Abstract
Synthetic cationic lipids are widely used component of non-viral gene carriers and the factors regulating their transfection efficiency are subject of considerable interest. In view of the important role that electrostatic interactions with the polyanionic nucleic acids play in formation of lipoplexes, a common empirical approach to improving transfection has been the synthesis and testing of amphiphiles with new versions of positively charged polar groups, while much less attention has been given to the role of the hydrophobic lipid moieties. On basis of data for ~20 cationic phosphatidylcholine (PC) derivatives, here we demonstrate that hydrocarbon chain variations of these lipids modulate by over two orders of magnitude their transfection efficiency. The observed molecular structure-activity relationship manifests in well expressed dependences of activity on two important molecular characteristics - chain unsaturation and total number of carbon atoms in the lipid chains, which is representative of the lipid hydrophobic volume and hydrophilic-lipophilic ratio. Transfection increases with decrease of chain length and increase of chain unsaturation. Maximum transfection was found for cationic PCs with monounsaturated 14:1 chains. It is of particular importance that the high-transfection lipids strongly promote cubic phase formation in zwitterionic membrane phosphatidylethanolamine (PE). These remarkable correlations point to an alternative, chain-dependent process in transfection, not related to the electrostatic cationic-anionic lipid interactions.
Keywords: cationic phospholipid, phosphatidylethanolamine, hydrophobic chain, lipoplex, cubic phase, hexagonal phase, transfection
INTRODUCTION
As non-viral carriers of nucleic acids into cells, synthetic cationic lipids are being developed for tasks of utmost importance in the laboratory and clinic, such as gene transfection and gene silencing 1-4. They readily form complexes (lipoplexes) with the polyanionic nucleic acids, protecting the latter until entry into cells. However, a critical obstacle for application of lipid-mediated DNA delivery (lipofection) is its unsatisfactory efficiency for many cell types. Since the specific delivery routes by cationic lipid vectors are mostly unknown, the efforts to improve lipoplex transfection have been largely empirical. In view of the important role of electrostatic interactions with DNA taking place at the level of the lipid headgroups, most of these efforts have been focused on synthesis of amphiphiles with new versions of positively charged polar groups 5,6. Moreover, it is known that cationic lipids interact also with anionic membrane lipids and, in certain cases, their mixtures form nonlamellar phases that may be involved in lipoplex fusion with cellular membranes and release of encapsulated nucleic acid 7-10. Much less attention has been given to investigating the role of the hydrophobic portion of the lipid molecules 11-13. Our studies on a specific class of cationic lipids, cationic phosphatidylcholine (PC) derivatives, indicate, however, that substantial enhancement of lipoplex gene delivery could be attained by variations in the lipid hydrocarbon chains. These studies also provided evidence for a relationship between transfection success and lipid phase behavior 10,14-17. As is well known, the latter is strongly modulated by the hydrophobic portions of the lipid molecules.
Here we report data demonstrating that certain relationship exists between cationic lipid hydrocarbon chain molecular structure and transfection activity. It is of particular importance that high transfection rates correlate with enhanced cubic phase formation in cationic PC mixtures with membrane phosphatidylethanolamine (PE). This behavior indicates that enhanced transfection may be mediated by cationic lipid interactions with the zwitterionic membrane PEs in an alternative transfection pathway that does not involve interactions with anionic membrane lipids.
EXPERIMENTAL METHODS
Lipids and DNA
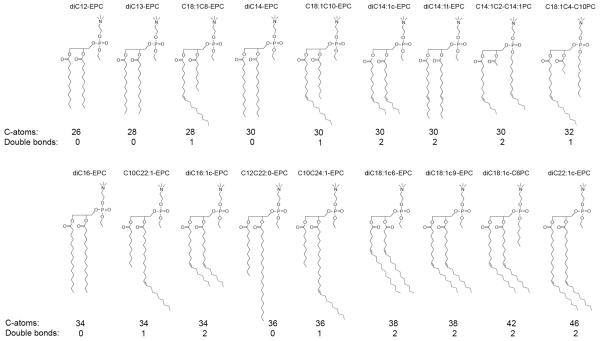
The cationic phospholipids, triflate derivatives of phosphatidylcholines (Fig. 1), were synthesized in house as previously described 18,19, or were custom synthesized by Avanti Polar Lipids, Inc (Alabaster, AL), and were stored in chloroform solution at −20 °C. Dipalmitoleoylphosphatidylethanolamine (DPoPE) was from Avanti Polar Lipids. All lipids were checked for purity by mass spectroscopy and TLC and found to contain no detectable impurities. Herring sperm DNA was from Invitrogen. CMV-β-galactosidase plasmid was purchased from Clontech Laboratories Inc. (Palo Alto, CA) and propagated and purified by Bayou Biolabs (Harahan, LA). HBSS (HEPES buffered saline solution) and Opti-MEM were obtained from Cambrex (Walkersville, MD) and Invitrogen, respectively.
