Abstract
The oddity task (e.g. A−, A−, B+) is classified as a conjunctive or relational task in which accurate performance depends upon learning to attend to stimulus relationships, not stimulus identity, and has no retention component as stimuli are presented simultaneously. It has been suggested that the hippocampus may play a particular role in learning this type of task in humans and animals. To test this, we trained adult rhesus macaques with selective neurotoxic damage to the hippocampal formation on their ability to learn and apply an oddity rule. The results suggest that the monkeys were able to adapt simple strategies to solve variations of the oddity task, however as the opportunity for such strategies was reduced, monkeys with hippocampal damage were increasingly impaired.
Introduction
The development of conjunctive or relational learning abilities in humans and animals is a protracted process. For example, children younger than 7 years of age perform poorly on spatial navigation tasks (Overman et al., 1996b) and those younger than 4.5 years are impaired on transverse patterning (Rudy et al., 1993). Because this age range is concurrent with final maturational events in the dentate gyrus and trisynaptic circuit connectivity in humans (for review see Seress, 2001), and because performance on these tasks activates the hippocampus in humans and is impaired in humans with medial temporal lobe damage (e.g. Maguire et al., 2006; Rickard et al., 2006; Moses et al., 2009), it has been suggested that the behavioral onset of these conjunctive or relational memory abilities marks the functional maturation of the hippocampal formation (Rudy, 1991).
Another late-developing task with a long history in both the human and animal literature is the oddity task. The oddity task belongs to the conjunctive or relational class of memory problems because the correct choice is determined by the relations among stimuli, rather than associative strength of individual items. In its most basic format, the oddity task requires subjects to choose one of three simultaneously presented items, A, A, or B. Whether by instruction or trial and error, subjects must learn to choose the odd item, B in this example. Using a paradigm adapted from non-human primate testing, Overman and colleagues (1996a) reported that children under 7 years old had great difficulty learning the oddity rule when all three items were presented simultaneously (see Figure 1a, “one-part” oddity) and they were required to learn the rule by trial-and-error. By contrast, they readily mastered the task when the items were presented sequentially (Figure 1b, “two-part” oddity) in a manner that is similar to the standard nonmatching-to-sample task, with one member of the identical pair serving as the “sample”, which is then re-presented along with the other member and the odd item in the choice phase. This format may have allowed a simpler “novelty” or “non-matching” solution which young children mastered easily without instruction. However, when retrained on the one-part oddity task, those under 5 years of age again failed to reach criterion. Thus, the ability to learn the oddity rule is late-developing in human and non-human primates (Gollin & Schadler, 1972; Harlow, 1959; Harlow et al., 1964; Hill, 1965; Overman et al., 1996a) and it has been suggested that this developmental delay is tied to maturational events in the hippocampal formation in both macaques and humans (Alvarado & Bachevalier, 2000; Overman et al., 1996a). More recently, the task has been used to explore the effects of medial temporal lobe damage on perception in rodents (Bartko et al, 2007), monkeys (Buckley et al., 2001) and humans (Barense et al., 2009: Lee et al, 2006), however in these and related studies, the role of specific brain structures in learning the oddity rule was not assessed, rather the subjects performed either a spontaneous oddity task, (rodents, Bartko et al., 2007), mastered the oddity rule pre-operatively (monkeys, Buckley et al., 2001) or were instructed as to which rule to use at the time of testing (humans, Lee et al., 2006). Thus, it is unclear whether the hippocampus or another structure contributes specifically to the ability to learn the oddity rule. To that end, we trained adult macaques with neurotoxic damage to the hippocampal formation and their controls on the oddity task using the same paradigm described by Overman et al. (1996a). Following that training we tested them on several variations of the task to better determine the strategies used to solve the task.
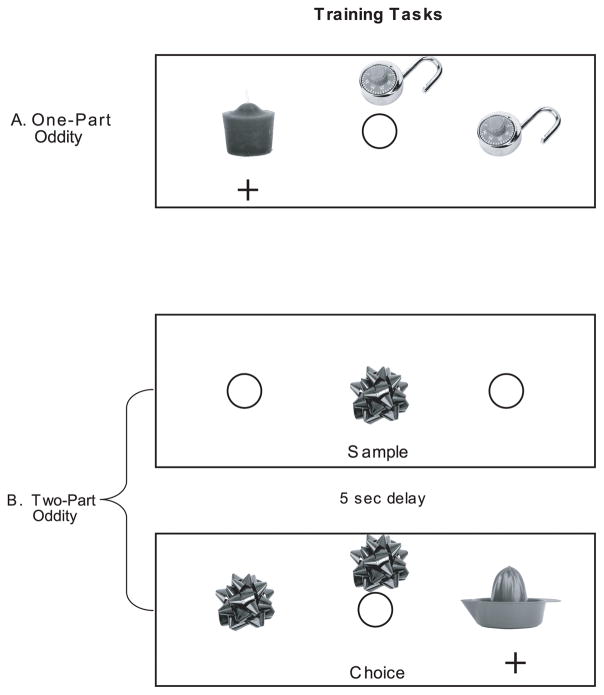
Figure 1.
Oddity Training. A) “One-part” oddity: One member of an identical pair is presented behind the central food well. Monkeys are given a choice between the other member of the pair and an “odd” object. Displacing the odd object reveals a reward. B) “Two-part” oddity: One member of the identical pair is presented alone, covering a baited well. The monkey displaces the object to reveal a food reward. The tray is shielded from view and after a 5-sec delay, the displaced object is positioned behind the central well, and its identical pair and an odd object are placed over the lateral wells, in the same manner as for one-part oddity. The animal must select the odd (i.e. “nonmatching”) object. Two-part oddity differs from delayed nonmatching-to-sample in that the monkey is not required to retain the sample in memory. That is, the sample is present at the time of choice.
