Abstract
Background and Purpose
Stroke of the right MCA is common. Such strokes often have consequences for emotional experience, but these can be subtle. In such cases diagnosis is difficult because emotional awareness (limiting reporting of emotional changes) may be affected. The present study sought to clarify the mechanisms of altered emotion experience after right MCA stroke. It was predicted that after right MCA stroke the anterior cingulate cortex (ACC), a brain region concerned with emotional awareness, would show reduced neural activity.
Methods
Brain activity during presentation of emotional stimuli was measured in six patients with stable stroke, and in 12 age and gender matched non-lesion comparisons using positron emission tomography and the [15O]H2O autoradiographic method.
Results
MCA stroke was associated with weaker pleasant experience and decreased activity ipsilaterally in the ACC. Other regions involved in emotional processing including thalamus, dorsal and medial prefrontal cortex showed reduced activity ipsilaterally. Dorsal and medial prefrontal cortex, association visual cortex and cerebellum showed reduced activity contralaterally. Experience from unpleasant stimuli was unaltered and was associated with decreased activity only in the left midbrain.
Conclusions
Right MCA stroke may reduce experience of pleasant emotions by altering brain activity in limbic and paralimbic regions distant from the area of direct damage, in addition to changes due to direct tissue damage to insula and basal ganglia. The knowledge acquired in this study begins to explain the mechanisms underlying emotional changes following right MCA stroke. Recognizing these changes may improve diagnoses, management and rehabilitation of right MCA stroke victims.
Keywords: Stroke, neuroimaging, alexithymia, depression, nondysphoric depression, positron emission tomography, middle cerebral artery
The middle cerebral artery (MCA) is the vessel most commonly affected by cerebrovascular accidents.(1) Because the damaged area may include striate/lenticular nuclei and frontal/temporal cortices (e.g., insula),(1, 2) it is no surprise that the clinical picture following MCA stroke often includes emotional changes. Emotional symptoms following left MCA stroke are frequent and may cluster in syndromes consistent with the diagnosis of major depression (3, 4) which is easily recognized and treated.(5)
Right MCA strokes may represent a challenge for the clinician and therefore often go unrecognized (6). This may be due to absence of striking cognitive deficits (e.g., language disturbance). Neglect (7) and disturbance in emotions including reduced emotional awareness may further complicate their recognition.(8) The neuropsychiatric picture following damage in the territory of the right MCA may consist of alexithymia (8) and apathy.(9) Alexithymia comprises deficits in awareness and description of personal emotions (often misrepresented as somatic complaints) (10, 11). Alexithymia ensuing after stroke may result in significant interpersonal and psychiatric difficulties, and most importantly in excessive demand for medical care which may interfere with rehabilitation efforts and increase the duration of hospitalization.(8) Disinhibition ranging from alteration in social behavior to mania is less common (12) and follows damage to more medial and ventral frontal structures.(13)
The mechanisms of emotional blunting after right MCA are poorly understood. While apathy and lack of energy can be explained by tissue damage to insula (14) or basal ganglia, (15) emotional blunting may originate from activity changes in distant brain regions.(16) Functional and structural changes in the anterior cingulate cortex (ACC) have been implicated in the mechanisms of alexithymia.(10, 17, 18) Therefore, a candidate brain region to explain the alexithymic features(8) following right MCA damage is the ACC.
In the present study, this relatively unexamined issue was addressed measuring whole brain activity in subjects with right MCA stroke exposed to visual stimuli aimed at evoking pleasant, unpleasant, and neutral emotional responses using positron emission tomography (PET) and the [15O]H2O autoradiographic method. Based on the prevalent clinical presentation of patients with right MCA stroke it was expected that blunted emotional responses would be associated with reduced activity in the ACC. It was however chosen not to limit our analyses to ACC ROI data. Such an analysis would have narrowed the ability to examine the functionality of distant brain regions. A brain-wide analysis testing the specific ACC hypothesis while not missing activity changes distant from the region of interest was preferred. One advantage of this approach stems from the suggestion that alexithymia with or without brain damage is associated with language deficits. (19-21) Based on this information reduced activity in left hemisphere regions was expected in association with right MCA stroke. Pleasant and unpleasant emotions were manipulated to determine the emotional specificity of the findings in light of the two prevalent hypotheses on laterality and emotion experience.(19, 22-24) One hypothesis identifies the right hemisphere as dominant for experiencing all emotions (dominance model), (19-21) whereas in the other the right hemisphere subserves experiencing only unpleasant emotions (laterality model).(22-24) Based on the dominance model, right MCA stroke was expected to result in blunting of both positive and negative emotions while the laterality model would predict blunted responses to unpleasant stimuli and normal responses to pleasant stimuli.
Methods
Subjects
All experimental subjects had stable stroke lesions and were recruited based on clinical and magnetic resonance imaging (MRI) findings showing right MCA stroke with involvement of the lenticulostriate beds (Figure 1). Healthy volunteers were recruited through newspaper advertisements and were excluded if they had any history of prior stroke or other abnormal structural pathology on MRI scan. Additional exclusionary criteria were: history of other brain injury/trauma, life threatening physical illness or illness interfering with daily activities, active psychotropic medications or history of psychiatric disorder, inability to complete Part One of the Token test (25) or a score below 23 on the Mini-Mental Status Exam.(26) Six patients with stroke [1/6 (16.7%) female] and twelve healthy, age matched volunteers [4/12 (33.3%) female] were recruited. The University of Iowa Institutional Review Board approved this study. All participants gave informed consent and were financially compensated.
Figure 1. Lesion Location.
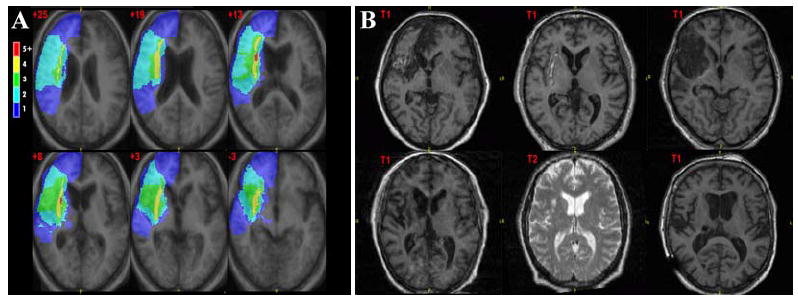
Scans follow radiological convention. Panel A shows topographical maps of brain damage in MNI305space using a variation of the lesion overlap method.(76) Color bars indicate the number of overlapping lesion at each voxel. Talairach z coordinates are indicated at the top-left corner of each slice. Lesions included both subcortical structures and cortex; the areas greatest overlap were in the caudate and putamen. Panel B shows axial slices of the individual MRI scans (T1 or T2-weighted is indicated).