Figure 1.
Structures of the studied cationic PC derivatives.
Cell Culture
Human umbilical artery endothelial cells (HUAECs) were obtained from Cambrex (Walkersville, MD) and maintained in EGM-2 MV containing 5% fetal bovine serum (FBS) (Cambrex) at 37°C with 5% CO2. At confluence, the cells were passaged using 0.25 mg/ml Trypsin/EDTA (Cambrex) and were used at passages 5-10 for these experiments.
Transfection
The cells were seeded in 96-well plates at 24 h before transfection at densities appropriate to give about 80% confluence at the time of transfection. Chloroform solutions of cationic lipids were mixed in glass vials at the desired ratios, after which the chloroform was removed under an Argon or N2 stream. The vials were placed under high vacuum for several hours to remove the last traces of solvent. The lipid mixtures were hydrated in HBSS at 1 mg/ml to form liposomes. Liposomes and plasmid DNA were each diluted in OptiMEM to 160 μg/ml for lipid and to 40 μg/ml for DNA, and liposomes were pipetted into an equal volume of plasmid DNA solution at a 4:1 weight ratio and mixed gently. The resultant plasmid-lipid complexes were incubated at room temperature for 15 min, after which 50 μl per well were added to cells that were either in medium lacking serum or in medium containing serum. Two hours after their addition, the lipoplexes were removed and fresh medium containing serum was added. Cells were assayed for β-galactosidase activity 24 h after transfection using a microplate fluorometric assay. Briefly, the latter procedure was as follows: Subsequent to aspiration of the medium from each well, the cells were washed once with PBS and then lysed by addition of 100 μl lysis buffer (0.03% Triton X-100 in 100 mM HEPES, 8 mM MgSO4, pH 7.8). Ten μl of 100 μM FDG (fluorescein di-β-D-galactopyranoside) were added into each well and the fluorescence that had developed at 20 hours was measured with a microplate fluorometer (Model 7620, Cambridge Technology Inc.).
X-ray diffraction measurements
Pure cationic lipid dispersions, lipoplexes and mixtures with DPoPE were prepared using previously described procedures 17,20,21. Briefly, lipids were dissolved in chloroform, the chloroform removed with a stream of argon, and the mixtures were subjected to high vacuum for 24-36 h. The dispersions were prepared by hydrating and vortexing at room temperature, followed by 5 freeze-thaw cycles between dry ice and room temperature, all accompanied by vortexing during the thawing steps. Measurements were carried out on dispersions with lipid contents of 10% (w/v) for the DPoPE/cationic lipid mixtures and 40% (w/v) for pure cationic lipids. The dispersion medium used was Dulbecco’s Phosphate-Buffered Saline (PBS, Gibco; pH 7.2). The samples were loaded into X-ray capillaries and flame sealed before measurement. Low-angle X-ray diffraction patterns were recorded at stations 5IDD, DND-CAT and 18D, BioCAT, APS, Argonne, using 2-D 2048×2048 MAR detectors at a sample-to-detector distance of ~200 cm, as described earlier 17.
