Abstract
Background
Reports have identified cells capable of osteogenic differentiation in bone marrow, muscle, and adipose tissues, but there are few direct comparisons of these different cell-types. Also, few have investigated the potential connection between a tissue-specific pathology and cells derived from seemingly unrelated tissues. Here, we compare cells isolated from wild-type rabbits or rabbits with nonsyndromic craniosynostosis, defined as the premature fusion of one or more of the cranial sutures.
Methods
Cells were derived from bone marrow, adipose, and muscle of 10 day-old wild-type rabbits (WT; n=17) or from age-matched rabbits with familial nonsyndromic craniosynostosis (CS; n=18). Cells were stimulated with bone morphogenetic protein 4 (BMP4) and alkaline phosphatase expression and cell proliferation were assessed.
Results
In WT rabbits, cells derived from muscle had more alkaline phosphatase activity than cells derived from either adipose or bone marrow. The cells derived from CS rabbit bone marrow and muscle were significantly more osteogenic than WT. Adipose-derived cells demonstrated no significant differences. While muscle-derived cells were most osteogenic in WT rabbits, bone marrow-derived cells were most osteogenic in CS rabbits.
Conclusions
Results suggest that cells from different tissues have different potentials for differentiation. Furthermore, cells derived from rabbits with craniosynostosis were different from wild-type derived cells. Interestingly, cells derived from the craniosynostotic rabbits were not uniformly more responsive compared with wild-type cells, suggesting that specific tissue-derived cells may react differently in individuals with craniosynostosis.
Keywords: craniosynostosis, adipose tissue, muscle, bone marrow, rabbit, variability, tissue engineering
Introduction
For many challenges in plastic and reconstructive surgery, it may be necessary to use tissue engineering strategies to improve the body's ability to heal. The capacity of endogenous progenitor cells to repair and regenerate tissues has garnered significant attention in recent years.1, 2 Evidence supports the use of progenitor cells as a novel means to combat a variety of human diseases.3, 4 Progenitor cell transplantation is already the standard practice in the treatment of certain hematopoietic diseases.5 Furthermore, clinical trials are underway to investigate other applications for progenitor/stem cell therapy in several fields spanning cardiology,6-8 hepatology,9 wound healing,10 immunology,11 transplantation,12 neurology,13, 14 ophthalmology,15 autoimmune disorders,16-18 urology,19 orthopedics,20 and craniofacial biology.21 The tissue sources of the multipotent stem cells and tissue specific progenitor cells used for these therapies include adipose, muscle, and bone marrow tissues.3, 22-30 However, further research is necessary to elucidate the mechanism of progenitor cell effectiveness.
The current paradigm for the development of tissue engineering strategies involves having a cellular component surrounded by a supportive scaffold and growth factors appropriate for the desired tissue formation. Though much work has been performed to perfect these methodologies, therapies based on these scaffolds and growth factors may be less desirable if the response of the cellular component is not consistent or reproducible in different patients. Therefore, a better understanding of the variability among different tissue-derived progenitor cell populations is necessary. Many patients in need of therapies involving progenitor cells may have a disease that affects their response to a particular therapy. To date, the effects of disease on progenitor cell response to a therapeutic stimulus has just begun to be directly investigated.31
Our group has developed a colony of New Zealand White (NZW) rabbits with familial nonsyndromic craniosynostosis.32-36 This specific rabbit colony has coronal suture synostosis with no other major pathological phenotype. These rabbits provide an opportunity to study multiple progenitor cell populations in a model that has an anatomically isolated pathology.
This study directly compared progenitor cells derived from adipose, muscle, and bone marrow tissues in craniosynostotic (CS) and wild-type (WT) rabbits. The hypothesis was that the effects of craniosynostosis would be restricted to the phenotypically affected tissues and cells taken from phenotypically unaffected sites would behave identically, regardless of whether they were derived from CS or WT rabbits. Specifically, we tested the response of progenitor cells derived from adipose, muscle, and bone marrow in CS rabbits to osteogenic stimulation compared to similar cells derived from WT rabbits. We chose to compare the alkaline phosphatase expression (ALP; as a single outcome measure) in response to recombinant human bone morphogenetic protein 4 (BMP4; as a single stimulation). BMP4 was chosen because of its known agonistic affects on osteogenic differentiation.37
Materials and Methods
Tissue Harvest
Adipose, muscle, and bone marrow tissues were harvested from WT and CS rabbits housed at the University of Pittsburgh under an IACUC approved protocol. The craniosynostosis in the rabbit colony is the product of an autosomal dominant mutation with variable morphological expression.32, 34 Tissues were collected from a subpopulation of the rabbits that has complete obliteration of the coronal suture, diagnosed by the absence of a patent coronal suture at 10 days of age.