Psychiatric and Neuropsychological Assessment
All participants received a neurological exam, and a psychiatric and neuropsychological assessment examining general intelligence, attention, language, visuospatial skills, visual and verbal memory, and executive functions. Depressive and anxiety symptoms were measured using the Hamilton Depression Scale (HamD) (27) and the Hamilton Anxiety Scale.(28) Other neuropsychiatric symptoms were coded using the Present State Exam.(29) The 20 item Toronto Alexithymia scale was administered.(30, 31) Global functioning (Global Assessment of Functioning - GAF), (3) functioning on activities of daily living (Johns Hopkins Functioning Inventory - JHFI), functional independence (Functional Independence Measure - FIM) (32), strengths of social ties (Social Ties checklist) and functioning (Social Functioning Exam – SFE) were also measured.(33, 34) The Wechsler Adult Intelligence Scale-Revised (WAIS-R) (35) and the National Adult Reading Test (NART) (36) were used to assess current and premorbid general intelligence, respectively. Visuospatial processing was assessed with the Rey Complex Figure Test (RCFT) (37) and the Facial Recognition Test.(38) Visual memory was assessed with the RCFT immediate and delayed recall conditions, (37) and the Benton Visual Retention Test.(39) Attention was assessed with the WAIS-R Digit Span subtest.(35) Language was evaluated with a short form of the Boston Naming Test (40) and Controlled Oral Word Association (COWA).(41) Executive functions were assessed by examining the number of perseverative errors made on the Wisconsin Card Sorting Test (WCST).(42) Visual neglect was assessed with the line bisection task constituted by 18 staggered lines of 20-mm, 40-mm, and 60-mm in length.(43) The distance between the left edge of each line and the subject’s mark showing the subjective midpoint was measured to the closest millimeter.
Activation stimuli
Emotions were induced using stimuli chosen from an extensive database of emotionally evocative color pictures previously normed for emotional intensity and valence.(44) Each stimulus set contained eighteen pictures displayed for six seconds each. Sets were equivalent based on the ratings of normal subjects for mean valence and intensity of emotion. The stimuli and detailed protocol have been described previously.(45) Prior to viewing each image set, subjects were instructed they would be watching a series of emotionally-laden and neutral pictures (scenes and objects) and they should allow the stimuli to influence their emotional state. Subjects were told that at the end of each stimulus set they would be asked to rate the intensity of their feelings with the aid of a visual Likert scale ranging from 0 to 10, with zero being the absence of that specific emotion and ten being a very intense emotional experience. Each set of stimuli was shown once in a pseudo-random order on a computer monitor. The emotions tested for intensity of experience were happiness, amusement, anger, disgust, sadness and fear. Amusement and happiness were analyzed as the main dependent variables in response to positive stimuli. For the negative stimuli, the main dependent variables were fear, disgust, and anger.
It is plausible that participants may have experienced emotions inconsistent with the valence of the stimuli (e.g., anger during the presentation of positive stimuli). Emotions inconsistent with negative stimuli were tested using amusement and happiness variables as dependent measures, whereas emotions inconsistent with positive stimuli were tested using anger, disgust, and fear.
MRI Imaging Acquisition and Processing
T1-, T2-, and proton density (PD)-weighted images were acquired using a 1.5 tesla GE Signa Scanner. The field of view for all images was 26×26cm and the matrix was 256×192. Technical parameters for T1-weighted images included 1.5mm slice thickness, SPGR sequence, flip angle= 40 degrees, echo time (TE)= 5ms, repetition time (TR)= 24ms, and number of excitations (NEX)= 2. Two-dimensional PD and T2 sequences were acquired with the following parameters: slice thickness= 3.0mm for PD and 4.0mm for T2, TE= 36ms for PD and 96ms for T2; TR= 3000ms, NEX= 1. MR images were analyzed with locally developed software (BRAINS2).(46) All MRI scans were realigned parallel to the anterior-posterior commissure line and interhemispheric fissure and placed into standard Talairach space.(47)
PET Imaging Acquisition and Processing
PET data were collected with a GE-4096 PLUS scanner using the [15O] water method (50 mCi/injection).(48, 49) Fifteen slices with an intrinsic in-plane resolution of 6.5mm FWHM and a 10cm axial field-of-view were acquired. Images were reconstructed using a Butterworth filter (cutoff frequency= 0.35 Nyquist). A “scout” injection (15 mCi) estimated bolus arrival time for each participant; stimulus onset was adjusted accordingly to begin fifteen seconds prior to estimated bolus arrival.(50) Functional imaging began at the time of injection and lasted for 100 seconds. Count activity was collected in five second bins; a 40-second summed image containing the bins immediately post bolus transit were utilized in the analyses. Each subject’s PET and MR images were co-registered using previously described methods.(51) The hand-traced outlines of lesion extent from the MRI were used to exclude damaged regions from the PET analyses. PET count images were normalized to a value of 1 by dividing by the global activity. An 18mm Hanning filter was applied to the PET images.
Data Analysis
Clinical Data
Demographic, psychiatric, neuropsychological, psychosocial and physical functioning data, and emotional responses were examined with nonparametric tests: Mann Whitney U tests for between group differences and Wilcoxon Signed Rank Testing for within group differences. Spearman’s correlation coefficient was computed to determine association between variables.
Emotion Induction and PET Imaging Data
Amusement and happiness were the dependent variables in response to positive stimuli. For the negative stimuli, the dependent variables were fear, disgust, and anger. Brain-wide voxel-wise between-group differences were examined using a non-parametric randomization method described in detail elsewhere.(52-55) This analysis compared lesion and healthy subjects on functional images obtained in response to emotional stimuli controlling for functional activity obtained in response to neutral stimuli. An uncorrected p-value of 0.005 was used as significance threshold as in our previous studies.(52, 55) Only areas exceeding 50 contiguous voxels were included in order to omit isolated outlying values. Peaks are identified based on visual inspection of coregistered MRI scans and PET images. Interpretation of the direction of the change in activity in each group was determined by examining the T-max for each significant peak.
Results
Demographic, psychiatric, psychosocial and physical functioning variables
All MCA stroke subjects were aware of having suffered a stroke. Stroke and comparison groups did not differ on age or education. Significant group effects were found for GAF, JHFI and FIM. Subjects with stroke showed more severe psychopathology (PSE) in general, and depressive symptoms (HamD) in particular (Table 1).
Table 1.
