Abstract
Objective
To investigate the mechanism for lupus acceleration by interferon alpha (IFNα) in NZB/W mice.
Methods
NZB/W mice were treated with an adenovirus expressing IFNα. T cells were depleted in some mice with an anti-CD4 antibody. The production of anti-dsDNA antibodies was measured by ELISA and ELISpot assays. Germinal centers and antibody-secreting cells (ASCs) in spleens and IgG deposition and leukocyte infiltrates in kidneys were visualized by immunofluorescence staining. The phenotype of splenic cells was determined by flow cytometry. Finally, somatic hypermutation and gene usage in heavy chain variable regions of IgG2a and IgG3 were studied by single cell PCR.
Results
IFNα accelerated lupus in NZB/W mice is associated with elevated serum levels of IgG2 and IgG3 anti-dsDNA antibodies, and accumulation of many IgG ASCs in the spleen, which do not develop into long-lived plasma cells. Furthermore, IgG2a and IgG3 antibodies in these mice are highly somatically mutated and use distinct repertoires of VH genes. The induction of SLE in these mice is associated with an increase in B cell TLR7 expression, increased serum levels of BAFF, IL-6 and TNFα, and induction of T cells expressing IL-21. Although IFNα drives a T-independent increase in serum levels of IgG, autoantibody induction and the development of nephritis are both completely dependent on CD4 T cell help.
Conclusion
Our study shows that although IFNα activates both innate and adaptive immune responses in NZB/W mice, CD4 T cells are necessary for IFNα driven induction of anti-dsDNA antibodies and clinical SLE.
Keywords: SLE, B cells, Cytokines, Rodent, Interferon alpha
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the production of pathogenic autoantibodies specific for nuclear components. Immune complexes containing nucleic acids are endocytosed by B cells and dendritic cells (DCs) that express intracellular Toll like receptors (TLRs) specific for nucleic acids (1). TLR ligation on B cells enhances proliferation and production of autoantibodies and cytokines (2). TLR ligation on plasmacytoid dendritic cells (pDCs) induces them to secrete interferon alpha (IFNα) (3). IFNα induces maturation of myeloid DCs that activate naïve CD4+ T cells to provide help for B cells (4). Activated myeloid DCs also produce B cell-activating factor of the TNF Family (BAFF), a cytokine that enhances selection and survival of autoreactive B cells (5) and promotes isotype switching, giving rise to more immune complexes (6). BAFF transgenic mice develop SLE independently of T cells suggesting that T cells are dispensable for disease initiation if TLR-activating immune complexes are present.
In humans, IFNα can induce autoantibodies and clinical lupus (7). Furthermore, peripheral blood mononuclear cells (PBMCs) from active lupus patients have upregulated expression of a group of Type I IFN induced genes (8–10). IFNα is therefore deemed an important cytokine in SLE pathogenesis. In young lupus prone NZB/W mice, but not in BALB/c mice, administration of adenovirus expressing IFNα rapidly induces anti-dsDNA antibodies, proteinuria and glomerulonephritis (11). Since some of the immunologic effects of IFNα are mediated independently of T cells, we wished to determine whether IFNα could bypass the need for T cells in the induction of SLE. Our data show that although IFNα induces T-independent class-switching and increases circulating IL-6 and BAFF, the generation of pathogenic autoantibodies still requires CD4 T cells.
MATERIALS AND METHODS
IFNα adenovirus treatment of NZB/W mice
12 week NZB/W females (Jackson Laboratory Bar Harbor, ME) were treated with a single i.v. injection of 3.3 × 108 particles of IFNα adenovirus (Ad-IFNα, Qbiogene Morgan Irvine, CA) that reproducibly induced proteinuria within 22–30 days. Controls received the same dose of β-galactosidase-expressing adenovirus (Ad-LacZ) or no treatment. Mice were bled and urine was tested weekly for proteinuria by dipstick (Multistick; Fisher Scientific, Pittsburg, PA). Groups of five Ad-IFNα treated mice were sacrificed at 13, 14, 15, 16, 17, 19, and 23w of age, and controls were sacrificed at 12 and 20w of age. Groups of 5 mice received 1 mg of anti-CD4 (GK1.5) antibody (BioXCell, West Lebanon, NH) intraperitoneally weekly for 7 weeks starting the day of adenovirus injection. All experiments using animals were carried out according to protocols approved by the Institutional Animal Care and Use Committees of Columbia University and the Feinstein Institute.
Serum immunoglobulin and anti-dsDNA antibody levels
Serum immunoglobulin levels were measured as previously described (12). Standard curves were established using serial dilutions of murine IgM, IgG1, IgG2a, IgG2b, or IgG3 (Sigma-Aldrich, St. Louis, MO).
Serum levels of anti-dsDNA antibodies were measured as previously described (12). ELISA data was normalized to a high-titer serum assigned an arbitrary level of 512 U and run in serial dilution on each plate.
Serum cytokine levels
Serum levels of IL-6, IL-17, IL-21, BAFF, IFNγ and TNFα were measured using a commercial multiplex assay (Assaygate, Inc. Ijamsville, MD). BAFF levels were also measured using an ELISA kit specific for murine BAFF (Axxora, LLC, San Diego, CA).
ELISpot assay
ELISpot assays for total ASCs and for anti-dsDNA ASCs were performed as previously described (12).
FACS analysis of spleen and peripheral blood cells
Spleen and PBMCs were analyzed for cell surface markers as previously described (13). Follicular helper T (TFH) cells were gated as previously described (14).
Immunohistochemistry and Immunofluorescence
H&E sections were scored for renal damage as previously described (15). Cryosections (5 µm) of kidney and spleen were stained (13) using: FITC-conjugated anti-mouse IgG2a, IgG3 (Southern Biotech, Birmingham, Alabama) or peanut agglutinin (PNA – Vector Laboratories, Inc. Burlingame, CA), PE-conjugated anti-mouse IgD, CD4, B220, CD11c (BD Pharmingen, San Diego, CA) or F4/80 (Invitrogen, Carlsbad, California). Images were captured using a Zeiss Axiocam digital camera connected to a Zeiss Axioplan2 microscope. Glomerular Ig deposition was scored on a 1–4 scale by an observer blinded to the treatment groups.
BrdU feeding and detection
7 or 21 days after Ad-IFNα injection, mice were loaded i.p. with 10mg of bromodeoxyuridine (BrdU, Sigma-Aldrich, St. Louis, MA) followed by feeding with water containing 1 mg/ml BrdU for 10 days. Groups of five mice were sacrificed upon completing BrdU feeding or two weeks after stopping BrdU. Spleen cells were stained with anti-B220-APC, anti-CD138-PE (Southern Biotechnology Association, Birmingham, AL) or for intracellular IgG2a and then with anti-BrdU (BD Pharmingen, San Diego, CA) according to manufacturer’s instructions.
