecipitation. Rgs6−/− mice were generated by gene targeting, and the cardiac effects of Rgs6 ablation were analyzed by whole-cell recordings in isolated cardiomyocytes and electrocardiogram (ECG) telemetry. Loss of Rgs6 yielded profound delays in m2R-IKACh deactivation kinetics in both neonatal atrial myocytes and adult sino-atrial nodal cells. Rgs6−/− mice exhibited mild resting bradycardia and altered heart rate responses to pharmacologic manipulations that were consistent with enhanced m2R-IKACh signaling.
Conclusions
The cardiac Rgs6/Gβ5 complex modulates the timing of parasympathetic influence on atrial myocytes and heart rate in mice.
Keywords: G protein, muscarinic, knockout, cardiac, GIRK, Regulators of G protein signaling
Introduction
Cardiac output is shaped to a great extent by sympathetic and parasympathetic influences. Parasympathetic input tempers heart rate (HR) and counteracts the pro-arrhythmic effects of sympathetic activation, and is mediated by acetylcholine (ACh)1. ACh is released from post-ganglionic parasympathetic neurons and binds to m2 muscarinic receptors (m2R) on pacemaker cells and atrial myocytes, triggering activation of pertussis toxin-sensitive (Gi/o) heterotrimeric G proteins2. Once activated, G proteins dissociate into Gα-GTP and Gβγ subunits, leading to modulation of adenylyl cyclase and multiple ion channels. Central among these reactions is the binding of Gβγ to the atrial potassium channel IKACh, a heterotetramer composed of Girk1 and Girk4 subunits3. Binding of Gβγ to IKACh enhances its gating which leads to cell hyperpolarization and ultimately, decreased HR4.
The duration of G protein signaling is controlled by members of the Regulator of G protein Signaling (RGS) family5. RGS proteins stimulate inactivation of Gα-GTP, facilitating its re-assembly with Gβγ. RGS proteins play a critical role in shaping bradycardic effects of M2R receptor activation6–8. Indeed, eliminating RGS influence by expressing Gα subunits insensitive to RGS action results in a substantial enhancement of IKACh regulation by m2R signaling, via both Gαo and Gαi2 pathways7,8. Although more than 30 RGS proteins have been identified, the involvement of specific RGS proteins in the regulation of parasympathetic input is not fully-understood. Here, we report an unexpected role of the Rgs6/Gβ5 complex, previously thought to be neuron-specific regulator, in the temporal regulation of m2R-IKACh signaling.
Methods
Littermate mice were used for all experiments in this study. All procedures were carried out in accordance with the NIH guidelines and were approved by the Institutional Animal Care and Use Committee of the University of Minnesota. A detailed Methods section is available as Supplementary Material.
Results
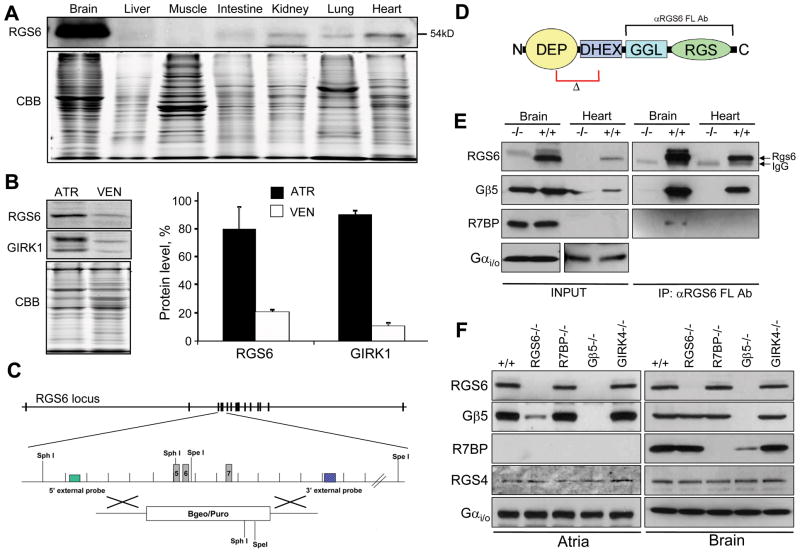
Profiling Rgs6 protein expression across mouse tissues revealed its readily detectable levels in the heart in addition to abundant presence in the brain (Fig. 1A; Online Fig. I). Rgs6 protein was enriched in atria, where it was found predominantly in myocytes (Online Fig. II), consistent with a recent report9, and similar to the distribution of Girk1, an integral subunit of IKACh (Fig. 1B). To begin exploring the role of Rgs6 in cardiac physiology, we obtained Rgs6−/− mice where exons 5–7 encoding the critical N-terminal portion of the protein were eliminated (Fig. 1C, D). Immunoblotting verified the complete absence of Rgs6 protein in the hearts of Rgs6−/− mice (Fig. 1E).