Demographic, psychiatric, physical independence, psychosocial functioning data
| HV | MCA | |||||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | U | p | |
| Age | 65.5 | (6.3) | 66.5 | (8.5) | 33.0 | 0.82 |
| Education | 15.6 | (4.1) | 12.5 | (2.8) | 19.0 | 0.13 |
| GAF | 83.3 | (7.7) | 71.8 | (11.5) | 14.0 | 0.04 |
| PSE | 3.2 | (2.8) | 14.3 | (12.6) | 7.5 | 0.005 |
| Ham-D | 3.8 | (2.7) | 16.3 | (9.5) | 4.0 | 0.001 |
| Ham-A | 6.8 | (5.2) | 12.7 | (7.2) | 17.5 | 0.08 |
| JHFI | 0.0 | (0.0) | 5.0 | (1.3) | 0.0 | <0.001 |
| FIM | 71.3 | (1.2) | 57.8 | (8.1) | 0.0 | <0.001 |
| SFE | 0.06 | (0.05) | 0.13 | (0.09) | 17.0 | 0.08 |
| STC | 2.5 | (0.91) | 3.5 | (1.5) | 21.5 | 0.18 |
| TAS-20 | 41.6 | (9.8) | 52.2 | (7.3) | 7.0 | 0.007 |
| TAS-F1 | 9.7 | (3.3) | 13.6 | (4.2) | 13.0 | 0.048 |
| TAS-F2 | 10.1 | (2.1) | 12.6 | (2.2) | 13.0, | 0.048 |
| TAS-F3 | 10.6 | (1.9) | 12.2 | (2.1) | 14.0 | 0.062 |
Legend
GAF: Global Assessment of Functioning
PSE: Present State Exam
Ham-D: Hamilton Depression Scale
Ham-A: Hamilton Anxiety Scale
JHFI: Johns Hopkins Functioning Inventory
FIM: Functional Independence Measure
SFE: Social Functioning Exam
STC: Social Ties Checklist
TAS-20: Toronto Alexithymia Scale – 20 items
TAS-F1 = difficulty identifying feelings factor
TAS-F2 = difficulty describing feelings factor
TAS-F3 = externally-oriented thinking factor
Patients with right MCA infarcts (MCA), Healthy Volunteers (HV).
Total alexithymia score showed a significant group effect. Factors representing difficulty identifying feelings and difficulty describing feelings were significantly greater among stroke patients, while externally oriented thinking just failed to reach significant difference (Table 1). Based on PSE symptoms, two subjects with right MCA met criteria for major depression (3) and two met criteria for nondysphoric depression.(56, 57) No healthy volunteers met such criteria. While group differences on pathological emotions assessed using the PSE did not reach statistical significance, qualitative inspection of the data indicated that stroke subjects were two times more likely to be irritable (33% vs. 16.7% in healthy volunteers), were four times more likely to show mood lability (33% vs. 8.3% in healthy volunteers) and showed blunted affect (16.7% vs. 0% in healthy volunteers). While none of the healthy volunteers reported loss of interest, 50% of patients with stroke showed moderate or severe anhedonia. Three subjects with MCA stroke reported listlessness and lack of energy compared with one healthy volunteer. Slowness and underactivity were not observed in either stroke patients or comparison subjects. Neither group had altered speech in terms of speed, volume, quantity or quality.
Neuropsychological Assessment
Neuropsychological data are shown in Table 2. Significant group differences were found on verbal, performance, and full scale IQ. Difference in estimated premorbid intelligence (NART) did not reach statistical significance. No significant group effect was found for attention (WAIS-R Digit Span), but stroke subjects showed weakness in some language (Boston Naming Test, but not Multilingual Aphasia Exam Token Test) and executive functioning measures (WCST- Perseverative Errors, but not COWA), as well as visuospatial encoding and immediate recall (Benton Visual Retention and RCFT-immediate recall). There were no significant group differences on visual constructional abilities (RCFT-copy), visual recognition for faces (Facial Recognition) and delayed figure recall (RCFT–delayed condition). A significant group difference was found for the 60-mm line bisection but not for the 40- or 20-mm length conditions.
Table 2.
Neuropsychological Functioning
| HV (N=12) | MCA (N=6) | |||||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | U | p | |
| General Intelligence | ||||||
| WAIS-R VIQ | 110.8 | (14.2) | 86.0 | (2.8) | 0.0 | <0.001 |
| WAIS-R PIQ | 121.7 | (21.1) | 84.4 | (9.6) | 3.0 | 0.003 |
| WAIS-R FIQ | 118.1 | (20.0) | 94.4 | (5.0) | 0.0 | <0.001 |
| NART | 115.0 | (11.4) | 105.0 | (7.8) | 14.5 | 0.06 |
| Visuospatial Functions | ||||||
| RCFT -Copy | 26.6 | (6.2) | 22.3 | (4.2) | 14.5 | 0.06 |
| Facial Recognition | 46.6 | (3.3) | 46.0 | (5.7) | 32.0 | 0.96 |
| Line Bisection 2 | 9.6 | (0.5) | 9.8 | (1.0) | 25.5 | 0.62 |
| Line Bisection 4 | 19.25 | (0.7) | 19.8 | (0.8) | 18.0 | 0.19 |
| Line Bisection 6 | 28.9 | (1.2) | 30.2 | (0.7) | 11.0 | 0.04 |
| Visual Memory | ||||||
| RCFT – Immediate | 12.5 | (3.3) | 7.4 | (4.2) | 11.5 | 0.03 |
| RCFT – Delayed | 11.5 | (3.8) | 7.4 | (4.9) | 20.0 | 0.22 |
| BV – Total | 29.55 | (2.4) | 23.8 | (5.6) | 9.0 | 0.04 |
| Attention | ||||||
| Digit Span | 9.3 | (2.1) | 8.2 | (1.5) | 18.5 | 0.15 |
| Language | ||||||
| Boston Naming Test | 27.1 | (3.0) | 21.3 | (6.6) | 11.5 | 0.03 |
| MAE Token Test | 42.3 | (1.7) | 38.0 | (6.2) | 19.5 | 0.18 |
| Executive Functions | ||||||
| WCST Pers. Errors | 8.5 | (5.3) | 25.0 | (16.3) | 6.5 | 0.005 |
| MAE COWA | 32.7 | (10.9) | 24.3 | (11.4) | 17.5 | 0.12 |
Legend
WAIS-R VIQ: Wechsler Adult Intelligence Scale Verbal Intelligence Quotient
WAIS-R PIQ: Wechsler Adult Intelligence Scale Performance Intelligence Quotient
WAIS-R FIQ: Wechsler Adult Intelligence Scale Full Scale Intelligence Quotient
NART: North American Reading Test
RCFT: Rey-Osterrieth Complex Figure Test
BV: Benton Visual Retention Test
MAE Token Test: Multilingual Aphasia Exam Token Test
WCST Pers. Errors: Wisconsin Card Sorting Test Perseverative Errors
MAE COWA: Multilingual Aphasia Exam Controlled Oral Word Association
Patients with right MCA infarcts (MCA), Healthy Volunteers (HV)
Emotional Experience and Arousal
The pleasant stimuli evoked moderate to high levels of happiness and amusement in healthy comparison subjects. Consistent with previous studies (45) and with older age “positivity bias”, (58) healthy volunteers showed mild/moderate levels of happiness and amusement in response to the neutral stimuli (Table 3). Stroke subjects showed low levels of happiness and amusement in response to neutral stimuli and essentially no emotional changes in response to pleasant stimuli, consistent with an emotional blunting observed on clinical exam. Significant group differences were found for the experience of amusement in response to pleasant stimuli, and happiness in response to neutral stimuli (both greater in the comparison group). Unpleasant stimuli were experienced with moderate levels of fear, anger and disgust by healthy comparisons and stroke subjects with no significant group differences. There was no significant group effect on emotions evoked inconsistently with the valence of the stimuli (data not shown). No group effects on subjective arousal reached statistical significance [Positive stimuli healthy volunteers (HV) mean = 7, SD=2.2 - rMCA mean = 5.2 SD=3.7 (U=24, p>.2); negative stimuli HV mean = 6.1 SD=2.6 - rMCA mean = 6.5 SD=4.1 (U=29.5, p>.5); neutral stimuli HV mean = 4.8 SD=2.1 - rMCA mean = 2.7 SD=3.2 (U=22.5, p>.2)].