Materials and Methods
Subjects
Fourteen rhesus monkeys (Macaca mulatta), 4–10 years of age served as subjects in the present study. Of these, 8 males (Group H-Ibo) received injections of ibotenic acid targeting the hippocampus. The control group (Group N) consisted of 4 males, who were operated controls, and 1 male and 1 female served as unoperated controls. Six monkeys died in the flooding resulting from Tropical Storm Allison. Three of these (1 from Group N, 2 from Group H-Ibo) had not completed the last phase of testing. Their data are included for the completed phases only. Animals were housed individually and maintained on a diet of Purina Monkey Chow, supplemented with fresh fruit and multi-vitamins. During behavioral testing, the food ration was given once daily, immediately following training, and the rations were adjusted individually to allow for rapid responding during test sessions, while maintaining the animal at 90% or greater of their free-feeding weight. Water was always available. This study was approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Houston.
Surgery
Damage to the hippocampus was made by MRI-guided injections of the neurotoxin, ibotenic acid, and was intended to include the hippocampus proper, the dentate gyrus, and the subiculum. One to three weeks prior to surgery, each monkey was anesthetized, placed in a non-ferromagnetic stereotaxic apparatus (Crist Instrument Co., Inc., Damascus MD) and received a T1-weighted magnetic resonance scan. The stereotaxic frame was aligned to the magnet, a 5″ surface coil (GE Medical Systems) was placed over the monkey’s head and the brain was imaged on a GE Signa 1.5 Tesla magnet (Milwaukee, WI) in each of two stereotaxic planes (sagittal and coronal). The MR images were obtained with a T1-weighted inversion recovery pulse sequence (TI = 600 msec, TR = 2500 msec, TE = 25 msec, FOV = 10 cm). Using the earbars and the midline sinus as referents, stereotaxic coordinates for the injection sites per hippocampus were determined on the coronal images for each animal. On the day of surgery, the monkey was sedated (ketamine hydrochloride, 10mg/kg) and repositioned into the stereotaxic frame. Surgical anesthesia was induced and maintained with isoflurane gas (1–2%, v/v, to effect) and heart rate, respiration rate, blood pressure, body temperature and expired CO2 were monitored throughout surgery, which was performed under aseptic conditions.
Following a midline incision through the skin and retraction of the temporalis muscles, a craniotomy was made through the skull above the injection site of each hemisphere. The dura was opened in a line connecting the injection sites. Bilateral injections of ibotenic acid (Biosearch Technologies, Novato CA, 10/mg/ml in phosphate buffered saline, pH 7.4) were made simultaneously through two, 10 μl Hamilton syringes held in Kopf electrode manipulators (David Kopf Instruments, Tujunga CA). Each was lowered slowly to the injection target where 1.5 to 2.4 μl of ibotenic acid was slowly injected (0.4μl/minute). The needles were allowed to remain in place for an additional 3 minutes to allow diffusion of the drug. After the injections were completed, the wound was closed in anatomical layers. Operated controls received the same opening, craniotomy and closing, but the needles were not lowered. All monkeys received pre- and postoperative treatment consisting of dexamethasone sodium phosphate (0.4 mg/kg i.m.) and cefazolin (25 mg/kg i.m.) 1 day before surgery and for 1 week after surgery to reduce swelling and to prevent infection, respectively. They also received acetaminophen for 7 days after surgery for relief of pain.
One week after surgery, all monkeys in Group H-Ibo received a second MRI-scan, using both a T1-weighed sequence and a Fluid Attenuated Inversion Recovery (FLAIR) protocol to visualize areas of edema caused by cell death. A comparison of the post-surgery FLAIR images depicting extent of brain edema at the injection sites was used to determine the accuracy of the injections and the extent of damage as well as to indicate whether the drug or needle passage caused any unintended cell death along the path of penetration.
Apparatus
Training was conducted in a standard Wisconsin General Testing Apparatus (WGTA), located inside a darkened, sound-shielded room. An opaque screen separated the animal from the testing tray, and was raised or lowered to control access. A one-way screen prevented the monkey from seeing the experimenter. Ambient noise was masked by the use of a white-noise generator. The testing trays (30 cm × 63 cm) contained either three, 1-inch diameter food wells, placed two inches apart on-center in the middle of the tray or 19 wells, arranged in three rows of 6, 7 and 6 wells, two inches apart on-center. Food rewards consisted of banana flavored monkey chow (300 mg Noyes), raisins, unsalted peanuts or Mini-M&M candies, according to the monkey’s preference. Visual discriminanda for the Tasks 1–3 were junk objects drawn from a pool of 300 identical pairs, and 300 “odd” items (Training Set). These were each divided into ten groups of 30 (pairs or individual items). For each training session, one group of pairs and one group of odd items was used. Items in the pairs group were consistently non-rewarded, whereas items in the odd group were always rewarded. In neither group were items re-presented until all ten groups had been used. Thus, 300 trials separated consecutive presentations of individual items or pairs, that is for every 1000 trials, each object was presented 3.3 times. However, with each successive cycle, the pairing order was advanced so that 3000 trials would have to occur before the same group of pairs appeared with a given group of odd items. The Training Set was used for one-part oddity, two-part oddity and the first two performance tasks. For performance Task 3, an entirely novel set of 10 identical pairs was used (Probe Set). None of the items forming the pairs had appeared in any previous test. For this group of pairs (A–J), a given item could appear as a member of a pair, or as an odd item on any given trial. For example, “A” could appear with “E” as an odd item, e.g., [E− E− A+], or as a pair [A− A− E+].
Behavioral Testing
Training
Prior to formal training, monkeys were given several pre-training sessions in which they were acclimated to the apparatus and trained to displace objects in order to retrieve food rewards. They were then trained on the one-part oddity task. Monkeys failing to reach criterion were then trained on the remedial two-part oddity task. After reaching criterion, they were retrained on one-part oddity (“one-part repeated”). Those subjects again failing to reach criterion on one-part repeated ceased training at that point. Those meeting criterion in the training or re-testing phase, continued on to the three performance tasks.