RESULTS
Transfection activity
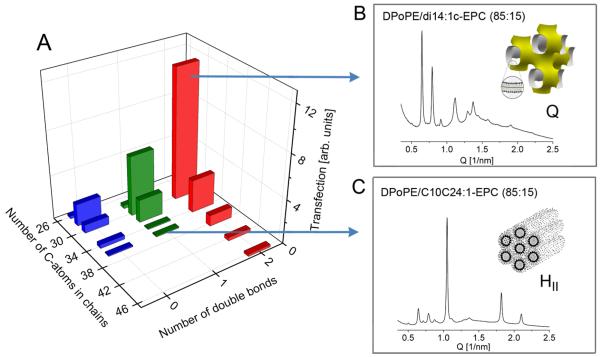
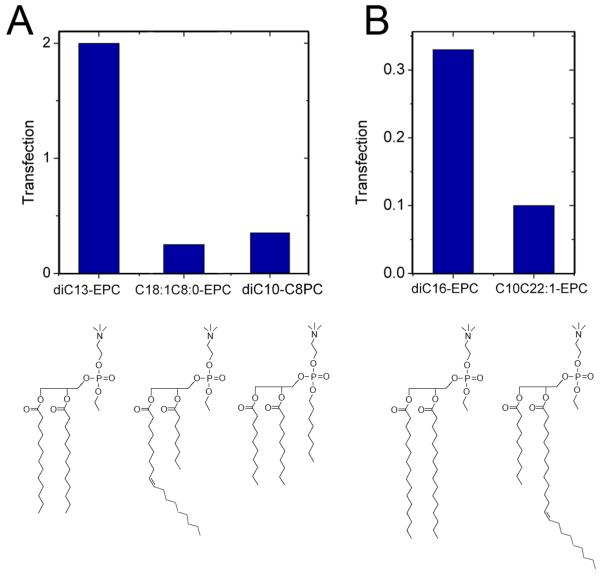
The cationic lipids examined are phosphatidylcholine (PC) derivatives, in which the PC phosphate is esterified, generating triesters and converting the zwitterionic head group to a cation. The hydrophobic moieties of the lipids synthesized thus far vary in the range of medium chain lengths and 0-1 double bonds per lipid chain (Fig. 1). Lipids with more than one double bond per chain (linoleoyl, arachidonoyl) have been also tested, but found to exhibit unsatisfactory transfection (data not shown). The transfection activities of the cationic PC lipoplexes are shown in Fig. 2 as a function of lipid chain length and unsaturation. To ensure compatibility, these activities are given in units of the activity obtained for dioleoyl ethylphosphatidylcholine (diC18:1-EPC; EDOPC), the best studied and historically the first representative of the cationic PCs 18,22. According to early studies, EDOPC transfection activity for BHK cells is similar to that of Lipofectamine 22. As seen from Fig. 2, transfection varied in a range of over two orders of magnitude and displayed a clearly expressed maximum for lipids with total of 30 C-atoms and 2 double bonds in the chains. This behavior was represented by three lipids, each comprising two 14:1 (myristoleoyl or myristelaidoyl) hydrocarbon chains: diC14:1c-EPC, diC14:1t-EPC and C14:1C2:0-C14:1PC. Increasing the chain length and/or decreasing the number of double bonds away from this maximum resulted in strongly reduced transfection activity. Introducing chain asymmetry also diminished transfection activity at the same total C-atom number C=28 (cf. diC13-EPC vs. C18:1C8:0-EPC, Fig. 3A), or C=34 (cf. diC16-EPC vs. C10C22:1-EPC, Fig. 3B); a specific three-chain configuration is also typified by weaker activity than its two-chain counterpart with the same overall number of carbon atoms (Fig. 3A).
Figure 2.
Correlations between transfection efficiency, hydrocarbon chain structure and phase behavior of the cationic PC derivatives. (A) Transfection activity as a function of the total number of carbon atoms and double bonds in the lipid chains (the three lipids with 30 C-atoms and 2 double bonds, diC14:1c-EPC, diC14:1t-EPC, and C14:1C2-C14:1PC, as well as the two lipids with 34 C-atoms and 2 double bonds, di18:1c9-EPC and di18:1c6-EPC (Fig. 1), exhibit virtually identical transfection activity and are represented by a single bar on the plot); (B) Cubic phase formed in mixture of DPoPE with a superior transfection agent, diC14:1c-EPC (85:15 molar ratio); (C) Hexagonal HII (with a small amount of cubic phase) formed in a mixture of DPoPE with a weak transfection agent, C18:1C4-C10EPC (85:15 molar ratio); the phase behaviors shown in (B) and (C) are representative for the strong and weak transfection agents, respectively 17.
Figure 3.
Effect of lipid molecular shape on transfection activity as demonstrated by three lipids with total of 28 C-atoms in the chains (A) (diC10 C8PC, which is effectively triple-chained, is not included in Figs. 1 and 2), and two lipids with 34 C-atoms in the chains (B).