Following diagnosis, we harvested inguinal adipose tissue, masseter muscle, and bone marrow from the femur of each rabbit. The collected tissues from each individual were placed in sterile collection tubes with cold phosphate buffered saline (PBS) until the conclusion of the tissue harvest. The tissues from each individual rabbit were kept separated for cell isolation and subsequent culture, meaning that samples were not pooled.
Adipose Cell Isolation
Adipose tissue was excised from WT (n=14) or CS (n=12) rabbits and minced. Adipose cells were released through a 40 minute digestion in a 0.1% collagenase I (Sigma) solution in Hank's balanced salt solution (HBSS). After centrifugation, the pellets were resuspended in Red Blood Cell Lysing Buffer (Sigma). Cell suspensions were vigorously vortexed and again centrifuged. The subsequent pellets were resuspended in proliferation medium (PM; Dulbecco's modified Eagle's medium [DMEM] supplemented with 10% fetal bovine serum [CellGro] and 1% penicillin/streptomycin [Invitrogen]) and plated.
Muscle Cell Isolation
Muscle cells were isolated from WT (n=17) or CS (n=13) rabbits as previously described.38 Briefly, cells were released through serial digestions in 0.2% Collagenase XI (Sigma) solution in HBSS, 2.4u/ml Dispase (Gibco) solution in HBSS and 0.1% Trypsin-EDTA solution in HBSS. The digests were centrifuged and the pellets were resuspended in PM and plated.
Bone Marrow Cell Isolation
Excised femora from WT (n=9) or CS (n=18) rabbits were removed from the cold PBS and further dissected under sterile conditions. The epiphyses were removed and the bone marrow was isolated using a 23-gauge needle and a syringe of sterile PBS. Resulting cell suspensions were centrifuged and the pellets were resuspended in Red Blood Cell Lysing Buffer (Sigma). Cell suspensions were vigorously vortexed and again centrifuged. The subsequent pellets were resuspended in PM and plated.
All adipose, muscle, and bone marrow derived cells were cultured by feeding every 2-3 days and passaging as needed. Cells were used before their 4th passage.
Alkaline Phosphatase (ALP) Assays
To characterize and quantify cellular response to BMP4 stimulation (rhBMP4, R&D Systems), ALP activity was used as an outcome measure. Cells were seeded in triplicate onto tissue culture grade plastic 24-well plates at a density of approximately 5,000cells/well. Cells were treated with 0 or 50ng/ml of BMP4 in PM for 3 or 7 days at which point, they were stained for ALP or lysed for quantitative ALP (QALP) analysis. ALP staining was performed according to the manufacturer's protocol (Sigma-Aldrich, cat #86C-1KT). QALP activity was measured via absorbance of cell lysate after treatment with SIGMA FAST p-Nitrophenyl Phosphate (Sigma, cat #N2770). Measures were normalized to amount of total protein per volume of lysate. Total protein was determined in identical volumes of cell lysate with a BCA Protein Assay (Pierce, cat #23227). Normalized QALP activity was calculated with the following formula: QALP activity (μmol pNPP/ml) / protein (μg/ml) × 1000μg/mg × 1000nmol/1μmol = nmol pNPP/mg protein. All measures were completed in triplicate for each animal.
Cell Proliferation
The effects of BMP4 stimulation on cell proliferation were assessed via CellTiter96 Aqueous One Solution Cell Proliferation Assay (Promega, cat #G3581). Cells were seeded in triplicate into flat-bottom 96-well plates at a density of approximately 1,000cells/well and treated with 0ng/ml or 50ng/ml BMP4 in PM for 3 or 7 days. After 3 or 7 days of treatment, cells were incubated for one hour with 20μl/well of CellTiter96 Aqueous One Solution according to manufacturer's instructions. The absorbance at 490nm was recorded with a 96-well plate reader (Benchmark Plus, BioRad). Because the CellTiter system is based on the total number of cells within the plate, it was expected that, regardless of the treatment, there would always be more cells on day 7 compared to day 3. Therefore, the 3 day and 7 day results were analyzed separately because a statistically significant time effect would not be biologically significant.
Statistical Analyses
Means and standard deviations for optical densities in ALP and cell number assays were collected. To perform the appropriate two-way (phenotype vs. time) or three-way (treatment vs. phenotype vs. time) ANOVA, we tested each data set for homogeneity of variance. In cases where variance was not homogeneous, the data were transformed to achieve statistical homogeneity distinguished by non-significant Brown-Forsythe test (p>0.05). Significant ANOVAs (p<0.05) were further analyzed using post-hoc Bonferroni and group differences were considered significant when p<0.05.
