Abstract
Background
Opiate abuse is a chronic relapsing disorder and maintaining prolonged abstinence remains a major challenge. Protracted abstinence is characterized by lowered mood and clinical studies show elevated co-morbidity between addiction and depressive disorders. At present, their relationship remains unclear and has been little studied in animal models. Here we investigated emotional alterations during protracted abstinence, in mice with a history of chronic morphine exposure.
Methods
C57BL6J mice were exposed to a chronic intermittent escalating morphine regimen (20-100mg/kg). Physical dependence (naloxone-precipitated withdrawal), despair-related (tail suspension test) and social behaviors were examined after 1 or 4 weeks of abstinence. Stress hormones and forebrain bioamine levels were analyzed at the end of morphine regimen and after 4 weeks abstinence. Finally, we examined the effects of chronic fluoxetine during abstinence on morphine-induced behavioral deficits.
Results
Acute naloxone-induced withdrawal was clearly measurable after 1 week, and became undetectable after 4 weeks. In contrast, social and despair-related were unchanged after 1 week, but low sociability and despair-like behavior became significant after 4 weeks. Chronic morphine regimen increased both corticosterone levels and forebrain serotonin turnover, but only serotonergic activity in the dorsal raphe remained impaired after 4 weeks. Remarkably, chronic fluoxetine prevented depressive-like behavioral deficits in 4-week abstinent mice.
Conclusions
During protracted abstinence, the immediate consequences of morphine exposure attenuate while fluoxetine-sensitive emotional alterations strengthen with time. Our study establishes a direct link between morphine abstinence and depressive-like symptoms, and strongly suggests that serotonin dysfunction represents a main mechanism contributing to mood disorders in opiate abstinence.
Keywords: Opiates, incubation, depression, serotonin, fluoxetine, dorsal raphe
Addiction is a chronic relapsing disorder (1-4). A major issue in recovering from addiction is to maintain a drug free or abstinent state. For drug abusers, achieving this goal permanently involves several steps. Acute withdrawal from chronic opiates is considered as a detoxification period during which the drug is cleared from the body, and is completed within a few days. This process produces a well-characterized aversive emotional state and physical symptoms, which occur spontaneously but can also be precipitated by opiate receptor antagonists. Alleviation of this acute aversive episode via drug intake (i.e. negative reinforcement) has been implicated in the maintenance of an established addiction (5, 6). Yet, acute withdrawal does not solely account for the relapse process. Reinstatement of drug abuse often occurs after prolonged periods of abstinence, while withdrawal symptoms are no longer detectable. This particular condition, classically defined as protracted abstinence, is characterized by symptoms reminiscent of anxiety and depression, including restlessness, high emotionality and lowered mood (7, 8). Epidemiological retrospective studies report a clear association between opiate addiction and major depressive disorders (9, 10) that likely contribute to relapse. Few animal studies have modelled the emotional consequences of protracted opiate abstinence following spontaneous withdrawal. In rodents, behavioral data indicate that motivation for natural reinforcers decreases (11-13), while conditioning for drug-associated compartments or cues strengthens with time, up to 4-5 weeks following drug discontinuation (14, 15) while relapse-tendencies persist up to 1 year (16).
Other studies have also demonstrated increased vulnerability to stress in rats spontaneously withdrawn from morphine for 7 days, as measured by decreased social interaction after restraint stress (17) and after intra-cerebro-ventricular corticotropin-releasing-factor administration (18), or increased shock probe-induced defensive burying after a 2-week abstinence period (19, 20). In the forced swim test, rats showed increased depressive-like behavior after a short morphine withdrawal period (3 days, (21, 22)), and one study only reported detectable depressive-like behavior following prolonged morphine abstinence (2 and 3 weeks, (23)).
Potential mechanisms underlying the negative emotional aspects of opiate dependence and acute withdrawal involve stress systems and aminergic neurotransmission (24-27). In rats and mice ((28, 29) respectively), acute systemic administration of morphine enhances serotonin (5-HT) concentration in the dorsal raphe nucleus (DR), where 5-HT neurons originate, and in several forebrain regions. Although 5-HT is not mandatory for morphine reinforcement (30), or withdrawal (31), electrical stimulation of the DR reduces morphine self-administration and withdrawal symptoms (32). In addition, acute (33, 34) or subchronic serotonin reuptake inhibitors administration reduces physical (35, 36) and negative motivational withdrawal symptoms (37) following chronic morphine. Hence interactions exist between opioids, stress and 5-HT system in dependence and acute withdrawal processes. How these interactions evolve after protracted abstinence, and whether long-term neuroadaptations in those systems contribute to aberrant emotional-like behaviors associated to protracted abstinence, is unknown.
In this study, we used a classical mouse model of morphine dependence and examined long-term consequences of chronic exposure to the drug. We analyzed physical dependence to morphine, changes in social, anxiety and despair-related behaviors, as well as circulating stress hormones and biogenic amines levels in limbic structures in the course of withdrawal, and after a short (1 week) or prolonged period of abstinence (4 weeks). Our data show major modifications of emotional-like responses and serotonergic function in abstinent animals. Importantly, the development of behavioral alterations was prevented by fluoxetine treatment during the 4-week abstinence period, strongly supporting the notion that serotonergic mechanisms underlie the depressive-like features that characterize protracted abstinence from chronic morphine.
Methods and Materials
Animals
Eight-week-old male C57BL/6J mice (Charles Rivers Laboratories, St-Germain-sur-l'Arbresle, France) were habituated to housing conditions 2 weeks before testing. Animals were housed 4/cage and were maintained under standard laboratory conditions. All experimental procedures were performed according to standard ethical guidelines (European Community Guidelines on the Care and Use of Laboratory Animals 86/609/EEC).
Drugs
Morphine sulfate (Francopia, Gentilly, France) and Naloxone hydrogen chloride (RBI, Natick, USA) were prepared in saline (0.9% sodium chloride) and injected at a volume of 10 ml/kg. Fluoxetine hydrochloride (Sigma-Aldrich, Lyon, France) was supplemented in food pellets. See Supplemental Methods & Materials in Supplement 1.
General Procedure
Dependence
Twice daily escaladating doses of morphine (20, 40, 60, 80, 100 mg/kg) or saline were administered intraperitoneally (i.p.) for 5 days, followed by a single 100 mg/kg injection on day 6. To confirm the induction of physical dependence, withdrawal was precipitated with naloxone (1 mg/kg) administered sub-cutaneously (s.c.) 2 h after the last morphine or saline injection (n=4/group). Somatic withdrawal signs (jumps, paw tremors, wet dog shakes, sniffings) were scored for 25 min (38, 39).