Table 3.
Responses to Emotion Evoking Stimuli
| Experienced Emotion | Affective Stimuli | Healthy Volunteers (N=12) | Right MCA Stroke (N=6) | Between-Group | Within-Group HV | Within-Group MCA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | U | p | Z | p | Z | P | ||
| Positive Emotion | |||||||||||
| Amusement | Neutral | 3.4 | (2.2) | 2.5 | (2.8) | 30.5 | 0.62 | -1.87 | 0.06 | -0.18 | 0.85 |
| Pleasant | 6.1 | (3.1) | 2.3 | (2.7) | 12.5 | 0.02 | |||||
| Happiness | Neutral | 6.0 | (2.8) | 2.7 | (2.8) | 12.5 | 0.02 | -2.53 | 0.01 | -1.48 | 0.14 |
| Pleasant | 8.1 | (1.5) | 5.7 | (3.6) | 21.5 | 0.18 | |||||
| Negative Emotion | |||||||||||
| Fear | Neutral | 1.4 | (1.8) | 0.5 | (1.2) | 22.5 | 0.21 | -2.85 | 0.004 | -2.03 | 0.04 |
| Unpleasant | 5.9 | (3.8) | 4.3 | (2.9) | 27.0 | 0.44 | |||||
| Disgust | Neutral | 1.2 | (2.3) | 0.7 | (1.2) | 35.0 | 0.96 | -2.94 | 0.003 | -2.02 | 0.04 |
| Unpleasant | 6.8 | (3.0) | 4.7 | (3.5) | 22.5 | 0.21 | |||||
| Anger | Neutral | 0.3 | (0.9) | 1.0 | (2.4) | 35.0 | 0.96 | -2.68 | 0.007 | -1.83 | 0.07 |
| Unpleasant | 5.1 | (3.7) | 4.7 | (4.0) | 33.0 | 0.82 | |||||
Abbreviations: Healthy Volunteers (HV), Right MCA stroke (MCA).
Because stroke patients experienced depression of mild to moderate severity (mean HamD scores = 16), the lack of pleasant experience to positive stimuli could be the result of depression. Therefore, the data were reexamined in two ways. In one analysis only the three patients with lower HamD scores (<14) were included. In another analysis, HamD scores were used as a covariate. In both analyses, results remained essentially the same, signifying that depressed mood or anhedonia was minimally responsible for changes in emotional experience. Notably depression severity (HamD) among MCA patients did not show significant associations with happiness or amusement responses (.13<rs<.5, all ps >.4). In addition, the responses of six patients with left focal basal ganglia lesions were examined (Paradiso et al., under review) and may help resolve this question. Five out of six (83%) patients with focal left basal ganglia damage suffered significant depression (HamD scores ranged from 16 to 25) and participated in the same experiment as the one reported in the present study but did not experience reduction in positive or negative emotion. This suggests that by and large the changes in emotions experienced by subjects with right MCA damage may not originate from depression per se.
Associations experienced emotion/neuropsychological functioning
Correlations between emotion responses and neuropsychological variables with significant between group effects (Tables 2 & 3) were computed among patients with MCA. In response to pleasant stimuli, FSIQ (r=-.89, p<.05) was strongly and indirectly correlated with experienced happiness, signifying that after MCA stroke greater emotional responses were associated with poorer global neurocognitive functioning. No other association reached statistical significance.
Brain activity in response to emotional stimuli
As predicted, subjects with right MCA stroke showed lower brain activity in relationship with healthy comparison subjects in the right anterior cingulate cortex (ACC) in response to positive emotional stimuli. This brain-wide analysis of brain activity in response to pleasant stimuli revealed additional regions of lower brain activity consistent with the emotional nature of the stimuli in the right MCA patients. These regions included dorsal and medial prefrontal cortex bilaterally, right thalamus, left primary and association visual cortices (BA 17/19) and left cerebellum. Frontal regions included the left superior frontal gyrus (BA 10), the left and right medial frontal gyrus (BA 11). It is noteworthy that reduction of brain activity was not limited to the side of the lesion but included the contralateral (left) hemisphere. In response to pleasant stimuli there were also brain regions of relatively increased activity in the right MCA patients [e.g., bilaterally in the parahippocampal gyrus (BA 19 and 36) and in the left visual striate and extrastriate cortex (BA 17, 18, and 31)].
Significant changes in the ACC were not observed in response to unpleasant stimuli as well as other changes in the frontal-thalamic-cerebellar system. Indeed in response to the negative stimuli the changes in brain activity were rather limited as the essentially intact emotional responses in right MCA stroke patients may have predicted. Subjects with right MCA stroke showed decreased activity only in the left midbrain, but there were also regions of increased activity (e.g., the left cerebellum) (Table 4).
Table 4.