Results
Adipose
ALP activity
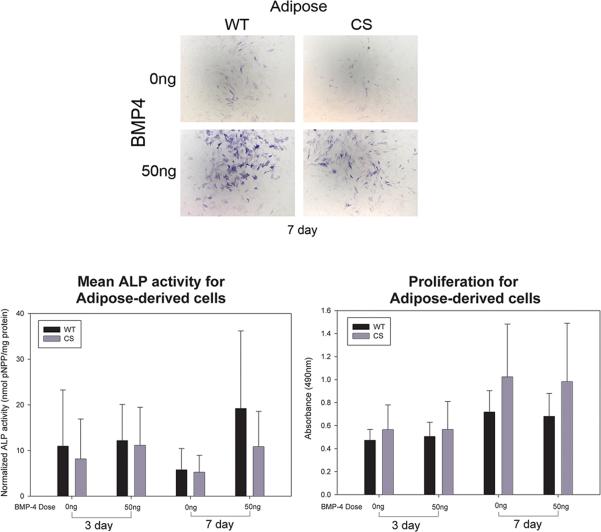
Regardless of diagnosis, adipose cells showed very little baseline ALP expression and did not seem to have a large response to BMP4 stimulation (7 day, Figure 1A). Analysis of both phenotypes showed a significant interaction between BMP4 stimulation and time in culture (F=3.96; p<0.05) with mean ALP activity decreasing between days 3 and 7 for cultures not treated with BMP4 (0ng, Figure1B) and increasing over time in cultures treated with BMP4 (50ng, Figure 1B). Also, a BMP4 stimulation main effect (F=9.77; p<0.01) was identified with 50ng of BMP4 inducing more ALP activity than was found in untreated (0ng) cultures (Figure 1B). There were no significant differences between CS (n=14) or WT (n=12) adipose-derived cells.
Figure 1.
A) Histophotomicrographs showing ALP-stained (blue) adipose-derived cells isolated from wild-type (WT) or craniosynostotic (CS) rabbits after 7 days in culture either in the absence (0ng) or presence (50ng) of BMP4. Notice that the cells were responsive to BMP4 after 7 days in culture. B) Graph depicting the mean quantified ALP activity (error bar depicts standard deviation) of adipose cells derived from WT (n=14) or CS (n=12) rabbits. BMP4 increased ALP activity overall, however, there were no significant differences between CS and WT derived cells. C) Graph depicting the means and standard deviations of absorbance collected as a measure of total cell number. No statistically significant differences were identified after either 3 or 7 days in culture.
Proliferation
There were no significant differences in the cell number when we considered the stimulation by phenotype interaction, the simulation main effect, or between WT and CS rabbit adipose-derived cells after 3 days in culture or 7 days in culture (Figure 1C).
Muscle
ALP activity
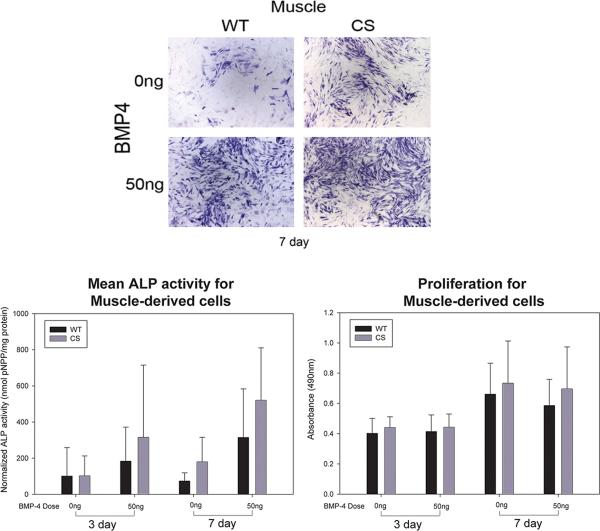
Rabbit muscle-derived cells showed some baseline ALP staining after both 3 and 7 days in culture and increased ALP staining when cultured with BMP4 (Figure 2A). Quantitative analysis showed significant main effects of rabbit diagnosis (F=8.921; p=0.003), culture time (F=7.240; p=0.008), and BMP4 stimulation (F=30.023; p<0.001). CS muscle-derived cells (n=13, 279.35 ± 30.1 SEM) showed more ALP activity compared to WT cells (n=17, 168.35 ± 26.8 SEM). Cells from both phenotypes were more ALP positive after 7 days (272.04 ± 28.2 SEM) compared to 3 days (175.66 ± 28.8 SEM) of culture. Additionally, QALP data supported the qualitative assessment that 50ng/ml BMP4 induced more ALP expression (333.51 ± 28.5 SEM) than 0ng/ml BMP4 (114.19 ± 28.5 SEM) (Figure 2B).
Figure 2.