Single cell PCR of GC B cells or plasma cells (PCs)
cDNA was synthesized from single GC B cells (CD19+IgM−IgD−PNA+) and PCs (IgD−B220intCD138hi) from spleens of 17 week old IFNα treated NZB/W mice using SUPERSCRIPT (Invitrogen). PCR (40 cycles of 30 sec at 95°C, 30 sec at 58°C, and 1 min at 72°C) was performed in 10 uL reactions containing 5 uL of FastStart PCR mastermix (Roche, Indianapolis, IN), 0.5 uM of primers (IgG2a: 5’- AAC TAC AAG AAC ACT GAA CCA GTC C and AAC TGG GTG GAA AGA AAT AGC TAC T - 3’. IgG3: 5’- GGA CAA CAA AGA AGT ACA CAC AGC and AAC TTC TTC TCT GAA GCC ATC AGT - 3’) and 1 uL of cDNA. Cells producing a single band were then used to perform PCR of the variable region of heavy chain as previously described (16). PCR products were sequenced (Genewiz, Southern Plainfield, NJ) and VH regions were compared with the germ line sequences using Ig Blast (http://www.ncbi.nlm.nih.gov/igblast/). Polymerase error rate of < 0.2% was calculated from 40 independent sequences (10,000bp) of a germline encoded heavy chain obtained from single cell sorted marginal zone and follicular B cells from a transgenic NZB/W mouse.
Real-time PCR of sorted splenic B cells and total spleen and bone marrow cells
Real-time PCR was performed in triplicates as previously described (17). Additional primers are: TACI (5’-GAG CTC GGG AGA CCA CAG, 3’-TGG TCG CTA CTT AGC CTC AAT), TLR7 (5’-TGA TCC TGG CCT ATC TCT GAC, 3’-CGT GTC CAC ATC GAA AAC AC), TLR9 (5’-GAA TCC TCC ATC TCC CAA CA, 3’-CCA GAG TCT CAG CCA GCA CT), CXCL12 (5’ - GAG CCA ACG TCA AGC ATC TG, 3’ -TCT TCA GCC GTG CAA CAA TC), VLA4 (5’ -CAA ACC AGA CCT GCG AAC A, 3’ - TGT CTT CCC ACA AGG CTC TC). The average of the raw data for each sample (Ct value) was normalized to the internal control (housekeeping gene β-actin). Normalized expression data was log2-transformed and scaled to the expression value for a single naïve mouse given an arbitrary value of 1 (0 by log scale). For display on the graphs the mean value for the naïve controls was given an arbitrary value of 1.
Statistics
Survival data in Figure 1 was analyzed using Kaplan-Meier curves and log-rank test. Comparisons in the other figures and tables were performed using Mann-Whitney test. p values ≤ 0.05 were considered significant.
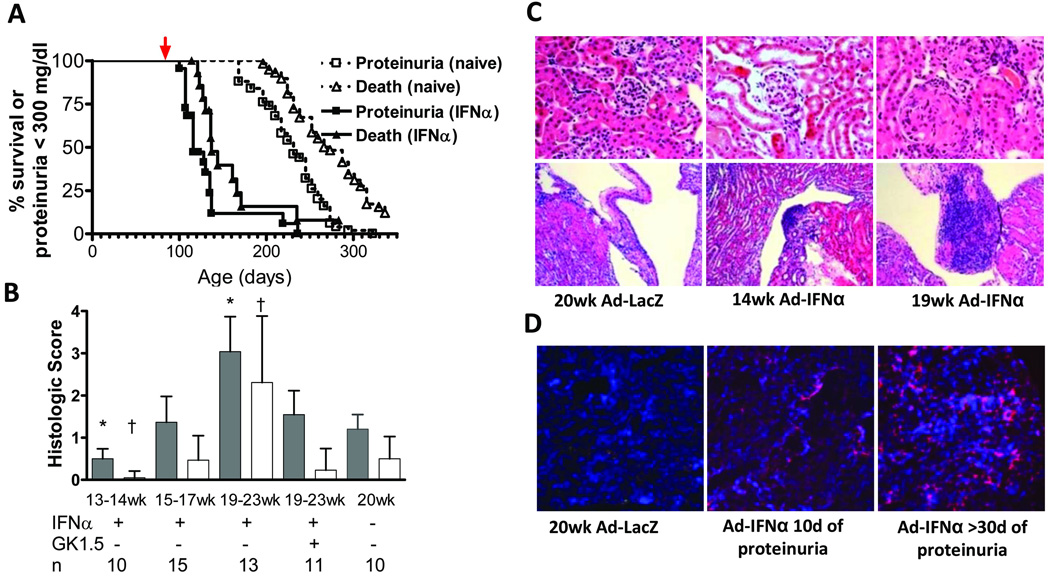
Figure 1.
Ad-IFNα treatment induces glomerulonephritis. A: survival (∆) and proteinuria (□) of Ad-IFNα treated (closed symbols) and control (open symbols) NZB/W mice. Red arrow indicates day of Ad-IFNα treatment. p < 0.0001, proteinuria onset and death. Data are representative of 2 experiments with 10–15 mice per group. B: renal glomerular damage (filled bars) and interstitial inflammation (open bars) in Ad-IFNα treated, Ad-IFNα/anti-CD4 treated and control naïve NZB/W mice. Two mice in the 19–23w group that died before tissue could be harvested were assigned a histological score of 4 for statistical analysis. p values are compared with 20 week old naïve controls. Mean + 1SD shown †: p < 0.05; *: p < 0.001. C: H&E staining of renal cortex (upper, 40× magnification) and pelvis (lower, 10× magnification). D: renal immunofluorescence staining with anti-F4/80 (red) and 4'-6-Diamidino-2-phenylindole (blue) (5× magnification). C–D: Data are representative of 4–5 mice per group.
RESULTS
Ad-IFNα treatment induces glomerulonephritis in NZB/W mice
Ad-IFNα treated NZB/W mice became proteinuric within 3–4 weeks, followed by rapid death (Figure 1A). Lymphocytic infiltrates appeared in the renal pelvis of Ad-IFNα treated mice at week 14 and had enlarged by week 19 (Figure 1C). Glomerular enlargement and damage with crescent formation (18, 19) occurred by week 19–23 (p=0.001) (Figure 1B and C). By immunofluorescence staining, interstitial infiltrates of F4/80hi mononuclear cells were visible after the onset of proteinuria and continued to increase until death (Figure 1D). In accordance with previous findings in this model (19), small infiltrates of CD4 T cells and B cells appeared in the perivascular areas only in the late stages of disease (data not shown). Serum levels of BAFF increased starting 2 weeks after Ad-IFNα treatment (8.1 ± 2.0 ng/mL vs. 17.8 ± 1.4 ng/mL, 12w naïve vs. 14w Ad-IFNα treated, p=0.0025).