One-part Oddity
Using the standard three-well tray, a training trial consisted of simultaneous presentation of an identical pair and one odd item. One member of the pair (the exemplar) was always located behind the unbaited central well, while the other member of the pair covered either the left or right well (unbaited) and the odd item covered the remaining, baited well (See Figure 1A). Left-right placement of the odd item was balanced and predetermined pseudorandomly. Monkeys were trained to a criterion of 90 correct out of 100 consecutive trials. Those failing to achieve criterion received the two-part oddity correction procedure.
Two-part Oddity
This task was very similar to the delayed-nonmatching-to-sample procedure in that a sample trial preceded the usual presentation. In the sample trial, the exemplar object was presented alone over the baited central well (Figure 1B, “Sample”). Once the animal displaced this object, the screen was closed and after a 5 sec interval, the exemplar, its mate and the odd item were presented simultaneously (Figure 1B, “Choice”), as in one-part oddity.
Oddity Repeated
Once criterion was reached on two-part Oddity, the monkey was retrained on the standard one-part oddity task to the same criterion. Monkeys failing to learn the repeated task did not proceed further.
Performance Tests
These three tasks (Figure 2) were used to confirm that the monkeys had truly learned an oddity rule and were not using another strategy to solve the task. Each of the three tasks increased in difficulty in that the subject’s attention was not directed towards a specific sample, as in the training phase, but rather they were required to search all three objects to determine which one was odd. The last of these required them to transfer the rule to a novel set of objects. Criterion for each of these tasks was set at 27/30 for two consecutive sessions, or a maximum of 510 trials.
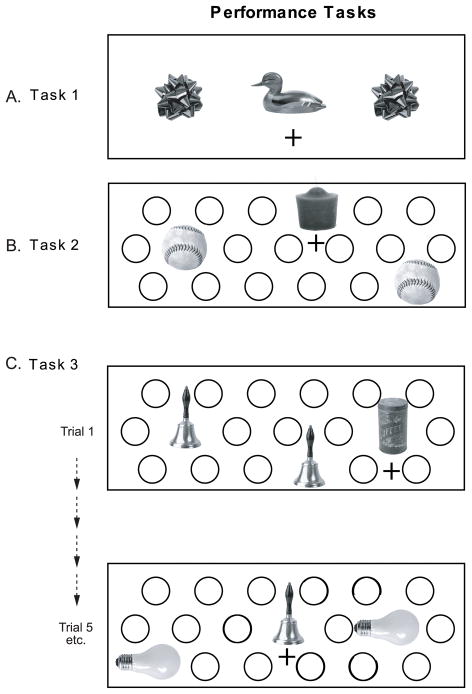
Figure 2.
Performance Tasks. A) Task 1: Identical to one-part oddity, except each object covers a food well and the odd object can appear over the central well as well as either lateral well. Training Set of objects was used. B) Task 2: 19-well tray, objects can appear at any position during testing. Training Set of objects was used. C) Task3: Same as Task 2, however Probe Set of 10 identical pairs is used. On any trial there is an equal chance for an object to be presented with its pair or as an odd item (e.g. A A B, or A E E), all objects appear equally as pairs and odds within a session, and each object (as a pair or an “odd” item) is presented equally with the other 9.
Task 1
For this task (Figure 2A), each of the three objects covered a well, and the odd item could appear over any of the three wells, with the only restriction being that the odd item appear equally often in each position, thus, the central position was now a possible response. Objects were drawn from the Training Set.
Task 2
The 19-well tray was used and objects could cover any well with the restriction that the odd item appear equally often in each row and to the left, right or center of the tray (Figure 2B). The Training Set stimuli were used.
Task 3
The final task tested whether monkeys could transfer the oddity rule to an entirely novel set of items (Probe Set). (Figure 2C). The 19-well tray was also used in this phase, but the objects were drawn from the completely novel Probe Set. To ensure that subjects could not form a learning set from the pool of rewarded items, on any given trial, one member of a set served as the odd item, while both members of another set served as foils (e.g., <A B B>, <A A D>, <B B D>, <B I I > etc. for a total of 90 combinations). Additionally, each set appeared equally 3 times as a pair and 3 times as the odd item per session, and each set appeared with all other sets equally across sessions. In other words, the accrued associative strength for any individual object in the Probe Set would be 50% because all objects are equally often rewarded and non-rewarded. Subjects who have learned to use the oddity rule should show good transfer to the novel Probe Set. Those who had developed a learning set for the Training Set objects might be expected to show poor transfer and slow acquisition of Task 3.
Lesion Evaluation
Four of the monkeys in Group H-Ibo died in the flooding of Tropical Storm Allison, and so their brains were not available for histological processing. Their lesion extents were evaluated using the postoperative FLAIR scans, using methods established in our laboratory and which have been shown to accurately estimate the extent of cell loss (Málková et al., 2001; Nemanic et al., 2002). The extent of the damage in these cases has been reported elsewhere (Bachevalier & Nemanic, 2009). Of the remaining 4 monkeys in Group H-Ibo, 2 have been processed histologically, and the two remaining animals were used in a tracer injection study and those brains await final processing. For histological processing, the monkeys were sedated and given a lethal dose of sodium pentobarbital, then perfused intracardially with 0.9% saline followed by 4% paraformaldehyde. The brains were photographed, cryoprotected and cut frozen at 50 μm in the coronal plane. Every 5th section was mounted and stained with thionin to visualize cell bodies. Histological sections were microscopically analyzed to determine extent of cell loss and presence of gliosis, including any potential damage to overlying structures along the injection paths.
Results
Lesion Extents
The MRI-estimated extent of intended and unintended damage of H-Ibo-1-8 and the histologically evaluated damage of H-Ibo-9 and H-6 is summarized in Table 1 using methods described. The lesion evaluation of all but three monkeys has been described elsewhere (H-Ibo-1-4 in Bachevalier & Nemanic, 2009; H-6 in Alvarado & Bachevalier, 2005). The three currently unpublished subjects will be briefly described here. Coronal FLAIR images from the postsurgical MR scan through three levels of the hippocampus of each of these subjects are shown in Figure 3.