Development of abstinence
Mice were maintained drug-free after chronic treatment, and experienced spontaneous withdrawal in their home cages. One or 4 weeks following the last injection, emotional-like and social behaviors of abstinent mice (1 week, n=8; 4 weeks, n=8) and saline controls (1week, n=8; 4 weeks, n=8) were evaluated. To monitor physical dependence throughout the abstinence period, withdrawal was precipitated in a separate cohort of animals 2 h after a single morphine injection (50 mg/kg) in abstinent mice (40) (1 week, n=5; 4 weeks, n=5) or saline controls (1week, n=5; 4 weeks, n=5). We used a subeffective dose of naloxone (41) so that mice previously exposed to saline showed very few withdrawal signs.
Sensitivity to fluoxetine
Two independent sets of experiments were conducted with n=9-10 mice/group, and data were pooled (n=19-20). Fluoxetine was administered 4 weeks per os (0 or 10 mg/kg/24h, see Supplemental Methods & Materials in Supplement 1). Behavioral analysis was performed drug free. The 10 mg/kg dose was based on pilot studies (see Supplemental Methods & Materials and Figure S1 in Supplement 1).
Behavioral testing
Animals previously exposed to chronic morphine or saline (1 week or 4 weeks after treatment) underwent openfield, social interaction and tail suspension tests, in that order. The light-dark test was performed on a separate group of animals. See detailed procedures in Supplemental Methods & Materials in Supplement 1.
Biochemical analysis
Experiments were performed following chronic morphine treatment (2 h) or after 4 weeks of spontaneous withdrawal, on separate cohort of animals, which did not undergo behavioral testing (n=6/group). Adrenocorticotropic hormone and corticosterone plasma levels were measured using radioimmunoassays. Bioamine levels and their metabolites were measured in prefrontal cortex (PFC), hippocampus (HIPP), central amygdala (CeA) and DR using high performance liquid chromatography performed on microdissected brain samples (See Supplemental Methods and Materials in Supplement 1 for details). 5-HT turnover rate was determined as 5-HIAA/5-HT. For both hormonal and neurochemical assesments one mouse was excluded from the study as it presented abnormal values in all parameters in both assays (n=5-6/group).
Statistical analysis
All data are expressed as mean±sem. Statistical analysis was performed using one and two-way analysis of variance (ANOVA) with independent and repeated measures, or unpaired t-tests in accordance with the experimental design. Between subjects two-way ANOVA was used to analyze the effect of morphine treatment and time in time-course experiments, or morphine and fluoxetine treatments. In case of significant main effect or interactions following ANOVA, multiple group comparisons were performed using Fischer's post-hoc analysis. Statistical significance was defined as p<0.05.
Results
Chronic morphine treatment induces physical dependence
The experimental design is shown on Figure 1A. A weight curve is shown for a group of 4-week abstinent mice (n=14) that were used for behavior (n=8) or neurochemical assessment (n=6). Repeated ANOVA indicated that morphine treatment reduced body weight (F(1,26)=6.8; p<0.01) over time (interaction: F(14,308)=32.3; p<0.0001). This decrease was significant as of the second day of injections (post-hoc analysis, p<0.01) and persisted for 2 days after the last morphine injection on day 6 (post-hoc analysis, p<0.0001 from days 3 to 8). After 1 and 4 weeks of abstinence, morphine-treated mice recovered body weight similar to saline-treated mice (post-hoc analysis, p> 0.05 for both).
Figure 1.
Physical dependence and HPA axis hyperactivity decline during protracted abstinence. (A) Time line of the experiment. Dashed arrows indicate morphine doses injected twice daily. Black arrows indicate chronic, 1-week and 4-week abstinence time points under study. Morphine treatment reduced body weight over time (2 experiments pooled, n=14/group). (B) Withdrawal signs following naloxone injection (s.c. 1 mg/kg) 2 h after the last morphine administration (n=4 mice/group). (C) Withdrawal signs measured following morphine re-exposure (50 mg/kg) after either 1 or 4 weeks of abstinence (n=5/group). Physical symptoms decreased after 4 weeks. (D) Corticosterone and ACTH levels measured in chronic and 4-week abstinent groups (n=5-6/group). HPA axis was activated after chronic treatment but not after 4 weeks. Values are mean ± sem. *p<0.05; **p<0.01; ***p<0.0001 morphine versus saline controls. #p<0.05 comparison between 1-week and 4-week abstinence groups within each treatment condition.
We verified that physical dependence had established following chronic morphine exposure (n=4/group) by measuring naloxone-precipitated withdrawal symptoms (Figure 1B). Two hours after the last morphine or saline injection on day 6, a single naloxone dose was administered. Morphine-treated mice showed significantly more withdrawal signs in response to naloxone, including jumps, paw tremors, sniffings and wet dog shakes (unpaired t-test, p<0.01 for all parameters).
Physical dependence decreases during protracted abstinence
We monitored the evolution of physical dependence during spontaneous withdrawal after drug cessation. Figure 1C shows somatic symptoms induced by naloxone following a single morphine reintiation (42), in animals exposed to chronic saline or morphine and maintained abstinent for 1 or 4 weeks (n=5/group).
The number of wet dog shakes and sniffing behaviors were affected by morphine treatment only (ANOVA: F(1,16)=28.0; p<0.0001, F(1,16)=18.0; p<0.01, respectively). Post-hoc analysis showed that previous exposure to morphine increased the number of shakes (p<0.01) and sniffings (p<0.05) at both time points. Two-way ANOVA revealed a main effect of morphine treatment F(1,16)=16.1; p<0.0001 and time F(1,16)=26.0; p<0.0001, as well as a significant interaction between these factors (p<0.0001) on the number of jumps. Morphine abstinent mice jumped significantly more than saline controls at the 1-week time point (p<0.0001), and this difference was no longer significant after 4 weeks of abstinence (p>0.05). The number of paw tremors were also affected by treatment (ANOVA: F(1,16)=18.3 p<0.01) and time (ANOVA: F(1,16)=5.6; p<0.05), and were more abundant in mice with a previous history of morphine after 1 (p<0.01) and 4 weeks (p<0.05) of abstinence. In addition, as for jumps, post-hoc analysis showed that 4-week abstinent mice exhibited significantly less paw tremors than 1-week abstinent mice (p<0.05). In conclusion, both jumping behavior and paw tremor parameters decrease with increasing abstinence duration, indicating that physical dependence attenuates as abstinence unfolds.
