Abstract
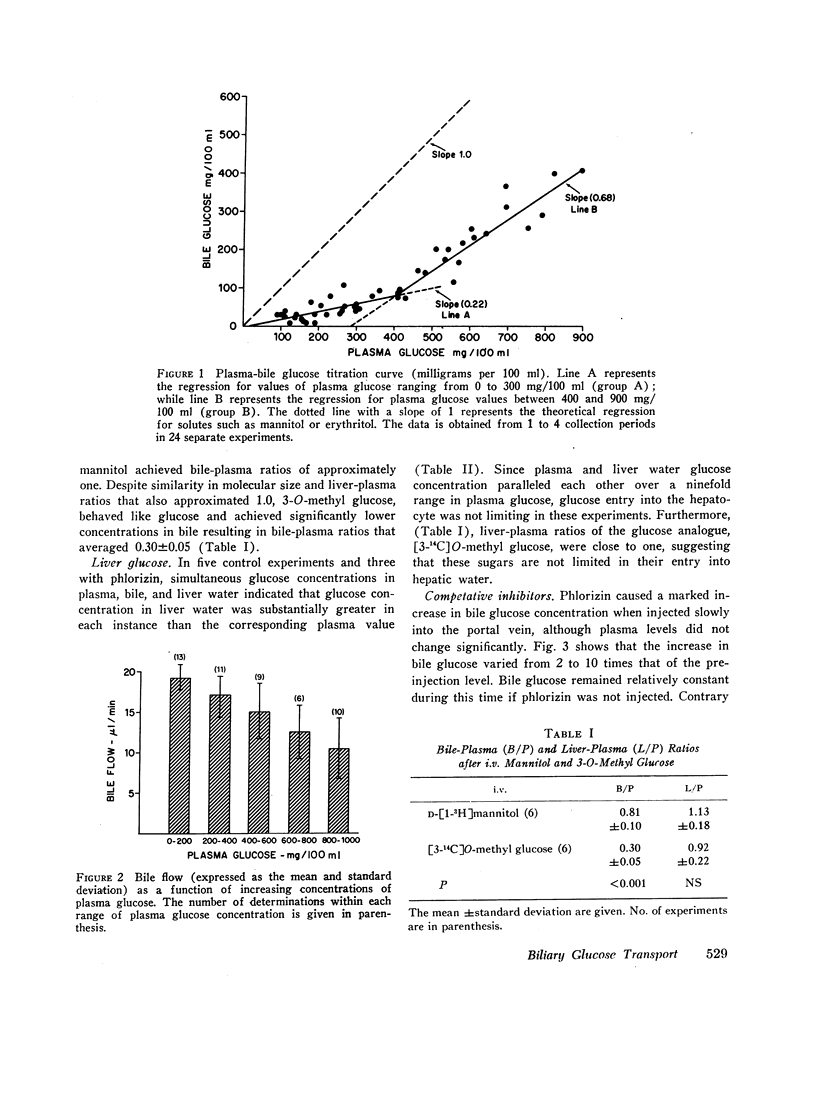
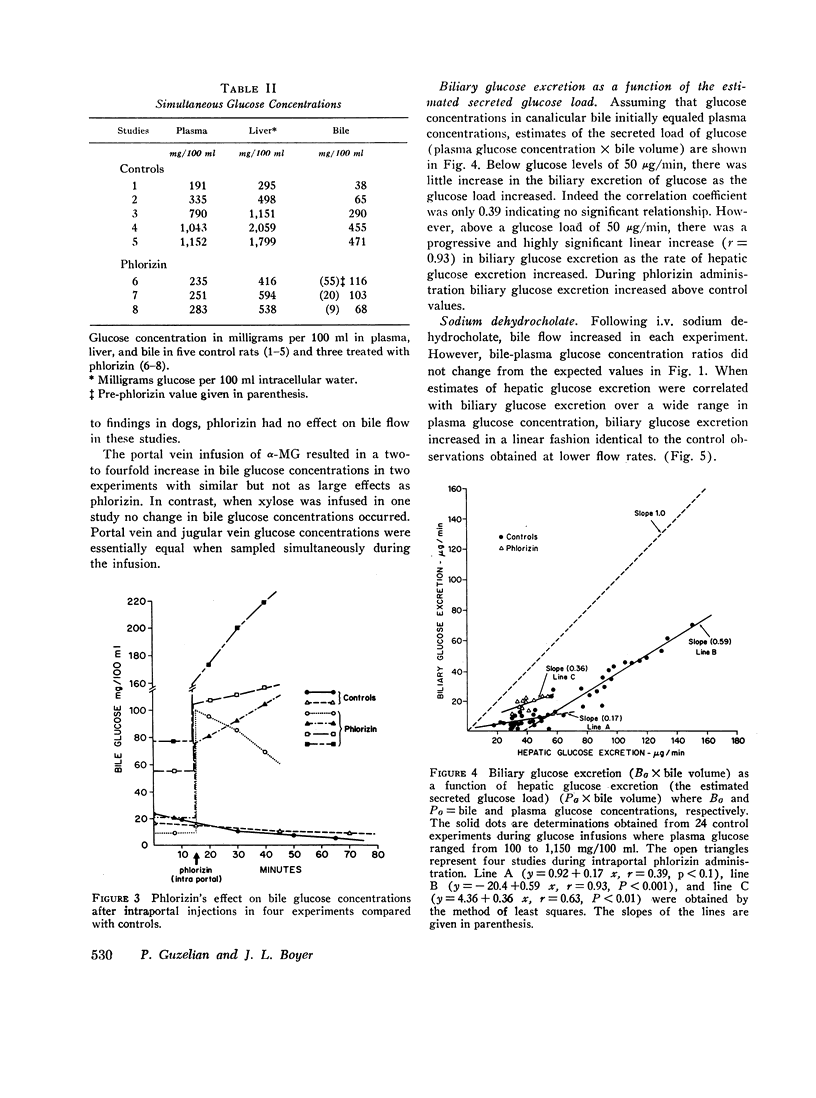
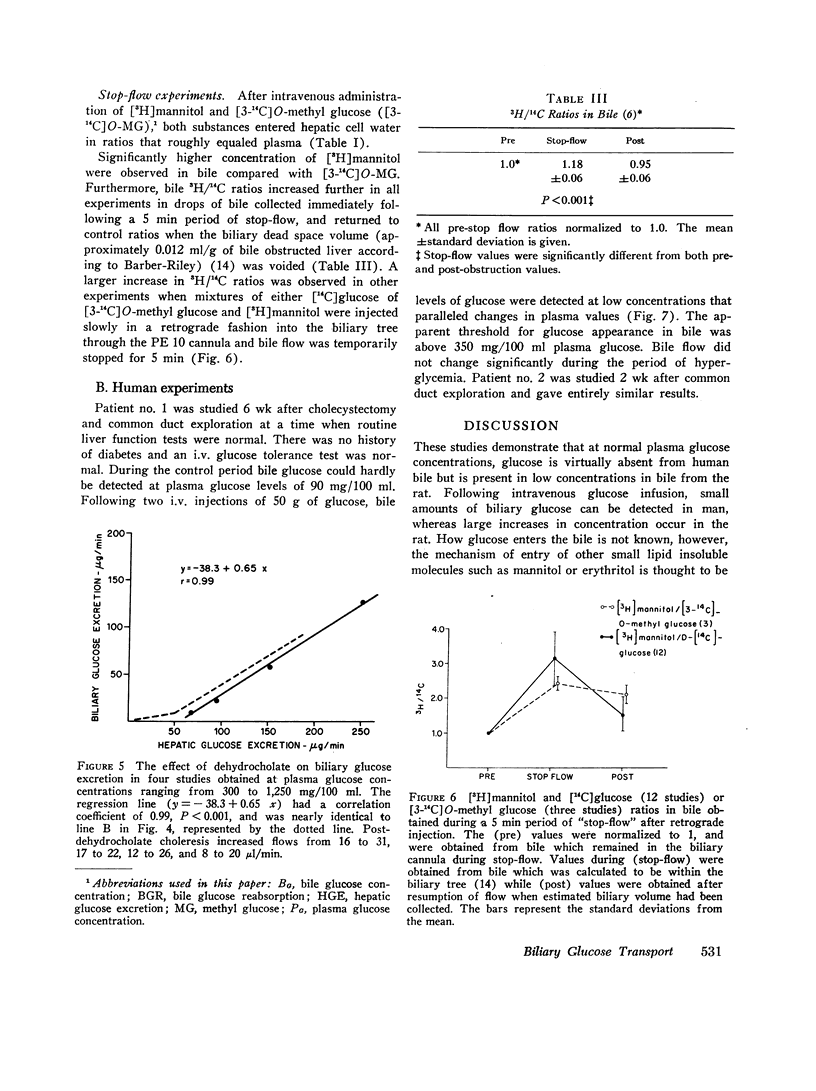
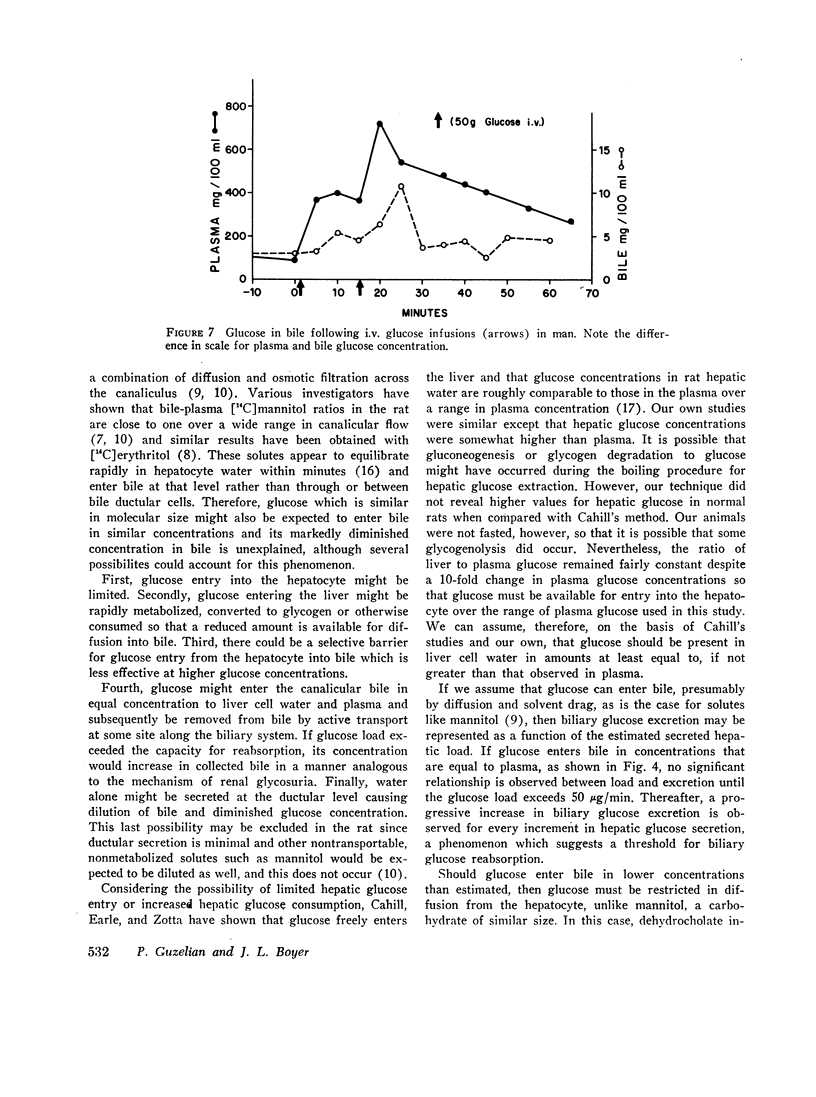
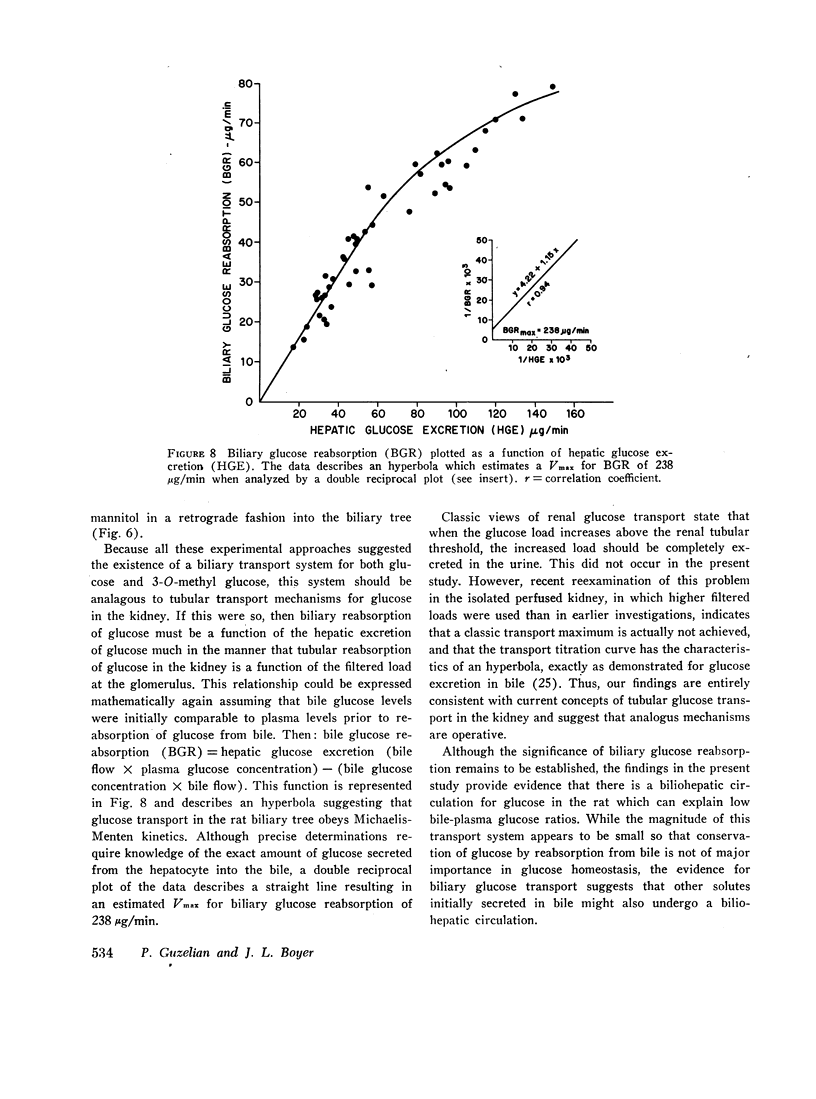
Glucose is absent from human bile and present in low concentrations in bile from the rat. To study the mechanisms of this blood-bile glucose concentration difference, infusions of glucose were administered i.v. to 300-400 g male Sprague-Dawley rats with ligated renal pedicles and to two postcholecystectomy patients with indwelling t-tubes. Glucose was assayed in plasma, bile, and rat liver by a hexokinase method specific for D-glucose. In man, glucose was detected in bile when plasma glucose increased above 350 mg/100 ml. In animals studies, low concentrations of bile glucose were observed at plasma levels between 100 and 300 mg/100 ml. However, when plasma concentrations increased between 400 and 900 mg/100 ml, glucose appeared more rapidly in bile, defining by extrapolation an apparent plasma glucose threshold of 280 mg/100 ml. Intraportal phlorizin, a competitive inhibitor of glucose transport, significantly increased bile glucose concentrations. Plasma-bile concentration differences were also observed in rats after i.v. [3-14C]O-methyl