A) Histophotomicrographs showing ALP-stained (blue) muscle-derived cells isolated from wild-type (WT) or craniosynostotic (CS) rabbits after 7 days in culture. Muscle-derived cells showed low background ALP staining (0ng) and responded to BMP4 stimulation (50ng). B) Graph depicting the mean quantified ALP activity (error bar depicts standard deviation) of muscle-derived cells from WT (n=17) or CS (n=13) rabbits. Analysis showed significant differences between WT and CS cells (p<0.01), between 3 and 7 days (p<0.01), and between BMP4 treated and unstimulated control cells (p<0.001). C) Graph depicting the means and standard deviations of absorbance collected as a measure of total cell number. No statistical differences were identified due to BMP4 or donor diagnosis after either 3 or 7 days in culture.
Proliferation
We found no significant differences in cell number due to treatment, cell type by treatment, or between CS and WT muscle cell proliferation over time (at 3 or 7 days in culture) or with BMP4 treatment (Figure 2C).
Bone Marrow
ALP activity
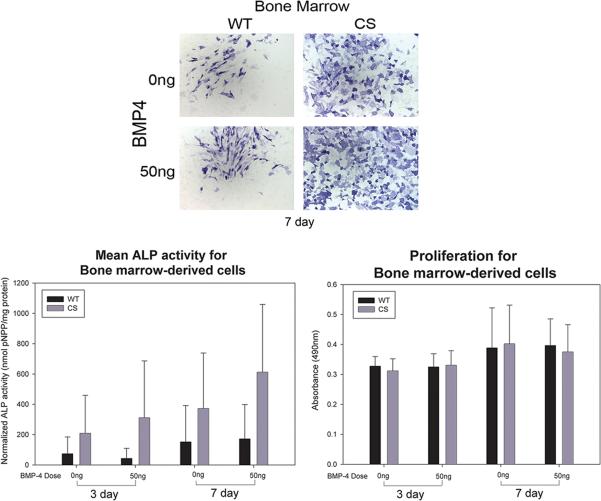
ALP staining was observed in all bone marrow-derived cells without BMP4 treatment (Figure 3A, 0ng) and treatment had variable effects on ALP staining (Figure 3A, 50ng). Three-way analysis of variance for mean ALP activity by phenotype (WT or CS), time (3 or 7 days in culture) and stimulation (no BMP4 or BMP4 administration) showed significant effects of time in culture (F=6.16; p<0.05) and rabbit phenotype (F=15.44; p<0.001) on ALP staining. CS cells (n=18) showed significantly more ALP staining (376.00 ± 38.3 SEM) than WT (n=9) cells (110.37 ± 55.7 SEM). QALP increased between day 3 (159.31 ± 50.4 SEM) and day 7 (327.06 ± 45.1 SEM) in culture (Figure 3B).
Figure 3.
A) Histophotomicrographs showing ALP-stained (blue) bone marrow-derived cells isolated from wild-type (WT) or craniosynostotic (CS) rabbits after 7 days in culture. Bone marrow-derived cells derived from CS rabbits showed more ALP staining than WT counterparts. B) Graph depicting the mean quantified ALP activity (error bar depicts standard deviation) of bone marrow-derived cells from WT (n=9) or CS (n=18) rabbits. Analysis showed significant differences between WT and CS cells (p<0.001) and between 3 and 7 days (p<0.05). C) Graph depicting the means and standard deviations of absorbance collected as a measure of total cell number. No statistical differences were identified due to BMP4 or donor diagnosis after either 3 or 7 days in culture.
Proliferation
No significant differences for the phenotype by treatment interaction, the treatment main effect, or between CS and WT bone marrow-derived cell proliferation over time (3 or 7 days in culture) or following BMP4 treatment were detected (Figure 3C).
Comparison of ALP activity among Different Tissues
For direct statistical comparison among the different cell types (adipose, muscle, and bone marrow) by phenotype (WT versus CS), a two-way ANOVA design was used (Figure 4). A significant cell type by phenotype interaction was identified, F (2,317) =8.147, p<0.001. The cell type main effect was significant, F (2,317) =145.576, p<0.001. There was a significant phenotype main effect F (1,317) =10.372, p=0.001 with CS derived cells (χ=228.50, SEM 326.75) exhibiting greater measures than the WT derived cells (χ=107.90, SEM 176.62). The significant interaction term appears to be the result of the WT having the greatest mean value for the muscle-derived stem cell, whereas the synostotic have the greatest measure for the bone marrow. The post-hoc Bonferonni multiple comparison test for cell type revealed that the bone marrow (χ=287.61, SEM 352.60) did not differ significantly from the muscle cells (χ=218.86, SEM 255.80). However, both cell types demonstrated significantly greater measures than adipose derived cells (χ=10.28, SEM 9.98), p<0.001.
Figure 4.