Structural studies
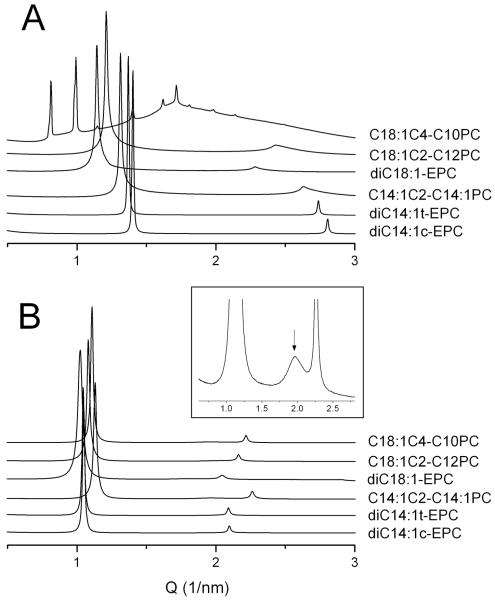
In order to gain insight into the mechanism underlying the observed dependence of transfection efficiency on the hydrocarbon chain structure, we used X-ray diffraction to characterize the physical properties of the lipids. Studies on pure cationic PCs showed however that most of the cationic PCs, in particular, all ethylphosphatidylcholines (EPCs), arrange into bilayers and readily form dispersions of uni- or oligolamellar liposomes in water. At higher electrolyte concentrations at which electrostatic repulsion is largely screened, e.g., in physiological solutions, and at sufficiently high lipid concentrations that prevent caused by electrostatic repulsion unbinding and disordering of the lipid phase (> 30-40 wt% of lipid), lipids of low, moderate and high transfection were found to typically form well-correlated liquid crystalline lamellar Lα phases. This behavior is illustrated in Fig. 4A (bottom five patterns). Some lipids may form non-lamellar phase, e.g., a cubic phase (Fig. 4A, top pattern); however, in presence of DNA all these lipids formed lamellar lipoplexes (Fig. 4B). In summary, the highly efficient transfection agents diC14:1c-EPC, diC14:1t-EPC and C14:1C2:0-C14:1PC, as well as most lipids of low and moderate transfection activity such as C18:1C2-C12PC and EDOPC, respectively, formed lamellar Lα phases of similar structure, and the same was valid also for their lipoplexes (Fig. 4). Such measurements have been carried out for large number of other cationic PCs 19,22-25. Similarly to the results shown in Fig. 4, the structural data obtained from these studies revealed no correlations between transfection activity and structural parameters of both lipoplexes and pure cationic lipid phases.
Figure 4.
Small-angle X-ray diffraction patterns of cationic PC dispersions (A), and lipoplexes prepared with herring sperm DNA (B) at 20°C. The inset shows enlarged view of an example of the DNA diffraction peak in the C18:1C2-C12PC lipoplexes.
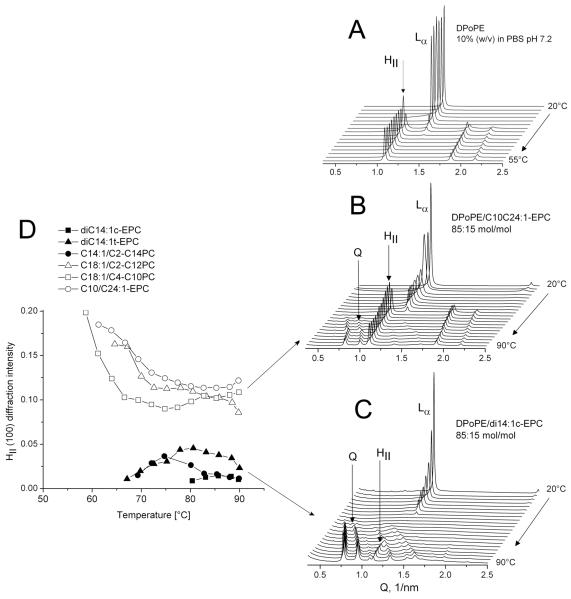
Based on the premise for the important role of lipoplex interactions with cellular membranes, a different approach to clarifying the underlying structural mechanism is provided by X-ray studies on cationic PCs mixtures with membrane lipids and membrane-mimicking lipid compositions 10,15-17,20,21,24,26. No correlations of the mixture phase behavior with the cationic PC hydrocarbon chain structure have been sought in previous work; however, such correlations appear to exist, in particular, in mixtures with non-lamellar membrane lipids. Fig. 5 presents the effects of cationic PCs with different chains and transfection efficiency on the lamellar – inverted hexagonal (Lα-HII) transition of DPoPE (see also ref. 17). Pure DPoPE dispersions are known to exhibit a direct Lα-HII transition at 42°C (Fig. 5A) 27. Noteworthy, strong transfection agents such as diC14:1c-EPC eliminated the direct Lα-HII phase transition and promoted formation of inverted cubic phase of Pn3m type between the Lα and HII phases, almost eliminating the formation of the HII phase (Fig. 5C); similar is the effect of other strong transfection agents, e.g., diC14:1t-EPC and C14:1C2:0-C14:1PC. By contrast, weak transfection agents retained the Lα-HII transition (Fig. 5B; the same holds also for other weak transfection agents, e.g., C18:1C2-C12PC and C18:1C4-C10PC). Plots of HII phase diffraction intensity and temperature range of existence clearly illustrate the extent of HII phase suppression by the high-efficiency cationic lipids and clearly distinguish between strong and weak transfection agents (Fig. 5D).