Brain activity in stroke patients compared to healthy volunteers
| Activity Δ (positive - neutral) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Activity Δa | Tal. Coor (mm)b | Volumec | ||||||||
| Region / Structure | BA | MCA | HV | MCA-HV | x | y | z | (cc) | (voxels) | |
| Superior frontal lobe | 10 | -2.44 | 0.33 | -3.11 | -19 | 44 | 16 | 0.3 | 162 | |
| -3.18 | -0.11 | -4.38 | -19 | 50 | -9 | 0.3 | 118 | |||
| Medial frontal lobe | 10,11 | -3.46 | -0.08 | -2.88 | 6 | 53 | -9 | 0.2 | 80 | |
| Anterior cingulate | 32 | -2.25 | 1.27 | -3.12 | 10 | 44 | 8 | 0.6 | 291 | |
| 2.77 | -0.55 | 3.55 | -26 | -52 | 0 | 0.2 | 116 | |||
| Parahippocampus | 3.31 | -1.25 | 3.34 | 36 | -30 | -17 | 0.1 | 63 | ||
| Thalamus | -1.69 | 1.92 | -3.59 | 14 | -26 | -3 | 0.9 | 437 | ||
| Visual cortex | 17,19 | -4.20 | -0.34 | -3.72 | -7 | -83 | 13 | 1.3 | 587 | |
| -2.62 | 1.83 | -3.17 | -25 | -76 | 31 | 0.1 | 62 | |||
| 1.46 | -0.67 | 3.44 | 19 | -70 | 0 | 0.4 | 181 | |||
| 2.88 | 0.06 | 3.66 | 47 | -60 | -9 | 0.8 | 360 | |||
| 1.04 | 0.26 | 3.78 | 24 | -56 | 39 | 0.4 | 180 | |||
| 1.41 | 0.23 | 3.56 | 17 | -46 | 32 | 0.2 | 96 | |||
| Cerebellum | -4.41 | 0.90 | -3.81 | -3 | -62 | -6 | 0.6 | 256 | ||
| Activity Δ (negative - neutral) | ||||||||||
| Midbrain | N/A | -1.74 | 3.03 | -2.97 | -5 | -16 | -12 | 0.3 | 133 | |
| Cerebellum | N/A | 2.56 | -1.74 | 2.96 | -29 | -76 | -18 | 0.1 | 52 | |
Legend
Brain regions where the rCBF in patients with stroke significantly differs from that of control subjects are shown (p ≤ 0.005, Vol.≥ 50 voxels).
T(max), the highest t value identified in the peak
Talairach coordinates indicate the location of the highest peak within the region of significance.
Indicates the volume and number of voxels in the peaks exceeding α = 0.005.
Abbreviations: Brodmann’s Area (BA), patients with right MCA infarcts (MCA), Healthy Volunteers (HV).
Discussion
This study combined lesion and neuroimaging methods to examine the effects of lateralized right MCA stroke on activity in neural nodes distant from the damaged area. Damage in the territory of the right MCA affected pleasant emotional experience but did not alter experiential responses to unpleasant stimuli. As predicted, blunted emotional response to pleasant stimuli was associated with reduced activity in the right ACC. In addition several other regions contralateral (i.e., dorsal and medial prefrontal cortex, visual association cortex and cerebellum) and ipsilateral (i.e., thalamus, and dorsal prefrontal cortex) to the damage showed reduced activity. Areas of increased activity included the parahippocampal gyri.
The main finding in this study is that blunted response to positive emotional stimuli following right MCA stroke may be attributed in part to reduced activity in brain regions distant from the site of direct damage. Based on its functions, the brain region that has the strongest bearing on blunted emotional experience as a consequence of right MCA stroke is the ACC. Structural or functional changes in the ACC have been associated with alexithymia.(59, 60) The acquired form of alexithymia can occur after lesions to brain regions in the territory of the right MCA (8) and is significant because it can alter the traditional presentation of depression.(56) Consistent with this view, patients with right MCA stroke in this study showed greater alexithymia scores than comparison subjects. In particular, significant effects were found for inability to recognize and describe feelings. The ACC contains evolutionary novel spindle-shaped neurons which may represent the neural underpinning of the interaction between emotion and perception, (61, 62) and may be particularly vulnerable to anoxic conditions.(63)
Whereas the ipsilateral thalamus and dorsal prefrontal cortex also showed lower activity, other regions showed reduced activity contralateral (left dorsal and medial prefrontal cortex, visual association cortex and cerebellum) to the lesion site. Therefore, impairment in the experience of pleasant emotions following right MCA stroke may result from additional dysfunction in frontal (and cerebellar (64, 65)) networks normally promoting linguistic labeling of emotions.(66-68) While this functional impairment to the left hemisphere may raise concern that subjects were unable to appropriately speak their emotional reactions due to impairment in language (while experience was intact), this possibility is low in light of the little verbal output needed to express the emotion, the exclusion of subjects with significant language difficulties, and especially because of the relative lack of alteration of MCA stroke subjects’ responses to negative stimuli. Nonetheless, the lower activity in the left hemisphere is consistent with the language-based conceptualization of acquired alexithymia.(10, 59, 69, 70)
Brain laterality and experienced emotions
It is well-established that right hemisphere damage causes significantly greater alexithymia and apathy compared to left hemisphere damage.(8, 9) The present study was not concerned with replicating this phenomenon, but with the mechanisms associated with changes in emotional experience after right MCA stroke. For this reason and because subjects with comparable lesion size stroke in the territory of the left MCA would have had language impairments not allowing them to meet inclusion criteria, comparison subjects with left hemisphere damage were not included. In addition, subjects with damage restricted to left striate/lenticular nuclei have shown a different pattern of changes in emotion experience (Paradiso et al., under review) but would not have been appropriate for direct comparison with subjects in this study due to the difference in the size of brain tissue damage. It should be also noted that the findings in the present study cannot be attributed to any brain lesion because studies using the same design but lesions in other parts of the brain have reported differing results.(71, 72) Finally, emotions were evoked using scenes and objects including no depictions of humans; different results may have been obtained with human stimuli or with a stimulus modality other than visual.