Table 1.
| Intended Damage |
Unintended Damage |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hippocampal Formation |
TH |
TF |
||||||||||
| R | L | Avg | W | R | L | Avg | W | R | L | Avg | W | |
| H-Ibo-1 | 97.94 | 76.27 | 87.11 | 74.70 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| H-Ibo-2 | 81.27 | 75.75 | 78.51 | 61.56 | 20.08 | 53.09 | 36.59 | 10.66 | 27.59 | 60.31 | 43.95 | 16.64 |
| H-Ibo-3 | 74.13 | 67.46 | 70.80 | 50.01 | 15.27 | 26.65 | 20.96 | 4.07 | 44.02 | 29.90 | 36.96 | 13.16 |
| H-Ibo-4 | 76.25 | 56.21 | 66.23 | 42.86 | 27.83 | 13.61 | 20.72 | 3.79 | 19.39 | 18.50 | 18.94 | 3.59 |
| H-Ibo-7 | 59.02 | 12.68 | 35.85 | 7.48 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| H-Ibo-8 | 98.39 | 79.78 | 89.09 | 78.50 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| H-Ibo-9 | 89.40 | 90.95 | 90.18 | 81.31 | 0.00 | 0.00 | 0.00 | 0.00 | 1.31 | 0.92 | 1.11 | 0.01 |
| H-6 | 69.58 | 79.45 | 74.52 | 55.28 | 0.00 | 4.68 | 2.34 | 0.00 | 37.93 | 3.41 | 20.67 | 1.29 |
| Mean | 80.75 | 67.32 | 74.03 | 56.46 | 7.90 | 12.25 | 10.08 | 2.31 | 16.28 | 14.13 | 15.20 | 4.34 |
Note: Percentage of intended damage to Hippocampus (*CA fields, dentate gyrus and subiculum) and unintended damage to subjacent cortical areas. TH, TF,= areas TH, TF, of von Bonin & Bailey (1947). R = percentage of damage to the right hemisphere; L = percentage of damage to the left hemisphere; Avg = average of L and R; W = (L × R)/100 (weighted index as defined by Hodos & Bobko, 1984).
Figure 3.
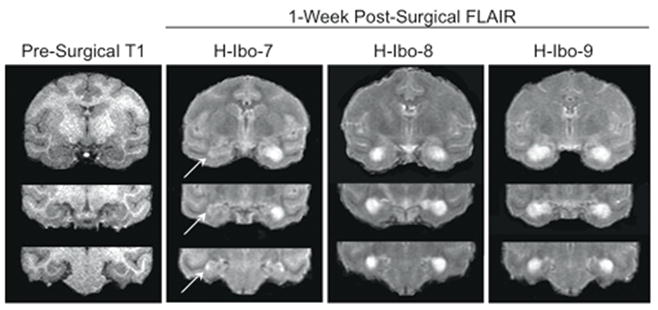
Lesion extents for H-Ibo-7-9. Left panel is a presurgical, T1-weighted structural MR image at three levels through the hippocampus. Remaining Panels: one-week post-surgical FLAIR (fluid attenuated inversion recovery) images at the same three levels depicting lesion extent for H-Ibo-7, H-Ibo-8 & H-Ibo-9. Hypersignal indcates edema resulting from targeted ibotenic acid injections. Arrows indicate areas of hypersignal (less obvious at this resolution) indicating damage limited to CA1 and CA2 in the left hippocampus for H-Ibo-7.
H-Ibo-1-4
In these cases the extent of hippocampal damage was largely as intended and bilaterally symmetrical, averaging 66–87% complete. Unintended damage in three cases was evident in the parahippocampal cortical areas TH/TF (see Table 1).
H-Ibo-7-9
As shown in Figure 3 and Table 1, damage to the hippocampus in H-Ibo-7 was asymmetrical and greater on the right than on the left side. Damage to the left hippocampus was largely limited to CA1 and CA2 along the full length of the hippocampus. There was no unintended cortical damage in this subject, however there was approximately 11% damage to the right posterior amygdala (not shown). Damage in the remaining two monkeys was bilateral and 79% or better on each side. H-Ibo-9 had minimal unintended damage to entorhinal cortex (< 0.7%) and area TF (< 1.2%).
Behavioral Testing
Performance results for all phases of testing are summarized in Tables 2 and 3.
Table 2.
| Oddity Training | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| One-Part | Two-Part | One-Part Repeated | |||||||
| TRL | ERR | % CRCT | TRL | ERR | % CRCT | TRL | ERR | % CRCT | |
| C-1 | 60 | 161 | 91.7 | ||||||
| C-4 | 970 | 319 | 91.7 | ||||||
| C-7 | 1000 | 323 | 80 | 0 | 0 | 91 | 690 | 110 | 90 |
| C-9 | 1000 | 236 | 83 | 30 | 4 | 91 | 180 | 36 | 91 |
| C-10 | 1000 | 306 | 78 | 20 | 2 | 90 | 620 | 110 | 90 |
| C-11 | 830 | 199 | 91 | ||||||
| Mean | 900 | 256 | 85.9 | 16.7 | 2 | 90.7 | 497 | 95.7 | 90.3 |
| H-Ibo-1 | 590 | 130 | 95 | ||||||
| H-Ibo-2 | 630 | 177 | 90 | ||||||
| H-Ibo-3 | 1000 | 361 | 90 | ||||||
| H-Ibo-4 | 1000 | 293 | 91.7 | ||||||
| H-Ibo-7 | 1000 | 342 | 74 | 130 | 36 | 91 | 200 | 42 | 90 |
| H-Ibo-8 | 1000 | 272 | 81 | 0 | 0 | 95 | 180 | 39 | 90 |
| H-Ibo-9 | 1000 | 344 | 69 | 10 | 1 | 90 | 1000 | 156 | 82 |
| H-6 | 1000 | 363 | 76 | 80 | 28 | 91 | 1000 | 207 | 78 |
| Mean | 903 | 285 | 83.3 | 55 | 16.3 | 91.8 | 595 | 111 | 85 |
Note: Individual scores for Oddity Training tasks. TRL= trials to criterion; ERR = errors to criterion; % CRCT = percentage correct during criterion run, or the equivalent number of trials at the end of a task. Bold-type numbers indicate failure to reach criterion.