Figure 1. Rgs6 protein level and complex formation in the mouse heart.
A, Rgs6 protein levels across mouse tissues as analyzed by Western blotting. Equal amounts of total protein (20 μg) were loaded in each lane. Coomasie staining (CBB) was used as a loading control. B, Rgs6 is co-enriched with Girk1 in the atria. Atrial (ATR) and ventricular (VEN) lysates were analyzed by Western blotting (upper panels). CBB staining confirms equal protein loading. Graph: Quantification of Rgs6 and Girk1 band densities. C, Strategy for Rgs6 ablation by homologous recombination. D, Structural organization of Rgs6. Frames designate the deleted region (Δ) and recognition site of the RGS6 FL antibody (αRGS6 FL Ab) used throughout the study. Colored boxes designate known structural domains. E, Co-immunoprecipitation of Rgs6 with Gβ5 and R7BP from heart and brain tissues. F, Co-dependence of Rgs6 and Gβ5 expression in the heart and brain as analyzed by Western blotting.
Rgs6 interacts with the type 5 G protein β subunit (Gβ5) and the R7 Binding Protein (R7BP) in the CNS10 (Fig. 1F). In the mouse heart, however, only Gβ5 is available for the interaction with Rgs6 (Fig. 1E). Rgs6 was undetectable in hearts from Gβ5−/− mice, indicating that the physical association with Gβ5 is critical for the expression and/or stability of Rgs6 (Fig. 1G). Similarly, Gβ5 levels were dramatically reduced in the Rgs6−/− heart but not brain, indicating that in the heart Rgs6 is the predominant RGS bound to Gβ5. No effect on Rgs6 or Gβ5 levels was observed upon elimination of R7BP or Girk4. Notably, we detected no compensatory changes in either Gαi/o proteins or RGS4, a protein previously implicated in regulation of the m2R-IKACh signaling6.
Given the co-enrichment of Rgs6 and IKACh in atria and the role of R7 RGS/Gβ5 complexes in GPCR-GIRK signaling in the CNS11, we next measured the impact of Rgs6 ablation on m2R-IKACh signaling in neonatal atrial myocytes, which exhibit robust inward current triggered by the non-selective muscarinic agonist carbachol (CCh). While CCh evoked currents with comparable potency in atrial myocytes from wild-type mice, current deactivation kinetics were notably slower across all CCh concentrations tested in myocytes from Rgs6−/− mice (Online Fig. III). Current activation kinetics were also delayed in Rgs6−/− myocytes, though only for the lower CCh concentrations tested.