glucose (3-O-MG) but not after [3H]mannitol. Hepatic glucose levels were never lower than plasma levels and liver-plasma 3-O-MG ratios were 0.92 +/- 0.22 indicating that entry of glucose and 3-O-MG into hepatocyte water was not limiting. Furthermore, when sodium dehydrocholate augmented canalicular secretion, biliary glucose excretion increased proportionally suggesting that glucose entry into bile was not impeded. When estimates of hepatic glucose secretion were compared with biliary glucose excretion, the latter increased progressively when estimated secretion rates exceeded 50 micrograms/min or when phlorizin was given. Finally, during bile stop-flow experiments, [3-14C]O-MG and [14C]glucose were selectively removed from bile compared with [3H]mannitol. The findings suggest that glucose and 3-O-MG are reabsorbed from bile after entry at the hepatocyte, accounting for their low bile-plasma ratio. The biliary glucose transport process may be described by Michaelis-Menten kinetics and is analogous to recently defined kinetics for renal tubular reabsorption of glucose. These studies provide evidence that certain products of bile secretion may undergo a "biliohepatic" circulation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARBER-RILEY G. MEASUREMENT OF CAPACITY OF BILIARY TREE IN RATS. Am J Physiol. 1963 Dec;205:1122–1126. doi: 10.1152/ajplegacy.1963.205.6.1122. [DOI] [PubMed] [Google Scholar]