Limitations notwithstanding, the findings in the present study have bearing on the emotion laterality debate and are therefore discussed in light of two predominant models of laterality and emotion experience.(19, 22-24) Briefly, one model asserts that the right hemisphere is dominant for the experience of all emotions, (19-21) while the competing other posits a right hemisphere specialization for unpleasant emotions and a left hemisphere specialization for positive emotions.(22-24) Based on the right hemisphere model (i.e., dominance for all emotion) subjects with right MCA stroke are expected to show blunted emotions in response to both pleasant and unpleasant stimuli. Based on the laterality hypothesis (i.e., right hemisphere dominance for negative emotions), subjects with right MCA stroke are expected to show blunted responses to unpleasant stimuli and normal responses to pleasant stimuli. The results of the present study are consistent with the right hemisphere hypothesis of emotion but surprisingly only in part. These conclusions are limited to brain regions affected by occlusion of the right MCA (e.g., frontal polar regions are excluded).(22) Parsimoniously, these results support the complexity of the brain substrates engaged in emotional experience,(73) highlighting the putative presence of redundant circuits (e.g., the parahippocampal region) supporting processing of emotional material after brain damage, similarly to what occurs to language after left hemisphere lesions.(74)
Cognitive impairment
As expected, stroke victims showed greater cognitive deficits including some degree of visual neglect (but not deficit in visual constructional abilities or visual recognition). Notably, while cognitive deficits were present in stroke patients, their affective responses to unpleasant stimuli were similar to those of volunteers. Therefore while the findings in this study warrant replication with a larger sample of MCA stroke subjects, a provisional conclusion is that the cognitive deficits associated with right MCA stroke do not preclude experiencing congruent emotions in response to stimuli designed to evoke negative affective states. Nonetheless it should be noted that measuring the quantity of an emotional response captures only in part the extent to which a response is normal. Among those patients with MCA stroke with the greater positive emotional responses were those with poorer FSIQ. This raises the possibility that the emotional responses measured in patients as quantitatively different from those of comparison subjects were indeed also qualitatively different representing perhaps an aspect of undue cheerfulness.(75)
Depression and right hemisphere damage
We did not interview the subjects’ families to assess whether anhedonia was present before the ischemic event. Anhedonia was present in 50% of subjects with right MCA stroke and none of the age-matched volunteers. One caveat in the interpretation of the findings in this study concerns the extent to which the impairment in emotional experience observed in patients with right MCA stroke may be due to the brain damage per se or to depression. First among MCA patients, severity of depression did not show significant associations with positive emotional responses. Analyses controlling for severity of depression, and excluding subjects with moderate depression essentially did not influence the findings concerning emotional responses to pleasant stimuli. In addition, lack of reduction in positive or negative emotion in response to pleasant or unpleasant stimuli in five patients with clinically significant depression and left focal basal ganglia lesions (Paradiso et al., under review) suggests that the changes in emotions experienced by subjects with right MCA damage may not be attributable to depression per se.
Nonetheless the mild to moderate severity of depression in our sample of MCA stroke subjects requires some elaboration. More than one third of patients experience depression following stroke (76) but mania or other psychiatric disorders are rare.(77) Damage to anterior (cortical and subcortical) left hemisphere regions is known to be associated with depression (5), but variability in depression severity exists within the left hemisphere depending in the anterior-posterior location of the lesion.(77) Right hemisphere damage is more rarely associated with major depression (4), but is often the underpinning of a condition known as nondysphoric depression (56) comprised of ideational (e.g., lack of self-esteem, loss of hope, thoughts of death) and vegetative symptoms of depression and characterized by the paradoxical absence of emotional symptoms (e.g., sad mood or anhedonia).(57) Stroke may therefore predispose to depression which may conceivably take differing phenomenological features depending on the functions of the brain regions that were damaged.
Clinical relevance
The MCA is the brain vessel most commonly affected by ischemic stroke with more than 50% of all cerebral vascular accidents in this territory.(1) Right hemisphere strokes are greatly under-recognized, (6) perhaps because emotional deficits are not routinely assessed. Damage to the territory of the right MCA may include the lenticular/striate nuclei and lead to emotional incontinence, (78) but apathy and alexithymia are decisively more common.(8) Consistent with these reports (8, 9), the patients with MCA stroke in the present study showed varying degrees of irritability, mood lability depression, anhedonia, and blunted affect. This study offers a preliminary explanation of the mechanisms of emotional changes following right-sided MCA damage with dysfunction of the right ACC leading to emotional blunting (10, 56) and ineffective modulation of emotional responses subsequent to failing frontal-thalamic-cerebellar networks leading to emotional incontinence.(71, 72) Alerting patients to the blunted emotional arousal to positive stimuli following right MCA stroke and instructing them to focus on the hedonic valence of situations may reduce nondysphoric depression and improve recovery.
Acknowledgments
Dr. Paradiso was supported by the Edward J. Mallinckrodt Jr. Foundation, the Dana Foundation and an NIH Career development award (5K23AG027837). Dr. Robinson was supported by NIMH research scientist award MH-00163 and NIMH grant MH52879. Dr. Ponto was supported in part by NIH P50DC03189, RO1 DA10551, NIH 1 R01 MH60990-01, NIH 7 R01 CA74959-04. Dr. Tranel was supported in part by Program Project Grant NINDS NS19632 and NIDA DA022549. The authors thank Albert Abreu, Teresa Kopel, Natalie Robinson, Erika Holm, Dimitrios Kozoukas, Brad McConville and Gene Zeien for assistance with this research.
Footnotes
The authors have no conflict of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ng YS, Stein J, Ning M, Black-Schaffer RM. Comparison of clinical characteristics and functional outcomes of ischemic stroke in different vascular territories. Stroke. 2007;38:2309–2314. doi: 10.1161/STROKEAHA.106.475483. [DOI] [PubMed] [Google Scholar]