Table 3.
| Performance Tasks | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Task 1 | Task 2 | Task 3 | |||||||
| TRL | ERR | % CRCT | TRL | ERR | % CRCT | TRL | ERR | % CRCT | |
| C-1 | 360 | 77 | 95 | 480 | 127 | 91.7 | 150 | 420 | 510 |
| C-4 | 270 | 57 | 91.7 | 480 | 125 | 90 | – | – | – |
| C-7 | 80 | 13 | 91 | 60 | 10 | 90 | 420 | 107 | 91.7 |
| C-9 | 0 | 0 | 90 | 30 | 8 | 95 | 300 | 92 | 90 |
| C-10 | 60 | 12 | 90 | 90 | 14 | 90 | 360 | 120 | 90 |
| C-11 | 240 | 41 | 93.3 | 60 | 28 | 90 | 450 | 90 | 90 |
| Mean | 168 | 33.3 | 91.8 | 200 | 52 | 91.1 | 336 | 88.4 | 91 |
| H-Ibo-1 | 510 | 97 | 80 | 90 | 27 | 91.7 | 510 | 114 | 67 |
| H-Ibo-2 | 420 | 127 | 90 | 510 | 132 | 73 | 510 | 88 | 83 |
| H-Ibo-3 | 330 | 68 | 95 | 480 | 95 | 91.7 | – | – | – |
| H-Ibo-4 | 270 | 48 | 90 | 480 | 92 | 91.7 | – | – | – |
| H-Ibo-7 | 90 | 22 | 90 | 510 | 102 | 80 | 510 | 159 | 75 |
| H-Ibo-8 | 240 | 42 | 90 | 30 | 6 | 91.7 | 180 | 45 | 91.7 |
| H-Ibo-9 | 240 | 45 | 92 | 60 | 17 | 90 | 510 | 163 | 80 |
| H-6 | |||||||||
| Mean | 300 | 64.1 | 89.6 | 309 | 67.3 | 87.3 | 444 | 114 | 79.3 |
Note: Individual scores for Oddity Performance tasks. TRL= trials to criterion; ERR = errors to criterion; % CRCT = percentage correct during criterion run, or the equivalent number of trials at the end of a task; – indicates the animal died before completion of the task. Bold-type numbers indicate failure to reach criterion
Oddity Training
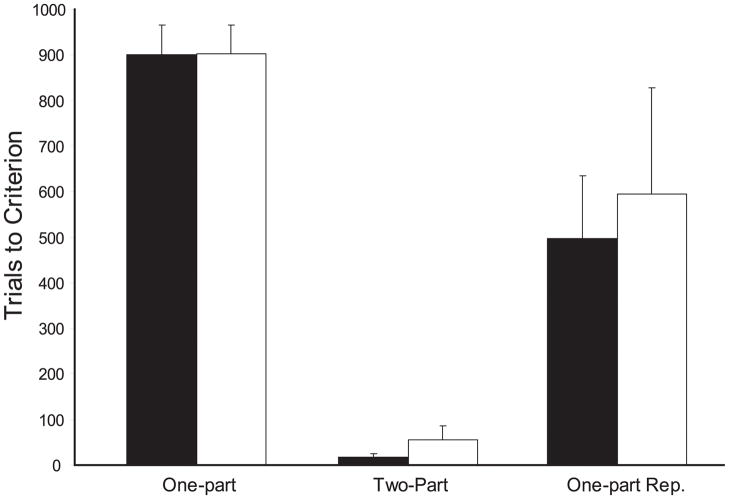
Trials to criterion, errors to criterion and final performance for the training tasks are shown in Table 2 and illustrated in Figure 4. Monkeys in both groups had a difficult time learning the initial oddity rule. Performance averaged 900 trials and 255 errors for Group N, and 902 trials and 285 errors for Group H-Ibo, with only half of the subjects in either group reaching criterion. For monkeys that did solve the task, Group N averaged 800 trials and 223 errors, and Group H-Ibo averaged 805 trials and 240 errors. For those monkeys who failed to reach criterion, Group N was performing at 80% correct at the end of testing (288 errors) and Group H-Ibo were performing at 75% correct (330 errors). Confirming this description, comparisons of trials and errors to criterion at the end of training did not differ between the two groups [Mann-Whitney U = 20, and 17 respectively, p’s > 0.05].
Figure 4.
Trials to criterion for One-part, Two-part, and One-part Repeated oddity training. Solid bars are Group N, open bars are Group H-Ibo. Error bars are standard error of the mean.
Two-part Oddity and Retraining
Figure 4 and Table 2 show the performance levels of the six monkeys requiring remedial training on the oddity task. Separating the example from the choices greatly simplified the task as illustrated by the ease of acquisition. The three animals in Group N learned two-part oddity in an average of 16.67 trials and made 5.33 errors on average. Similarly, the four animals in Group H-Ibo learned in an average of 55 trials and made 16.25 errors to criterion on average. The performance differences were not reliable [Mann-Whitney U = 4.5 and 4.5 respectively, p’s >0.05].