- BRAUER R. W., LEONG G. F., HOLLOWAY R. J. Mechanics of bile secretion; effect of perfusion pressure and temperature on bile flow and bile secretion pressure. Am J Physiol. 1954 Apr;177(1):103–112. doi: 10.1152/ajplegacy.1954.177.1.103. [DOI] [PubMed] [Google Scholar]
- Bowman R. H., Maack T. Glucose transport by the isolated perfused rat kidney. Am J Physiol. 1972 Jun;222(6):1499–1504. doi: 10.1152/ajplegacy.1972.222.6.1499. [DOI] [PubMed] [Google Scholar]
- Boyer J. L. Canalicular bile formation in the isolated perfused rat liver. Am J Physiol. 1971 Oct;221(4):1156–1163. doi: 10.1152/ajplegacy.1971.221.4.1156. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Klatskin G. Canalicular bile flow and bile secretory pressure. Evidence for a non-bile salt dependent fraction in the isolated perfused rat liver. Gastroenterology. 1970 Dec;59(6):853–859. [PubMed] [Google Scholar]
- CAHILL G. F., Jr, ASHMORE J., EARLE A. S., ZOTTU S. Glucose penetration into liver. Am J Physiol. 1958 Mar;192(3):491–496. doi: 10.1152/ajplegacy.1958.192.3.491. [DOI] [PubMed] [Google Scholar]