- 2.Ture U, Yasargil MG, Al-Mefty O, Yasargil DC. Arteries of the insula. J Neurosurg. 2000;92:676–687. doi: 10.3171/jns.2000.92.4.0676. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : Dsm-iv. Washington, DC: American Psychiatric Association; 1994. American Psychiatric Association. Task Force on DSM-IV. [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : Dsm-iv-tr. Washington, DC: American Psychiatric Association; 2000. American Psychiatric Association. Task Force on DSM-IV. [Google Scholar]
- 5.Robinson RG. The clinical neuropsychiatry of stroke. Cambridge ; New York: Cambridge University Press; 2006. [Google Scholar]
- 6.Foerch C, Misselwitz B, Sitzer M, Berger K, Steinmetz H, Neumann-Haefelin T. Difference in recognition of right and left hemispheric stroke. Lancet. 2005;366:392–393. doi: 10.1016/S0140-6736(05)67024-9. [DOI] [PubMed] [Google Scholar]
- 7.Manes F, Paradiso S, Springer JA, Lamberty G, Robinson RG. Neglect after right insular cortex infarction. Stroke. 1999;30:946–948. doi: 10.1161/01.str.30.5.946. [DOI] [PubMed] [Google Scholar]
- 8.Spalletta G, Pasini A, Costa A, De Angelis D, Ramundo N, Paolucci S, Caltagirone C. Alexithymic features in stroke: Effects of laterality and gender. Psychosom Med. 2001;63:944–950. doi: 10.1097/00006842-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Brodaty H, Sachdev PS, Withall A, Altendorf A, Valenzuela MJ, Lorentz L. Frequency and clinical, neuropsychological and neuroimaging correlates of apathy following stroke - the sydney stroke study. Psychol Med. 2005;35:1707–1716. doi: 10.1017/S0033291705006173. [DOI] [PubMed] [Google Scholar]
- 10.Lane RD, Ahern GL, Schwartz GE, Kaszniak AW. Is alexithymia the emotional equivalent of blindsight? Biol Psychiatry. 1997;42:834–844. doi: 10.1016/s0006-3223(97)00050-4. [DOI] [PubMed] [Google Scholar]
- 11.Sifneos PE. The prevalence of ‘alexithymic’ characteristics in psychosomatic patients. Psychother Psychosom. 1973;22:255–262. doi: 10.1159/000286529. [DOI] [PubMed] [Google Scholar]
- 12.Robinson RG, Boston JD, Starkstein SE, Price TR. Comparison of mania and depression after brain injury: Causal factors. Am J Psychiatry. 1988;145:172–178. doi: 10.1176/ajp.145.2.172. [DOI] [PubMed] [Google Scholar]
- 13.Starkstein SE, Robinson RG. Mechanism of disinhibition after brain lesions. J Nerv Ment Dis. 1997;185:108–114. doi: 10.1097/00005053-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Manes F, Paradiso S, Robinson RG. Neuropsychiatric effects of insular stroke. J Nerv Ment Dis. 1999;187:707–712. doi: 10.1097/00005053-199912000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Habib M. Athymhormia and disorders of motivation in basal ganglia disease. J Neuropsychiatry Clin Neurosci. 2004;16:509–524. doi: 10.1176/jnp.16.4.509. [DOI] [PubMed] [Google Scholar]
- 16.Andrews RJ. Transhemispheric diaschisis. A review and comment. Stroke. 1991;22:943–949. doi: 10.1161/01.str.22.7.943. [DOI] [PubMed] [Google Scholar]
- 17.Berthoz S, Artiges E, Van De Moortele PF, Poline JB, Rouquette S, Consoli SM, Martinot JL. Effect of impaired recognition and expression of emotions on frontocingulate cortices: An fmri study of men with alexithymia. Am J Psychiatry. 2002;159:961–967. doi: 10.1176/appi.ajp.159.6.961. [DOI] [PubMed] [Google Scholar]
- 18.Paradiso S, Vaidya JG, McCormick LM, Jones A, Robinson RG. Aging and alexithymia - association with reduced right rostral cingulate volume. American Journal of Geriatric Psychiatry. 2008 doi: 10.1097/JGP.0b013e31817e73b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 20.Sackeim HA, Gur RC, Saucy MC. Emotions are expressed more intensely on the left side of the face. Science. 1978;202:434–436. doi: 10.1126/science.705335. [DOI] [PubMed] [Google Scholar]
- 21.Levine SC, Levy J. Perceptual asymmetry for chimeric faces across the life span. Brain Cogn. 1986;5:291–306. doi: 10.1016/0278-2626(86)90033-3. [DOI] [PubMed] [Google Scholar]
- 22.Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-t. [DOI] [PubMed] [Google Scholar]
- 23.Robinson RG, Kubos KL, Starr LB, Rao K, Price TR. Mood disorders in stroke patients. Importance of location of lesion. Brain. 1984;107(Pt 1):81–93. doi: 10.1093/brain/107.1.81. [DOI] [PubMed] [Google Scholar]
- 24.Sackeim HA, Greenberg MS, Weiman AL, Gur RC, Hungerbuhler JP, Geschwind N. Hemispheric asymmetry in the expression of positive and negative emotions. Neurologic evidence. Arch Neurol. 1982;39:210–218. doi: 10.1001/archneur.1982.00510160016003. [DOI] [PubMed] [Google Scholar]
- 25.De Renzi E, Vignolo LA. The token test: A sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 29.Wing JK, Cooper JE, Sartorius N. Measurement and classification of psychiatric symptoms; an instruction manual for the pse and catego program. New York: Cambridge University Press; 1974. [Google Scholar]
- 30.Bagby RM, Parker JD, Taylor GJ. The twenty-item toronto alexithymia scale--i. Item selection and cross-validation of the factor structure. J Psychosom Res. 1994;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 31.Bagby RM, Taylor GJ, Parker JD. The twenty-item toronto alexithymia scale--ii. Convergent, discriminant, and concurrent validity. J Psychosom Res. 1994;38:33–40. doi: 10.1016/0022-3999(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 32.FIM GA. Buffalo: State University of New York at Buffalo; 1993. [Google Scholar]
- 33.Robinson RG, Szetela B. Mood change following left hemispheric brain injury. Ann Neurol. 1981;9:447–453. doi: 10.1002/ana.410090506. [DOI] [PubMed] [Google Scholar]
- 34.Starr LB, Robinson RG, Price TR. Reliability, validity, and clinical utility of the social functioning exam in the assessment of stroke patients. Exp Aging Res. 1983;9:101–106. doi: 10.1080/03610738308258434. [DOI] [PubMed] [Google Scholar]
- 35.Wechsler D. Wechsler adult intelligence scale - revised. New York: Psychological Corp; 1981. [Google Scholar]
- 36.Nelson HE. National adult reading test (nart) test manual. Windsor Berks: NFER-NELSON; 1982. [Google Scholar]
- 37.Meyers JE, Meyers KR. Rey complex figure test under four different administration procedures. Clinical Neuropsychologist. 1995;9:63–67. [Google Scholar]
- 38.Benton AL. Contributions to neuropsychological assessment : A clinical manual. New York: Oxford University Press; 1994. [Google Scholar]
- 39.Sivan AB. Benton visual retention test. New York: Harcourt Brace Jovanovich; 1992. [Google Scholar]
- 40.Kaplan E, Goodglass H, Weintraub S. The boston naming test. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 41.Benton AL, Hamsher K, Sivan AB. Multilingual aphasia examination. Iowa City, IA: AJA Associates; 1994. [Google Scholar]
- 42.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin card sorting test manual: Revised and expanded. Odessa, FL: Psychological Assessment Resources, Inc; 1981. [Google Scholar]
- 43.Lezak MD. Neuropsychological assessment. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 44.Lang PJ, Bradley MA, Cuthbert BN. International affective picture system (iaps): Technical manual on affective rating. Gainsville: University of Florida; 1995. [Google Scholar]