Serum antibody titers in Ad-IFNα treated NZB/W mice
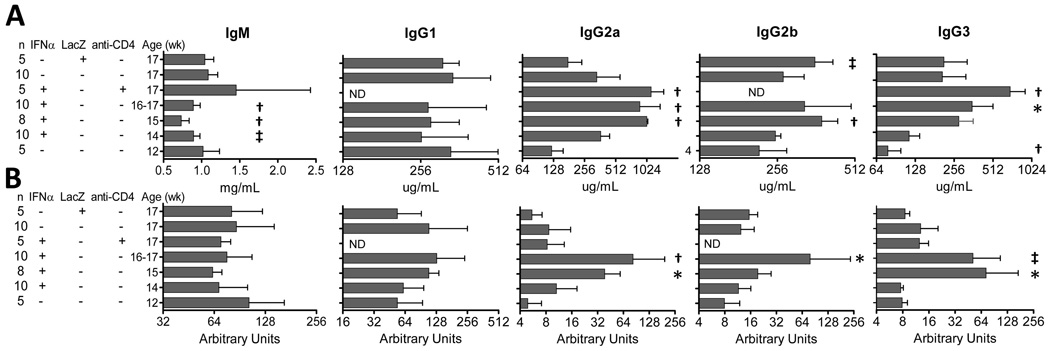
A previous study showed that Ad-IFNα treatment increases serum IgG levels in NZB/W mice (11). We found that this is due to an increase of IgG2 and IgG3, but not of IgG1. Serum levels of IgG2a, IgG2b and IgG3 were higher in Ad-IFNα treated mice than in naïve or Ad-LacZ treated controls (Figure 2A). Similarly, significant increases of serum IgG anti-dsDNA antibodies were detected in Ad-IFNα treated mice at week 15 and 17 (Figure 2B). In contrast, serum IgM levels decreased in Ad-IFNα treated mice compared to 17 week old controls (Figure 2A) and treatment did not affect circulating IgM anti-dsDNA antibodies (Figure 2B).
Figure 2.
Ad-IFNα treatment increases serum IgG2 and IgG3 levels. Serum Ig (A) and anti-dsDNA Ig (B) levels in Ad-IFNα treated, Ad-IFNα/anti-CD4 antibody treated, Ad-LacZ treated, and naïve NZB/W mice were quantitated by ELISA. p values are compared with 17-week-old naïve controls. Mean + 1SD shown *: p < 0.05, ‡: p < 0.02, †: p < 0.01. Data are representative of 3 experiments of 4 –10 mice per group. ND: not done
Formation of germinal centers and generation of ASCs in Ad-IFNα treated NZB/W mice
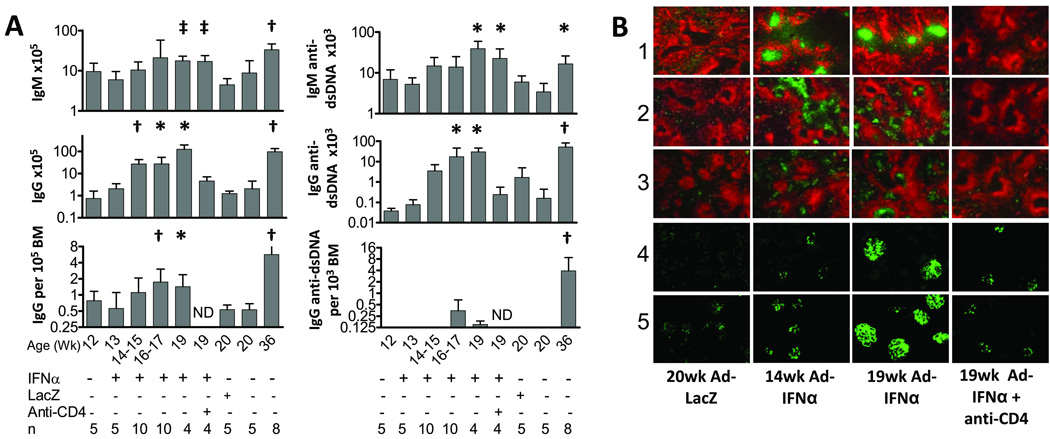
Germinal centers (GCs) appeared in the spleens two weeks after Ad-IFNα treatment and were sustained throughout the disease course (Figure 3B). Large numbers of IgG2a and IgG3 ASCs were found in extra-follicular areas and the splenic red pulp. Only a few small GCs and IgG ASCs were observed in spleens of 20 week old Ad-LacZ treated controls (Figure 3B). IgG2a and IgG3 deposits appeared in the glomeruli of treated mice at week 14 (Figure 3B). By week 19, heavy IgG deposition was found in the glomeruli of treated mice whereas minimal IgG deposits were found in the kidneys of the control mice (Figure 3B).
Figure 3.
Ad-IFNα induces germinal centers and accumulation of IgG plasma cells in the spleen. A: the number of IgG or IgM plasma cells per spleen and frequency of IgG plasma cells in bone marrows from different groups of mice were determined by ELISpot assay. p values for spleens are compared with 20 week LacZ controls and p values for BMs are compared with all controls (20 week naïve and Ad-LacZ treated controls). Mean + 1SD shown *: p < 0.05, ‡: p < 0.02, †: p < 0.01. Experiments were repeated twice and data are pooled from 2 representative experiments of 5–6 mice per group. B: immunofluorescence staining of spleens (rows 1–3, 5× magnification) and kidneys (rows 4–5, 10× magnification) with anti-IgD (red), peanut agglutinin (green; row 1), anti-IgG2a (green; rows 2 and 4) and anti-IgG3 (green; rows 3 and 5). Data are representative of two experiments of 5–6 mice per group.
A 13.1-fold increase in the number of splenic IgG ASCs was observed at week 14–15 in treated mice (p=0.0007), and increased over time (Figure 3A) compared with pretreatment mice. The number of splenic anti-dsDNA IgG ASCs increased 10.4- and 17.9- fold at weeks 16–17 and 19, respectively, in treated mice compared to 20 week old Ad-LacZ controls (p=0.036, week 16–17; p=0.029, week 19; Figure 3A). Ad-IFNα also induced a modest increase of anti-dsDNA IgM ASCs in the spleen (Figure 3A) that was not accompanied by an increase in circulating IgM anti-DNA antibodies (Figure 2B). The vast increase of IgG ASCs in the spleen was not accompanied by a similar increase of these cells in the bone marrows of the treated mice. The frequency of total bone marrow ASCs in the treated mice was only 3.3- and 2.7-fold over that of the naïve controls at weeks 16–17 (p=0.0046) and 19 (p=0.0242), respectively (Figure 3A). A low frequency of anti-dsDNA ASCs appeared in the bone marrows of treated mice after 16 weeks (Figure 3A) but this was not statistically significant. Furthermore, although 19 week old Ad-IFNα treated mice had the same number of IgG ASCs in the spleen as untreated aged nephritic NZB/W mice, IgG ASCs were 3-fold less frequent in the bone marrow (Figure 3A). Similarly, while the Ad-IFNα treated mice produced half as many IgG anti-dsDNA ASCs in the spleen as the untreated aged nephritic controls, they contained a much lower frequency of IgG anti-dsDNA spots in the bone marrow (Figure 3B). Taken together, our data demonstrated that fewer long-lived plasma cells are present in Ad-IFNα treated mice compared to spontaneously nephritic aged NZB/W controls.
