Abstract
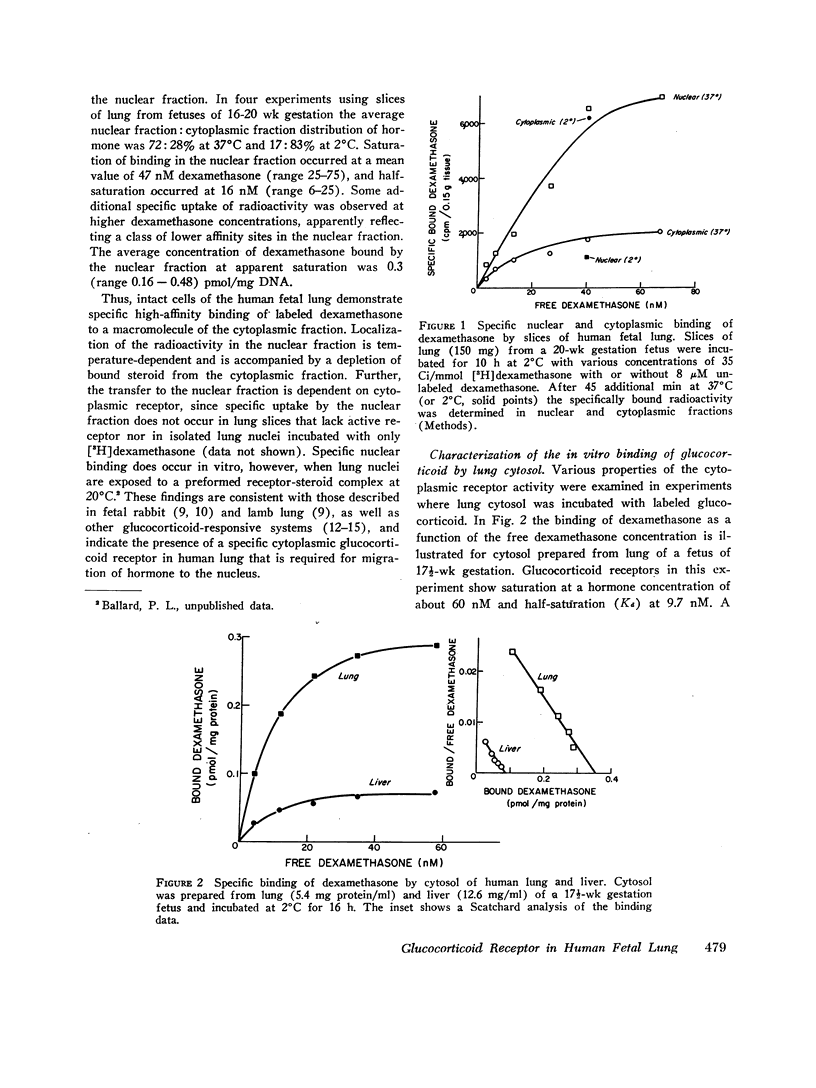
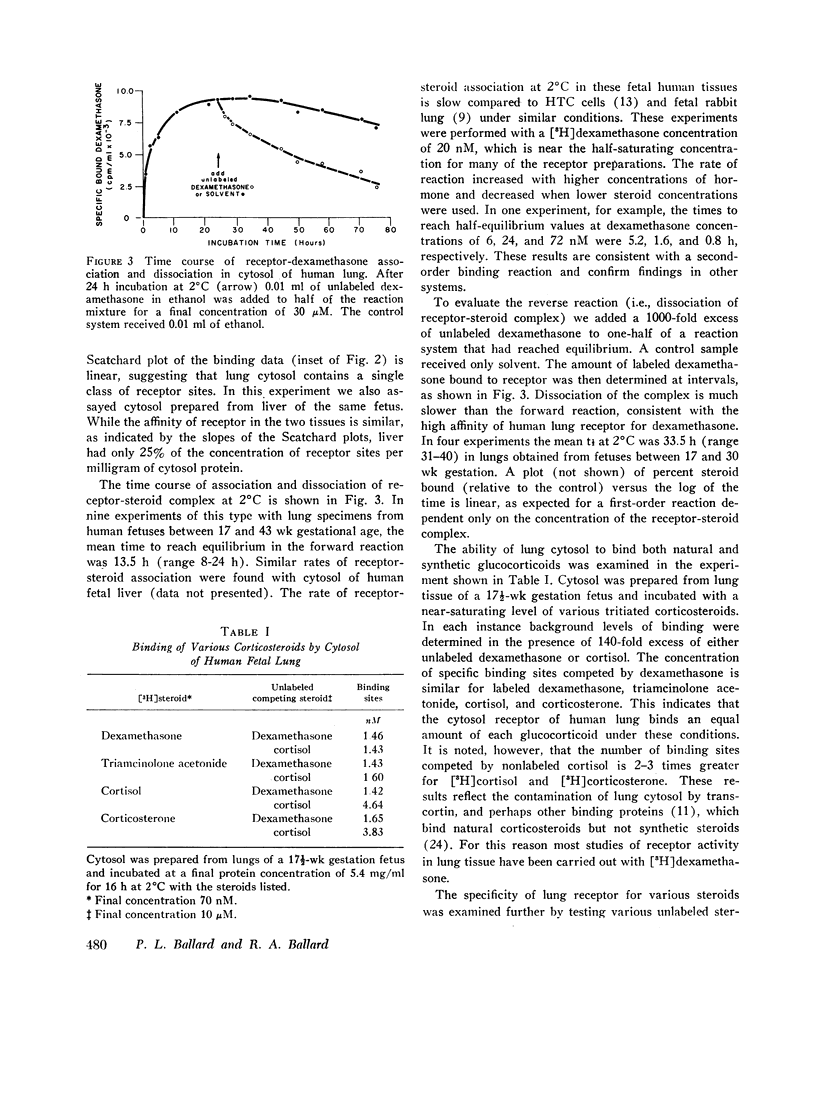
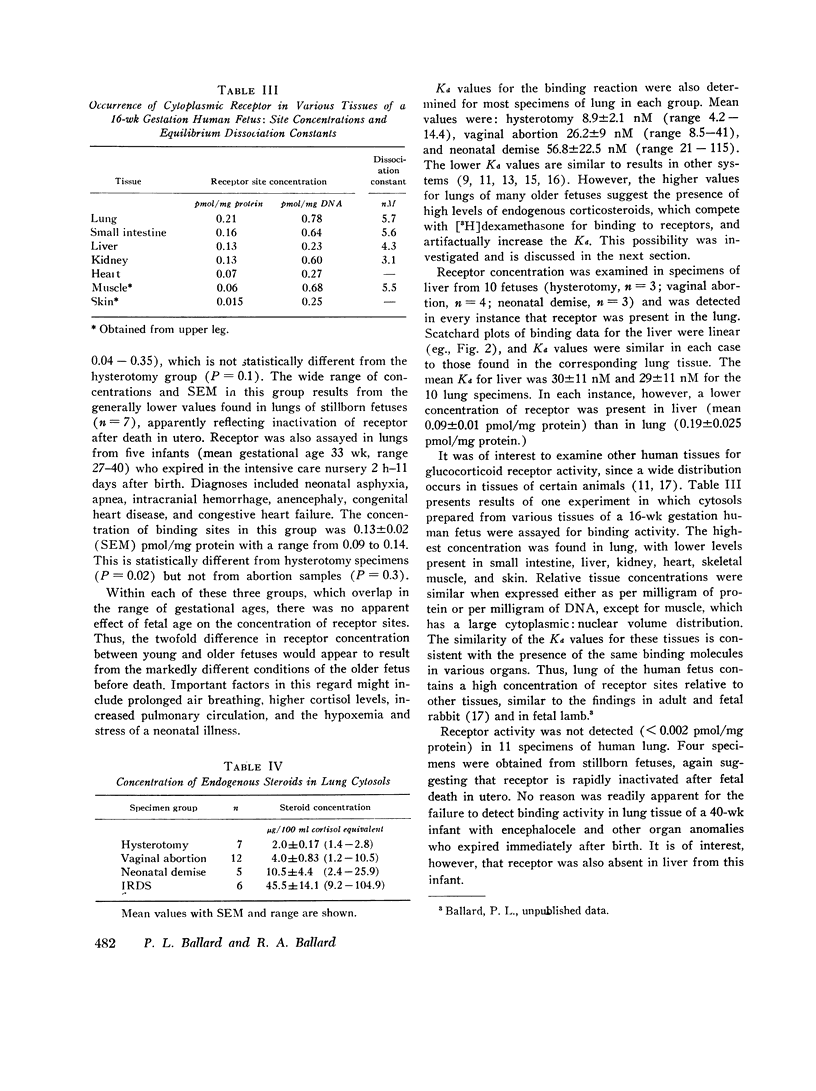
In fetal animals, glucocorticoids accelerate development of the lung and cause precocious appearance of alveolar surfactant. To determine if the human lung also can respond to corticosteroids, we examined lungs of the human fetus and neonate for both cytoplasmic binding and nuclear uptake of glucocorticoids. In slices of fetal lung incubated with [3H]dexamethasone at 2 degrees C, specific macromolecular binding occurs primarily in the "cytoplasmic" fraction. After further incubation at 37 degrees C. nearly 75% of the radioactivity localizes in the "nuclear" fraction with a concentration of 0.3 pmol/mg DNA at apparent dexamethasone saturation (47 nM). The cytoplasmic receptor binds dexamethasone in vitro with high affinity (dissociation constant = 8.9 nM), and the affinity of various other steroids correlates with their glucocorticoid potency. Receptor was present in lungs of fetuses and neonates of gestational age 12-43 wk, with a mean concentration in hysterotomy specimens of 0.24 pmol sites/mg cytosol protein. Similar binding activity was present at lower concentration in fetal liver, gut, kidney, heart, muscle, and skin. Cytoplasmic receptor was not detected in lung and liver of premature infants with respiratory distress syndrome. This deficit appears to result from increased levels of endogenous steroids (mean cortisol 45.5 micrograms/100 ml cytosol) as well as inactivation of receptor secondary to the illness. Thus, the lung of the human fetus and neonate contains the receptor mechanism necessary for direct responsiveness to glucocorticoids. These findings support the potential usefulness of these hormones in prevention of respiratory distress syndrome in the premature infant.