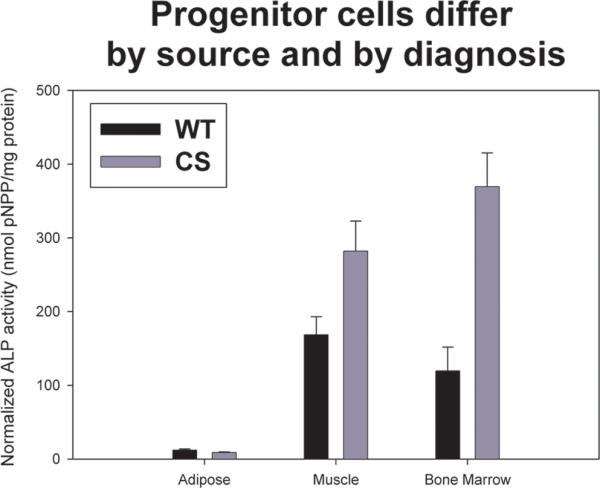
Graph showing the mean and standard errors for quantitative ALP activity for adipose-, muscle-, and bone marrow-derived cells from either wild-type (WT) or craniosynostotic (CS) rabbits (error bar depict standard error). Statistical analysis showed a significant cell type by phenotype interaction (p<0.001), suggesting that cells derived from WT and CS rabbits were different from each other. For WT rabbits, cells derived from muscle showed the most ALP activity, whereas in CS rabbits, cells derived from bone marrow had the highest ALP activity. Adipose-derived cells were similar between WT and CS rabbits.
Discussion
In this study, both WT and CS adipose-derived cells showed low levels of ALP activity, regardless of time in culture or BMP4 stimulation. These results are consistent with the majority of the reports showing a limited ability for adipose-derived cells to express ALP after exposure to osteogenic medium27, 39-42 and support the previous observation that rabbit adipose-derived cells did not show a marked ALP response to BMP4 stimulation.43 Although adipose cells showed relatively poor ALP expression, they did react similarly between WT and CS rabbits. This similarity among adipose cells from rabbits with different diagnoses suggests that adipose cells may be suitable candidates for designing cell-based therapies that work consistently among different patients.
ALP activity has been consistently demonstrated in bone marrow-derived cells.3, 39-42, 44 The data presented here suggest that rabbit bone marrow-derived cells did not have significantly increased ALP activity following stimulation with 50ng/ml BMP4, presumably due to the high baseline ALP activity (unstimulated). Bone marrow-derived cells were different between CS and WT rabbits. CS bone marrow cells showed higher ALP activity compared to WT bone marrow-derived cells in all treatment conditions.
Muscle-derived cells showed relatively low baseline (0ng/ml BMP4) ALP activity and demonstrated an approximate 4-fold increase in ALP activity after 7 days of stimulation with BMP4. Similar results have been noted in mouse muscle-derived stem cell populations.45, 46 These characteristics make muscle-derived cells an attractive target for bone tissue engineering applications because it would be beneficial to have cells that are highly responsive to a stimulating factor (in this case, BMP4). However, there were significant differences noted between CS and WT muscle-derived cells. Craniosynostotic muscle cells had higher mean ALP expression than WT cells in all conditions studied. Therefore, the reaction of muscle-derived cells to a specific treatment may not be consistent enough to use as a basis for a cellular therapy.
Comparison among the different tissues showed a significant phenotype by diagnosis interaction suggesting that cells derived from CS rabbits were not related to each other in the same way as cells from WT rabbits. In the cells derived from WT rabbits, muscle-derived cells had the highest overall ALP expression. Bone marrow-derived cells had the highest ALP expression in cells derived from CS rabbits. Both types of animals had similarly inactive adipose-derived cells. Therefore, it was not found that all cells from CS rabbits were simply more ALP-positive.
Together these data suggest there is not a uniform alteration in the CS progenitor cells that would manifest as increased osteogenic response in every cell population tested. Rather, 1) there are cells within specific tissues that act differently in the CS rabbits, and 2) the isolated CS phenotype plays some role in the relationship among cells from specific tissues.
The distinctions between the cells derived from CS rabbits and WT rabbits are important because they offer insight into larger questions such as 1) growth factor and scaffold therapies developed using normal cells may not function correctly when used in a patient with a seemingly unrelated pathology, 2) if stem cell function is affected, other processes such as healing after injury may also be affected, and 3) because of variability, it may be very difficult to identify appropriate sources for cells within any given patient population for consistent cell-based therapies.