Figure 5.
Lamellar-nonlamellar phase transformations in pure DPoPE dispersion (A) and 85:15 (mol/mol) mixtures of DPoPE with C10C24:1-EPC (B) and diC14:1c-EPC (C). Diffraction patterns were recorded every minute during heating scans at 3°C/min. (D) Diffraction intensity and temperature ranges of existence of the HII phase in DPoPE/cationic lipid mixtures. The intensity of the HII (100) reflection is normalized to the intensity of the initial lamellar phases of the mixtures 17.
DISCUSSION
The cationic PCs are attractive drug and gene delivery carriers because they are biodegradable and have remarkably low toxicities 18,22. We have tested their potency to transfect the β-galactosidase reporter gene most frequently with HUAEC 16,17,20,24,28. This choice of cell culture was influenced by the fact that the vascular endothelial cells are of interest as a gene therapy target. They act as an organ and tissue interface with circulating blood and, even though readily accessible, their gene therapy with non-viral vectors has been hampered because these cells are very difficult to transfect 29. The present results, which show strong increase of HUAEC transfection caused by specific variations of the hydrophobic moieties of the cationic PC carriers, can be of considerable interest.
The data presented in Figs. 2 and 3 demonstrate that the hydrocarbon chain structure of the cationic PCs is an important determinant of their transfection activity. Fig. 2 shows well expressed dependences of activity on two important molecular characteristics - chain unsaturation and total number of carbon atoms in the 3 lipid chains. The chain unsaturation is given by the number of double bonds per lipid and assumes typical for phospholipids values of 0, 1 and 2 double bonds, and the total number of carbon atoms in the chains is representative of the lipid hydrophobic volume. Because the cationic PCs have identical hydrophilic groups, the latter parameter may be considered also as a measure of the cationic PC hydrophilic-lipophilic balance (HLB). In addition to these two parameters, another characteristic of importance appears to be related to the lipid molecular shape, which may be varied by varying the chain asymmetry at constant values of HLB. Examples of the large effect that variations in molecular shape have on transfection are shown in Fig. 3. However, the limited number of such examples available at present precludes further analysis and establishment of quantitative representations of this effect.
The mechanism underlying the prominent effects of the lipid chain structure is of considerable interest. It is clearly not of electrostatic nature because the studied here cationic PCs have identical surface choline groups and cannot be distinguished at the level of electrostatic interactions. Factors related to the phase behavior of the pure lipids can also be excluded from consideration on basis of the results shown in Fig. 4. As seen in Fig. 4, both high- and low-transfection lipids as well as their lipoplexes form similar, well-correlated lamellar liquid crystalline phases and the structural parameters of these phases appear to display no correlations with the lipid transfection activity. This behavior gives no grounds to expect relationships with the properties of the lipoplexes per se but rather shifts the attention to the role of their interactions with the intracellular membrane structures. These interactions are long considered an important stage of transfection, for example, it is commonly accepted that lipoplex interactions with anionic intracellular lipids are essential for lipoplex disassembly and DNA release. Along this line of reasoning, a number of model studies on cationic/membrane lipid mixtures have been carried out in the recent years in order to shed light on the mechanisms of intracellular lipoplex disassembly 10,14-16,21. With respect to the very strong hydrocarbon chain effects demonstrated in the present work, of particular interest become studies on model systems, in which electrostatic cationic-anionic lipid interactions are not present. The binary mixtures of the zwitterionic phospholipid DPoPE with a set of high- and low-transfection cationic PCs are actually the first example of such system (Fig. 5). DPoPE represents the most abundant class of nonlamellar-forming membrane lipids, the zwitterionic phosphatidylethanolamines (PEs), which are believed to be involved in lipidic fusion events. It is of significant interest that the effects of the cationic PCs on the DPoPE Lα - HII transition correlate very well with their transfection efficiency. X-ray diffraction measurements showed that high-transfection agents eliminated the direct Lα → HII phase transition and promoted formation of an inverted cubic phase between the Lα and HII phases, which replaced the HII phase in a wide temperature range. In contrast, moderate and weak transfection agents retained the Lα → HII transition. The hydrocarbon chain structures of cationic PCs exhibiting highest transfection thus appear to facilitate formation of inverted cubic phases in nonlamellar phase-forming lipid mixtures.