- 45.Paradiso S, Robinson RG, Boles Ponto LL, Watkins GL, Hichwa RD. Regional cerebral blood flow changes during visually induced subjective sadness in healthy elderly persons. J Neuropsychiatry Clin Neurosci. 2003;15:35–44. doi: 10.1176/jnp.15.1.35. [DOI] [PubMed] [Google Scholar]
- 46.Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural mr image processing using the brains2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 47.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: An approach to cerebral imaging. Stuttgart: Thieme; 1988. [Google Scholar]
- 48.Herscovitch P, Markham J, Raichle ME. Brain blood flow measured with intravenous h2(15)o. I. Theory and error analysis. J Nucl Med. 1983;24:782–789. [PubMed] [Google Scholar]
- 49.Hichwa RD, Ponto LLB, Watkins GL. Clinical blood flow measurements with [15o[water and positron emission tomography. In: Emran AM, editor. Chemists’ views of imaging centers. New York: Plenum Press; 1995. pp. 401–417. [Google Scholar]
- 50.Hurtig RR, Hichwa RD, O’Leary DS, Boles Ponto LL, Narayana S, Watkins GL, Andreasen NC. Effects of timing and duration of cognitive activation in [15o]water pet studies. J Cereb Blood Flow Metab. 1994;14:423–430. doi: 10.1038/jcbfm.1994.53. [DOI] [PubMed] [Google Scholar]
- 51.Woods RP, Mazziotta JC, Cherry SR. Mri-pet registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Arndt S, Cizadlo T, Andreasen NC, Heckel D, Gold S, O’Leary DS. Tests for comparing images based on randomization and permutation methods. J Cereb Blood Flow Metab. 1996;16:1271–1279. doi: 10.1097/00004647-199611000-00023. [DOI] [PubMed] [Google Scholar]
- 53.Arndt S, Cizadlo T, O’Leary D, Gold S, Andreasen NC. Normalizing counts and cerebral blood flow intensity in functional imaging studies of the human brain. Neuroimage. 1996;3:175–184. doi: 10.1006/nimg.1996.0019. [DOI] [PubMed] [Google Scholar]
- 54.Holmes AP, Blair RC, Watson JD, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab. 1996;16:7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 55.Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD. Schizophrenia and cognitive dysmetria: A positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci U S A. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paradiso S, Vaidya J, Tranel D, Kosier T, Robinson RG. Nondysphoric depression following stroke. J Neuropsychiatry Clin Neurosci. 2008;20:52–61. doi: 10.1176/jnp.2008.20.1.52. [DOI] [PubMed] [Google Scholar]
- 57.Gallo JJ, Rabins PV, Lyketsos CG, Tien AY, Anthony JC. Depression without sadness: Functional outcomes of nondysphoric depression in later life. J Am Geriatr Soc. 1997;45:570–578. doi: 10.1111/j.1532-5415.1997.tb03089.x. [DOI] [PubMed] [Google Scholar]
- 58.Mezulis AH, Abramson LY, Hyde JS, Hankin BL. Is there a universal positivity bias in attributions? A meta-analytic review of individual, developmental, and cultural differences in the self-serving attributional bias. Psychol Bull. 2004;130:711–747. doi: 10.1037/0033-2909.130.5.711. [DOI] [PubMed] [Google Scholar]
- 59.Paradiso S, Vaidya JG, McCormick LM, Jones A, Robinson RG. Aging and alexithymia - association with reduced right rostral cingulate volume. American Journal of Geriatric Psychiatry. doi: 10.1097/JGP.0b013e31817e73b0. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci. 1998;10:525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- 61.Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci. 2001;935:107–117. [PubMed] [Google Scholar]
- 62.Nimchinsky EA, Vogt BA, Morrison JH, Hof PR. Spindle neurons of the human anterior cingulate cortex. J Comp Neurol. 1995;355:27–37. doi: 10.1002/cne.903550106. [DOI] [PubMed] [Google Scholar]
- 63.Vaidya JG, Paradiso S, Boles Ponto LL, McCormick LM, Robinson RG. Aging, grey matter, and blood flow in the anterior cingulate cortex. Neuroimage. 2007;37:1346–1353. doi: 10.1016/j.neuroimage.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 64.Paradiso S, Andreasen NC, O’Leary DS, Arndt S, Robinson RG. Cerebellar size and cognition: Correlations with iq, verbal memory and motor dexterity. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:1–8. [PubMed] [Google Scholar]
- 65.Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993;16:444–447. doi: 10.1016/0166-2236(93)90072-t. [DOI] [PubMed] [Google Scholar]
- 66.Matsumoto D, Assar M. The effects of language on judgments of universal facial expressions of emotion. Journal of Nonverbal Behavior. 1992;16:85–99. [Google Scholar]
- 67.Van Lancker D, Sidtis JJ. The identification of affective-prosodic stimuli by left- and right-hemisphere-damaged subjects: All errors are not created equal. J Speech Hear Res. 1992;35:963–970. doi: 10.1044/jshr.3505.963. [DOI] [PubMed] [Google Scholar]
- 68.Glozman ZM, Koviazina MS, Ermolaev DV. The role of the right and left hemispheres in the emotional evaluation of visual stimuli. Human Physiology. 2000;26:400–404. [PubMed] [Google Scholar]
- 69.Lamberty GJ, Holt CS. Evidence for a verbal deficit in alexithymia. J Neuropsychiatry Clin Neurosci. 1995;7:320–324. doi: 10.1176/jnp.7.3.320. [DOI] [PubMed] [Google Scholar]
- 70.Wood RL, Williams C. Neuropsychological correlates of organic alexithymia. J Int Neuropsychol Soc. 2007;13:471–479. doi: 10.1017/S1355617707070518. [DOI] [PubMed] [Google Scholar]
- 71.Turner BM, Paradiso S, Boles Ponto LL, Hichwa RD, Robinson RG. The cerebellum and emotional experience. Neuropsychologia. 2007;45:1331–1341. doi: 10.1016/j.neuropsychologia.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parvizi J, Anderson SW, Martin CO, Damasio H, Damasio AR. Pathological laughter and crying: A link to the cerebellum. Brain. 2001;124:1708–1719. doi: 10.1093/brain/124.9.1708. [DOI] [PubMed] [Google Scholar]
- 73.Stone VE, Nisenson L, Eliassen JC, Gazzaniga MS. Left hemisphere representations of emotional facial expressions. Neuropsychologia. 1996;34:23–29. doi: 10.1016/0028-3932(95)00060-7. [DOI] [PubMed] [Google Scholar]
- 74.Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke. 1999;30:749–754. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- 75.Starkstein SE, Robinson RG, Honig MA, Parikh RM, Joselyn J, Price TR. Mood changes after right-hemisphere lesions. Br J Psychiatry. 1989;155:79–85. doi: 10.1192/bjp.155.1.79. [DOI] [PubMed] [Google Scholar]
- 76.Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: A systematic review of observational studies. Stroke. 2005;36:1330–1340. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- 77.Paradiso S, Robinson RG. Minor depression after stroke: An initial validation of the dsm-iv construct. Am J Geriatr Psychiatry. 1999;7:244–251. doi: 10.1097/00019442-199908000-00009. [DOI] [PubMed] [Google Scholar]
- 78.Kim JS, Choi-Kwon S. Poststroke depression and emotional incontinence: Correlation with lesion location. Neurology. 2000;54:1805–1810. doi: 10.1212/wnl.54.9.1805. [DOI] [PubMed] [Google Scholar]