Nonsyndromic CS is characterized by an isolated pathology, meaning that there is a fusion of bones in the skull but there are no other related pathologies. In cases of nonsyndromic disease, we would expect to see no alterations in cells from tissues that have no pathology in the patient. Non-cranial bone growth (from metacarpal bone growth measurements) has been found to be the same between CS and WT rabbits.33 The results presented here suggest that CS and WT rabbits have cells in their bone marrow and muscle that do not react similarly to BMP4 stimulation. This unexpected result begs the larger stem cell biology question, “How many of the diseases that are characterized as “nonsyndromic” have related but currently unidentified stem cell issues?” Most studies of animal models of human disease are to better understand the pathology of interest and have a focus limited to the target organ or tissue. In our rabbit model of craniosynostosis, we have identified differences in progenitor cells from tissues that were thought to be unaffected in the animals. It is necessary to continue studying the effects that a disease has on an individual's progenitor cells.31 It is imperative to isolate postnatal progenitor cells from currently available animal models (knock-out, knock-in, deficient mutants, etc) to confirm whether seemingly isolated pathologies might influence stem cell activity.
Results presented here also shed light on the issue of variability. We found high levels of variability among cells derived from postnatal rabbit tissues. It was interesting to observe such variability within the relatively closed population, genetically speaking, of CS NZW rabbits. Other groups have also reported high variability in rabbit bone marrow-derived cells.47 If such variability exists among NZW rabbits, the more genetically diverse human population is bound to exhibit higher variability. In fact, variability within mesenchymal stem cells has been identified in patient-derived populations.48-50 Therefore, we are confident that such variability is endemic in primary cell isolations and not an artifact of isolation technique. With this in mind, caution should be used when interpreting results of studies on human tissues that involve very small sample sizes.51-54
The results presented in this report are strengthened by the sample size used for each analysis. The large sample sizes of either WT or CS rabbits allowed for a better understanding of cellular characteristics. High cellular variability between individuals is a major problem that plagues the development of effective cell-based therapies. Much of the focus in tissue engineering so far has been in identifying cells, scaffolds, or growth factors that lead to specific tissue regeneration. However, high variability between patient responses to therapy, either due to variability in individual patient healing (innate stem cell differences) or to the effects of different diseases (disease-specific changes in stem cells), would complicate designing therapies that consistently achieve the desired results. One solution to this issue may be to focus on developing patient-based, customized therapies. To that end, it may be helpful to identify cells within a patient population that may serve as an in vitro proxy to test therapies and give insight into how a therapy may function when applied in vivo.
Overall, the results presented here do not support the hypothesis that cells from unaffected tissues in CS and WT rabbits react similarly to BMP4 stimulation. They do, however, suggest that adipose-derived cells are not as ALP-positive or as BMP4-responsive as their muscle- or bone marrow-derived counterparts. The results also suggest that outcomes of a particular therapy may, in part, depend on the tissues being treated. Finally, due to the high level of variability observed between donors, it may be beneficial to focus future research on the development of customized therapies, rather than traditional “off-the-shelf” therapies.
Acknowledgements
This work was funding in part from NIH/NIDCR DE-019430 (GMC), the Plastic Surgery Education Foundation (PSEF), the American Cleft Palate Association (ACPA), and the American Society of Oral and Maxillofacial Surgeons (ASMS). Also, thank you to Dr. Christopher Kinsella and Ms. Laurie Meszaros for critical review of this manuscript.
Funding for this project from NIH/NIDCR 1-R01-DE019430-02 (GMC), the Plastic Surgery Education Foundation (PSEF), the American Cleft Palate Association (ACPA), and the American Society of Oral and Maxillofacial Surgeons (ASMS).
Footnotes
Conflict of Interest Statement:
All authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mikos AG, Herring SW, Ochareon P, et al. Engineering complex tissues. Tissue Eng. 2006;12:3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vacanti CA. The history of tissue engineering. J Cell Mol Med. 2006;10:569–576. doi: 10.1111/j.1582-4934.2006.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 4.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shizuru JA, Negrin RS, Weissman IL. Hematopoietic stem and progenitor cells: clinical and preclinical regeneration of the hematolymphoid system. Annu Rev Med. 2005;56:509–538. doi: 10.1146/annurev.med.54.101601.152334. [DOI] [PubMed] [Google Scholar]