Corticosterone decreases during protracted abstinence
To investigate the impact of the morphine regimen on stress systems, as well as the influence of the 4-week abstinence period, we measured plasmatic ACTH and corticosterone concentrations 2 h after the last morphine injection or 4 weeks later (Figure 1D). Two-way ANOVA indicated that corticosterone concentration was affected by morphine treatment (F(1,19)=35.9 ; p<0.0001) as well as time (F(1,19)=40.2 ; p<0.0001) with a significant interaction between these factors (p<0.0001). Chronic morphine injections increased corticosterone concentrations compared to saline-treated group (p<0.0001), but this difference was no longer present in 4-week abstinent mice (p>0.05). For ACTH, two-way ANOVA detected a main effect of treatment (F(1,19)=5.6 ; p<0.05), and time (F(1,19)=4.3 ; p<0.05) but post hoc analysis failed to reach statistical significance at each separate time point (p>0.05). Altogether corticosterone levels, and to a lesser extent those of ACTH, indicate that activity of the hypothalamo-pituitary-adrenal (HPA) axis was increased after the chronic morphine treatment, but that this dysregulation was restored after 4 weeks (Figure 1D).
Depressive-like behaviors develop during protracted abstinence
Next we investigated emotional-like responses of morphine- or saline-exposed animals either 1 or 4 weeks after the chronic treatment.
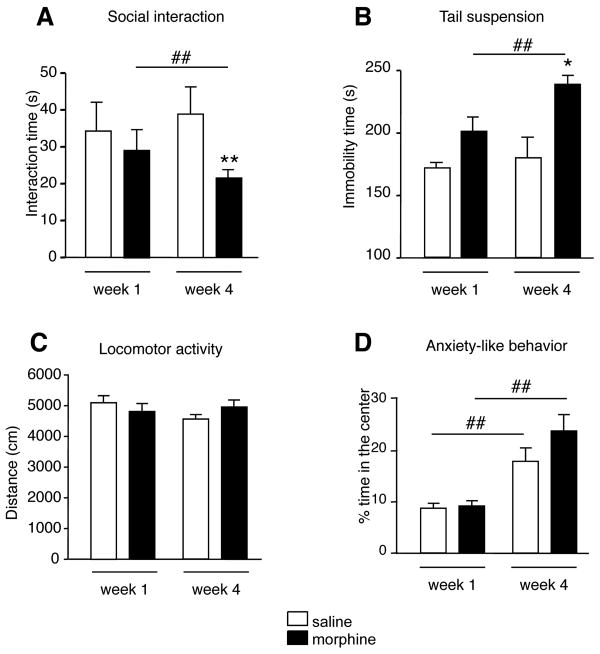
First we assessed social interactions (Figure 2A). Two-way ANOVA showed that that the total social exploration time for pairs of same treatment was affected by morphine pre-exposure (F(1,14)=9.0; p<0.01) and by the duration of abstinence (F(1,14)=0.1; p<0.05), and a significant interaction was detected between these factors (p<0.05). Post hoc analysis revealed that morphine-treated pairs of mice interacted less than saline-treated pairs after the 4-week abstinence period (p<0.01). This group also interacted less than the 1-week morphine abstinent pairs (p<0.01).
Figure 2.
Social interaction deficits and depressive-like behavior develop during protracted abstinence. (A) Social interaction test. Social behaviors decreased after 4 weeks of abstinence. (B) Tail suspension test. Immobility duration increased after 4 weeks abstinence. (C, D) Open-field test. Morphine abstinence had no effect on locomotor activity (C) or anxiety-like behavior (D). Values are mean ± sem. *p<0.05; **p<0.01 morphine versus saline controls. #p<0.05; ##p<0.01 comparison between 1-week and 4-week abstinence groups within each treatment condition (n=8/group).
Second we evaluated despair behavior in the TST (Figure 2B). Two-way ANOVA detected a main effect of treatment (F(1,28)=4.4; p<0.05 and time F(1,28)=16.4; p<0.01) on immobility time. Thus, morphine treatment significantly increased immobility at the 4-week time point only, compared to saline-treated counterparts (p<0.05) and to 1-week morphine abstinent mice (p<0.01). Altogether, social interactions were impaired and despair behavior was enhanced after pre-exposure to chronic morphine. Importantly these effects were strong only after a prolonged abstinence period.
Third we examined locomotor activity and anxiety-like behaviors in the open-field test. Total distance travelled in the arena was neither influenced by previous morphine treatment (F(1,28)=0.1; p>0.05), nor by the duration of abstinence (F(1,28)=0.8; p>0.05, Figure 2C) indicating that behavioral modifications are not secondary to changes in activity. The percentage of time spent in the center of the arena, did not differ between saline and morphine treated animals (F(1,28)=2.2; p>0.05 figure 2D). However, ANOVA detected a significant effect of abstinence time in both saline and morphine-exposed groups (F(1,28)=31.0; p<0.01), which both spent less time in the center at the 1-week time point, as compared to 4 weeks (p< 0.01 for both groups, post-hoc analysis).
Serotonin turnover is increased in the dorsal raphe after 4 weeks abstinence
HPLC analysis was conducted to determine 5-HT, 5-HT metabolite (5-HIAA), noradrenaline (NA) and dopamine (DA) levels in the PFC, HIPP, CeA and DR, either 2 h after the last morphine injection or 4 weeks later. All values are shown in Table I. Two-way ANOVA was performed for each amine, in each structure. Statistical analysis of treatment and time effects and their interactions are summarized in Table II. Post-hoc analysis of amine contents indicates that chronic morphine, compared to chronic saline, altered 5-HT, but not NA or DA concentrations in the several limbic brain regions investigated. 5-HT concentrations were significantly reduced in PFC (p<0.05), HIPP (p<0.05) and CeA (p<0.01) in morphine-treated mice. After 4 weeks of abstinence concentrations were similar in groups previously exposed to morphine or saline, indicating recovery of aminergic transmission in all three regions (p>0.05 for all). In the DR however, a decrease was obvious in morphine abstinent mice for both 5-HT and NA concentrations. This decrease was restricted to the DR (p<0.01 for both 5-HT and NA), suggesting that long-term adaptations take place in this brain structure.
Table I. Bioamine levels in the forebrain and brainstem following chronic morphine administration and protracted abstinence.