- CRANE R. K. Intestinal absorption of sugars. Physiol Rev. 1960 Oct;40:789–825. doi: 10.1152/physrev.1960.40.4.789. [DOI] [PubMed] [Google Scholar]
- Chenderovitch J., Phocas E., Rautureau M. Effects of hypertonic solutions on bile formation. Am J Physiol. 1963 Nov;205(5):863–867. doi: 10.1152/ajplegacy.1963.205.5.863. [DOI] [PubMed] [Google Scholar]
- Chenderovitch J. Stop-flow analysis of bile secretion. Am J Physiol. 1968 Jan;214(1):86–93. doi: 10.1152/ajplegacy.1968.214.1.86. [DOI] [PubMed] [Google Scholar]
- DEWEY W. C. Vascular-extravascular exchange of II31 plasma proteins in the rat. Am J Physiol. 1959 Aug;197:423–431. doi: 10.1152/ajplegacy.1959.197.2.423. [DOI] [PubMed] [Google Scholar]
- Forker E. L. Hepatocellular uptake of inulin, sucrose, and mannitol in rats. Am J Physiol. 1970 Dec;219(6):1568–1573. doi: 10.1152/ajplegacy.1970.219.6.1568. [DOI] [PubMed] [Google Scholar]
- Forker E. L., Hicklin T., Sornson H. The clearance of mannitol and erythritol in rat bile. Proc Soc Exp Biol Med. 1967 Oct;126(1):115–119. doi: 10.3181/00379727-126-32380. [DOI] [PubMed] [Google Scholar]
- Forker E. L. Two sites of bile formation as determined by mannitol and erythritol clearance in the guinea pig. J Clin Invest. 1967 Jul;46(7):1189–1195. doi: 10.1172/JCI105612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENNER F. A., SMYTH D. H. The excretion of phlorrhizin. J Physiol. 