Abstract
We used integrin αLβ2 heterodimers containing I domains locked open (active) or closed (inactive) with disulfide bonds to investigate regulatory interactions among domains in integrins. mAbs to the αL I domain and β2 I-like domain inhibit adhesion of wild-type αLβ2 to intercellular adhesion molecule-1. However, with αLβ2 containing a locked open I domain, mAbs to the I domain were subdivided into subsets (i) that did not inhibit, and thus appear to inhibit by favoring the closed conformation, and (ii) that did inhibit, and thus appear to bind to the ligand binding site. Furthermore, αLβ2 containing a locked open I domain was completely resistant to inhibition by mAbs to the β2 I-like domain, but became fully susceptible to inhibition after disulfide reduction with DTT. This finding suggests that the I-like domain indirectly contributes to ligand binding by regulating opening of the I domain in wild-type αLβ2. Conversely, locking the I domain closed partially restrained conformational change of the I-like domain by Mn2+, as measured with mAb m24, which we map here to the β2 I-like domain. By contrast, locking the I domain closed or open did not affect constitutive or Mn2+-induced exposure of the KIM127 epitope in the β2 stalk region. Furthermore, locked open I domains, in αLβ2 complexes or expressed in isolation on the cell surface, bound to intercellular adhesion molecule-1 equivalently in Mg2+ and Mn2+. These results suggest that Mn2+ activates αLβ2 by binding to a site other than the I domain, most likely the I-like domain of β2.
Integrins are large heterodimeric adhesive glycoproteins that convey signals bidirectionally across the plasma membrane. The integrin αLβ2 [lymphocyte function-associated (LFA)-1, CD11a/CD18], regulates cell adhesive functions and migration of lymphocytes and most other leukocytes (1, 2). αLβ2 binds to intercellular adhesion molecules (ICAMs) that are cell surface glycoproteins containing Ig superfamily domains. Conformational change in extracellular domains as well as clustering in the membrane have been proposed as mechanisms to regulate adhesiveness of integrins (3, 4); however, there is little information on how conformational signals are communicated among the many domains present in integrins.
The integrin α and β subunits are each type I transmembrane glycoproteins with a large extracellular domain, a single transmembrane region, and a short cytoplasmic tail (4). The N-terminal region of the integrin α subunit has been predicted to fold into a seven-bladed β-propeller domain (5). Half of the 18 known vertebrate integrin α subunits, including αL, contain an inserted domain or I domain of about 200 aa (2). The I domain is predicted to be inserted between β-sheets 2 and 3 of the β-propeller domain (5). Three-dimensional structures of the I domain show that it adopts the dinucleotide-binding fold like small G proteins and has a unique divalent cation coordination site designated the metal ion-dependent adhesion site (MIDAS) (6). Integrin β subunits contain an N-terminal cysteine-rich plexin semaphorin integrin (PSI) domain (7), a highly evolutionarily conserved I-like domain, and a C-terminal cysteine-rich region. The β subunit I-like domain has a MIDAS-like motif, and experimental evidence supports a protein fold similar to that of the α subunit I domain (8). Electron microscopy of integrins reveals a ligand-binding globular head piece that is connected to the membrane by two rod-like stalks about 16 nm long (9). In the headpiece, the β subunit I-like domain associates with the α subunit β-propeller domain near its linkage to the I domain (10, 11). The C-terminal region of the α subunit and the N- and C-terminal cysteine-rich regions of the β subunit each form stalks that connect the headpiece to the plasma membrane. The C-terminal cysteine-rich region of the β subunit appears to be important in the regulation of integrin function because a number of activating and activation-dependent antibodies to the β1, β2, and β3 subunits bind to this region (4).
All of the domains present in the globular headpiece of αLβ2 have been reported to contribute to ligand binding. Many types of experiments underscore the importance of the I domain for binding to ICAMs, including mutation of the MIDAS and surrounding residues (12–14), function blocking mAbs that map to the I domain (13), deletion of the I domain (15, 16), and functional activity of the isolated I domain (17, 18). However, many findings suggest an equal contribution of the I-like domain of the β2 subunit to ligand binding (4), including mapping of function-blocking mAbs to different epitopes within this domain (8), and abolition of ligand binding by mutation of residues in the MIDAS-like motif (19). Whether the domains of the integrin headpiece all contribute portions of the ligand binding site, or whether some directly bind ligand and others regulate binding to ligand, has been unclear for αLβ2 and integrins in general.
There is growing evidence that the I domain of integrins undergoes conformational change upon activation and that this regulates cell adhesion (20–25). The I domains of the α2 and αM subunits have been crystallized in both “open” and “closed” conformations, and it has been hypothesized that these conformations represent the “high-affinity” and “low-affinity” conformations, respectively (6, 26, 27). In the open conformation, there is a 10-Å movement of the C-terminal helix down the body of the I domain, and a large rearrangement and downward movement of the loop connecting this helix to the preceding β-strand (26).
Despite these advances on I domain structure, it remains unclear how conformational rearrangements in the I domain are linked to conformational changes in other domains within integrins. Here, we examine conformational interactions between domains in integrins and whether different domains have direct or regulatory roles in ligand binding. We use in these studies αLβ2 heterodimers that contain I domains that are locked in the open or closed conformations with disulfide bonds (25, **).
Methods
The sources of the mouse anti-human αL mAbs (28, 29) and anti-β2 mAbs (8) have been described. mAbs m24 (30) and KIM127 (31) were kind gifts of N. Hogg (Celltech, Slough, U.K.) and M. Robinson (Imperial Cancer Research Fund, London), respectively. mAbs were used as 1:20 hybridoma supernatants, 10 μg/ml purified IgG, or 1:200 ascites.