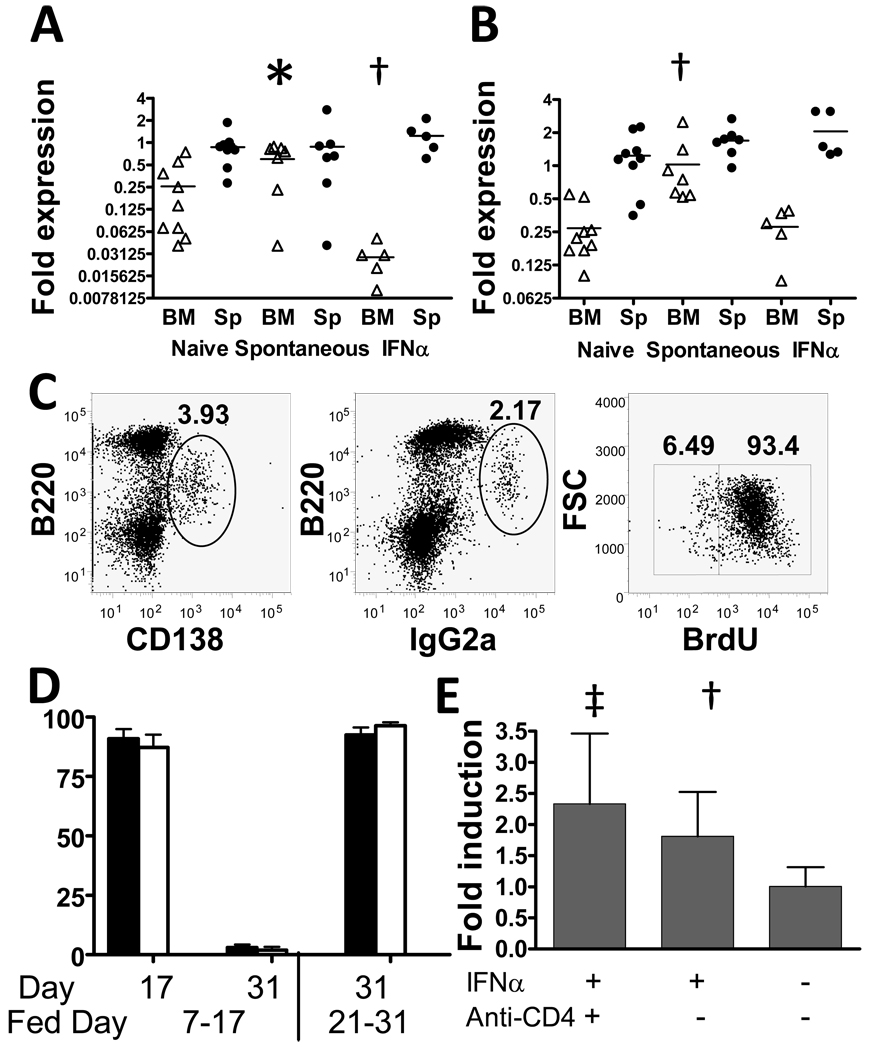
To determine whether this was due to alterations in the bone marrow environment we performed quantitative PCR for expression of CXCR4, CXCL12, VCAM1 and VLA4, the main molecules that attract and retain plasma cells in the bone marrow. There was a two-fold increase in CXCL12 in the bone marrows of aged NZB/W mice compared with 14–20 week naïve mice (p = 0.05). In contrast, CXCL12 expression was decreased in the bone marrows of 19w old Ad-IFNα treated mice (Figure 4A, p = 0.002). Similarly, VCAM-1 expression increased in the bone marrows of aged NZB/W mice compared with naïve mice (p < 0.001) but this increase did not occur in the bone marrows of 19w old Ad-IFNα treated mice (Figure, 4B). No differences were detected between groups in expression of VLA4 or CXCR4.
Figure 4.
Ad-IFNα treatment induces production of short-lived PCs. A–B: Expression of CXCL12 (A) and VCAM1 (B) in spleens (Sp) and bone marrows (BM) of NZB/W mice with spontaneous or IFNα induced nephritis. p values are compared with 19 week old naïve controls. Bars indicate mean values. C: PCs and IgG2a producing cells were identified as B220intCD138hi (left panel) and B220intIgG2ahi (middle panel), respectively. BrdU incorporation was determined by intracellular staining (right panel). D: percentage of BrdU positive cells in B220intCD138hi (filled bars) and B220intIgG2ahi (open bars) cells. Days after Ad-IFNα injection of BrdU feeding and harvest are as indicated. E: relative expression of TLR7 in CD19+ splenic B cells isolated from 19 week old Ad-IFNα treated, Ad-IFNα/anti-CD4 antibody treated, and naïve control NZB/W mice. Expression levels were normalized to the mean of naïve controls. p values are compared with 19 week old naïve controls. Mean + 1SD shown *: p < 0.05, ‡: p < 0.02, †: p < 0.01. Data are representative of 2 experiments of 4–10 mice per group.
Longevity of PCs in Ad-IFNα treated NZB/W mice
We used BrdU incorporation to investigate whether the PCs in the spleens of Ad-IFNα treated mice are long-lived. More than 90% of CD138+ PCs became BrdU positive after 10 days of BrdU feeding (Figures 4C, 4D). The vast majority of these cells, however, disappeared 2 weeks after BrdU withdrawal, leaving only 3% of CD138+ PCs positive for BrdU (Figure 4D). Similarly, the percentage of BrdU labeled IgG2a PCs (all of which were positive both for intracellular IgG2a and for CD138) declined from ~87% before BrdU withdrawal to ~2% 2 weeks after BrdU withdrawal (Figure 4D). Similar results were found when BrdU feeding was delayed until day 21 after Ad-IFNα injection, at which time PCs were already present in large numbers in the spleen (Figures 3, 4D). Taken together, our results show that almost no terminally differentiated long-lived PCs are present in the spleens during this time window.
We next investigated VH gene usage and somatic hypermutation of GC B cells and PCs of Ad-IFNα treated mice using single cell PCR. IgG2a producing PCs were twice as frequent as IgG3 producing ones (IgG2a/IgG3=227/90) whereas GC B cells predominantly produced IgG2a (IgG2a/IgG3=101/21, p<0.02). The VH regions of 118 PC (65 IgG2a and 53 IgG3) and 48 GC (37 IgG2a and 11 IgG3) were analyzed. Of 51 unique genes identified, 27 were found only in PCs, 20 were shared between PCs and GCs and 4 were found only in GCs. VH gene usage was distinct between IgG2a and IgG3 secreting PCs (Table 1). Of the 47 genes found among PCs, 22 were found only in IgG2a PCs, 11 were found only in IgG3 PCs, and 14 were found in both IgG2a and IgG3 PCs. Seven overrepresented genes were examined for clonal expansion as evidenced by use of the same VDJ junction in more than 2 sequences and this was found in 2/7 (data not shown). There was a high rate of somatic mutation among GCs: this was somewhat less in PCs but there was no difference in the mutation rates between IgG2a and IgG3 PCs (Table 1). A higher replacement/silent mutation ratio in the CDR regions compared with the framework regions suggests that both GCs and PCs were subject to post-somatic mutation selection (Table 1).
Table 1.