The phase behavior of the DPoPE/cationic PC mixtures is not unusual in principle, because both introduction of double bonds and variations in the chain lengths are known to have considerable effect on the lamellar-nonlamellar transitions in lipids. A specific structural characteristic of lipids that exhibits distinct change around the chain length of 14 carbons is the formation of bicontinuous inverted cubic phases QII. Such phases tend to be found with diacyl or dialkyl phospholipids and glycolipids that have chain lengths of C14 or shorter; the longer-chain lipids typically experience direct lamellar-inverted hexagonal Lα-HII phase transitions 27,30,31. The rationale for this tendency is that longer hydrocarbon chains can relieve packing frustration by filling the “voids” between parallel adjacent HII phase cylinders, consequently, lipids with longer chains are able to more easily form the HII phase 30,32-34. In contrast, the HII phase gives way to formation of inverse bicontinuous cubic phases in shorter-chain lipids because the latter lipids are unable to extend easily to fill the HII voids 32,34. Although cationic lipids with even shorter chains (<14), respectively, with lower total number of carbon atoms in their hydrocarbon chains (<30), are expected to even more promote formation of cubic phases, we did not observe a corresponding high transfection efficiency with these lipids. The reason for diminished activity of these shorter-chain compounds is almost certainly related to their toxicity 24.
The relationship between the ability of the cationic lipids to induce cubic phase and their transfection activity may be related to the molecular details of lipoplex fusion with cellular membranes. Indeed, multiple studies have demonstrated a relationship between transfection success and enhanced lipoplex-membrane fusion, the latter being required also for neutralization of the cationic lipid charge and subsequent DNA release 22,24,35,36. Although the mechanism of lipid membrane fusion is still not fully understood, there is now sound evidence that it is related to the mechanism of lamellar-cubic phase transitions 37.
CONCLUSIONS
The data presented here demonstrate that: (i) a clearly expressed relationship between cationic PC hydrocarbon chain structure and transfection efficiency manifests as transfection increase with decrease of chain length and increase of chain unsaturation; (ii) the hydrophobic lipid moiety modulates by over two orders of magnitude the transfection potency of cationic PCs; (iii) cationic PCs with two 14-C monounsaturated (myristoleoyl or myristelaidoyl) chains exhibit maximum activity; (iv) the superior transfection agents strongly promote formation of cubic phase at the expense of the inverted hexagonal phase in a membrane PE. The latter effect points to an alternative transfection process mediated by interactions with zwitterionic membrane PEs that does not originate from electrostatic interactions of the cationic lipids with anionic membrane lipids. Such effect is consistent with enhanced lipoplex – membrane fusion and provides a plausible physical explanation of the observed hydrocarbon chain effect on transfection.
ACKNOWLEDGMENTS
We thank the Argonne National Laboratory for the use of the APS, supported by the US Department of Energy. This work was supported in part by the Center for Cancer Nanotechnology Excellence (CCNE), Northwestern University, initiative of the National Institutes of Health’s National Cancer Institute.