Human-mouse αL and β2 chimeras and methods used in epitope mapping were exactly as described (11).
The construction of mutant I domains, and stable K562 cell transfectants expressing mutant αLβ2 heterodimers or isolated αL I domains has been described (25, 32, **).
Immunofluorescence flow cytometry was as described (32). For staining by mAbs KIM127 or m24, cells were washed with Hepes/NaCl/glucose/BSA (20 mM Hepes, pH 7.4/140 mM NaCl/2 mg/ml glucose/1% BSA) in the presence or absence of 2 mM MnCl2, resuspended in 100 μl of the same buffer, and incubated with 15 μg/ml of mAb for 30 min at 37°C.
Cell adhesion to ICAM-1 purified from human tonsil and coated on 96-well plates was as described (25, 32).
Results
Binding of Antibodies to Conformationally Constrained αLβ2 Heterodimers.
Disulfide bonds have previously been introduced into the αL I domain to lock it in the open or closed conformations (25, **). When residues Lys-287 and Lys-294 are mutated to cysteine to lock the I domain open, the affinity for ICAM-1 is increased 10,000-fold for the soluble open I domain compared with wild type.** When an isolated I domain or αLβ2 complex containing this mutation is expressed on the cell surface, adhesiveness for ICAM-1 is increased equivalently to maximally activated αLβ2 (25). Conversely, when residues Leu-289 and Lys-294 are mutated to cysteine to lock the I domain closed, the isolated I domain and αLβ2 are inactive and resistant to activation (25). Formation of a disulfide bond was confirmed by a dramatic decrease in mobility in SDS/PAGE after reduction with DTT for both the open and closed mutants; no change in mobility was seen for wild type (**). Reduction with DTT of the open mutant surface expressed or soluble I domains abolished adhesive function and reduced affinity for ICAM-1 10,000-fold, respectively (25, **).
We examined a panel of antibodies mapped to different regions of αL and β2 (8, 28, 29) for binding to locked open (K287C/K294C), locked closed (L289C/K294C), and control single cysteine substitution mutants K287C, L289C, and K294C (Table 1). αL subunits containing mutated I domains were coexpressed with the integrin β2 subunit and binding to mAbs was determined by immunofluorescence flow cytometry. Binding of mAb CBR LFA-1/1 to the open I domain K287C/K294C αLβ2 mutant was reduced to 40–50% of wild type, whereas this antibody reacted with the closed I domain L298C/K294C mutant and the single cysteine substitution mutants as well as wild type (Table 1). CBR LFA-1/1 was mapped to residues 301–359 by using human-mouse αL chimeras (13) and reacts with isolated I domains containing residues 130–338 (see below). Therefore, this mAb recognizes the I domain C-terminal α-helix, or the subsequent segment that connects to the β-propeller domain. The reactivity of all other mAbs, including 10 mAbs to the I domain, two to the β-propeller domain, and eight to the β2 subunit I-like and C-terminal domains (Table 2) was unaffected by the mutations (Table 1). Thus, the structural integrity of the αLβ2 molecule was not disturbed by the mutations.
Table 1.
Reactivity of antibodies with LFA-1 cysteine substitution mutants
| mAb | % Wild-type binding
|
||||||
|---|---|---|---|---|---|---|---|
| K287C/K294C
|
L289C/K294C
|
K287C 293T | L289C 293T | K294C 293T | |||
| 293T | K562 | 293T | K562 | ||||
| BL5 | 92 ± 11 | 92 | 86 ± 16 | 98 | 93 | 92 | 88 |
| F8.8 | 94 | 102 | 84 | 94 | 96 | 100 | 95 |
| CBR LFA-1/9 | ND | 85 | ND | ND | ND | ND | ND |
| TS2/6 | 85 ± 6 | 89 | 79 ± 3 | 96 | 91 | 88 | 92 |
| May.035 | 93 ± 8 | 93 | 82 ± 14 | 101 | 96 | 95 | 106 |
| TS1/11 | 94 | 96 | 94 | 105 | ND | ND | ND |
| TS1/12 | 89 | 87 | 102 | 106 | 99 | 104 | 94 |
| TS1/22 | 96 ± 12 | 93 | 91 ± 8 | 110 | 103 | 96 | 92 |
| TS2/14 | 86 ± 9 | 95 | 83 ± 11 | 103 | 103 | 100 | 103 |
| 25.3.1 | 93 | 88 | 91 | 86 | ND | ND | ND |
| CBR LFA-1/1 | 44 ± 1 | 56 | 96 ± 8 | 118 | 86 | 93 | 89 |
| S6F1 | 89 | 97 | 95 | 86 | 98 | 86 | 92 |
| TS1/18 | 100 ± 10 | 97 | 96 ± 5 | 106 | 88 | 87 | 108 |
| YFC51 | 103 | 101 | 95 | 111 | ND | ND | ND |
| CLBLFA-1/1 | ND | 96 | ND | 101 | ND | ND | ND |
| May.017 | ND | 109 | ND | 111 | ND | ND | ND |
| 6.5e | ND | 84 | ND | 96 | ND | ND | ND |
| CBR LFA-1/7 | 95 | 95 | 92 | 97 | ND | ND | ND |
| CBR LFA-1/2 | ND | 86 | ND | 86 | ND | ND | ND |
| YTA-1 | ND | 111 | ND | 108 | ND | ND | ND |
Wild-type or mutant αL and wild-type β2 were coexpressed transiently on the surface of 293T cells or stably on K562 cells. Reactivity with antibodies was determined by flow cytometry. Mean fluorescence of each antibody binding was normalized to the mean fluorescence of mAb TS2/4 binding. The results are expressed as percent of wild-type binding ± difference from the mean. For some antibodies, only one experiment was done. ND, not determined.