Serotonin (5-HT), noradrenaline (NA) and dopamine (DA) concentrations (fmole/μg) were determined either 2 h (chronic) or 4 weeks (abstinent) after chronic morphine (mor) or saline (sal) treatment. Chronic morphine significantly decreased serotonin in the prefrontal cortex, central amygdala and hippocampus, but not NA or DA levels. After 4 weeks of abstinence, 5-HT and NA levels were decreased in the dorsal raphe. Values represent mean ± sem. For each structure, statistical significance was determined by two-way ANOVA (cf. Table II) followed by multiple group comparison when relevant. *p<0.05; **p<0.01 indicate morphine versus saline comparison for each timepoint and #p<0.05; ##p<0.01 comparison between chronic and abstinent groups for each treatment (n=6/group).
| Prefrontal Cortex | Hippocampus | Central Amygdala | Dorsal Raphe | ||
|---|---|---|---|---|---|
| 5-HT | Sal-chronic | 249.2±16.9 | 486.4±69.2 | 44.6±6.6 | 9.6±3.3 |
| Mor-chronic | 172.3±10.0* | 350.2±37.6*# | 28.0±3.2** | 7.0±2.0 | |
| Sal-abstinent | 244.8±23.6 | 257.1±17.## | 24.0±3.5## | 13.2±1.7 | |
| Mor-abstinent | 221.9±45.6 | 283.6±34.8 | 21.7±3.3 | 5.4±2.0** | |
| NA | Sal-chronic | 279.9±8.9 | 285.6±46.3 | 24.2±2.3 | 10.5±2.1 |
| Mor-chronic | 265.8±15.9 | 273.6±8.4 | 24.1±2.3 | 6.4±1.6 | |
| Sal-abstinent | 276.0±22.8 | 280.3±20.4 | 19.0±2.8 | 23.8±3.7## | |
| Mor-abstinent | 251.9±44.7 | 191.5±24.2 | 22.2±4.4 | 8.7±3.0** | |
| DA | Sal-chronic | 102.1±8.1 | nd | 148.8±7.2 | 2.7±1.0 |
| Mor-chronic | 95.1±6.9 | nd | 135.0±12.1 | 2.4±0.6 | |
| Sal-abstinent | 107.3±12.2 | nd | 115.2±12.4 | 3.4±0.4 | |
| Mor-abstinent | 89.0±18.0 | nd | 110.3±16.8 | 1.8±0.6 |
Table II. Summary of two-way analysis of variance of bioamine contents in the brain.
This analysis is based on data presented in Table I. In each structure, two-way analysis of variance for treatment (morphine, saline) and time factors (chronic, abstinent) was performed. The table represent F(1,19); p values for each factor, and p value for interaction between factors. In case of significant interaction or significant main effects, the results of post-hoc analysis are described in the text. ns: non significant and nd: non determined. n=5-6/group.
| Prefrontal Cortex | Hippocampus | Central Amygdala | Dorsal Raphe | ||
|---|---|---|---|---|---|
| 5-HT | Treatment | 4.1; <0.05 | 5.3; <0.05 | 4.3; <0.05 | 4.5; <0.05 |
| Time | 0.7; ns | 19.0; <0.001 | 8.6; <0.05 | 2; ns | |
| Interaction | ns | ns | ns | ns | |
| NA | Treatment | 0.5; ns | 3.0; ns | 0.3; ns | 12.0; <0.01 |
| Time | 0.1; ns | 2.2; ns | 1.3; ns | 7.9; <0.05 | |
| Interaction | ns | ns | ns | <0.05 | |
| DA | Treatment | 0.4; ns | nd | 1.9; ns | 1.2; ns |
| Time | 2.2; ns | nd | 3.1; ns | 0.1; ns | |
| Interaction | ns | nd | ns | ns | |
Because 5-HT content was most affected by morphine treatment, we analyzed 5-HT turnover, as a measure of serotonergic activity in chronic and abstinent conditions (Figure 3). In the PFC, two-way ANOVA revealed a main effect of treatment (F(1,19)=11.2; p<0.01) and time (F(1,19)=39.3; p<0.0001), with a significant interaction between factors (p<0.01). 5-HT turnover was higher in morphine-treated group as compared to saline control group in the chronic condition only (p<0.0001). In the HIPP, 5-HT turnover was also affected by morphine treatment (F(1,19)=3.9; p<0.05) and time (F(1,19)=8.4; p<0.01), with a significant within-factor interaction (p<0.05). Post hoc analysis showed that chronic morphine treatment increased the turnover rate, compared to chronic saline treatment (p<0.01). In the CeA, no effect of treatment (F(1,19)=3.6; p>0.05) or condition (F(1,19)=3.4; p>0.05) was detected, but revealed a significant interaction between these factors (F(1,19)=12.8; p<0.01). Post hoc analysis showed that 5-HT turnover was enhanced following chronic morphine treatment (p<0.01), and restored in the abstinent condition (p>0.05). Finally we examined the turnover in the DR, the main source of 5-HT in the brain. Two-way analysis of variance revealed a main effect of treatment (F(1,19)=10.6; p<0.01). Post-hoc analysis showed that 5-HT turnover was higher in morphine treated groups compared to saline controls, although the effect was not significant in the chronic condition (p<0.05). In contrast with limbic structures, high turnover rates were maintained throughout abstinence in morphine-treated animals and reached statistical significance in the abstinent condition (Figure 3D, p<0.01). Together these data show major morphine-induced alterations of serotonergic activity in both limbic areas and brainstem. The data also indicate that these modifications evolve with time, decreasing in 5-HT neuron projections areas while strengthening at the level of 5-HT neurons.
Figure 3.
5-HT turnover is increased in the dorsal raphe after protracted abstinence. 5-HT turnover, defined as the 5-HIAA/5-HT ratio, is shown. Chronic morphine treatment significantly increased 5-HT turnover in the prefrontal cortex (A), central amygdala (B) and hippocampus (C) but not in the dorsal raphe (D). After 4 weeks of abstinence, 5-HT turnover was increased specifically in the dorsal raphe. Values represent mean ± sem. **p<0.01; ***p<0.001 morphine versus saline controls. (n=5-6/group).
Chronic fluoxetine administration prevents the development of depressive-like deficits during protracted abstinence
We next examined effects of chronic fluoxetine treatment (FLX, 10 mg/kg/24h) along the abstinence period (see Supplemental Methods and Materials and Results in Supplement 1) to evaluate depressive features in the observed behavioral deficits, and test whether enhancement of 5-HT function could prevent their incubation.
We first evaluated fluoxetine effect on locomotor activity. Neither previous morphine (F(1,74)=0.2; p>0.05) nor fluoxetine treatment (F(1,74)= 3.3; p>0.05) affected total distance travelled in the open-field (saline/chow: 10205 ± 321 ; morphine/chow : 10226 ± 299 ; saline/FLX : 9686 ± 554 morphine/FLX : 9294 ± 381).