REFERENCES
- 1.Felgner PL, Ringold GM. Cationic Liposome-Mediated Transfection. Nature. 1989;337:387–388. doi: 10.1038/337387a0. [DOI] [PubMed] [Google Scholar]
- 2.Leventis R, Silvius JR. Interactions of Mammalian-Cells with Lipid Dispersions Containing Novel Metabolizable Cationic Amphiphiles. Biochim. Biophys. Acta. 1990;1023:124–132. doi: 10.1016/0005-2736(90)90017-i. [DOI] [PubMed] [Google Scholar]
- 3.Gao X, Huang L. A Novel Cationic Liposome Reagent for Efficient Transfection of Mammalian-Cells. Biochem. Biophys. Res. Commun. 1991;179:280–285. doi: 10.1016/0006-291x(91)91366-k. [DOI] [PubMed] [Google Scholar]
- 4.Felgner PL. Prospects for synthetic self-assembling systems in gene delivery. J. Gene Medicine. 1999;1:290–292. doi: 10.1002/(SICI)1521-2254(199907/08)1:4<290::AID-JGM47>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Ilies MA, Seitz WA, Balaban AT. Cationic lipids in gene delivery: Principles, vector design and therapeutical applications. Curr. Pharm. Des. 2002;8:2441–2473. doi: 10.2174/1381612023392748. [DOI] [PubMed] [Google Scholar]
- 6.Gascon AR, Pedraz JL. Cationic lipids as gene transfer agents: a patent review. Expert Opinion Ther. Patents. 2008;18:515–524. [Google Scholar]
- 7.Tarahovsky YS, Arsenault AL, MacDonald RC, McIntosh TJ, Epand RM. Electrostatic control of phospholipid polymorphism. Biophys. J. 2000;79:3193–3200. doi: 10.1016/S0006-3495(00)76552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis RNAH, McElhaney RN. Surface charge markedly attenuates the nonlamellar phase-forming propensities of lipid bilayer membranes: Calorimetric and P-31-nuclear magnetic resonance studies of mixtures of cationic, anionic, and zwitterionic lipids. Biophys. J. 2000;79:1455–1464. doi: 10.1016/S0006-3495(00)76397-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hafez IM, Maurer N, Cullis PR. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 2001;8:1188–1196. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- 10.Tarahovsky YS, Koynova R, MacDonald RC. DNA release from lipoplexes by anionic lipids: Correlation with lipid mesomorphism, interfacial curvature, and membrane fusion. Biophys. J. 2004;87:1054–1064. doi: 10.1529/biophysj.104.042895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heyes J, Palmer L, Bremner K, MacLachlan I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J. Controlled Release. 2005;107:276–287. doi: 10.1016/j.jconrel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 12.Liu D, Qiao D, Li Z, Chen Y, Cui X, Li K, Yu L, Yan K, Zhu L, Guo Y, Cheng L. Structure–Function Relationship Research of Glycerol Backbone-Based Cationic Lipids for Gene Delivery. Chem. Biol. & Drug Design. 2008;71:336–344. doi: 10.1111/j.1747-0285.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 13.Balasubramaniam RP, Bennett MJ, Aberle AM, Malone JG, Nantz MH, Malone RW. Structural and functional analysis of cationic transfection lipids: The hydrophobic domain. Gene Ther. 1996;3:163–172. [PubMed] [Google Scholar]
- 14.Zuhorn IS, Bakowsky U, Polushkin E, Visser WH, Stuart MCA, Engberts JBFN, Hoekstra D. Nonbilayer phase of lipoplex-membrane mixture determines endosomal escape of genetic cargo and transfection efficiency. Mol. Ther. 2005;11:801–810. doi: 10.1016/j.ymthe.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Koynova R, Wang L, Tarahovsky Y, MacDonald RC. Lipid phase control of DNA delivery. Bioconjug. Chem. 2005;16:1335–1339. doi: 10.1021/bc050226x. [DOI] [PubMed] [Google Scholar]
- 16.Koynova R, Wang L, MacDonald RC. An intracellular lamellar - nonlamellar phase transition rationalizes the superior performance of some cationic lipid transfection agents. Proc. Natl. Acad. Sci. U. S. A. 2006;103:14373–14378. doi: 10.1073/pnas.0603085103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenchov BG, Wang L, Koynova R, MacDonald RC. Modulation of a membrane lipid lamellar-nonlamellar phase transition by cationic lipids: a measure for transfection efficiency. Biochim. Biophys. Acta-Biomembranes. 2008;1778:2405–2412. doi: 10.1016/j.bbamem.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 18.MacDonald RC, Rakhmanova VA, Choi KL, Rosenzweig HS, Lahiri MK. O-ethylphosphatidylcholine: A metabolizable cationic phospholipid which is a serum-compatible DNA transfection agent. J. Pharm. Sci. 1999;88:896–904. doi: 10.1021/js990006q. [DOI] [PubMed] [Google Scholar]