Table 2.
Differential inhibition by antibodies of binding of wild-type and open mutant αLβ2 to immobilized ICAM-1
| mAb | Epitope | % Inhibition
|
|||
|---|---|---|---|---|---|
| Wild-type
αLβ2*
|
K287C/K294C
|
||||
| 293T | K562 | 293T | K562 | ||
| RR1/1 | ICAM-1 | 96 | ND | 98 | ND |
| αL I domain | |||||
| BL5 | 119–153, 185–215 | 97 ± 1 | 98 | 91 ± 3 | 91 ± 4 |
| F8.8 | 119–153, 185–215 | 95 | 98 | 92 | 98 |
| TS2/6 | 154–183 | 97 ± 1 | 92 ± 3 | 79 ± 7 | 88 ± 5 |
| May.035 | 185–215 | 96 ± 0 | 96 ± 1 | 97 ± 1 | 93 ± 2 |
| TS1/11 | 185–215 | 94 | 97 | 45 | 41 |
| TS1/12 | 185–215 | 96 ± 3 | 97 ± 0 | 49 ± 7 | 64 ± 6 |
| TS1/22 | 250–303 | 96 | 97 ± 0 | 95 | 94 ± 3 |
| TS2/14 | 250–303 | 94 ± 2 | 96 ± 1 | 3 ± 7 | 9 ± 0 |
| 25.3.1 | 250–303 | 90 | 92 ± 0 | 4 | 3 ± 3 |
| CBR LFA-1/1 | 301–338 | 93 ± 1 | 95 ± 4 | 9 ± 2 | 3 ± 3 |
| αL β-propeller | |||||
| S6F1 | 1–57 | ND | 6 | ND | 10 |
| TS2/4 | 1–57 | ND | 7 | ND | 3 |
| β2 I-like domain | |||||
| TS1/18 | R122 or H332 | ND | 98 | ND | 6 |
| YFC51 | R122 and H332 | ND | 98 | ND | 0 |
| CLBLFA-1/1 | H332 and N339 | ND | 95 | ND | 7 |
| May.017 | E175 and ? | ND | 98 | ND | 3 |
| 6.5e | E175 | ND | 98 | ND | 6 |
| β2 C-terminal | |||||
| CBR LFA-1/7 | 345–612 | ND | 5 | ND | 6 |
| YTA-1 | β-propeller and I-like domain | ND | 99 | ND | 7 |
Binding of the transfectants to immobilized ICAM-1 was determined in the presence of the indicated antibodies. The amount of control binding was similar to that shown in Fig. 2A. Data are % inhibition ± difference from the mean of two independent experiments. For some antibodies, only one experiment was done. However, in each experiment, each antibody was repeated in triplicate, and the standard deviation of % bound cells of the triplicate samples was <4%. ND: not determined.
Wild-type αLβ2 is constitutively active in 293T cells; wild-type αLβ2 in K562 transfectants was activated by preincubation with mAb CBR LFA-1/2 at 10 μg/ml for 30 min (25).
αLβ2 with the Open, Mutant I Domain Is Resistant to Inhibition by a Subset of mAbs.
The same panel of mAbs was tested for inhibition of binding to ICAM-1 of αLβ2 heterodimers containing wild type or open, K287C/K294C mutant I domains (Table 2). Ten different antibodies to the I domain of the αL subunit inhibited ligand binding by wild-type αLβ2 (Table 2). Seven of these antibodies also inhibited binding by αLβ2 containing the mutant, open I domain. On the other hand, three mAbs to the I domain, TS2/14, 25.3.1, and CBR LFA-1/1, completely inhibited binding to ICAM-1 of wild-type αLβ2, but did not inhibit binding of mutant, open αLβ2 (Table 2). The results with CBR LFA-1/1 may be partially ascribed to its reduced binding to open αLβ2; however, mAbs TS2/14 and 25.3.1 bound equally well to open and wild-type αLβ2 (Table 1). Thus, locking open αLβ2 renders it resistant to inhibition by these mAbs.
Five different antibodies to the I-like domain of the β2 subunit, TS1/18, YFC51, CLBLFA-1/1, May.017, and 6.5e, potently inhibited ligand binding by wild-type αLβ2 (Table 2). However, none of these antibodies blocked ligand binding by αLβ2 containing the mutant K287C/K294C open I domain. Notably, these mAbs bound to at least four different epitopes as shown by mapping to specific mouse-human amino acid substitutions (8) (Table 2).
YTA-1 is a function-blocking mAb that recognizes an activation epitope that maps to residues in the top of the β2 subunit I-like domain and to the side of the αL subunit β-propeller domain at β-sheets 2 and 3, and thus defines a site of association between these domains (29). This mAb inhibited binding to ICAM-1 of wild-type αLβ2, but not of the open K287C/K294C mutant αLβ2 (Table 2). It thus behaved similarly to the mAbs to the I-like domain.