We then examined the effect of chronic fluoxetine treatment in mice previously exposed to saline or morphine, on social (Figure 4A) and individual (Figure 4B) parameters in the social interaction test. Two-way ANOVA revealed that both morphine (F(1,37)=5.5; p<0.05) and fluoxetine (F(1,37)=5.8; p<0.05) modified social interaction time, with a significant interaction between treatments (p<0.05). Consistent with our previous results (Figure 2A), morphine/chow pairs spent significantly less time interacting than saline/chow pairs in control groups (p<0.01). Chronic fluoxetine administration fully prevented this morphine-induced deficit, as interaction times between morphine/FLX pairs were significantly increased compared to the morphine/chow abstinent pairs (p<0.01). During the social encounter individual grooming was also modified by morphine treatment (F(1,37)=12.5; p<0.01, but not by fluoxetine (F(1,37)=3.4; p>0.05), although a significant interaction was detected between treatments (Figure 4B). Inter-group comparisons indicated that morphine-induced increase in individual grooming time in chow mice (p<0.01), was reduced following fluoxetine treatment. Indeed, morphine/FLX groups exhibited less self-grooming behaviors than morphine/chow groups (p<0.05). For this parameter, fluoxetine did not fully prevent the behavioral modification, as morphine/FLX mice still spent more time self-grooming than the saline/FLX control group (p<0.05).
Figure 4.
Fluoxetine prevents the development of depressive-like and social deficits. (A, B) Social interaction test. Fluoxetine prevented protracted morphine effects on social (A) and individual (B) behaviors. (C, D) Tail suspension test. Fluoxetine also normalized passive (C) and active (D) behaviors in morphine abstinent animals. Values represent mean ± sem. *p<0.05; **p<0.01 morphine versus saline controls. #p<0.05; ##p<0.01 comparison between morphine/chow and morphine/FLX groups. (n=19-20/group).
We next investigated the effect of chronic fluoxetine treatment in the TST, (Figure 4C and D). Two-way ANOVA detected a significant effect of fluoxetine (F(1,74)=3.9; p<0.05) on immobility time, as well as an interaction with previous exposure to morphine (p<0.05). In accordance with our previous results (Figure 2A), morphine/chow mice showed higher immobility than saline/chow controls (Figure 4C, p<0.05). Fluoxetine abolished this difference, so that morphine/FLX mice showed reduced immobility time compared to morphine/chow group (p<0.05). Finally, high activity time was affected by both morphine (F(1,74)=4.9 ; p<0.01) and fluoxetine treatments (F(1,74)=8.2 ; p<0.05 ; Figure 4D). Morphine/chow animals were less active compared to saline/chow controls (p<0.05), and fluoxetine treatment normalized this effect, as active times no longer differed in morphine/FLX and saline/FLX groups after chronic fluoxetine treatment (Figure 4B, p>0.05). Moreover, active time in morphine/FLX group was significantly increased compared to morphine/chow (p<0.01).
Together these experiments indicate that fluoxetine administered during the 4-week abtinence period is able to prevent the development of aberrant social and despairlike behaviors.
Discussion
Opiate addiction and depression show a high degree of co-morbidity (43-45). The lifetime prevalence of major depression is approximately 30-50% in post-dependent subjects undergoing maintenance therapy (46, 47). This strong association suggests that opiate abuse may induce depressive symptoms or conversely, that depressed mood can contribute to drug abuse (9) in a self-medication hypothesis. In the latter case, antidepressants could contribute to alleviate both illnesses (48). Depression, when resulting from addiction, is observed long after acute aspects of withdrawal have dissipated and mood alterations are not reversed by opioid maintenance therapy, suggesting that long-term adaptive processes develop also outside the opioid system. At present, direct links between these two severe and frequent psychiatric disorders remain unclear, and have been little studied in animal models.
In our model of morphine abstinence, we show that abstinent mice develop depressive-like deficits, which increase with the duration of abstinence, while sensitivity to naloxone-precipitated withdrawal declines. Early studies in the field failed to reveal increased sensitivity to precipitated withdrawal in post-dependent animals (49, 50) likely due to differences in methodology, and led to the conclusion that relapse may result from psychological factors. Differences between physical and motivational aspects of acute withdrawal have since been well documented (51, 52). Here we examined both physical withdrawal and emotional alterations during protracted abstinence, and demonstrate a time-dependent dichotomy in the two processes.
Thus, increased immobility in the TST appeared as a trend after 1 week of abstinence, and became significant after 4 weeks. In this inescapable situation, enhanced immobility reflects a passive coping strategy associated with resignation, a behavioral measure of emotional despair (53). Concurrently, low sociability, reflected by reduced sniffing, following and contacts between pairs of mice, became evident during protracted abstinence. At 4 weeks also, mice exhibited a strong increase in self-grooming behavior, executed in a stereotyped and incomplete sequence which could reflect displacement behavior, a compulsive-type behavior to avoid coping (54). Similar social interaction deficits were reported in rats submitted to a chronic mild stress model (55) in the absence of anxiety-like behavior, further supporting this behavioral alteration as a relevant readout for reduced social interest in animal models of depression. Thus, our results highlight the emergence of gradual social withdrawal that parallels the development of despair behaviors in animals experiencing protracted morphine abstinence.
It could be argued here that decreased social interactions may also result from increased levels of anxiety, but this seems unlikely under our experimental conditions. We deliberately used low light and familiar condition that are not expected to generate anxiety (56, 57). In addition, anxiety-related behaviors assessed in the open-field test and independently confirmed in the light-dark test, were comparable between saline and morphine treated groups after 4 weeks of abstinence. It should be noted that heightened anxiety level was observed in both saline and morphine treated animals at the 1-week timepoint. However, this anxiogenic-like effect did not influence social or despair behaviors, as saline-treated mice showed similar social interaction times and immobility in the TST at both timepoints. This may result from an anxiogenic-like consequence of the twice-daily i.p. injection protocol still detectable 1 week later, but is anyhow unrelated to morphine treatment per se. Thus, in our conditions, protracted morphine abstinence does not seem to involve a major modification of basal anxiety.
Abnormal HPA axis activity has been reported in post-dependent heroin addicts (58, 59). Recent clinical studies have shown that HPA activity and anxiety, but not negative mood ratings, could be normalized by opiate maintenance treatment, suggesting that depressive-like symptoms and basal HPA axis are not necessarily associated during abstinence (60). In accordance with previous studies, we found a transient increase in corticosterone levels after the last morphine injection (61) that normalized after 4 weeks, indicating that HPA axis activity was dissociable from depressive-like symptoms in our model. This transient corticosterone increase could nonetheless contribute to the setting of depressive-like behavior during protracted abstinent, by triggering neuroadaptations in extrahypothalamic neuropeptidic stress circuits (3, 24, 25, 62, 63) or by influencing the serotoninergic tone, at pre-synaptic DR level or in forebrain projection structures (64).