Oddity Repeated
Despite the relative ease with which they acquired two-part oddity, monkeys in both groups required additional training to re-learn the standard Oddity task. The three monkeys in Group N learned in an average of 496.67 trials and 95.67 errors. Only 2 of the four monkeys in Group H-Ibo reached criterion on the retrained task, and the group required 595 trials and 111 errors on average. A two-way ANOVA with repeated measures comparing performance on the two tasks confirmed that both groups found the two-part task to be easier than retraining on one-part oddity, revealing a main effect of Task [F(1, 5)= 10.312, p < 0.05], but no Group effect and no interaction [F’s < 1].
Performance Tests
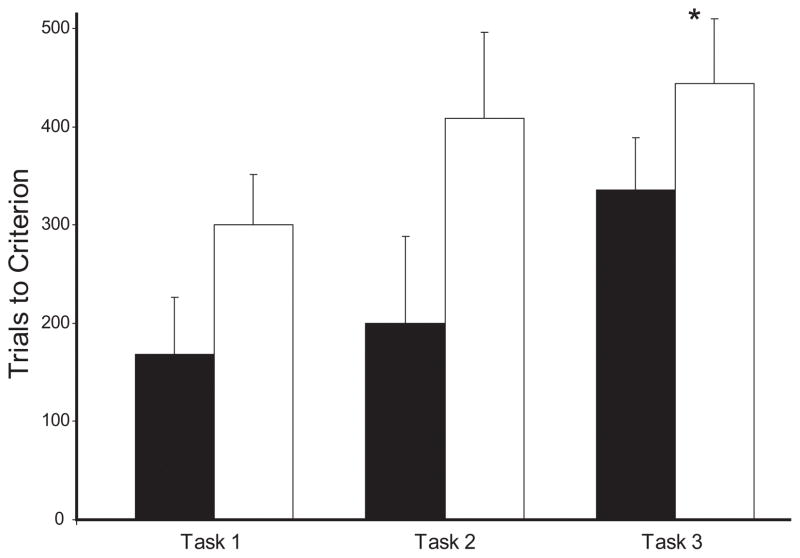
All monkeys in Group N and Group H-Ibo, except for H-6, were given the three performance tasks. The decision to include H-Ibo-9, who failed to reach criterion on oddity-repeated, was made because this monkey had two sessions of 90% or better in the last four training sessions. Results for all performance tasks are depicted in Table 3 and Figure 5. Comparisons across the three performance tasks were made using one-tailed Mann-Whitney U tests to compare whether Group H-Ibo required significantly more trials to reach criterion for each task and an overall ANOVA with task as the repeated measure was used to compare errors, performance levels and task difficulty across the three tasks.
Figure 5.
Trials to criterion for performance Tasks 1, 2 and 3. Conventions as in Figure 4. * indicates significant Group difference, p < 0.05. See text for discussion.
Task 1
Allowing the odd item to appear at any of the three locations did not impact performance for Group N, which made on average 1.5 additional errors on Task 1 as compared to the end of training on one-part oddity. The average performance level at criterion was 91.8%. Group H-Ibo also showed minimal impact transfer, increasing an average of only 4.1 errors on the first day of transfer and only one monkey failed to reach criterion in the time allotted. The differences between groups in transfer performance were not significant (p > 0.05). The trials to criterion approached significance [Mann-Whitney U = 10.5, p = 0.069], however there was no difference in the number of errors to criterion between groups [F(1,11) = 2.85, p > 0.05].
Task 2
Increasing the number of food wells from 3 to 19 affected performance equally on the first day of transfer for both Groups N and H-Ibo (average increase in errors 6.2 and 6.0 respectively). All monkeys in Group N reached criterion, four of them within 100 trials (Table 3) but the remaining two narrowly reached criterion at 480. Their performance at the end of training was 91.12% on average. For Group H-Ibo, two monkeys failed to reach criterion, and the group required 308 trials and 81 errors to reach criterion. Their performance at the end of training was 91.2%. Despite the better performance of Group N, the group variance prevented any significant difference between the groups [Mann-Whitney U = 14.0, p>0.05].
Task 3
Finally, reducing the pool of items to 10 novel pairs, and allowing each object to be either the target or the foils impacted performance of both groups. On average, Group N showed an increase of 5.8 errors on the first day of transfer, reached criterion in 336 trials and made 88 errors (Table 3). Their performance at the end of testing was 91%. By contrast, Group H-Ibo decreased performance by 8.2 errors on the transfer day and four of the five subjects in Group H-Ibo failed to reach criterion, requiring 444 trials and committing 114 errors on average. The errors on the first day of transfer or to criterion did not differ between groups (p’s > 0.05), but overall, Group H-Ibo required significantly more trials to reach criterion on Task 3 [Mann-Whitney U = 4.0, p = 0.048].
Two-way ANOVAs with repeated measures comparing Group performance and errors across the three Task levels revealed a main effect of Group for overall performance [F(1,8) = 6.27, p = 0.037] but not for the number of errors to criterion [F(1,8) = 3.36, p > 0.05], a main effect of Task for performance [F(2,16) = 6.84, p = 0.007], but not errors [F(2,16) = 3.071, p = 0.07] and a Group × Task interaction for performance [F(2,16) = 5.6, p = 0.014] but not errors [F<1]. Finally, an overall comparison of the effects of Task on errors for the first transfer day confirmed an effect of Task [F(2,16) = 4.05, p < 0.04] but not of Group [F(1,8) = 2.38, p > 0.05], nor was there any interaction.
Correlations
Despite the range of performance deficits within Group H-Ibo, we did not observe any correlations between the extent or specificity of brain damage and performance.
Discussion
In the present study, we tested adult monkeys with neurotoxic lesions of the hippocampus and their controls on the standard oddity task and a number of permutations of the paradigm. Lesioned and control monkeys were first trained on the standard one-part oddity paradigm using pseudo-trial-unique stimuli. Those that failed to reach criterion received remedial training on the two-part oddity task. If they reached criterion on this version, they were retrained on one-part oddity to determine whether they had in fact learned the oddity principle as opposed to the simpler nonmatching strategy. To the degree that the hippocampus is specifically required for relational or conjunctive learning, we predicted that damage to this structure should impact learning of the one-part oddity task, but should have little or no impact on the ability to perform two-part oddity. Assuming that the strategy learned to solve two-part oddity cannot be used to perform one-part, as was suggested by the Overman et al. (1996a) study, these animals should again have been impaired when retrained on the second round of one-part oddity training. Finally, to further explore the nature of the solution used by the monkeys to perform the task, we trained the monkeys on three performance tasks intended to reduce the availability of alternative strategies, and thus be increasingly susceptible to hippocampal damage.