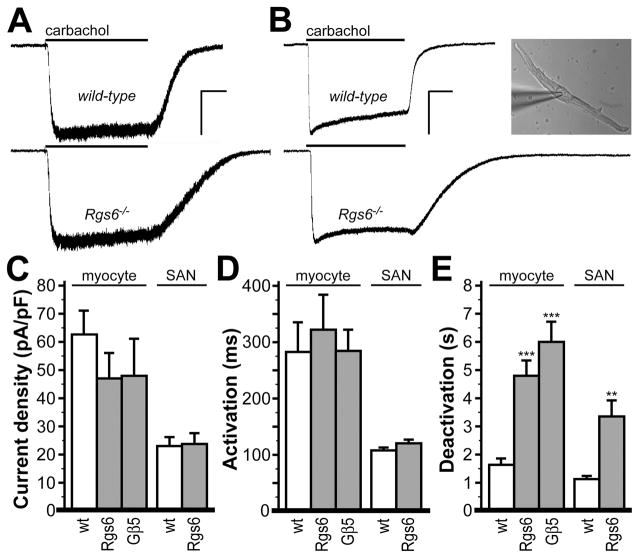
We next compared CCh-induced currents in sino-atrial node (SAN) cells, the key anatomic substrate for parasympathetic control of heart rate (Fig. 2). While some differences in the density and kinetics of CCh-induced responses between adult SAN cells and neonatal atrial myocytes were evident, Rgs6 ablation correlated with significantly delayed deactivation rates in both cell types. Under the same conditions, no differences in CCh-induced steady-state current density or activation kinetics were observed between genotypes in either atrial myocytes or SAN cells (Fig. 2). Furthermore, deletion of the Gβ5 replicated prolonged deactivation kinetics seen in Rgs6−/− myocytes (Fig. 2E), indicating that regulation of the m2R-IKACh signaling in heart atria is mediated by the Rgs6/Gβ5 complex rather than Rgs6 by itself.
Figure 2. Impact of Rgs6 ablation on m2R-IKACh signaling in atrial myocytes and SAN cells.
A, Inward currents evoked by CCh (10 μmol/L) in atrial myocytes from wild-type and Rgs6−/− mice. Scale bars: 5 s/200 pA. B, Inward currents evoked by CCh (10 μmol/L) in SAN cells from wild-type and Rgs6−/− mice. Scale bars: 5 s/400 pA. Inset: image of the wild-type SAN cell evaluated in the adjacent trace. Summary of steady-state CCh-induced current density (C), activation kinetics (D), and deactivation kinetics (E) in wild-type, Rgs6−/−, and Gβ5−/− atrial myocytes (n=5–11 per group), and in wild-type and Rgs6−/− SAN cells (n=10–12 per genotype). Genotype did not impact current density (atrial myocytes: F2,21=1.9, P=0.18; SAN cells: t(20)=0.13, P=0.90) or activation kinetics (atrial myocytes: F2,21=1.0, P=0.38; SAN cells: t(20)=1.69, P=0.11), but did influence deactivation kinetics (atrial myocytes: F2,21=24.8, P<0.001; SAN cells: t(20)=3.71, P<0.01). Symbols: **, *** P<0.01 and 0.001, respectively, vs. wild-type (within cell type).
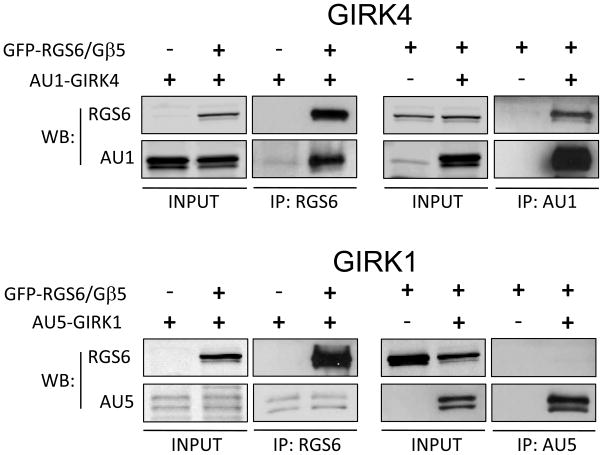
The striking impact of Rgs6 ablation on m2R-IKACh signaling kinetics in atrial myocytes and SAN cells, prompted us to test whether Rgs6/Gβ5 can physically associate with the IKACh channel. In transfected HEK293 cells, we detected robust co-immunoprecipitation of the Rgs6/Gβ5 complex with Girk4 but not Girk1 by both forward and reverse precipitation strategies (Fig 3). Thus, the involvement of Rgs6/Gβ5 in m2R-IKACh signaling is likely aided by a direct protein-protein interaction mediated by the cardiac-specific Girk subunit, Girk4.
Figure 3. RGS6/Gβ5 forms a complex with Girk4.