We tested whether the resistance to inhibition by mAbs to the I-like domain depended on a disulfide in the open mutant αLβ2. Studies with isolated I domains show that the disulfide forms and is required for high affinity binding of the K287C/K294C mutant to ICAM-1 (25, **). We pretreated cells with 10 mM DTT, then added mAbs and tested for inhibition of binding to ICAM-1 (Table 3). Reduction with DTT rendered the open mutant susceptible to inhibition by mAbs TS1/18 and YFC51 to the β2 I-like domain. By contrast, DTT did not relieve inhibition by the TS1/22 mAb to the αL I domain (Table 3). The amount of control adhesion was unaffected by DTT. Thus, the disulfide bond is required for resistance to inhibition by mAbs to the I-like domain.
Table 3.
αLβ2 containing the mutant open I-domain can be inhibited by mAbs to the β2-I-like domain after DTT treatment
| mAb | Epitope | %
Inhibition
|
|||
|---|---|---|---|---|---|
| Wild-type αLβ2
|
Open
αLβ2
|
||||
| −DTT | +DTT | −DTT | +DTT | ||
| TS1/22 | αL I-domain | 92 ± 1 | 92 ± 2 | 87 ± 7 | 94 ± 1 |
| TS1/18 | β2 I-like domain | 89 ± 3 | 97 ± 0.5 | 0 ± 0 | 97 ± 2 |
| YFC51 | β2 I-like domain | 85 ± 1 | 88 ± 1 | 1 ± 1 | 90 ± 1 |
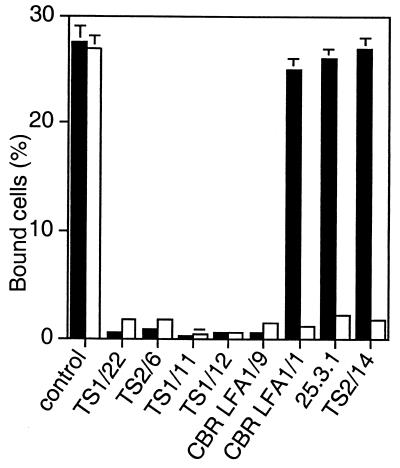
The I domain can be expressed in isolation from other integrin domains on the cell surface by using a platelet-derived growth factor receptor transmembrane domain (25). The antibodies to the I domain were tested for inhibition of ligand binding by the isolated, locked open I domain side by side with activated, wild-type αLβ2 (Fig. 1). All antibodies to the I domain, except for CBR LFA-1/1, bound to the mutant open I domain as well as the wild-type I domain as determined by flow cytometry (data not shown). Binding of CBR LFA-1/1 was only slightly reduced; it bound 80% as well to the open, mutant I domain as to the wild-type I domain. I domain antibodies CBR LFA-1/1, 25.3.1, and TS2/14 that failed to inhibit ligand binding by the open, mutant αLβ2 heterodimer as shown in Table 2 also failed to inhibit binding by the isolated, open I domain (Fig. 1). Conversely, antibodies that blocked binding by open, mutant αLβ2 (Table 2) also blocked binding by the open, mutant I domain in isolation (Fig. 1).
Figure 1.
Binding to ICAM-1 of the isolated, locked open αL I domain is resistant to inhibition by a subset of mAbs to the I domain. Binding to ICAM-1 was measured of K562 transfectants expressing wild-type αLβ2 activated with mAb CBR LFA-1/2 (open bars) or K562 transfectants expressing the isolated, open K287C/K294C mutant I domain (black bars). Binding to ICAM-1 was performed in the presence of control X63 myeloma IgG or the indicated mAbs to the I domain. Results are mean ± SD of three independent experiments in duplicate.
Ligand Binding by αLβ2 Containing Locked Open or Closed I Domains Is Not Modulated by Mn2+.
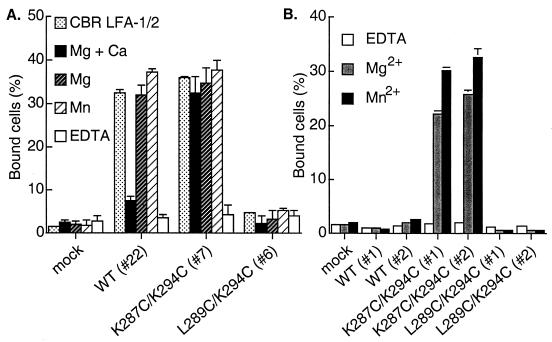
The divalent cation Mn2+ has been found to activate adhesiveness by almost all integrins, including αLβ2 (33). Ligand binding by wild-type αLβ2 was activated by Mn2+, and in the combined presence of Mg2+ and absence of Ca2+, as described (33) (Fig. 2A). Mn2+ activated ligand binding by wild-type αLβ2 to the same extent as the activating mAb CBR LFA-1/2. The open K287C/K294C mutant was already maximally active in Mg2+ and Ca2+ and could not be further activated by withdrawal of Ca2+ or addition of Mn2+, confirming its constitutive activity. However, withdrawal of Ca2+ or addition of Mn2+ did not activate the closed L289C/K294C mutant (Fig. 2A). Thus, locking the I domain closed was dominant over Mn2+ in its effect on ligand binding.
Figure 2.