- 6.Herbots L, D'Hooge J, Eroglu E, et al. Improved regional function after autologous bone marrow-derived stem cell transfer in patients with acute myocardial infarction: a randomized, double-blind strain rate imaging study. Eur Heart J. 2009;30:662–670. doi: 10.1093/eurheartj/ehn532. [DOI] [PubMed] [Google Scholar]
- 7.Yeo C, Mathur A. Autologous bone marrow-derived stem cells for ischemic heart failure: REGENERATE-IHD trial. Regen Med. 2009;4:119–127. doi: 10.2217/17460751.4.1.119. [DOI] [PubMed] [Google Scholar]
- 8.Kirillov AM, Fatkhudinov T, Dyachkov AV, et al. Transplantation of allogenic cells in the therapy of patients with dilated cardiomyopathy. Bull Exp Biol Med. 2007;144:635–639. doi: 10.1007/s10517-007-0392-4. [DOI] [PubMed] [Google Scholar]
- 9.Pai M, Zacharoulis D, Milicevic MN, et al. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952–1958. doi: 10.1111/j.1572-0241.2008.01993.x. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 11.Qasim W, Cavazzana-Calvo M, Davies EG, et al. Allogeneic hematopoietic stem-cell transplantation for leukocyte adhesion deficiency. Pediatrics. 2009;123:836–840. doi: 10.1542/peds.2008-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutchinson JA, Brem-Exner BG, Riquelme P, et al. A cell-based approach to the minimization of immunosuppression in renal transplantation. Transpl Int. 2008;21:742–754. doi: 10.1111/j.1432-2277.2008.00692.x. [DOI] [PubMed] [Google Scholar]
- 13.Silani V, Cova L, Corbo M, et al. Stem-cell therapy for amyotrophic lateral sclerosis. Lancet. 2004;364:200–202. doi: 10.1016/S0140-6736(04)16634-8. [DOI] [PubMed] [Google Scholar]
- 14.Burt RK, Loh Y, Cohen B, et al. Autologous non-myeloablative haemopoietic stem cell transplantation in relapsing-remitting multiple sclerosis: a phase I/II study. Lancet Neurol. 2009;8:244–253. doi: 10.1016/S1474-4422(09)70017-1. [DOI] [PubMed] [Google Scholar]
- 15.Di Girolamo N, Bosch M, Zamora K, et al. A contact lens-based technique for expansion and transplantation of autologous epithelial progenitors for ocular surface reconstruction. Transplantation. 2009;87:1571–1578. doi: 10.1097/TP.0b013e3181a4bbf2. [DOI] [PubMed] [Google Scholar]
- 16.Voltarelli JC, Couri CE, Stracieri AB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–1576. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 17.Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301:1573–1579. doi: 10.1001/jama.2009.470. [DOI] [PubMed] [Google Scholar]
- 18.Burt RK, Traynor A, Statkute L, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006;295:527–535. doi: 10.1001/jama.295.5.527. [DOI] [PubMed] [Google Scholar]
- 19.Strasser H, Marksteiner R, Margreiter E, et al. Transurethral ultrasonography-guided injection of adult autologous stem cells versus transurethral endoscopic injection of collagen in treatment of urinary incontinence. World J Urol. 2007;25:385–392. doi: 10.1007/s00345-007-0190-7. [DOI] [PubMed] [Google Scholar]
- 20.Marcacci M, Kon E, Moukhachev V, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13:947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 21.Gimbel M, Ashley RK, Sisodia M, et al. Repair of alveolar cleft defects: reduced morbidity with bone marrow stem cells in a resorbable matrix. J Craniofac Surg. 2007;18:895–901. doi: 10.1097/scs.0b013e3180a771af. [DOI] [PubMed] [Google Scholar]
- 22.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 23.Asakura A, Komaki M, Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation. 2001;68:245–253. doi: 10.1046/j.1432-0436.2001.680412.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee JY, Qu-Petersen Z, Cao B, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–1100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musgrave DS, Pruchnic R, Bosch P, et al. Human skeletal muscle cells in ex vivo gene therapy to deliver bone morphogenetic protein-2. J Bone Joint Surg Br. 2002;84:120–127. doi: 10.1302/0301-620x.84b1.11708. [DOI] [PubMed] [Google Scholar]
- 26.Seale P, Asakura A, Rudnicki MA. The potential of muscle stem cells. Dev Cell. 2001;1:333–342. doi: 10.1016/s1534-5807(01)00049-1. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa R, Mizuno H, Watanabe A, et al. Osteogenic and chondrogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice. Biochem Biophys Res Commun. 2004;313:871–877. doi: 10.1016/j.bbrc.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 30.Cowan CM, Shi YY, Aalami OO, et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560–567. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 31.El-Ftesi S, Chang EI, Longaker MT, et al. Aging and diabetes impair the neovascular potential of adipose-derived stromal cells. Plast Reconstr Surg. 2009;123:475–485. doi: 10.1097/PRS.0b013e3181954d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mooney MP, Aston CE, Siegel MI, et al. Craniosynostosis with autosomal dominant transmission in New Zealand white rabbits. J Craniofac Genet Dev Biol. 1996;16:52–63. [PubMed] [Google Scholar]