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVERY M. E., MEAD J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959 May;97(5 Pt 1):517–523. doi: 10.1001/archpedi.1959.02070010519001. [DOI] [PubMed] [Google Scholar]
- Baden M., Bauer C. R., Colle E., Klein G., Taeusch H. W., Jr, Stern L. A controlled trial of hydrocortisone therapy in infants with respiratory distress syndrome. Pediatrics. 1972 Oct;50(4):526–534. [PubMed] [Google Scholar]
- Ballard P. L., Ballard R. A. Glucocorticoid receptors and the role of glucocorticoids in fetal lung development. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2668–2672. doi: 10.1073/pnas.69.9.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. D., Rousseau G. G., Benson M. C., Garcea R. L., Ito J., Tomkins G. M. Role of DNA and specific cytoplasmic receptors in glucocorticoid action. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1892–1896. doi: 10.1073/pnas.69.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. D., Tomkins G. M. Specific cytoplasmic glucocorticoid hormone receptors in hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1971 May;68(5):932–937. doi: 10.1073/pnas.68.5.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M., Feigelson P. Glucocorticoid-binding proteins of rat liver cytosol. I. Separation and identification of the binding proteins. J Biol Chem. 1972 Dec 25;247(24):7890–7896. [PubMed] [Google Scholar]
- Clements J. A., Platzker A. C., Tierney D. F., Hobel C. J., Creasy R. K., Margolis A. J., Thibeault D. W., Tooley W. H., Oh W. Assessment of the risk of the respiratory-distress syndrome by a rapid test for surfactant in amniotic fluid. N Engl J Med. 1972 May 18;286(20):1077–1081. doi: 10.1056/NEJM197205182862004. [DOI] [PubMed] [Google Scholar]