Effect of divalent cations on binding of locked αLβ2 or isolated I domains to immobilized ICAM-1. (A) Binding of K562 transfectants expressing αLβ2 containing wild-type (WT) or locked I domains to immobilized ICAM-1 was determined in 20 mM Tris⋅HCl (pH 7.5), 150 mM NaCl supplemented with 1 mM Mg2+ and Ca2+, 1 mM Mg2+, 1 mM Mn2+, 5 mM EDTA, or in medium containing Ca2+ and Mg2+ in the presence of the activating mAb CBR LFA-1/2 at 10 μg/ml as indicated. Numbers in parentheses are clone numbers of the K562 stable transfectants. (B) Effect of divalent cations on binding to ICAM-1 of K562 transfectants expressing isolated I domains. Binding was performed in Hepes/NaCl/glucose/BSA (20 mM Hepes, pH 7.5/140 mM NaCl/2 mg/ml glucose/1% BSA) supplemented with 1 mM EDTA, 1 mM Mg2+, or 1 mM Mn2+. Results are mean ± SD of triplicate samples and are representative of at least three experiments; some error bars are too small to be visible.
For comparison, we examined the effect of divalent cations on binding of isolated, cell-surface expressed I domains to ICAM-1 (Fig. 2B). In contrast to results with wild-type αLβ2 heterodimers, Mn2+ did not activate ligand binding by the isolated, wild-type I domain. In similarity to results with locked αLβ2 heterodimers, Mn2+ did not activate binding by the locked closed I domain, and the activity of the locked open I domain was comparable in Mg2+ and Mn2+ (Fig. 2B).
Conformational Linkage of the αL I Domain with the β2 I-Like Domain and Cysteine-Rich Repeats.
To examine conformational interactions between the I domain and other integrin domains, we tested the effect of locking the I domain open or closed on the constitutive exposure of epitopes in the β2 subunit I-like domain and C-terminal cysteine-rich repeats. Furthermore, we examined whether Mn2+ would still be capable of inducing activation epitopes in these domains when the conformation of the I domain was locked. The KIM127 mAb to the cysteine-rich C-terminal stalk region of β2 can activate ligand binding (31). We recently have found that this mAb preferentially binds to the activated forms of β2 integrins (34), and thus is a reporter of integrin activation. This mAb maps to residues 504, 506, and 508 in cysteine-rich repeat 2 of β2 (34). The m24 mAb also selectively binds to activated β2 integrins and is a widely used reporter of integrin activation (30); however, its binding site on αLβ2 has not been localized.
To use m24 mAb in the analysis of domain interactions, we mapped its epitope. mAb m24 was specific for the human β2 subunit, because it reacted with human but not mouse β2 complexed with human αL in transfected cells (Fig. 3). Furthermore, m24 bound to human β2 complexed with mouse or human αL equally well (data not shown). Mouse-human β2 chimeras showed that m24 mapped to residues 1–254, and to two different subregions, as shown by a lack of reactivity with either chimera h162m or chimera m163h (Fig. 3). Examination of all human-mouse amino acid substitutions in the I-like domain showed that m24 mAb is specific for residues R122 and E175 (Table 4). These residues are predicted to be located on the top of the I-like domain, on the same face that bears the MIDAS-like motif and hence the predicted Mn2+/Mg2+ binding site (8). The I-like domain associates with the αL β-propeller domain near its β-sheets W2 and W3 as shown by binding of mAb YTA-1 to this subunit interface (29); mouse-human incompatibilities in this region appeared to conformationally affect m24 expression. Thus, chimeras with a species interface between W2 and W3 (αL m153h and h153m) or after β-strand 3 of W3 (αL m359h) showed little m24 expression, whereas reciprocal chimeras in the αL stalk region (αL m654h and h654m) and as mentioned above, wild-type mouse αL, showed good m24 expression when associated with human β2 (data not shown). Preincubation of cells at 37°C with m24 mAb completely blocked binding of biotinylated YTA-1 mAb, measured exactly as described (29). This finding confirms the mapping results, because YTA-1 binds to a loop predicted to be nearby residue R122 at the top of the I-like domain, and also recognizes the side of the β-propeller domain as mentioned above (29). Furthermore, YTA-1 also recognizes an activation-dependent epitope (29).
Figure 3.
Mapping of m24 mAb to residues 1–254 of the β2 subunit. The indicated human/mouse β2 chimeras and mutants were cotransfected with human αL into 293T cells (11). The transfectants were stained with m24 mAb or TS1/22 mAb to αL followed by immunofluorescence flow cytometry. m24 recognition was measured as specific mean fluorescence intensity and quantitated as a percentage of total αLβ2 expression defined by staining with TS1/22 mAb. Results are the mean and SD of three independent experiments.
Table 4.
m24 mAb maps to residues R122 and E175 of the β2 subunit
| β2 subunit mutation | m24 binding, % of TS1/22 |
|---|---|
| Human wild type | 60.0 ± 1.7 |
| Mouse wild type | 1.7 ± 1.9 |
| R122N | 1.8 ± 1.5 |
| R133Q | 54.1 ± 3.0 |
| R133Q/H332Q | 54.6 ± 2.9 |
| R133Q/N339Y | 59.8 ± 1.7 |
| R133Q/H332Q/N339Y | 53.6 ± 2.0 |
| D163E | 54.4 ± 2.2 |
| D163E/E175A | 0.3 ± 0.3 |
| E175A | 1.7 ± 0.8 |
| N190D | 54.1 ± 2.6 |
| L270M | 73.3 ± 3.3 |
| A290S | 67.8 ± 2.6 |
| S302K/R303K | 66.6 ± 1.9 |
| E325D | 59.7 ± 1.4 |
| H332Q | 66.5 ± 1.2 |
| N339Y | 70.9 ± 2.0 |
m24 binding to αLβ2 transfectants was determined as described in Fig. 3.