We found that total NA and DA contents are not altered following chronic morphine treatment, whereas 5-HT concentrations are significantly reduced in PFC, HIPP and DR. Interestingly, clinical studies in heroin addicts have also shown specific 5-HT activity deficiencies, in contrast to normal DA and NA function, with more profound changes in subjects having co-morbid affective disorders (65). In our study, 5-HT content and turnover rates returned to control levels after the 4-week abstinence period in all three limbic regions. Notably at this time point, 5-HT turnover was increased relative to saline-treated control group in the DR, a brain structure from which 5-HT neurons originate. Although measures of both intra- and extra-cellular 5-HT contents do not explain the exact nature of 5-HT activity alterations in the DR, our results clearly indicate that aberrant 5-HT activity persists and incubates during morphine abstinence.
Chronic morphine triggers broad adaptations at synaptic and transcriptional levels, which have been mainly investigated in mesocorticolimbic reward circuits (66-69), and affect monoaminergic function (70-72). Our data confirm that neuroplasticity takes place within 5-HT system in response to chronic morphine, and reveal that 5-HT dysfunction evolves after cessation of drug exposure and persists for extended period of time during protracted abstinence. Within this line, a key finding of our study is that both enhanced despair behaviors and decreased social interest were normalized by chronic fluoxetine administration. Importantly, fluoxetine did not affect despair or social-related behaviors of control animals, demonstrating that treatment with the antidepressant drug specifically influenced long-term neuroadaptations, which result from chronic exposure to morphine and incubate during abstinence. Antidepressant therapies acutely enhance 5-HT, an effect thought to be responsible for neuroplastic changes in the brain. These, in turn, may restore functional alterations in cortico-limbic circuits and reverse behavioral depressive-like symptoms (73, 74). Together therefore, our observations strongly support the notion that 5HT function is altered in protracted abstinence to morphine. We suggest that neuroplasticity within 5-HT systems is a major mechanism contributing to the development of mood-related deficits in opiate abstinence.
In conclusion, this study establishes a direct connection between protracted abstinence to opiates and depressive-like symptoms in mice. The data strongly suggest a causal effect of serotonergic dysfunction in mood disruption. Future studies using mouse models should help clarifying the molecular basis of long-term 5-HT neuroadaptations in morphine abstinence.
Supplementary Material
Acknowledgments
We thank the Mouse Clinical Institute, (Illkirch, France) in particular Marie-France Champy for biochemical analysis, David Reiss for technical assistance, and Valérie Simmoneaux, (INCI, University of Strasbourg) for help with HPLC analysis. We also thank Katia Befort for the time spent on manuscript corrections and submission. This work was supported by the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, and Université de Strasbourg. We thank the European Union (GENADDICT/FP6 005166), the Fondation de la Recherche Médicale (CG) and the National Institutes of Health (NIAAA AA-16658 and NIDA DA-16768), for financial support.
Footnotes
Financial disclosure. All authors report no biomedical financial interest or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 2.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat Neurosci. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulteis G, Koob GF. Reinforcement processes in opiate addiction: a homeostatic model. Neurochem Res. 1996;21:1437–1454. doi: 10.1007/BF02532385. [DOI] [PubMed] [Google Scholar]
- 7.Jaffe JH. Trivializing dependence. Br J Addict. 1990;85:1425–1427. doi: 10.1111/j.1360-0443.1990.tb01624.x. discussion 1429-1431. [DOI] [PubMed] [Google Scholar]
- 8.Martin WR, Jasinski DR. Physiological parameters of morphine dependence in man--tolerance, early abstinence, protracted abstinence. J Psychiatr Res. 1969;7:9–17. doi: 10.1016/0022-3956(69)90007-7. [DOI] [PubMed] [Google Scholar]
- 9.Cerda M, Sagdeo A, Galea S. Comorbid forms of psychopathology: key patterns and future research directions. Epidemiol Rev. 2008;30:155–177. doi: 10.1093/epirev/mxn003. [DOI] [PubMed] [Google Scholar]
- 10.Kosten TR, Rounsaville BJ, Kleber HD. A 2.5-year follow-up of depression, life crises, and treatment effects on abstinence among opioid addicts. Arch Gen Psychiatry. 1986;43:733–738. doi: 10.1001/archpsyc.1986.01800080019003. [DOI] [PubMed] [Google Scholar]
- 11.Harris GC, Aston-Jones G. Altered motivation and learning following opiate withdrawal: evidence for prolonged dysregulation of reward processing. Neuropsychopharmacology. 2003;28:865–871. doi: 10.1038/sj.npp.1300122. [DOI] [PubMed] [Google Scholar]
- 12.Lieblich I, Yirmiya R, Liebeskind JC. Intake of and preference for sweet solutions are attenuated in morphine-withdrawn rats. Behav Neurosci. 1991;105:965–970. doi: 10.1037//0735-7044.105.6.965. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D, Zhou X, Wang X, Xiang X, Chen H, Hao W. Morphine withdrawal decreases responding reinforced by sucrose self-administration in progressive ratio. Addict Biol. 2007;12:152–157. doi: 10.1111/j.1369-1600.2007.00068.x. [DOI] [PubMed] [Google Scholar]
- 14.Aston-Jones G, Harris GC. Review - Brain substrates for increased drug seeking during protracted withdrawal. Neuropharmacology. 2004;47 1:167–179. doi: 10.1016/j.neuropharm.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Sakoori K, Murphy NP. Maintenance of conditioned place preferences and aversion in C57BL6 mice: effects of repeated and drug state testing. Behav Brain Res. 2005;160:34–43. doi: 10.1016/j.bbr.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Wikler A, Pescor FT. Persistence of “relapse-tendencies” of rats previously made physically dependent on morphine. Psychopharmacologia. 1970;16:375–384. doi: 10.1007/BF00404848. [DOI] [PubMed] [Google Scholar]
- 17.Blatchford KE, Diamond K, Westbrook RF, McNally GP. Increased vulnerability to stress following opiate exposures: behavioral and autonomic correlates. Behav Neurosci. 2005;119:1034–1041. doi: 10.1037/0735-7044.119.4.1034. [DOI] [PubMed] [Google Scholar]