- DeLemos R. A., Shermeta D. W., Knelson J. H., Kotas R., Avery M. E. Acceleration of appearance of pulmonary surfactant in the fetal lamb by administration of corticosteroids. Am Rev Respir Dis. 1970 Sep;102(3):459–461. doi: 10.1164/arrd.1970.102.3.459. [DOI] [PubMed] [Google Scholar]
- Farrell P. M., Zachman R. D. Induction of choline phosphotransferase and lecithin synthesis in the fetal lung by corticosteroids. Science. 1973 Jan 19;179(4070):297–298. doi: 10.1126/science.179.4070.297. [DOI] [PubMed] [Google Scholar]
- Giannopoulos G., Mulay S., Solomon S. Cortisol receptors in rabbit fetal lung. Biochem Biophys Res Commun. 1972 Apr 28;47(2):411–418. doi: 10.1016/0006-291x(72)90729-2. [DOI] [PubMed] [Google Scholar]
- Gluck L., Kulovich M. V. Lecithin-sphingomyelin ratios in amniotic fluid in normal and abnormal pregnancy. Am J Obstet Gynecol. 1973 Feb 15;115(4):539–546. doi: 10.1016/0002-9378(73)90404-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liggins G. C., Howie R. N. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972 Oct;50(4):515–525. [PubMed] [Google Scholar]
- Lippman M. E., Halterman R. H., Leventhal B. G., Perry S., Thompson E. B. Glucocorticoid-binding proteins in human acute lymphoblastic leukemic blast cells. J Clin Invest. 1973 Jul;52(7):1715–1725. doi: 10.1172/JCI107353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama E. K., Orzalesi M. M., Kikkawa Y., Kaibara M., Wu B., Zigas C. J., Cook C. D. Effect of cortisol on the maturation of fetal rabbit lungs. Pediatrics. 1971 Oct;48(4):547–555. [PubMed] [Google Scholar]
