Abstract
Objective
RA is associated with the presence of anti-citrullinated protein antibodies (ACPA). Nearly two thirds of patients with ACPA-positive RA have immune complexes (IC) that contain citrullinated fibrinogen (cFb), and these cFb-containing IC (cFb-IC) can exacerbate disease in murine models of RA; however, the exact role of such ACPA IC in RA pathogenesis has remained elusive. Here, we investigate a novel mechanism by which ACPA specifically targeting citrullinated fibrinogen may directly stimulate macrophage TNF production.
Methods
Murine or human macrophages were stimulated with native fibrinogen (nFb), citrullinated fibrinogen (cFb) or in vitro-generated nFb-IC or cFb-IC, and TNF production was measured by ELISA. IC were generated with either polyclonal anti-Fb antibodies or pooled IgG from patients with ACPA-positive RA. To evaluate the role of the toll-like receptor 4 (TLR4)-MyD88 pathway and the Fcγ receptor (FcγR) pathway in the induction of TNF by Fb and Fb-IC, parallel experiments were performed using (i) TLR4-deficient or MyD88-deficient macrophages, and (ii) inhibitors of TLR4 or FcγR.
Results
cFb stimulated macrophage TNF production more potently than nFb. Incorporation of cFb into IC augmented its ability to stimulate macrophage TNF production. cFb stimulation of TNF was dependent on TLR4 and MyD88, while cFb-IC stimulation was dependent on both TLR4-MyD88 and FcγR.
Conclusion
We demonstrate that cFb-IC can co-stimulate macrophages via dual engagement of TLR4 and FcγR, resulting in the synergistic induction of TNF production. Our findings suggest a potential role for citrullination in increasing the potency of an endogenous innate immune ligand and provide insight into the mechanism by which anti-citrulline autoimmunity may contribute to the onset and propagation of inflammation in RA.
Rheumatoid arthritis (RA) is an autoimmune disease characterized by the presence of autoantibodies, specifically those targeting proteins that have undergone post-translational conversion of peptidyl-arginine to citrulline, a process known as citrullination. Although citrullination occurs at sites of inflammation in diverse inflammatory conditions (1, 2), the presence of anti-citrullinated protein antibodies (ACPA) is specific to RA. The physiological targets of ACPAs are many and diverse (3); nevertheless, nearly two thirds of ACPA-positive RA patients have immune complexes (IC) containing citrullinated fibrinogen (cFb), both circulating and deposited in the RA synovium (4). Although transfer of ACPA that target cFb has been shown to exacerbate disease in a murine model of RA (5), the role of citrullination in inflammation and the mechanism by which ACPA may contribute to RA pathogenesis has not been elucidated.
A role for fibrinogen (Fb) in the pathogenesis of autoimmune arthritis has been suggested by findings showing that Fb-deficient mice are protected against the development of collagen-induced arthritis (CIA) (6). In addition, impairment of resolution of synovitis during antigen-induced arthritis in urokinase-deficient mice is associated with excessive deposition of fibrin in the synovium and can be reversed with a fibrinolytic agent (7). Mice expressing a mutant form of Fb that is unable to interact with monocytes and macrophages, while maintaining full clotting function, were also found to be relatively resistant to CIA (6), suggesting that interaction with monocytes/macrophages is important in the pathogenicity of Fb.
Macrophages accumulate in the synovial lining and sublining in RA, where they produce cytokines and chemokines that mediate inflammation, as well as destruction of cartilage and bone (8). The most prominent of the macrophage-produced cytokines is TNF, a molecule that mediates much of the articular and extra-articular pathology associated with RA (9). Interestingly, Fb deficiency attenuated TNF expression in the joints of mice with CIA but could not protect against inflammatory arthritis in transgenic mice overexpressing TNF, suggesting that Fb acts upstream of TNF in the promotion of synovitis (6).