- 18.Blatchford KE, Choi EA, McNally GP. Altered responsivity to central administrations of corticotropin-releasing factor in rats with a history of opiate exposures. Behav Neurosci. 2006;120:1169–1174. doi: 10.1037/0735-7044.120.5.1169. [DOI] [PubMed] [Google Scholar]
- 19.Harris GC, Aston-Jones G. Beta-adrenergic antagonists attenuate withdrawal anxiety in cocaine- and morphine-dependent rats. Psychopharmacology (Berl) 1993;113:131–136. doi: 10.1007/BF02244345. [DOI] [PubMed] [Google Scholar]
- 20.Harris GC, Aston-Jones G. Augmented accumbal serotonin levels decrease the preference for a morphine associated environment during withdrawal. Neuropsychopharmacology. 2001;24:75–85. doi: 10.1016/S0893-133X(00)00184-6. [DOI] [PubMed] [Google Scholar]
- 21.Anraku T, Ikegaya Y, Matsuki N, Nishiyama N. Withdrawal from chronic morphine administration causes prolonged enhancement of immobility in rat forced swimming test. Psychopharmacology (Berl) 2001;157:217–220. doi: 10.1007/s002130100793. [DOI] [PubMed] [Google Scholar]
- 22.Molina VA, Heyser CJ, Spear LP. Chronic variable stress or chronic morphine facilitates immobility in a forced swim test: reversal by naloxone. Psychopharmacology (Berl) 1994;114:433–440. doi: 10.1007/BF02249333. [DOI] [PubMed] [Google Scholar]
- 23.Grasing K, Ghosh S. Selegiline prevents long-term changes in dopamine efflux and stress immobility during the second and third weeks of abstinence following opiate withdrawal. Neuropharmacology. 1998;37:1007–1017. doi: 10.1016/s0028-3908(98)00093-8. [DOI] [PubMed] [Google Scholar]
- 24.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maldonado R. Participation of noradrenergic pathways in the expression of opiate withdrawal: biochemical and pharmacological evidence. Neurosci Biobehav Rev. 1997;21:91–104. doi: 10.1016/0149-7634(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 27.Tassin JP. Uncoupling between noradrenergic and serotonergic neurons as a molecular basis of stable changes in behavior induced by repeated drugs of abuse. Biochem Pharmacol. 2008;75:85–97. doi: 10.1016/j.bcp.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 28.Fadda P, Scherma M, Fresu A, Collu M, Fratta W. Dopamine and serotonin release in dorsal striatum and nucleus accumbens is differentially modulated by morphine in DBA/2J and C57BL/6J mice. Synapse. 2005;56:29–38. doi: 10.1002/syn.20122. [DOI] [PubMed] [Google Scholar]
- 29.Tao R, Auerbach SB. Involvement of the dorsal raphe but not median raphe nucleus in morphine-induced increases in serotonin release in the rat forebrain. Neuroscience. 1995;68:553–561. doi: 10.1016/0306-4522(95)00154-b. [DOI] [PubMed] [Google Scholar]
- 30.Zhao ZQ, Gao YJ, Sun YG, Zhao CS, Gereau RWt, Chen ZF. Central serotonergic neurons are differentially required for opioid analgesia but not for morphine tolerance or morphine reward. Proc Natl Acad Sci U S A. 2007;104:14519–14524. doi: 10.1073/pnas.0705740104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caille S, Espejo EF, Koob GF, Stinus L. Dorsal and median raphe serotonergic system lesion does not alter the opiate withdrawal syndrome. Pharmacol Biochem Behav. 2002;72:979–986. doi: 10.1016/s0091-3057(02)00810-9. [DOI] [PubMed] [Google Scholar]
- 32.Alaei H, Pourshanazari AA, Rafati A. Electrical stimulation of nucleus raphe dorsalis changes morphine self-administration and withdrawal symptoms in rats. Pathophysiology. 2002;9:1. doi: 10.1016/s0928-4680(02)00050-0. [DOI] [PubMed] [Google Scholar]
- 33.Akaoka H, Aston-Jones G. Indirect serotonergic agonists attenuate neuronal opiate withdrawal. Neuroscience. 1993;54:561–565. doi: 10.1016/0306-4522(93)90227-7. [DOI] [PubMed] [Google Scholar]
- 34.Wu CC, Chen JY, Tao PL, Chen YA, Yeh GC. Serotonin reuptake inhibitors attenuate morphine withdrawal syndrome in neonatal rats passively exposed to morphine. Eur J Pharmacol. 2005;512:37–42. doi: 10.1016/j.ejphar.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Gray AM. The effect of fluvoxamine and sertraline on the opioid withdrawal syndrome: a combined in vivo cerebral microdialysis and behavioural study. Eur Neuropsychopharmacol. 2002;12:245–254. doi: 10.1016/s0924-977x(02)00028-7. [DOI] [PubMed] [Google Scholar]
- 36.Singh VP, Jain NK, Kulkarni SK. Fluoxetine suppresses morphine tolerance and dependence: modulation of NO-cGMP/DA/serotoninergic pathways. Methods Find Exp Clin Pharmacol. 2003;25:273–280. doi: 10.1358/mf.2003.25.4.769675. [DOI] [PubMed] [Google Scholar]
- 37.Rafieian-Kopaei M, Gray AM, Spencer PS, Sewell RD. Contrasting actions of acute or chronic paroxetine and fluvoxamine on morphine withdrawal-induced place conditioning. Eur J Pharmacol. 1995;275:185–189. doi: 10.1016/0014-2999(94)00770-8. [DOI] [PubMed] [Google Scholar]
- 38.Contet C, Filliol D, Matifas A, Kieffer BL. Morphine-induced analgesic tolerance, locomotor sensitization and physical dependence do not require modification of mu opioid receptor, cdk5 and adenylate cyclase activity. Neuropharmacology. 2008;54:475–486. doi: 10.1016/j.neuropharm.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 40.Ouagazzal AM, Kenny PJ, File SE. Stimulation of nicotinic receptors in the lateral septal nucleus increases anxiety. Eur J Neurosci. 1999;11:3957–3962. doi: 10.1046/j.1460-9568.1999.00823.x. [DOI] [PubMed] [Google Scholar]
- 41.Kest B, Palmese CA, Hopkins E, Adler M, Juni A. Assessment of acute and chronic morphine dependence in male and female mice. Pharmacol Biochem Behav. 2001;70:149–156. doi: 10.1016/s0091-3057(01)00600-1. [DOI] [PubMed] [Google Scholar]
- 42.Brase DA, Iwamoto ET, Loh HH, Way EL. Sensitization of mice to naloxone induced jumping after a single narcotic injection: effect of prior physical dependence. Proc West Pharmacol Soc. 1975;18:294–297. [PubMed] [Google Scholar]
- 43.Brooner RK, King VL, Kidorf M, Schmidt CW, Jr, Bigelow GE. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54:71–80. doi: 10.1001/archpsyc.1997.01830130077015. [DOI] [PubMed] [Google Scholar]