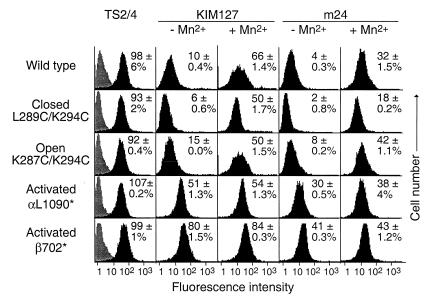
In the absence of activation with Mn2+, wild-type αLβ2 showed little expression of the KIM127 or m24 epitopes (Fig. 4). There was also little expression of the KIM127 or m24 epitopes in αLβ2 with closed or open I domains. By contrast, truncation of the αL cytoplasmic domain before the GFFKR sequence (αL1090*) or truncation of the β2 cytoplasmic domain (β2 702*) activated expression of both the KIM127 and m24 epitopes (Fig. 4). Both of these truncations activate ligand binding by αLβ2 (32) (C.L., J.T., and T.A.S., unpublished work).
Figure 4.
Linkage between the I domain and activation epitopes in other domains. K562 transfectants stably expressing wild-type αLβ2, or αLβ2 with mutant I domains or activating truncations of the cytoplasmic domains of the αL (αL1090*) or β2 (β702*) subunits were stained with control X63 myeloma IgGl (gray histograms), TS2/4 mAb to αL, TS1/18 mAb to β2 (not shown) in absence of MnCl2, or activation-dependent mAbs KIM127 or m24 in the presence or absence of 2 mM MnCl2 (black histograms), followed by immunofluorescence flow cytometry. For each mutant, specific fluorescence intensity was determined as the percentage of the average intensity of TS2/4 and TS1/18 staining, and the mean and difference from the mean in two independent experiments is shown in each panel.
Mn2+ induced expression of the KIM127 and m24 epitopes on wild-type αLβ2 to a level of 66% and 32%, respectively, of that of control constitutively expressed epitopes (Fig. 4). Furthermore, Mn2+ induced expression of the KIM127 or m24 epitopes on the closed and open αLβ2 mutants (Fig. 4), despite its lack of effect on ligand binding by these mutants. However, induction by Mn2+ of the m24 epitope was blunted on the closed mutant, as shown by comparison to wild type and the other mutants. This finding was reproducible in independent experiments and also contrasted with the good induction by Mn2+ of the KIM127 epitope on the closed mutant.
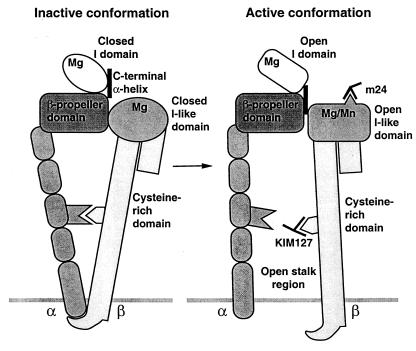
Thus our results suggest that activation of integrins by inside-out signaling, as reported previously and mimicked here by cytoplasmic domain deletions, is linked to opening of the stalk region and activation of ligand binding. By contrast, mutational opening of the I domain is not linked to opening of the stalk region (Fig. 5).
Figure 5.
Cartoon showing the approximate spatial relationship among integrin domains and the conformational linkages between them.
Discussion
Our study resolves a longstanding question on how multiple domains in the integrin headpiece contribute to ligand binding by demonstrating that the β2 subunit I-like domain has a regulatory rather than a direct role in ligand binding by αLβ2. Furthermore, our study defines linkages between the αL I domain, a regulatory Mn2+-binding site, and activation epitopes in the β2 I-like domain and cysteine-rich region (Fig. 5).
Our results suggest that the β2 I-like domain has an indirect role in ligand binding by αLβ2. mAbs to β2 are as effective as mAbs to αL in blocking all known functions of αLβ2, including antigen-specific T cell functions, homotypic adhesion, adhesion to endothelial cells, and T cell receptor-stimulated adhesion to ICAM-1 (1, 2). We now demonstrate that mAbs to the I-like domain are not inhibitory when the αL I domain is locked in the open conformation. These mAbs map to three widely separated sites in a model of the I-like domain (8). One site comprising three different epitopes involves residues 133, 332, and 339 and is located on the bottom half of the “front” face of the domain. Another site comprising two epitopes involves residue 175 and is located in a loop on the “top” face bearing the MIDAS-like site. The third site, defined by mAb YTA-1, is located in the model on the opposite side of the top face, in a loop bearing residues 302 and 303. This mAb recognizes an activation epitope and also recognizes residues 78–80, 365, and 367 located in the αL β-propeller domain, on either side of the I domain linkage between β-sheets 2 and 3 of the β-propeller (29). All of these mAbs bound equally well to wild-type and locked open αLβ2. Therefore, their ability to inhibit binding by wild-type αLβ2 but not by locked open αLβ2 suggests that binding of these mAbs favors the closed conformation of the I domain, and that the conformations of the β2 I-like domain and αL I domain are allosterically linked. Locking open the I domain disrupts this linkage. In further demonstration of this, after disulfide reduction, the locked open αLβ2 became susceptible to inhibition by mAbs to the I-like domain. Thus, in αLβ2, and perhaps in all I domain-containing integrins, the I-like domain regulates ligand binding by the I domain. In integrins that lack I domains, the I-like domain may either have a direct role in ligand binding or may regulate the conformation of adjacent ligand binding loops in W2 and W3 of the β-propeller domain (10).