One-part vs. Two-part Oddity
Contrary to our prediction, initial training on one-part oddity was equally difficult for both groups. Selective damage to the hippocampal formation did not appear to further impact subjects’ ability to learn the oddity task, although it may have altered the strategy they used to solve the task. Though proposed as a relational task (because the meaning of an individual stimulus depends upon the other two stimuli present), the manner in which the stimuli are presented may allow simpler solutions, such as nonmatch-to-sample, since the central object is never rewarded. That this is an easier strategy is exemplified by the animals’ performance on two-part oddity in which that very rule is emphasized and which they learned very quickly. Confirmation that different strategies were employed to solve standard and two-part oddity was evident when subjects in both groups required extensive retraining on one-part oddity to reach criterion. Had they been using the relational strategy to solve two-part oddity, they should have shown immediate transfer when retrained on one-part oddity. However, the fact that Group H-Ibo did learn it after extra training suggests either that the hippocampus is not required for performance on the oddity task, that another solution was available, or that other structures are sufficient for performance.
Performance Tasks
To explore the possibility of alternative performance strategies, we created three performance tests of increasing difficulty that nevertheless were solvable by using the oddity principle. The first two of these were designed to reduce the use of object position as a cue and encourage the exploration of all three objects. The third tested whether the rule could transfer to an entirely new set of objects.
Task 1
This task used the Training Set object pool and the odd object could appear over any of the three wells. Thus, the monkeys could no longer use the central object as an example to compare the two targets. However, animals in both groups learned this task faster than the original oddity, suggesting that they had successfully transferred the rule to the new task. Although subjects in Group N learned twice as quickly, monkeys with hippocampal damage also were able to reach criterion.
Task 2
Although Task 1 decreased the opportunity for nonmatching in that there was no longer a “sample” to compare to, there was an opportunity for side-by-side comparison with the three-well orientation. So to further examine how well the rule had been learned, in Task 2 we increased the object separation and orientation to each other using 19 possible locations that differed every trial, but continued to use the Training Set of stimuli. This change also impacted performance more for Group H-Ibo than N, with two subjects in Group H-Ibo failing to reach criterion, however the difference was not reliable, which is interesting in that this task could be seen as more spatial in nature than the previous ones.
Task 3
As a final test of rule learning, we tested whether performance would transfer to a novel set of stimuli. This last test was designed to ensure that the monkeys had not developed a learning set to the pool of odd stimuli, but rather were correctly applying the oddity rule. By the end of Task 2, it appeared that animals in both groups could be using simple associations formed to the items in the pool of Odd stimuli to guide performance, rather than using the oddity principle or even a non-matching strategy. Thus, in the final performance task, we used an entirely novel set of stimuli (Probe Set), and furthermore prevented any given item from acquiring positive or negative associative strength by allowing it to appear either with its identical mate in a pair, or as the odd item equally across trials.
The negative impact on performance by the switch for subjects in both groups suggests that the strategy used to solve Task 2 was not sufficient for performance on Task 3 and it is likely that this strategy was related to the pool of stimuli used. That is, the extensive training with the Training Set of stimuli may have allowed a simple associative strategy to the objects in the Odd pool of stimuli. Because the stimuli comprising the Probe Set were novel and non-differentially reinforced, no such associations could accrue to any subset of objects. Despite this change, all control subjects were able to reach criterion within the 510 trials allotted. By contrast, only one of the five subjects in Group H-Ibo reached criterion on Task 3. Thus, the data suggest that the hippocampus contributes to normal acquisition and use of the oddity principle, however the ability of one subject to learn Task 3 suggests that other structures, perhaps in the temporal lobe or prefrontal cortex, contribute to performance on this task, and may be sufficient for acquisition and/or performance once the rule is learned.
These findings have some support in the animal and human literature. Several investigators have used the oddity task to test the role of medial temporal lobe structures in perception. For example, Buckley and colleagues used 6-item oddity discriminations to test the role of medial temporal lobe structures in object and scene perception in monkeys. They demonstrated that damage to the perirhinal cortex impaired oddity judgments using different views of objects and scenes (Buckley et al., 2001) but not simple features. Whether the perirhinal cortex contributed to acquisition of the oddity rule was not directly assessed as the animals were pre-operatively trained on the oddity task. Similarly, Lee and colleagues (2005; 2006) have found that amnesic patients with hippocampal damage are impaired at spatial scene oddity judgments, but not objects (also Barense et al., 2007; 2009), whereas patients with damage to the perirhinal cortex have difficulty discriminating objects and faces. Similarly, imaging studies have indicated hippocampal activation during oddity judgments for scenes and faces, but not objects (Barense et al., 2009), which might suggest that the hippocampus only played a role depending on the items to be discriminated. However, it is important to point out that in none of these studies were the monkeys or humans required to derive the rule. Monkeys were trained preoperatively and humans were given verbal instructions to find the odd item, thus the data are more related to discrimination than learning ability. This is of interest because as described in Overman et al., (1996a), children who could not evoke the rule from the reinforcement contingencies, could nevertheless successfully apply it when it was provided by the experimenter.
Summary
We have shown that in a task in which alternative solutions to the oddity principle are limited, damage to the hippocampal formation significantly impairs group performance on an object oddity task. These results support the idea that the developmental delay in children and non-human primates’ ability to solve oddity tasks is due in part to the late maturation of the hippocampal formation and/or other structures that also develop postnatally (such as prefrontal cortical regions). This hypothesis needs to be addressed directly, however, and to that end we are currently exploring the normal development of performance on these tasks in infant macaques and the consequences of neonatal hippocampal or perirhinal damage on performance from infancy to adulthood.