Somatic mutation and VH gene usage of plasma cells in 17 week old Ad-IFNα treated mice.
| Average mutations per sequence* | % of sequences with noted numbers of mutations per sequence |
# of VH genes (# of seq)** |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| CDR replacement |
CDR silent |
FR replacement |
FR silent | Total | 0–2 | 3–10 | >11 | ||
| GC B | 3.2 ± 2.6 | 0.8 ± 1.2 | 2.9 ± 2.1 | 1.6 ± 1.3 | 8.5 ± 5.3 | 8.3 | 60.4 | 31.2 | |
| PC IgG2a | 1.9 ± 2.0c | 0.5 ± 0.7 | 1.8 ± 1.7c | 1.4 ± 1.6 | 5.6 ± 4.3c | 21.5 | 69.2 | 9.2 | 22 (37/65) |
| PC IgG3 | 2.5 ± 2.3 | 0.5 ± 0.8 | 1.9 ± 1.7c | 1.1 ± 1.3b | 6.0 ± 4.2a | 26.4 | 64.2 | 9.4 | 11 (28/53) |
Average number of mutations per sequence is shown as mean ± standard deviation. 48 sequences from GC B cells, 65 sequences from IgG2a plasma cells, and 53 sequences from IgG3 plasma cells were analyzed. P values are compared with GC B cells. Only significant p values are shown.
p < 0.05,
p < 0.02,
p < 0.01.
A total of 47 VH genes are detected in PCs. The numbers of genes that are exclusively present in IgG2a or IgG3 PCs are shown with the numbers of sequences that use these genes shown in the brackets.
Phenotype and gene expression profile of splenocytes in Ad-IFNα treated NZB/W mice
The first phenotypic change detected by flow cytometry was an increase in the number of class-switched B cells, seen one week after Ad-IFNα administration. Three weeks after virus administration further changes included an increase in spleen size and in the absolute number of activated (CD69+) CD4 T cells and B cells, and memory CD4 T cells. By 19 weeks, there was a marked increase in the absolute number of T cells and B cells of all subsets, except B1 B cells and naïve T cells. Splenic and peripheral blood DCs also increased in Ad-IFNα treated mice (Table 2).
Table 2.
Characterization of spleen cell subsetsa
| No. of cells per spleen (mean ± SD) |
Naïve 12 wk (n=5) |
IFNα 13 wk (n=5) |
IFNα 15 wk (n=5) |
IFNα 16 wk (n=5) |
IFNα 19 wk (n=6) |
IFNα 19 wk + anti-CD4 (n=4) |
Naïve 20 wk (n=5) |
Ad-LacZ 20 wk (n=5) |
|---|---|---|---|---|---|---|---|---|
| Total cell no. × 107 | 7.5 ± 2.6 | 7.8 ± 1.8 | 15.0 ± 4.2c | 12.0 ± 4.7 | 21.8 ± 7.7b | 7.2 ± 1.7b | 5.0 ± 0.6 | 4.3 ± 1.0 |
| CD19 × 107 | 2.7 ± 1.4 | 2.6 ± 0.5 | 4.7 ± 1.4 | 5.1 ± 2.3 | 10.8 ± 4.0b | 3.6 ± 0.7b | 2.2 ± 0.5 | 1.9 ± 0.8 |
| CD19/CD69 × 106 | 1.5 ± 1.4 | 1.7 ± 0.6 | 3.8 ± 1.5 | 6.1 ± 2.5c | 11.2 ± 4.7b | 1.5 ± 0.2d | 0.6 ± 0.7 | 0.6 ± 0.7 |
| Follicular × 107 | 1.5 ± 0.7 | 1.2 ± 0.3 | 2.0 ± 0.5 | 3.0 ± 1.6 | 5.2 ± 2.1b | 1.4 ± 0.4b | 1.2 ± 0.3 | 1.1 ± 0.4 |
| T1 × 106 | 1.8 ± 1.5 | 1.0 ± 0.8 | 1.8 ± 0.5 | 3.3 ± 2.3 | 7.6 ± 4.6c | 1.8 ± 0.2b | 1.3 ± 0.5 | 1.1 ± 0.5 |
| MZ × 106 | 2.5 ± 1.2 | 3.6 ± 0.4 | 5.2 ± 1.6d | 5.9 ± 2.1d | 9.3 ± 3.9b | 8.8 ± 2.9 | 3.0 ± 0.5 | 2.2 ± 0.7 |
| B1 × 106 | 5.0 ± 2.7 | 2.2 ± 0.6d | 2.2 ± 0.6c | 2.3 ± 1.0 | 7.5 ± 2.1 | ND | 1.2 ± 0.4b | 1.5 ± 0.4b |
| IgM−/IgD− × 106 (switched) | 0.4 ± 0.2 | 1.9 ± 0.4b | 3.4 ± 2.7 | 4.3 ± 2.0b | 11.8 ± 2.7b | 1.4 ± 0.2b | 0.9 ± 0.2d | 1.0 ± 0.3c |
| CD4 × 107 | 2.2 ± 0.6 | 2.6 ± 0.7 | 4.4 ± 1.0 | 3.8 ± 1.0 | 5.9 ± 2.4b | 0.0 ± 0.0b | 1.6 ± 0.3 | 1.1 ± 0.5 |
| CD4/CD69 × 107 | 2.1 ± 0.9 | 2.2 ± 0.5 | 7.3 ± 2.5c | 8.0 ± 2.7c | 14.0 ± 6.1b | ND | 0.9 ± 0.4 | 0.8 ± 0.5 |
| CD4/CD44+/CD62L− (memory) × 107 | 0.4 ± 0.1 | 0.5 ± 0.1 | 1.3 ± 0.4b | 1.2 ± 0.5b | 3.1 ± 1.1b | ND | 0.4 ± 0.1 | 0.3 ± 0.1 |
| CD4/CD44−/CD62L+(naive) × 107 | 1.5 ± 0.5 | 1.9 ± 0.5 | 2.3 ± 0.5 | 1.9 ± 0.3 | 1.8 ± 0.8 | ND | 0.9 ± 0.2 | 0.6 ± 0.4 |
| TFH × 106 | ND | ND | ND | ND | 1.2 ± 0.3 | ND | 0.2 ± 0.1b | ND |
| CD8 × 107 | 1.1 ± 0.4 | 1.4 ± 0.2 | 1.9 ± 0.4 | 1.5 ± 0.4 | 1.8 ± 0.7 | 2.0 ± 0.3 | 0.8 ± 0.2 | 0.6 ± 0.3 |
| CD11b/CD11c × 106 | 0.7 ± 0.2 | 0.1 ± 0.1b | 3.3 ± 1.1b | 2.5 ± 1.2d | 2.8 ± 2.0 | 0.7 ± 0.1b | 0.5 ± 0.3 | 0.5 ± 0.3 |
| % of CD11b/c in PBMCe | ND | ND | ND | ND | 3.1 ± 1.6b | 1.1 ± 0.6c | 0.7 ± 0.3 | ND |
Follicular: CD19+IgDhiIgMlo; T1: CD19+/CD23−/IgMhi/ CD21lo; MZ: CD19+/CD23−/IgMhi/CD21hi; B1: B220+/CD4−/CD5hi; TFH: B220−/CD11b−/CD4+/ CD44hi/CXCR5hi/PD-1hi
p values are compared with 12 week old naive NZB/W mice, except for IFNα + anti-CD4 group that is compared with IFNα 19wk group. Only significant p values are shown.
p < 0.01;
p < 0.02;
p < 0.05; ND: not done; Data are representative of 3 experiments of 4–6 mice per group.
percentage of CD11b+CD11c+ cells in lymphocytes.