Macrophages can be activated by T-cell contact or T-cell-released cytokines such as IFNγ or IL-17; IC, which trigger Fcγ receptor (FcγR) signaling; and innate immune receptors, such as the toll-like receptors (TLRs). TLRs are pattern-recognition receptors that recognize conserved microbial products known as pathogen-associated molecular patterns (PAMPs), as well as endogenous ligands known as damage-associated molecular patterns (DAMPs) (8).
Both TLR2 and TLR4 are highly expressed in RA synovial tissue (10, 11), and macrophages isolated from the joints of RA patients display increased levels of TLR2 and TLR4 and are more responsive to PAMPs (12). Furthermore, findings in animal models have implicated TLR4 in the pathogenesis of RA (13–15). Although bacterial TLR ligands have been reported in RA synovial tissue (16, 17), endogenous TLR ligands, such as HSP96 (18) and Tenascin-C (19), have also been implicated in RA pathogenesis. Fb, too, has been shown to serve as an endogenous activator of TLR4 (20, 21) and is highly expressed in the RA synovium (22)
Thus, Fb can induce TLR4 signaling, and cFb-IC can exacerbate murine arthritis. Unclear, however, is the extent to which these processes are interlinked and the role citrullination and ACPA play in RA. In this study, we investigate one mechanism by which cFb-IC can stimulate macrophage TNF production. We demonstrate that (i) citrullination renders fibrinogen a more potent stimulator of macrophage cytokine production; (ii) this stimulation is TLR4 dependent; and (iii) cFb-IC can co-stimulate macrophages via TLR4 and FcγR, resulting in synergistic induction of TNF production. These findings suggest that citrullination boosts the local inflammatory response at sites of damage or inflammation and provide the first explanation to date for the pathogenic specificity of an ACPA target.
MATERIALS AND METHODS
Sample collection
Serum was obtained from peripheral blood collected from patients with RA or osteoarthritis (OA). All RA patients met the criteria of the American College of Rheumatology (23). The patients were recruited from the Palo Alto, California VA Medical Center. Informed consent was obtained from all participating patients and these studies were approved by the institutional review board. Cell-free plasma was isolated by centrifugation and stored at −80°C.
Cell isolation and culture
Peritoneal exudate macrophages (PEM) were obtained from wild-type C57BL/6 mice (Jackson Laboratory, Bar Harbor, Maine), and from C57BL/6 TLR4Lps-n mice and C57BL/6 MyD88−/− mice (both a generous gift from Dr. Lawrence Steinman), by peritoneal lavage with 8 ml of cold DMEM media. PEM were allowed to adhere for 1 hour to plastic wells, washed with DMEM, and cultured overnight in DMEM supplemented with 10 ng/ml murine macrophage colony-stimulating factor (MCSF) (Peprotech). For co-stimulation experiments, PEM were pretreated for 12 hours with 100 U/ml murine interferon γ (IFN-γ) (Peprotech). The RAW 264.7 murine macrophage cell line was purchased from American Tissue Culture Center and used in experiments within 15 passages. RAW 264.7 macrophages were maintained in DMEM supplemented with 10% FCS, 100 U of penicillin, 100 μg/ml streptomycin, and 100μM glutamine.
For the generation of monocyte-derived macrophages, peripheral blood mononuclear cells (PBMCs) were obtained by density gradient centrifugation over Ficoll (Invitrogen) of buffy coats purchased from the Stanford Blood Center. Human monocytes were purified from PBMCs by negative selection, as recommended by the manufacturer (Miltenyi Biotec) and differentiated into macrophages by culture in RPMI containing 10% FCS and 50 ng/ml human MCSF for 7 days. Alternatively, monocytes were purified by adhesion for 2 hours, washed, and then cultured as described above. MCSF containing media was replaced at 3 days. After 7 days in MCSF culture, macrophage purity was observed to be similar with monocyte negative selection as compared with selection by adhesion. The experiments using animal or human materials were approved by the Stanford University Institutional Review Board.