- Munck A., Wira C., Young D. A., Mosher K. M., Hallahan C., Bell P. A. Glucocorticoid-receptor complexes and the earliest steps in the action of glucocorticoids on thymus cells. J Steroid Biochem. 1972 Apr;3(3):567–578. doi: 10.1016/0022-4731(72)90103-3. [DOI] [PubMed] [Google Scholar]
- Murphy B. E. Some studies of the protein-binding of steroids and their application to the routine micro and ultramicro measurement of various steroids in body fluids by competitive protein-binding radioassay. J Clin Endocrinol Metab. 1967 Jul;27(7):973–990. doi: 10.1210/jcem-27-7-973. [DOI] [PubMed] [Google Scholar]
- Piddington R., Moscona A. A. Precocious induction of retinal glutamine synthetase by hydrocortisone in the embryo and in culture. Age-dependent differences in tissue response. Biochim Biophys Acta. 1967 Jul 25;141(2):429–432. doi: 10.1016/0304-4165(67)90120-1. [DOI] [PubMed] [Google Scholar]
- Pratt W. B., Ishii D. N. Specific binding of glucocorticoids in vitro in the soluble fraction of mouse fibroblasts. Biochemistry. 1972 Apr 11;11(8):1401–1410. doi: 10.1021/bi00758a012. [DOI] [PubMed] [Google Scholar]
- Reynolds E. O. Hyaline membrane disease. Am J Obstet Gynecol. 1970 Mar;106(5):780–797. doi: 10.1016/0002-9378(70)90406-0. [DOI] [PubMed] [Google Scholar]
- Rosenau W., Baxter J. D., Rousseau G. G., Tomkins G. M. Mechanism of resistance to steroids: glucocorticoid receptor defect in lymphoma cells. Nat New Biol. 1972 May 3;237(70):20–24. doi: 10.1038/newbio237020a0. [DOI] [PubMed] [Google Scholar]
- Yalovsky U., Zelikson R., Kulka R. G. The effect of hydrocortisone on the accumulation of amylase in embryonic chick pancreas. FEBS Lett. 1969 Mar;2(5):323–326. doi: 10.1016/0014-5793(69)80054-2. [DOI] [PubMed] [Google Scholar]