Although most mAbs to the I domain blocked binding by αLβ2 with both wild-type and locked open I domains, locked open αLβ2 was completely resistant to inhibition by three mAbs. The same three mAbs bound to the isolated locked open I domain but failed to inhibit ligand binding by it. These mAbs therefore may favor the closed conformation of the I domain. Interestingly, one of these mAbs is CBR LFA-1/1, which binds to the region including the lower half of the I domain C-terminal α-helix, and the linker to β-propeller sheet 3. The other two mAbs, TS2/14 and 25.3.1, bind to the top of helix α5 (C.L., J.T., and T.A.S., unpublished work), close to the conformationally mobile β6-α6 loop.
The I domain and I-like domain appear to be nearby one another on the same side of the β-propeller domain (10, 11) (Fig. 5). The bottom of the I domain connects to the top of the β-propeller domain, between β-sheets 2 and 3 (5). The top of the I-like domain contacts β-sheets 2 and 3 at the side of the β-propeller domain (10, 11). Therefore, the bottom of the I domain is in close proximity to the top of the I-like domain. Our data suggest that the β2 I-like domain regulates ligand binding by the αL I domain. It is attractive to speculate that regulation is accomplished by structural linkage across an interface between the I domain and I-like domain near their interfaces with the β-propeller domain. Regulation of ligand binding could be accomplished by a downward pull on the I domain C-terminal α-helix exerted by the I-like domain.
Both integrins that contain and lack I domains can be activated with Mn2+ (2–4, 33). Often it has been assumed that I domain-containing integrins are activated by binding of Mn2+ to the MIDAS of the I domain. However, Mn2+ does not activate ligand binding by the wild-type, isolated I domain, as shown here and elsewhere (18). Furthermore, if Mn2+ activated by binding to the I domain, locking its conformation should abolish allosteric effects on other domains, yet Mn2+ could still activate exposure of the m24 epitope in the I-like domain and KIM127 epitope in cysteine-rich repeat 2. Moreover, Mn2+ did not affect ligand binding by αLβ2 containing locked closed or open I domains, but dramatically activated ligand binding by wild-type αLβ2. These findings strongly suggest that Mn2+ exerts its effect by binding elsewhere than the I domain. The MIDAS-like site of the β2 I-like domain is an excellent candidate for this site (Fig. 5). Allosteric regulation by Mn2+ at this site would be highly consistent with our finding that the β2 I-like domain regulates ligand binding by the αL I domain.
Activation of integrins in inside-out signaling, as mimicked here by mutation of the αL or β2 cytoplasmic domains, is associated not only with opening of the ligand binding site, but also in exposure of epitopes in other regions. KIM127 mAb selectively recognizes activated β2 integrins (31, 34) and the dissociated β2 subunit (35), and maps to cysteine-rich repeat 2 (24, 34). The m24 mAb also recognizes an activation epitope (33), which we now map to an epitope requiring residues R122 and E175 in the β2 subunit I-like domain. Interestingly, m24 mAb competes binding of YTA-1 mAb, which also recognizes an activation epitope and binds both to the top of the I-like domain and side of the β-propeller domain (29). Residues R122 and E175 also are predicted to be on the top face of the I-like domain, nearby the MIDAS-like, Mg2+/Mn2+ -binding site (8). Thus, during inside-out signaling, structural rearrangements occur both in the β subunit cysteine-rich region and near the MIDAS-like site of the I-like domain. These domains are intermediate between the membrane and the α subunit I domain (Fig. 5), and thus provide a mechanism for linking signals within the cell to conformational change within the I domain. A linkage between the I-like domain and I domain is supported by (i) their common association with β-propeller blades 2 and 3 (29); (ii) the demonstration here that binding of mAbs to the I-like domain regulates ligand binding by the I domain; and (iii) the demonstration here that Mn2+ does not activate ligand binding directly by binding to the I domain, but activates indirectly by binding elsewhere, with the best candidate being the MIDAS-like motif of the I-like domain. Is this linkage equivalent in both directions, i.e., does outside-in signaling work exactly like inside-out except in reverse? We believe that this is not necessarily so, because locking the I domain open did not induce the m24 or KIM127 epitopes, whereas inside-out signaling did. Furthermore, locking the I domain closed did not alter induction by Mn2+ of the KIM127 epitope, although it did lessen exposure of the m24 epitope, consistent with a closer linkage of the I domain to the I-like domain than to the cysteine-rich repeats. Further work is required to determine the effect of locking in specific I domain conformations on integrin clustering and downstream signaling pathways.
Acknowledgments
We thank Mike Dustin for reviewing the manuscript. This work was supported by National Institutes of Health Grant CA31798. C.L. was supported by a Cancer Research Institute Fellowship.
Abbreviations
- ICAM
intercellular adhesion molecule
- LFA
lymphocyte function-associated
- MIDAS
metal ion-dependent adhesion site
Footnotes
M.S., C.L., R. Paframan, U. H. von Andrian, J.T., and T.A.S., unpublished work.