To evaluate the effects of Ad-IFNα treatment on the production of pro-inflammatory cytokines, we performed real-time PCR on spleen cells from Ad-IFNα treated mice and age-matched naive NZB/W mice. Our data showed that the expression of IL-4, IL-6, IL-10, IL-21 and IFNγ were significantly elevated in the spleen cells from Ad-IFNα treated mice compared to naïve controls. No significant difference was detected between Ad-IFNα treated mice and naïve controls in the splenic expression of IL-12, IL-17, or TNFα (Supplementary Table 1). We also measured serum levels of IL-6, IL-10, BAFF and TNFα in naïve and 19wk-old Ad-IFNα treated mice and all were increased in the Ad-IFNα treated mice (Supplementary Table 2).
Using real-time PCR, we detected a 1.8-fold increase in TLR7 expression in sorted splenic CD19+ B cells from Ad-IFNα treated mice compared with naïve controls (p=0.0073, Figure 4E), but no difference in the expression of TLR9 or TACI (data not shown).
Effects of CD4 T cell depletion in Ad-IFNα treated NZB/W mice
To investigate the role of T cell help in this model, we depleted CD4 T cells with anti-CD4 antibody. 99% of CD4+ cells were depleted from peripheral blood within the first week of anti-CD4 antibody treatment (data not shown) and splenic CD4 T cells were still completely depleted after 7 weeks of continuous treatment (Table 2). The spleens of these mice were smaller and contained less B cells than those of age matched Ad-IFNα treated controls (Table 2). This was due to a significant decrease of follicular B cells with no effect on the IFNα driven expansion of MZ B cells. Furthermore, there was no increase of CD69 expression on B cells in anti-CD4 treated mice and class-switching was partially inhibited by the antibody (p=0.0159, vs. 12w or 20w naïve controls; Table 2). Expansion of splenic DCs was also inhibited by anti-CD4. Nevertheless, isolated B cells from anti-CD4 treated mice expressed increased levels of TLR7, similar to those from Ad-IFNα treated mice (Figure 4C).
We next investigated whether T cell help is required for immunoglobulin production in Ad-IFNα treated mice. Serum levels of IgM were higher in anti-CD4 antibody treated mice than in age matched Ad-IFNα treated controls with no difference in the serum levels of anti-dsDNA IgM (Figure 2A). Surprisingly, anti-CD4 did not prevent the increase in serum levels of total IgG2a and IgG3 detected at week 17 (Figure 2A), despite partial inhibition of class switching and almost complete blockade of PC formation in the spleens (Table 2 and Figure 3). This was not due to an increase in the frequency of either bone marrow or peritoneal plasma cells (data not shown). It is possible that the increase in IgG observed in Ad-IFNα treated mice is derived from class switched B cells that are producing low amounts of Ig per cell as a direct result of Ad-IFNα stimulation (20). In addition, loss of Ig in the urine may result in an underestimate of total Ig production in the control IFNα treated mice.
Despite the overall increase in serum IgG levels, serum levels of anti-dsDNA IgG2a and IgG3 antibodies in the anti-CD4 treated mice were significantly lower than in Ad-IFNα treated controls (p=0.0057, IgG2a; p=0.0168, IgG3). ELISpot analysis revealed significantly lower numbers of both total and anti-dsDNA IgG ASCs in the spleens of anti-CD4 treated mice compared with Ad-IFNα controls (Figure 3A). Immunofluorescence staining showed that germinal centers failed to form in the spleens of anti-CD4 treated mice and only a few IgG2a and IgG3 ASCs were detected (Figure 3B). Renal deposition of IgG2a and IgG3 was greatly decreased by anti-CD4 antibody treatment (Figure 3B – immunofluorescence score 1.0 +/− 0.7 in anti-CD4 treated mice vs. 3.6 +/− 0.5 in IFNα treated controls; p < 0.01) and the degree of renal damage was significantly less than in Ad-IFNα treated controls (p<0.0001, glomerular and interstitial damage; Figure 1B).
Ad-IFNα treated mice and naïve controls in the splenic expression of IL-12, IL-17, or TNFα (Supplementary Table 1 - www.feinsteininstitute.org/Feinstein/Autoimmune+Disease+Lab+Publications). We also measured serum levels of IL-6, IL-10, BAFF and TNFα in naïve and 19wk-old Ad-IFNα treated mice and all were increased in the Ad-IFNα treated mice (Supplementary Table 2 - www.feinsteininstitute.org/Feinstein/Autoimmune+Disease+Lab+Publications).
DISCUSSION
Our study explores the immunological events that underlie IFNα-induced acceleration of lupus in NZB/W mice. The earliest event we can detect is class-switching of splenic B cells that occurs before formation of GCs or an increase in circulating BAFF; this occurs even when T cells are depleted. Two weeks after Ad-IFNα injection large GCs and IgG ASCs appear in the spleens and a week later B and T cell activation become evident, accompanied by the appearance of serum autoantibodies, renal IgG deposition, glomerular damage, onset of proteinuria, and increasing renal infiltration with mononuclear phagocytes.
IFNα enhances GC formation and antigen-specific antibody responses in several experimental models (21, 22) especially in the context of weak immunogens (23). IFNα also induces differentiation of CD40-activated B cells into plasmablasts; terminal differentiation to Ig-secreting PCs is then mediated by IL-6 secreted in response to CD40 ligation on pDCs (24). However, expression of IL-6 by splenic cells in Ad-IFNα treated mice is only partly dampened by CD4 T cell depletion, suggesting that IL-6 is induced by other mechanisms in our model, such as enhanced TLR signaling or direct stimulation of B cells (20). IFNα enhanced TLR signaling also inhibits shedding of ICOS ligand from B cells, resulting in increased T cell help through ICOS within GCs (25). Finally, IFNα signaling on DCs is required for the development of TFH cells (26). In accordance with these mechanisms we noted the rapid appearance of enlarged GCs, increased numbers of TFH cells, elevated expression of IL-21, and the accumulation of large numbers of IgG secreting PCs in the spleens of Ad-IFNα treated mice.