- 44.Darke S, Mills K, Teesson M, Ross J, Williamson A, Havard A. Patterns of major depression and drug-related problems amongst heroin users across 36 months. Psychiatry Res. 2009;166:7–14. doi: 10.1016/j.psychres.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Teesson M, Havard A, Fairbairn S, Ross J, Lynskey M, Darke S. Depression among entrants to treatment for heroin dependence in the Australian Treatment Outcome Study (ATOS): prevalence, correlates and treatment seeking. Drug Alcohol Depend. 2005;78:309–315. doi: 10.1016/j.drugalcdep.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Nunes EV, Sullivan MA, Levin FR. Treatment of depression in patients with opiate dependence. Biol Psychiatry. 2004;56:793–802. doi: 10.1016/j.biopsych.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 47.Peles E, Schreiber S, Naumovsky Y, Adelson M. Depression in methadone maintenance treatment patients: rate and risk factors. J Affect Disord. 2007;99:213–220. doi: 10.1016/j.jad.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 48.Nunes EV, Quitkin FM, Donovan SJ, Deliyannides D, Ocepek-Welikson K, Koenig T, Brady R, McGrath PJ, Woody G. Imipramine treatment of opiate-dependent patients with depressive disorders. A placebo-controlled trial. Arch Gen Psychiatry. 1998;55:153–160. doi: 10.1001/archpsyc.55.2.153. [DOI] [PubMed] [Google Scholar]
- 49.Cheney DL, Goldstein A. Tolerance to opioid narcotics: time course and reversibility of physical dependence in mice. Nature. 1971;232:477–478. doi: 10.1038/232477a0. [DOI] [PubMed] [Google Scholar]
- 50.Wei E, Loh H. Morphine physical dependence unaltered by previous dependence on morphine. Nature. 1972;238:396–397. doi: 10.1038/238396a0. [DOI] [PubMed] [Google Scholar]
- 51.Frenois F, Cador M, Caille S, Stinus L, Le Moine C. Neural correlates of the motivational and somatic components of naloxone-precipitated morphine withdrawal. Eur J Neurosci. 2002;16:1377–1389. doi: 10.1046/j.1460-9568.2002.02187.x. [DOI] [PubMed] [Google Scholar]
- 52.Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186–191. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- 53.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 54.Kalueff AV, Tuohimaa P. The grooming analysis algorithm discriminates between different levels of anxiety in rats: potential utility for neurobehavioural stress research. J Neurosci Methods. 2005;143:169–177. doi: 10.1016/j.jneumeth.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Kompagne H, Bardos G, Szenasi G, Gacsalyi I, Harsing LG, Levay G. Chronic mild stress generates clear depressive but ambiguous anxiety-like behaviour in rats. Behav Brain Res. 2008;193:311–314. doi: 10.1016/j.bbr.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 56.File SE, Kenny PJ, Ouagazzal AM. Bimodal modulation by nicotine of anxiety in the social interaction test: role of the dorsal hippocampus. Behav Neurosci. 1998;112:1423–1429. doi: 10.1037//0735-7044.112.6.1423. [DOI] [PubMed] [Google Scholar]
- 57.File SE, Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463:35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 58.Li SX, Li J, Epstein DH, Zhang XY, Kosten TR, Lu L. Serum cortisol secretion during heroin abstinence is elevated only nocturnally. Am J Drug Alcohol Abuse. 2008;34:321–328. doi: 10.1080/00952990802013664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang GF, Ren YP, Sheng LX, Chi Y, Du WJ, Guo S, Jiang ZN, Xiao L, Luo XN, Tang YL, Smith AK, Liu ZQ, Zhang HX. Dysfunction of the hypothalamic-pituitary-adrenal axis in opioid dependent subjects: effects of acute and protracted abstinence. Am J Drug Alcohol Abuse. 2008;34:760–768. doi: 10.1080/00952990802385781. [DOI] [PubMed] [Google Scholar]
- 60.Kakko J, von Wachenfeldt J, Svanborg KD, Lidstrom J, Barr CS, Heilig M. Mood and neuroendocrine response to a chemical stressor, metyrapone, in buprenorphine-maintained heroin dependence. Biol Psychiatry. 2008;63:172–177. doi: 10.1016/j.biopsych.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Houshyar H, Gomez F, Manalo S, Bhargava A, Dallman MF. Intermittent morphine administration induces dependence and is a chronic stressor in rats. Neuropsychopharmacology. 2003;28:1960–1972. doi: 10.1038/sj.npp.1300271. [DOI] [PubMed] [Google Scholar]
- 62.Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Y, Proudnikov D, Yuferov V, Kreek MJ. Drug-induced and genetic alterations in stress-responsive systems: Implications for specific addictive diseases. Brain Res. 2010;1314C:235–252. doi: 10.1016/j.brainres.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev. 2008;32:1174–1184. doi: 10.1016/j.neubiorev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Gerra G, Zaimovic A, Timpano M, Zambelli U, Begarani M, Marzocchi GF, Ferri M, Delsignore R, Brambilla F. Neuroendocrine correlates of temperament traits in abstinent opiate addicts. J Subst Abuse. 2000;11:337–354. doi: 10.1016/s0899-3289(00)00031-6. [DOI] [PubMed] [Google Scholar]
- 66.Ammon-Treiber S, Hollt V. Morphine-induced changes of gene expression in the brain. Addict Biol. 2005;10:81–89. doi: 10.1080/13556210412331308994. [DOI] [PubMed] [Google Scholar]
- 67.Befort K, Filliol D, Darcq E, Ghate A, Matifas A, Lardenois A, Muller J, Thibault C, Dembele D, Poch O, Kieffer BL. Gene expression is altered in the lateral hypothalamus upon activation of the mu opioid receptor. Ann N Y Acad Sci. 2008;1129:175–184. doi: 10.1196/annals.1417.028. [DOI] [PubMed] [Google Scholar]
- 68.Befort K, Filliol D, Ghate A, Darcq E, Matifas A, Muller J, Lardenois A, Thibault C, Dembele D, Le Merrer J, Becker JA, Poch O, Kieffer BL. Mu-opioid receptor activation induces transcriptional plasticity in the central extended amygdala. Eur J Neurosci. 2008;27:2973–2984. doi: 10.1111/j.1460-9568.2008.06273.x. [DOI] [PubMed] [Google Scholar]
- 69.McClung CA, Nestler EJ, Zachariou V. Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area. J Neurosci. 2005;25:6005–6015. doi: 10.1523/JNEUROSCI.0062-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lanteri C, Salomon L, Torrens Y, Glowinski J, Tassin JP. Drugs of abuse specifically sensitize noradrenergic and serotonergic neurons via a non-dopaminergic mechanism. Neuropsychopharmacology. 2008;33:1724–1734. doi: 10.1038/sj.npp.1301548. [DOI] [PubMed] [Google Scholar]
- 71.Sastre-Coll A, Esteban S, Garcia-Sevilla JA. Supersensitivity of 5-HT1A autoreceptors and alpha2-adrenoceptors regulating monoamine synthesis in the brain of morphine-dependent rats. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:210–219. doi: 10.1007/s00210-001-0508-8. [DOI] [PubMed] [Google Scholar]
- 72.Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 74.D'Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disord. 2002;4:183–194. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.