References
- 1.Larson R S, Springer T A. Immunol Rev. 1990;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 2.Gahmberg C G, Tolvanen M, Kotovuori P. Eur J Biochem. 1997;245:215–232. doi: 10.1111/j.1432-1033.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- 3.Stewart M, Hogg N. J Cell Biochem. 1996;61:554–561. doi: 10.1002/(sici)1097-4644(19960616)61:4<554::aid-jcb8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 4.Humphries M J. Biochem Soc Trans. 2000;28:311–339. [PubMed] [Google Scholar]
- 5.Springer T A. Proc Natl Acad Sci USA. 1997;94:65–72. doi: 10.1073/pnas.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J-O, Rieu P, Arnaout M A, Liddington R. Cell. 1995;80:631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- 7.Bork P, Doerks T, Springer T A, Snel B. Trends Biochem Sci. 1999;24:261–263. doi: 10.1016/s0968-0004(99)01416-4. [DOI] [PubMed] [Google Scholar]
- 8.Huang C, Zang Q, Takagi J, Springer T A. J Biol Chem. 2000;275:21514–21524. doi: 10.1074/jbc.M002286200. [DOI] [PubMed] [Google Scholar]
- 9.Weisel J W, Nagaswami C, Vilaire G, Bennett J S. J Biol Chem. 1992;267:16637–16643. [PubMed] [Google Scholar]
- 10.Puzon-McLaughlin W, Kamata T, Takada Y. J Biol Chem. 2000;275:7795–7802. doi: 10.1074/jbc.275.11.7795. [DOI] [PubMed] [Google Scholar]
- 11.Zang, Q. & Springer, T. A. (2001) J. Biol. Chem., in press. [DOI] [PubMed]
- 12.Kamata T, Wright R, Takada Y. J Biol Chem. 1995;270:12531–12535. doi: 10.1074/jbc.270.21.12531. [DOI] [PubMed] [Google Scholar]
- 13.Huang C, Springer T A. J Biol Chem. 1995;270:19008–19016. doi: 10.1074/jbc.270.32.19008. [DOI] [PubMed] [Google Scholar]
- 14.Edwards C P, Fisher K L, Presta L G, Bodary S C. J Biol Chem. 1998;273:28937–28944. doi: 10.1074/jbc.273.44.28937. [DOI] [PubMed] [Google Scholar]
- 15.Leitinger B, Hogg N. Mol Biol Cell. 2000;11:677–690. doi: 10.1091/mbc.11.2.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yalamanchili P, Lu C, Oxvig C, Springer T A. J Biol Chem. 2000;275:21877–21882. doi: 10.1074/jbc.M908868199. [DOI] [PubMed] [Google Scholar]
- 17.Randi A M, Hogg N. J Biol Chem. 1994;269:12395–12398. [PubMed] [Google Scholar]
- 18.Knorr R, Dustin M L. J Exp Med. 1997;186:719–730. doi: 10.1084/jem.186.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman T G, Bajt M L. J Biol Chem. 1996;271:23729–23736. doi: 10.1074/jbc.271.39.23729. [DOI] [PubMed] [Google Scholar]
- 20.Oxvig C, Lu C, Springer T A. Proc Natl Acad Sci USA. 1999;96:2215–2220. doi: 10.1073/pnas.96.5.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDowall A, Leitinger B, Stanley P, Bates P A, Randi A M, Hogg N. J Biol Chem. 1998;273:27396–27403. doi: 10.1074/jbc.273.42.27396. [DOI] [PubMed] [Google Scholar]
- 22.Shimaoka M, Shifman J M, Jing H, Takagi J, Mayo S L, Springer T A. Nat Struct Biol. 2000;7:674–678. doi: 10.1038/77978. [DOI] [PubMed] [Google Scholar]
- 23.Xiong J-P, Li R, Essafi M, Stehle T, Arnaout M A. J Biol Chem. 2000;275:38762–38767. doi: 10.1074/jbc.C000563200. [DOI] [PubMed] [Google Scholar]
- 24.Stephens P, Romer J T, Spitali M, Shock A, Ortlepp S, Figdor C, Robinson M K. Cell Adhes Commun. 1995;3:375–384. doi: 10.3109/15419069509081292. [DOI] [PubMed] [Google Scholar]
- 25.Lu C, Shimaoka M, Ferzly M, Oxvig C, Takagi J, Springer T A. Proc Natl Acad Sci USA. 2001;98:2387–2392. doi: 10.1073/pnas.041606398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee J-O, Bankston L A, Arnaout M A, Liddington R C. Structure (London) 1995;3:1333–1340. doi: 10.1016/s0969-2126(01)00271-4. [DOI] [PubMed] [Google Scholar]
- 27.Emsley J, Knight C G, Farndale R W, Barnes M J, Liddington R C. Cell. 2000;101:47–56. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- 28.Huang C, Springer T A. Proc Natl Acad Sci USA. 1997;94:3162–3167. doi: 10.1073/pnas.94.7.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zang Q, Lu C, Huang C, Takagi J, Springer T A. J Biol Chem. 2000;275:22202–22212. doi: 10.1074/jbc.M002883200. [DOI] [PubMed] [Google Scholar]
- 30.Dransfield I, Hogg N. EMBO J. 1989;8:3759–3765. doi: 10.1002/j.1460-2075.1989.tb08552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson M K, Andrew D, Rosen H, Brown D, Ortlepp S, Stephens P, Butcher E C. J Immunol. 1992;148:1080–1085. [PubMed] [Google Scholar]
- 32.Lu C, Springer T A. J Immunol. 1997;159:268–278. [PubMed] [Google Scholar]
- 33.Dransfield I, Cabañas C, Craig A, Hogg N. J Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu, C., Ferzly, M., Takagi, J. & Springer, T. A. (2001) J. Immunol., in press. [DOI] [PubMed]
- 35.Huang C, Lu C, Springer T A. Proc Natl Acad Sci USA. 1997;94:3156–3161. doi: 10.1073/pnas.94.7.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]