IFNα also mediates T-independent class-switching by several mechanisms. It induces upregulation of TLRs on DCs and B cells (6, 27) in an IFNAR dependent fashion (20, 28). We show here that IFNα induces upregulation of TLR7, but not TLR9 expression in the splenic B cells of mice, similar to what has been reported in human B cells (20). Activation of murine B cells through TLR7 or TLR9 together with CD40 or cytokine mediated-costimulation promotes class-switching to IgG2a, G2b, G3, and inhibits the generation of IgG1 (29, 30). Second, IFNα amplifies the response of B cells to TLR mediated signals (31, 32), enhances B cell production of IL-6, and induces antibody secretion from both naïve and memory B cells. Furthermore, TLR-activating immune complexes direct the formation of extrafollicular foci in which somatic mutation can occur (33). Third, IFNα induces DCs to produce BAFF and APRIL that enhance T-independent class-switching and Ig secretion (5, 11, 34, 35). BAFF amplifies TLR expression on B cells and TLR ligation in turn upregulates expression of the BAFF receptor TACI (6, 36). Overexpression of BAFF induces class-switching in a MyD88 dependent manner; in BAFF transgenic mice that express a supra-physiologic 50–100 fold increase in serum BAFF this is sufficient to induce autoimmunity even in the absence of T cells (6). These findings suggest that a T-independent mechanism involving immune complexes, TLRs and BAFF might initiate or propagate disease and question the importance of T cells in the break of tolerance of autoreactive B cells and the value of T cell directed therapies in SLE. Our study in NZB/W mice showed that IFNα upregulated BAFF and IL-6 production, enhanced B cell TLR7 expression, promoted T-independent class-switching to IgG, and facilitated expansion of the marginal zone (MZ) B cell subset that is the source of pathogenic IgG autoantibodies in BAFF transgenic mice (6). Nevertheless, these events were not sufficient to initiate autoimmunity in the absence of CD4 T cells in our model.
The IgG PCs induced by IFNα in NZB/W mice may derive from GCs or extrafollicular foci. As shown by single cell PCR, GC B cells predominantly gave rise to IgG2a ASCs but the PC compartment contained a higher percentage of IgG3 ASCs, suggesting that some of the IgG3 PCs may be derived from the extrafollicular pathway. In addition, a significant proportion of PCs producing either IgG2a or IgG3 used VH genes that are not present in GC B cells, indicating that they may have arisen from outside the GCs. Furthermore IgG2a and IgG3 PCs have different repertoires suggesting different origins. Although 24% of the PCs had < 2 somatic mutations, compared with only 9% of the GC cells, most were extensively mutated suggesting that they had received help from T cells either inside or outside the GCs. The absence of autoreactive antibodies despite evidence of active class-switching in T cell depleted mice strongly suggests that clonal expansion and/or somatic mutation is required for the initiation of pathogenic autoreactivity in NZB/W mice.
Our data strongly suggest that the IgG PCs induced by IFNα in NZB/W mice are not long-lived PCs. Impaired homing of PCs to the bone marrow is characteristic of NZW and NZB/W mice (15, 37). Our studies show that the autoreactive plasma cells that are induced in the spleen by IFNα do not become long-lived and do not appear in the bone marrow. The mechanism for this is yet to be determined. One explanation is that IFNα affects the expression of critical chemoattractant molecules or the responsiveness of PCs to these molecules or to extrinsic signals provided in survival niches of bone marrow (38, 39). We observed a decrease of bone marrow expression of CXCL12 and a failure to upregulate VCAM1 in the bone marrow of IFNα treated mice compared with mice with spontaneous disease, suggesting that the bone marrows of Ad-IFNα treated mice do not provide optimal support for plasma cell survival. The downregulation of CXCL12 might be due to the observed increase in serum TNFα levels (40). Alternatively, sphingosine-1-phosphate (SIP) receptor is required for PC egress from the spleen (41) and IFNα inhibits SIP-mediated egress of CD69 positive lymphocytes from the spleen by modulating expression of the SIP1 receptor (42). Whether IFNα affects the expression of SIP1 receptor on PCs remains to be determined.
Survival niches for PCs also exist in the red pulp of spleen (43) and 40% of the PCs in the spleens of aged NZB/W mice are long-lived (44). It is possible that survival niches and soluble survival factors are limited in the spleen and therefore cannot accommodate the large numbers of PCs rapidly generated in IFNα treated NZB/W mice.
IFNα activates immature myeloid DCs and monocytes, causing them to up-regulate the expression of costimulatory molecules and produce inflammatory cytokines (27, 45, 46). Mature DCs activate naïve Th1 CD4+ T cells that secrete IFNγ and provide help to B cells (47). In accordance with this mechanism, we observed that CD11c+ DCs appeared in the spleens and the peripheral blood of Ad-IFNα treated NZB/W mice. However the expansion of the DCs occurs downstream of T cell activation as they failed to accumulate in the spleens of Ad-IFNα treated mice in the absence of T cells.
The NZB/W mouse is an excellent model for human SLE nephritis. However studies of therapeutic interventions in this mouse are hampered by the stochastic and delayed spontaneous disease onset. Because of the high reproducibility and the synchronized onset of disease, the IFNα induced model now allows study of the effects of therapies on defined stages of disease including remission induction studies, which pose a higher bar for therapeutic intervention. Such studies may help predict appropriate interventions for patients with an IFN signature.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Anton Kuratnik and Elif Alpoge for technical assistance.
This work was supported by grants from the NY SLE Foundation (RB), Rheuminations and NIH AI082037 and AR 049938-01
Footnotes
AUTHOR CONTRIBUTIONS
Liu and Davidson were involved in drafting the article or revising it critically for important intellectual content, and all authors edited and approved the final version to be published. Dr. Davidson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Liu, Bethunaickan, Davidson
Acquisition of data. Liu, Bethunaickan, Huang, Lodhi, Solano
Analysis and interpretation of data. Liu, Bethunaickan, Lodhi, Madaio, Davidson
REFERENCES
- 1.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6(11):823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202(9):1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronnblom L, Alm GV. An etiopathogenic role for the type I IFN system in SLE. Trends Immunol. 2001;22(8):427–431. doi: 10.1016/s1471-4906(01)01955-x. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 5.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199(12):1631–1640. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204(8):1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25(3):383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100(5):2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirou KA, Lee C, George S, Louca K, Papagiannis IG, Peterson MG, et al. Coordinate overexpression of interferon-alpha-induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50(12):3958–3967. doi: 10.1002/art.20798. [DOI] [PubMed] [Google Scholar]
- 11.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black × New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174(5):2499–2506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 12.Mihara M, Tan I, Chuzhin Y, Reddy B, Budhai L, Holzer A, et al. CTLA4Ig inhibits T cell-dependent B-cell maturation in murine systemic lupus erythematosus. J Clin Invest. 2000;106(1):91–101. doi: 10.1172/JCI9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramanujam M, Wang X, Huang W, Schiffer L, Grimaldi C, Akkerman A, et al. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J Immunol. 2004;173(5):3524–3534. doi: 10.4049/jimmunol.173.5.3524. [DOI] [PubMed] [Google Scholar]