- 19.Rosenzweig HS, Rakhmanova VA, McIntosh TJ, MacDonald RC. O-alkyl dioleoylphosphatidylcholinium compounds: The effect of varying alkyl chain length on their physical properties and in vitro DNA transfection activity. Bioconjug. Chem. 2000;11:306–313. doi: 10.1021/bc9901144. [DOI] [PubMed] [Google Scholar]
- 20.Koynova R, Tarahovsky Y, Wang L, MacDonald RC. Lipoplex formulation of superior efficacy exhibits high surface activity and fusogenicity, and readily releases DNA. Biochim. Biophys. Acta-Biomembranes. 2007;1768:375–386. doi: 10.1016/j.bbamem.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koynova R, MacDonald RC. Natural lipid extracts and biomembrane-mimicking lipid compositions are disposed to form nonlamellar phases, and they release DNA from lipoplexes most efficiently. Biochim. Biophys. Acta-Biomembranes. 2007;1768:2373–2382. doi: 10.1016/j.bbamem.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald RC, Ashley GW, Shida MM, Rakhmanova VA, Tarahovsky YS, Pantazatos DP, Kennedy MT, Pozharski EV, Baker KA, Jones RD, Rosenzweig HS, Choi KL, Qiu RZ, McIntosh TJ. Physical and biological properties of cationic triesters of phosphatidylcholine. Biophys. J. 1999;77:2612–2629. doi: 10.1016/S0006-3495(99)77095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rakhmanova VA, McIntosh TJ, MacDonald RC. Effects of dioleoylphosphatidylethanolamine on the activity and structure of O-alkyl phosphatidylcholine-DNA transfection complexes. Cell. Mol. Biol. Lett. 2000;5:51–65. [Google Scholar]
- 24.Wang L, Koynova R, Parikh H, MacDonald RC. Transfection Activity of Binary Mixtures of Cationic O-Substituted Phosphatidylcholine Derivatives: The Hydrophobic Core Strongly Modulates Their Physical Properties and DNA Delivery Efficacy. Biophys. J. 2006;91:3692–3706. doi: 10.1529/biophysj.106.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koynova R, Wang L, MacDonald RC. Cationic phospholipids forming cubic phases: lipoplex structure and transfection efficiency. Mol. Pharmaceutics. 2008;5:739–744. doi: 10.1021/mp800011e. [DOI] [PubMed] [Google Scholar]
- 26.Koynova R, MacDonald RC. Mixtures of cationic lipid O-ethylphosphatidylcholine with membrane lipids and DNA: Phase diagrams. Biophys. J. 2003;85:2449–2465. doi: 10.1016/s0006-3495(03)74668-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipid Data Bank 2000 http://www.lipidat.ul.ie/
- 28.Wang L, MacDonald RC. New strategy for transfection: mixtures of medium-chain and long-chain cationic lipids synergistically enhance transfection. Gene Ther. 2004;11:1358–1362. doi: 10.1038/sj.gt.3302297. [DOI] [PubMed] [Google Scholar]
- 29.Teifel M, Heine LT, Milbredt S, Friedl P. Optimization of transfection of human endothelial cells. Endothelium-New York. 1997;5:21–35. doi: 10.3109/10623329709044156. [DOI] [PubMed] [Google Scholar]
- 30.Seddon JM, Templer RH. Polymorphism of lipid-water systems. In: Lipowsky R, Sackmann E, editors. Handbook of Biological Physics. Elsevier Science; Amsterdam: 1995. pp. 97–160. [Google Scholar]
- 31.Koynova R, Tenchov B. Phase Transitions of Lipids. In: Begley TP, editor. Wiley Encyclopedia of Chemical Biology. Vol. 2. John Wiley & Sons; Hoboken: 2009. pp. 601–615. [Google Scholar]
- 32.Templer RH, Seddon JM, Duesing PM, Winter R, Erbes J. Modeling the phase behavior of the inverse hexagonal and inverse bicontinuous cubic phases in 2 : 1 fatty acid phosphatidylcholine mixtures. J. Phys. Chem. B. 1998;102:7262–7271. [Google Scholar]
- 33.Anderson DM, Gruner SM, Leibler S. Geometrical Aspects of the Frustration in the Cubic Phases of Lyotropic Liquid-Crystals. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5364–5368. doi: 10.1073/pnas.85.15.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shearman GC, Ces O, Templer RH, Seddon JM. Inverse lyotropic phases of lipids and membrane curvature. J. Phys. Condensed Matter. 2006;18:S1105–S1124. doi: 10.1088/0953-8984/18/28/S01. [DOI] [PubMed] [Google Scholar]
- 35.Xu YH, Szoka FC. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996;35:5616–5623. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 36.Zelphati O, Szoka FC. Mechanism of oligonucleotide release from cationic liposomes. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11493–11498. doi: 10.1073/pnas.93.21.11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siegel DP. The relationship between bicontinuous inverted cubic phases and membrane fusion. In: Lynch ML, Spicer PT, editors. Bicontinuous Liquid Crystals. Taylor & Francis Group, CRC Press; Boca Raton: 2005. pp. 59–98. [Google Scholar]