Rgs6 and Gβ5 were co-expressed with either AU1-tagged Girk4 or AU5-tagged Girk1 in HEK293 cells. Forward and reciprocal co-immunoprecipitation assays were performed as described in the Methods using indicated antibodies. Eluates were analyzed using Western blotting.
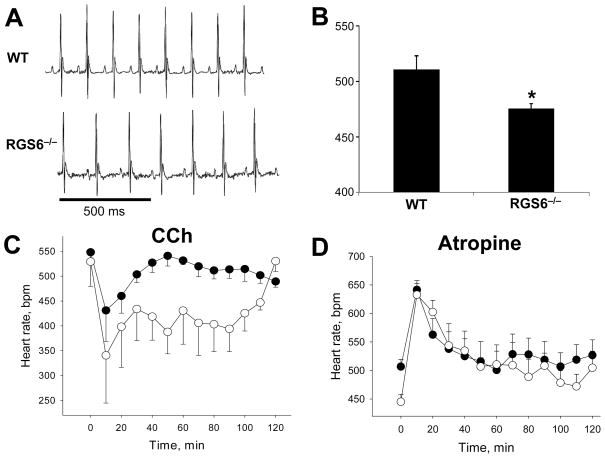
The delay in IKACh deactivation kinetics triggered by Rgs6/Gβ5 elimination is expected to enhance m2R-IKACh signaling as the channel would stay open longer which would potentiate the parasympathetic regulation of HR. We addressed this possibility by analyzing cardiac function in mice using ECG telemetry, at baseline and following pharmacologic manipulation. Analysis of ECG traces did not reveal gross abnormalities in cardiac physiology in Rgs6−/− mice (Fig. 4A and Online Fig. IV). Rgs6−/− mice did, however, display a mild resting bradycardia (511±13 vs. 476±4 bpm, P<0.05), consistent with the effect of Rgs6/Gβ5 ablation on m2R-dependent signaling in atrial myocytes (Fig. 4B). While CCh administration (0.1 mg/kg, i.p.) triggered a rapid decrease in HR in wild-type and Rgs6−/− animals, the effect was significantly larger and persisted longer in Rgs6−/− mice (Fig. 4C). Similarly, parasympathetic blockade with atropine (1 mg/kg, i.p.) had a positive chronotropic effect in both groups, with a significantly larger effect seen in Rgs6−/− mice (Fig. 4D). Importantly, there was no difference in HR immediately following atropine administration, indicating that the bradycardia seen in Rgs6−/− mice results from enhanced intrinsic m2R signaling.
Figure 4. Effect of Rgs6 ablation on resting HR and muscarinic regulation.
A, Baseline ECG recorded during light phase from conscious, unrestrained wild-type (WT, n=5) and Rgs6−/− mice (n=5). B, Average HR as determined from the analysis of the ECG recordings (6hr). Symbols: * P<0.05 vs. wild-type. C, Effect of CCh (0.1 mg/kg, i.p.) on HR, analyzed and plotted as simple moving average with a period of 10 min. HR at 0 min corresponds to the 30-min average baseline HR on the day of the experiment. Two-way ANOVA analysis (genotype and time) of the 120-min post-injection interval revealed main effects of genotype (F1,104=21.6; P<0.001). D, Effect of atropine (1 mg/kg, i.p.) on HR, analyzed and plotted as simple moving averages with a period of 10 min. No significant difference in HR was observed during the 60-min post-injection interval (F1,56=0.02; P=0.89).
Discussion
Here, we report that Rgs6/Gβ5 negatively regulates m2R-IKACh signaling in atrial myocytes by accelerating IKACh deactivation kinetics. These observations, together with the effect of Rgs6 ablation on HR and responses to pharmacologic manipulation, indicate that Rgs6/Gβ5 represents a key node of regulation in the parasympathetic control of cardiac output. Since dysregulation of the parasympathetic tone by deficiencies in IKACh function is increasingly accepted as a major factor in the pathogenesis of the atrial fibrillation1, our study introduces Rgs6/Gβ5 complex as an attractive candidate for better understanding of cardiac pathophysiology and development of corrective therapies.