Acknowledgments
We thank the staff of the Center for Laboratory Animal Management and Care at the University of Texas for their expertise and valuable help in the pre- and postoperative care of the monkeys, Belinda Rivera for the care and handling of the animals during MRI procedures, Zachary Torry for behavioral testing assistance.
Grant sponsor: NIMH; Grant Number: MH-58846; Grant Sponsor: NICHD; Grant Number: HD-35471.
References
- Alvarado MC, Bachevalier J. Revisiting the maturation of medial temporal lobe memory functions in primates. Learning & Memory. 2000;7:244–256. doi: 10.1101/lm.35100. [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Bachevalier J. Selective neurotoxic damage to the hippocampal formation impairs performance of the transverse patterning and location memory tasks in rhesus macaques. Hippocampus. 2005;15(1):118–31. doi: 10.1002/hipo.20037. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2009;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45(13):2963–74. doi: 10.1016/j.neuropsychologia.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Barense MD, Henson RN, Lee AC, Graham KS. Medial temporal lobe activity during complex discrimination of faces, objects, and scenes: Effects of viewpoint. Hippocampus. 2009 doi: 10.1002/hipo.20641. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perceptual functions of perirhinal cortex in rats: zero-delay object recognition and simultaneous oddity discriminations. J Neurosci. 2007;27(10):2548–59. doi: 10.1523/JNEUROSCI.5171-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MJ, Booth MC, Rolls ET, Gaffan D. Selective perceptual impairments after perirhinal cortex ablation. J Neurosci. 2001;21:9824–9836. doi: 10.1523/JNEUROSCI.21-24-09824.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollin ES, Schadler M. Relational learning and transfer by young children. J Exp Child Psychol. 1972;14:219–232. [Google Scholar]
- Harlow HF. The development of learning in the rhesus monkey. Am Sci Winter. 1959:459–479. [PubMed] [Google Scholar]
- Harlow HF, Akert K, Schiltz KA. The effects of bilateral prefrontal lesions on learned behavior of neonatal, infant, and preadolescent monkeys. In: Warren JW, Akert K, editors. The frontal granular cortex and behavior. New York: McGraw–Hill; 1964. pp. 126–148. [Google Scholar]
- Hill SD. The performance of young children on three discrimination tasks. Child Dev. 1965;36:425–436. [PubMed] [Google Scholar]
- Hodos W, Bobko PA. A weighted index of bilateral brain lesions. J Neurosci Methods. 1984;12(1):43–7. doi: 10.1016/0165-0270(84)90046-3. [DOI] [PubMed] [Google Scholar]
- Lee AC, Buckley MJ, Pegman SJ, Spiers H, Scahill VL, Gaffan D, Bussey TJ, Davies RR, Kapur N, Hodges JR, Graham KS. Specialization in the medial temporal lobe for processing of objects and scenes. Hippocampus. 2005;5(6):782–97. doi: 10.1002/hipo.20101. [DOI] [PubMed] [Google Scholar]
- Lee AC, Buckley MJ, Gaffan D, Emery T, Hodges JR, Graham KS. Differentiating the roles of the hippocampus and perirhinal cortex in processes beyond long-term declarative memory: a double dissociation in dementia. J Neurosci. 2006;6(19):5198–203. doi: 10.1523/JNEUROSCI.3157-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Nannery R, Spiers HJ. Navigation around London by a taxi driver with bilateral hippocampal lesions. Hippocampus. 2006;16(12):1091–101. doi: 10.1093/brain/awl286. [DOI] [PubMed] [Google Scholar]
- Málková L, Lex CK, Mishkin M, Saunders RC. MRI-Based evaluation of locus and extent of neurotoxic lesions in monkeys. Hippocampus. 2001;11(4):361–70. doi: 10.1002/hipo.1050. [DOI] [PubMed] [Google Scholar]
- Moses SN, Ryan JD, Bardouille T, Kovacevic N, Hanlon FM, McIntosh AR. Semantic information alters neural activation during transverse patterning performance. Neuroimage. 2009;46(3):863–73. doi: 10.1016/j.neuroimage.2009.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Price RE, Jackson EF, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: a replication. J Neurosci Methods. 2002;121(2):199–209. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- Overman W, Bachevalier J, Miller M, Moore K. Children’s performance on “animal tests” of oddity: implications for cognitive processes required for tests of oddity and delayed nonmatch to sample. J Exp Child Psychol. 1996a;62(2):223–42. doi: 10.1006/jecp.1996.0029. [DOI] [PubMed] [Google Scholar]
- Overman WH, Pate BJ, Moore K, Peuster A. Ontogeny of place learning in children as measured in the radial arm maze, Morris search task, and open field task. Behav Neurosci. 1996b;110(6):1205–28. doi: 10.1037//0735-7044.110.6.1205. [DOI] [PubMed] [Google Scholar]
- Rickard TC, Verfaellie M, Grafman J. Transverse patterning and human amnesia. J Cogn Neurosci. 2006;18(10):1723–33. doi: 10.1162/jocn.2006.18.10.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Elemental and configural associations, the hippocampus and development. Dev Psychobiol. 1991;24:221–236. [Google Scholar]
- Rudy JW, Keith JR, Georgian K. The effect of age on the child’s learning of tasks that require a configural association solution. Dev Psychobio. 1993;26:171–184. doi: 10.1002/dev.420260304. [DOI] [PubMed] [Google Scholar]
- Seress L. Morphological changes of the human hippocampal formation from midgestation to early childhood; In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge: The MIT Press; 2001. pp. 45–58. [Google Scholar]
- von Bonin G, Bailey P. The neocortex of Macaca mulatta. University of Illinois Press; Urbana, IL: 1947. [Google Scholar]