- 33.Mooney MP, Losken HW, Siegel MI, et al. Development of a strain of rabbits with congenital simple nonsyndromic coronal suture synostosis. Part II: Somatic and craniofacial growth patterns. Cleft Palate Craniofac J. 1994;31:8–16. doi: 10.1597/1545-1569_1994_031_0008_doasor_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 34.Mooney MP, Losken HW, Siegel MI, et al. Development of a strain of rabbits with congenital simple nonsyndromic coronal suture synostosis. Part I: Breeding demographics, inheritance pattern, and craniofacial anomalies. Cleft Palate Craniofac J. 1994;31:1–7. doi: 10.1597/1545-1569_1994_031_0001_doasor_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 35.Mooney MP, Losken HW, Tschakaloff A, et al. Congenital bilateral coronal suture synostosis in a rabbit and craniofacial growth comparisons with experimental models. Cleft Palate Craniofac J. 1993;30:121–128. doi: 10.1597/1545-1569_1993_030_0121_cbcssi_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 36.Mooney MP, Smith TD, Burrows AM, et al. Coronal suture pathology and synostotic progression in rabbits with congenital craniosynostosis. Cleft Palate Craniofac J. 1996;33:369–378. doi: 10.1597/1545-1569_1996_033_0369_cspasp_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 37.Peng H, Usas A, Hannallah D, et al. Noggin improves bone healing elicited by muscle stem cells expressing inducible BMP4. Mol Ther. 2005;12:239–246. doi: 10.1016/j.ymthe.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 38.Qu-Petersen Z, Deasy B, Jankowski R, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthritis Cartilage. 2005;13:845–853. doi: 10.1016/j.joca.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimura H, Muneta T, Nimura A, et al. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 41.Liu TM, Martina M, Hutmacher DW, et al. Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells. 2007;25:750–760. doi: 10.1634/stemcells.2006-0394. [DOI] [PubMed] [Google Scholar]
- 42.Hayashi O, Katsube Y, Hirose M, et al. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum, and adipose tissue. Calcif Tissue Int. 2008;82:238–247. doi: 10.1007/s00223-008-9112-y. [DOI] [PubMed] [Google Scholar]
- 43.Dudas JR, Losee JE, Penascino VM, et al. Leporine-derived adipose precursor cells exhibit in vitro osteogenic potential. J Craniofac Surg. 2008;19:360–368. doi: 10.1097/SCS.0b013e318163e17b. [DOI] [PubMed] [Google Scholar]
- 44.Krebsbach PH, Mankani MH, Satomura K, et al. Repair of craniotomy defects using bone marrow stromal cells. Transplantation. 1998;66:1272–1278. doi: 10.1097/00007890-199811270-00002. [DOI] [PubMed] [Google Scholar]
- 45.Wright V, Peng H, Usas A, et al. BMP4-expressing muscle-derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol Ther. 2002;6:169–178. doi: 10.1006/mthe.2002.0654. [DOI] [PubMed] [Google Scholar]
- 46.Phillippi JA, Miller E, Weiss L, et al. Microenvironments engineered by inkjet bioprinting spatially direct adult stem cells toward muscle- and bone-like subpopulations. Stem Cells. 2008;26:127–134. doi: 10.1634/stemcells.2007-0520. [DOI] [PubMed] [Google Scholar]
- 47.Solchaga LA, Johnstone B, Yoo JU, et al. High variability in rabbit bone marrow-derived mesenchymal cell preparations. Cell Transplant. 1999;8:511–519. doi: 10.1177/096368979900800506. [DOI] [PubMed] [Google Scholar]
- 48.Jaiswal N, Haynesworth SE, Caplan AI, et al. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. [PubMed] [Google Scholar]
- 49.Phinney DG, Kopen G, Righter W, et al. Donor variation in the growth properties and osteogenic potential of human marrow stromal cells. J Cell Biochem. 1999;75:424–436. [PubMed] [Google Scholar]
- 50.Siddappa R, Licht R, van Blitterswijk C, et al. Donor variation and loss of multipotency during in vitro expansion of human mesenchymal stem cells for bone tissue engineering. J Orthop Res. 2007;25:1029–1041. doi: 10.1002/jor.20402. [DOI] [PubMed] [Google Scholar]
- 51.De Pollack C, Renier D, Hott M, et al. Increased bone formation and osteoblastic cell phenotype in premature cranial suture ossification (craniosynostosis). J Bone Miner Res. 1996;11:401–407. doi: 10.1002/jbmr.5650110314. [DOI] [PubMed] [Google Scholar]
- 52.Coussens AK, Wilkinson CR, Hughes IP, et al. Unravelling the molecular control of calvarial suture fusion in children with craniosynostosis. BMC Genomics. 2007;8:458. doi: 10.1186/1471-2164-8-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coussens AK, Hughes IP, Morris CP, et al. In vitro differentiation of human calvarial suture derived cells with and without dexamethasone does not induce in vivo-like expression. J Cell Physiol. 2009;218:183–191. doi: 10.1002/jcp.21586. [DOI] [PubMed] [Google Scholar]
- 54.Coussens AK, Hughes IP, Wilkinson CR, et al. Identification of genes differentially expressed by prematurely fused human sutures using a novel in vivo - in vitro approach. Differentiation. 2008;76:531–545. doi: 10.1111/j.1432-0436.2007.00244.x. [DOI] [PubMed] [Google Scholar]