- 14.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, et al. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 207(2):353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramanujam M, Wang X, Huang W, Liu Z, Schiffer L, Tao H, et al. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J Clin Invest. 2006;116(3):724–734. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang X, Suzuki H, Hanai Y, Wada F, Hitomi K, Yamane T, et al. A novel strategy for generation of monoclonal antibodies from single B cells using rt-PCR technique and in vitro expression. Biotechnol Prog. 2006;22(4):979–988. doi: 10.1021/bp060092h. [DOI] [PubMed] [Google Scholar]
- 17.Schiffer L, Bethunaickan R, Ramanujam M, Huang W, Schiffer M, Tao H, et al. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol. 2008;180(3):1938–1947. doi: 10.4049/jimmunol.180.3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Triantafyllopoulou A, Franzke CW, Seshan SV, Perino G, Kalliolias GD, Ramanujam M, et al. Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proc Natl Acad Sci U S A. 2010;107(7):3012–3017. doi: 10.1073/pnas.0914902107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adalid-Peralta L, Mathian A, Tran T, Delbos L, Durand-Gasselin I, Berrebi D, et al. Leukocytes and the kidney contribute to interstitial inflammation in lupus nephritis. Kidney Int. 2008;73(2):172–180. doi: 10.1038/sj.ki.5002625. [DOI] [PubMed] [Google Scholar]
- 20.Bekeredjian-Ding IB, Wagner M, Hornung V, Giese T, Schnurr M, Endres S, et al. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J Immunol. 2005;174(7):4043–4050. doi: 10.4049/jimmunol.174.7.4043. [DOI] [PubMed] [Google Scholar]
- 21.Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14(4):461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 22.Le Bon A, Thompson C, Kamphuis E, Durand V, Rossmann C, Kalinke U, et al. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol. 2006;176(4):2074–2078. doi: 10.4049/jimmunol.176.4.2074. [DOI] [PubMed] [Google Scholar]
- 23.Bach P, Kamphuis E, Odermatt B, Sutter G, Buchholz CJ, Kalinke U. Vesicular stomatitis virus glycoprotein displaying retrovirus-like particles induce a type I IFN receptor-dependent switch to neutralizing IgG antibodies. J Immunol. 2007;178(9):5839–5847. doi: 10.4049/jimmunol.178.9.5839. [DOI] [PubMed] [Google Scholar]
- 24.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19(2):225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 25.Logue EC, Bakkour S, Murphy MM, Nolla H, Sha WC. ICOS-induced B7h shedding on B cells is inhibited by TLR7/8 and TLR9. J Immunol. 2006;177(4):2356–2364. doi: 10.4049/jimmunol.177.4.2356. [DOI] [PubMed] [Google Scholar]
- 26.Cucak H, Yrlid U, Reizis B, Kalinke U, Johansson-Lindbom B. Type I interferon signaling in dendritic cells stimulates the development of lymph-node-resident T follicular helper cells. Immunity. 2009;31(3):491–501. doi: 10.1016/j.immuni.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Mohty M, Vialle-Castellano A, Nunes JA, Isnardon D, Olive D, Gaugler B. IFN-alpha skews monocyte differentiation into Toll-like receptor 7-expressing dendritic cells with potent functional activities. J Immunol. 2003;171(7):3385–3393. doi: 10.4049/jimmunol.171.7.3385. [DOI] [PubMed] [Google Scholar]
- 28.Thibault DL, Chu AD, Graham KL, Balboni I, Lee LY, Kohlmoos C, et al. IRF9 and STAT1 are required for IgG autoantibody production and B cell expression of TLR7 in mice. J Clin Invest. 2008;118(4):1417–1426. doi: 10.1172/JCI30065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin L, Gerth AJ, Peng SL. CpG DNA redirects class-switching towards "Th1-like" Ig isotype production via TLR9 and MyD88. Eur J Immunol. 2004;34(5):1483–1487. doi: 10.1002/eji.200324736. [DOI] [PubMed] [Google Scholar]
- 30.Liu N, Ohnishi N, Ni L, Akira S, Bacon KB. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 2003;4(7):687–693. doi: 10.1038/ni941. [DOI] [PubMed] [Google Scholar]
- 31.Finkelman FD, Svetic A, Gresser I, Snapper C, Holmes J, Trotta PP, et al. Regulation by interferon alpha of immunoglobulin isotype selection and lymphokine production in mice. J Exp Med. 1991;174(5):1179–1188. doi: 10.1084/jem.174.5.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heer AK, Shamshiev A, Donda A, Uematsu S, Akira S, Kopf M, et al. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178(4):2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 33.Herlands RA, William J, Hershberg U, Shlomchik MJ. Anti-chromatin antibodies drive in vivo antigen-specific activation and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. Eur J Immunol. 2007;37(12):3339–3351. doi: 10.1002/eji.200737752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3(9):822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J Immunol. 2004;173(7):4479–4491. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 36.Treml LS, Carlesso G, Hoek KL, Stadanlick JE, Kambayashi T, Bram RJ, et al. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol. 2007;178(12):7531–7539. doi: 10.4049/jimmunol.178.12.7531. [DOI] [PubMed] [Google Scholar]
- 37.Erickson LD, Lin LL, Duan B, Morel L, Noelle RJ. A genetic lesion that arrests plasma cell homing to the bone marrow. Proc Natl Acad Sci U S A. 2003;100(22):12905–12910. doi: 10.1073/pnas.2131686100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minges Wols HA, Ippolito JA, Yu Z, Palmer JL, White FA, Le PT, et al. The effects of microenvironment and internal programming on plasma cell survival. Int Immunol. 2007;19(7):837–846. doi: 10.1093/intimm/dxm051. [DOI] [PubMed] [Google Scholar]
- 39.Cassese G, Lindenau S, de Boer B, Arce S, Hauser A, Riemekasten G, et al. Inflamed kidneys of NZB / W mice are a major site for the homeostasis of plasma cells. Eur J Immunol. 2001;31(9):2726–2732. doi: 10.1002/1521-4141(200109)31:9<2726::aid-immu2726>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 40.Zhang Q, Guo R, Schwarz EM, Boyce BF, Xing L. TNF inhibits production of stromal cell-derived factor 1 by bone stromal cells and increases osteoclast precursor mobilization from bone marrow to peripheral blood. Arthritis Res Ther. 2008;10(2):R37. doi: 10.1186/ar2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kabashima K, Haynes NM, Xu Y, Nutt SL, Allende ML, Proia RL, et al. Plasma cell S1P1 expression determines secondary lymphoid organ retention versus bone marrow tropism. J Exp Med. 2006;203(12):2683–2690. doi: 10.1084/jem.20061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440(7083):540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 43.Sze DM, Toellner KM, Garcia de Vinuesa C, Taylor DR, MacLennan IC. Intrinsic constraint on plasmablast growth and extrinsic limits of plasma cell survival. J Exp Med. 2000;192(6):813–821. doi: 10.1084/jem.192.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. 2004;199(11):1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294(5546):1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 46.Papewalis C, Jacobs B, Wuttke M, Ullrich E, Baehring T, Fenk R, et al. IFN-{alpha} Skews Monocytes into CD56+-Expressing Dendritic Cells with Potent Functional Activities In Vitro and In Vivo. J Immunol. 2008;180(3):1462–1470. doi: 10.4049/jimmunol.180.3.1462. [DOI] [PubMed] [Google Scholar]
- 47.Spranger S, Javorovic M, Burdek M, Wilde S, Mosetter B, Tippmer S, et al. Generation of Th1-polarizing dendritic cells using the TLR7/8 agonist CL075. J Immunol. 185(1):738–747. doi: 10.4049/jimmunol.1000060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.