Rgs6 belongs to the R7 family of RGS proteins, members of which were thought to be expressed exclusively in the nervous system, where they play roles in nociception, vision, reward behavior and locomotion10. Although Rgs6 expression was reported previously in the heart9,12,13, our study documents for the first time the functional relevance of Rgs6 to cardiac physiology. In the CNS, Rgs6 forms complexes with two proteins, Gβ5 and R7BP that specify its stability, subcellular distribution, and activity10. Here we show that cardiac Rgs6 forms a complex with Gβ5, but not with R7BP which is undetectable in the heart. The obligate and functionally-relevant nature of the Rgs6/Gβ5 interaction was underscored by the mutual dependence of Rgs6 and Gβ5 levels on their co-expression, and the phenotypic similarities in m2R-IKACh signaling in myocytes from Rgs6−/− and Gβ5−/− mice. In neurons, Gβ5 recruits R7 RGS proteins to GIRK channels, resulting in accelerated channel kinetics associated with GABAB receptor activation11. Thus, the present work reveals the conservation of this compartmentalization mechanism by showing that Rgs6/Gβ5 can likewise regulate m2R-IKACh signaling.
Previous work has identified Rgs4 as a critical regulator of m2R-IKACh signaling in sinoatrial nodal cells6. Indeed, the deficiencies in m2R-IKACh signaling linked to Rgs6 ablation reported herein are reminiscent of those reported in Rgs4−/− mice6. Therefore, murine sinoatrial nodal cells may employ parallel approaches involving Rgs4 and Rgs6/Gβ5 to regulate m2R-IKACh signaling. It is possible, for example, that Rgs4 and Rgs6/Gβ5 selectively regulate different G protein subtypes involved in IKACh gating. Indeed, studies with knock-in mice expressing RGS-insensitive G proteins reveal a differential contribution of Gαi2 and Gαo to m2R-dependent actions7,8. Furthermore, Rgs6/Gβ5 shows selectivity towards Gαo over Gαi2 in vitro14. However, while the role of Gαi2 in mediating m2R-IKACh coupling is well established8, the involvement of Gαo in this process is less certain. Moreover, it remains possible that other proteins of more than 30-member RGS family also play roles in this regulation. Delineating the mechanisms of the functional involvement of RGS proteins in controlling m2R-IKACh signaling in the mouse models and their relevance to human physiology will serve as an exciting future research direction.
Novelty and Significance.
What is known?
Activation of the parasympathetic branch of the autonomic nervous system decreases heart rate via the neurotransmitter acetylcholine.
Acetylcholine stimulates m2 muscarinic receptors (m2R) on sinoatrial nodal cells and atrial myocytes, leading to the G protein-dependent activation of the potassium channel IKACh.
Modulating m2R-IKACh signaling can impact heart rate.
What new information does this article contribute?
The Rgs6/Gβ5 protein complex is an essential modulator of m2R-IKACh signaling in cardiac myocytes and sinoatrial cells
Inactivation of the Rgs6 gene in mice results in a mild bradycardia and an enhanced effect of drug-induced parasympathetic stimulation.
Summary
m2R-IKACh signaling plays critical role in controlling heart rate and has been implicated in the pathogenesis of atrial fibrillation. The molecular mechanisms regulating this process, however, are not fully-understood. Here, we identify the Rgs6/Gβ5 protein complex as a key regulator of m2R-IKACh signaling in cardiomyocytes and sinoatrial cells. We show that Rgs6/Gβ5 can physically associate with IKACh and that deletion of the Rgs6 gene in mice significantly enhances the parasympathetic regulation of heart rate. Thus, Rgs6/Gβ5 may contribute to, or represent a novel therapeutic target for, pathological conditions characterized by abnormal regulation of cardiac output.
Supplementary Material
Acknowledgments
Sources of funding. This work was supported by NIH grants DA026405 (KAM), MH061933 (KW), DA011806 (KW), and T32 DA07234 (KA), and a McKnight Land–Grant Award (KAM).