1959 Jun 11;146(3):563–571. doi: 10.1113/jphysiol.1959.sp006211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHANKER L. S., HOGBEN C. A. Biliary excretion of inulin, sucrose, and mannitol: analysis of bile formation. Am J Physiol. 1961 May;200:1087–1090. doi: 10.1152/ajplegacy.1961.200.5.1087. [DOI] [PubMed] [Google Scholar]
- SEGATO G., ROSA G. RICERCHE SULLA BILE DRENATA ALL'ESTERNO MEDIANTE FISTOLA BILIARE. VARIAZIONI DELLA GLICOCOLIA E DELLA PROTEINOCOLIA. III. Acta Chir Ital. 1964 Jan-Feb;20:85–90. [PubMed] [Google Scholar]
- Sacktor B., Wormser-Shavit E. Regulation of metabolism in working muscle in vivo. I. Concentrations of some glycolytic, tricarboxylic acid cycle, and amino acid intermediates in insect flight muscle during flight. J Biol Chem. 1966 Feb 10;241(3):624–631. [PubMed] [Google Scholar]
- Schein C. J., Zumoff B., Kream J., Cassouto J., Hellman L. A blood-bile glucose barrier in man. Gastroenterology. 1968 Jun;54(6):1094–1097. [PubMed] [Google Scholar]
- WILSON T. H., LANDAU B. R. Specificity of sugar transport by the intestine of the hamster. Am J Physiol. 1960 Jan;198:99–102. doi: 10.1152/ajplegacy.1960.198.1.99. [DOI] [PubMed] [Google Scholar]