Abbreviations
- GIRK channel
G protein-gated inwardly-rectifying K+ channel
- CCh
carbachol
- RGS
Regulator of G protein Signaling
- HR
heart rate
- ECG
electrocardiogram
- m2R
type 2 muscarinic receptor
Footnotes
Disclosures. None
References
- 1.Li D, Sun H, Levesque P. Antiarrhythmic drug therapy for atrial fibrillation: focus on atrial selectivity and safety. Cardiovasc Hematol Agents Med Chem. 2009;7:64–75. doi: 10.2174/187152509787047621. [DOI] [PubMed] [Google Scholar]
- 2.Wickman K, Krapivinsky G, Corey S, Kennedy M, Nemec J, Medina I, Clapham DE. Structure, G protein activation, and functional relevance of the cardiac G protein-gated K+ channel, IKACh. Ann NYAcad Sci. 1999;868:386–98. doi: 10.1111/j.1749-6632.1999.tb11300.x. [DOI] [PubMed] [Google Scholar]
- 3.Mirshahi T, Jin T, Logothetis DE. G beta gamma and KACh: old story, new insights. Sci STKE. 2003;2003:PE32. doi: 10.1126/stke.2003.194.pe32. [DOI] [PubMed] [Google Scholar]
- 4.Wickman K, Nemec J, Gendler SJ, Clapham DE. Abnormal heart rate regulation in GIRK4 knockout mice. Neuron. 1998;20:103–14. doi: 10.1016/s0896-6273(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 5.Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54:527–59. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- 6.Cifelli C, Rose RA, Zhang H, Voigtlaender-Bolz J, Bolz SS, Backx PH, Heximer SP. RGS4 regulates parasympathetic signaling and heart rate control in the sinoatrial node. Circ Res. 2008;103:527–35. doi: 10.1161/CIRCRESAHA.108.180984. [DOI] [PubMed] [Google Scholar]
- 7.Fu Y, Huang X, Piao L, Lopatin AN, Neubig RR. Endogenous RGS proteins modulate SA and AV nodal functions in isolated heart: implications for sick sinus syndrome and AV block. Am J Physiol Heart Circ Physiol. 2007;292:H2532–9. doi: 10.1152/ajpheart.01391.2006. [DOI] [PubMed] [Google Scholar]
- 8.Fu Y, Huang X, Zhong H, Mortensen RM, D’Alecy LG, Neubig RR. Endogenous RGS proteins and Galpha subtypes differentially control muscarinic and adenosine-mediated chronotropic effects. Circ Res. 2006;98:659–66. doi: 10.1161/01.RES.0000207497.50477.60. [DOI] [PubMed] [Google Scholar]
- 9.McGrath MF, de Bold AJ. Transcriptional analysis of the mammalian heart with special reference to its endocrine function. BMC Genomics. 2009;10:254. doi: 10.1186/1471-2164-10-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson GR, Posokhova E, Martemyanov KA. The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling. Cell Biochem Biophys. 2009;54:33–46. doi: 10.1007/s12013-009-9052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie K, Allen KL, Kourrich S, Colon-Saez J, Thomas MJ, Wickman K, Martemyanov KA. Gbeta5 recruits R7 RGS proteins to GIRK channels to regulate the timing of neuronal inhibitory signaling. Nat Neurosci. 2010;13:661–663. doi: 10.1038/nn.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doupnik CA, Xu T, Shinaman JM. Profile of RGS expression in single rat atrial myocytes. Biochim Biophys Acta. 2001;1522:97–107. doi: 10.1016/s0167-4781(01)00342-6. [DOI] [PubMed] [Google Scholar]
- 13.Snow BE, Betts L, Mangion J, Sondek J, Siderovski DP. Fidelity of G protein beta-subunit association by the G protein gamma-subunit-like domains of RGS6, RGS7, and RGS11. Proc Natl Acad Sci USA. 1999;96:6489–94. doi: 10.1073/pnas.96.11.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooks SB, Waldo GL, Corbitt J, Bodor ET, Krumins AM, Harden TK. RGS6, RGS7, RGS9, and RGS11 stimulate GTPase activity of Gi family G-proteins with differential selectivity and maximal activity. J Biol Chem. 2003;278:10087–93. doi: 10.1074/jbc.M211382200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.