Antibodies and reagents
Lipopolysaccharide (LPS) was from Sigma-Aldrich, and CpG oligodeoxynucleotides (CPG ODN) and the TLR4 inhibitor CLI-095 were from Invivogen. Murine FcγRII/III blocking antibody (2.4G2) was from eBioscience. Anti-human CD32 (FcγRIIa) antibody (Clone IV.3) was from Stem Cell Technologies. Purified human fibrinogen depleted of Von Willibrand’s factor and fibronectin (Enzymes Research Labs, Inc) were used in either unmodified (nFb) or citrullinated (cFb) forms. In vitro citrullination of fibrinogen was performed as previously described (4) and confirmed by mobility shift in SDS-PAGE analysis and by dot blot analysis using human ACPA-positive RA sera, anti-fibrinogen antibodies (Dako Cytomation), and anti-modified citrulline antibodies (Millipore). nFb was subjected to sham citrullination, in which nFb was processed in an identical manner to cFb but without the addition of the peptidyl arginine deiminase (PAD) enzyme. PAD enzyme incubated with citrullination buffer and DTT but without Fb served as a control to assure no contribution or contamination from the small amount of enzyme remaining in cFb. For some experiments, cFb and nFb were dialyzed against PBS (Slidalyzer, Pierce); macrophage stimulation with dialyzed or non-dialyzed Fb produced identical results (data not shown), confirming that the citrullination buffer was not a confounding factor.
Macrophage stimulation
Murine macrophages (1×105) were incubated with nFb, cFb, nFb-IC, or cFb-IC for 16–18h, after which TNF levels in culture supernatants were determined by ELISA(PeproTech). The TLR4 ligand LPS (100 ng/ml) and the TLR9 ligand CpG ODN (1 μg/ml) were used as controls for induction of TNF production and TLR4 specificity. IC were generated in vitro by incubation of nFb or cFb with a polyclonal rabbit antibody against human fibrinogen (Dako Cytomation) or, as a control, with normal polyclonal rabbit IgG (Dako Cytomation) at 37°C for 45 minutes. Cross titration of antibody and antigen yielded an optimal ratio for formation of IC: a final concentration of 10 μg/ml of Fb and 50 μg/ml of antibody were used for IC stimulation of RAW 267.4 cells, while 50 μg/ml of Fb and 100 μg/ml of antibody were used for IC stimulation of PEM and human monocyte-derived macrophage. At final dilutions, all reagents used in the stimulation assays were tested for endotoxin contamination by the Limulus amebocyte assay (Associates of Cape Cod, Inc), according to the manufacturer’s instructions, and were shown to possess endotoxin levels below the detectable range (< 0.03 EU/ml). For further confirmation that endotoxin contamination was not a confounding factor, in some experiments fibrinogen was treated with Detoxigel™ endotoxin-removal resin (Pierce) or performed stimulations in the presence of 10 μg/ml of polymyxin B (Sigma Aldrich).
For IC stimulation of human macrophages, human IgG derived from patients with ACPA-positive RA was used to generate plate-bound human cFb-IC. IgG was purified from three pooled plasma samples shown by ELISA to contain high levels of anti-cFb antibodies (see Figure 5A). IgG was purified by affinity chromatography on protein G columns (Pierce), according to the manufacturer’s instructions. The eluted IgG fractions were concentrated by centrifugation with buffer exchange to PBS (Amicon ultra, Millipore) and were depleted of endotoxin by filtration through a Polymyxin B column (Detoxigel, Pierce.) IgG concentrations were estimated by optical density (OD) at 280 nm, aliquoted, and stored at −80°C. For generation of cFb-IC, flat-bottomed 96-well cell culture plates were coated overnight with 50 μl of cFb (20μg/ml) at 4°C and washed in PBS containing 0.05% Tween-20. and then incubated for 2 hours at 4°C with 100μl of anti-cFb-positive IgG (2.5 mg/ml) or, as a control, with citrullination buffer alone. Wells were again washed in PBS containing 0.05% Tween-20, and macrophages (50–75,000/well) were then added to the wells in 200 μl of RMPI containing 5% FCS.
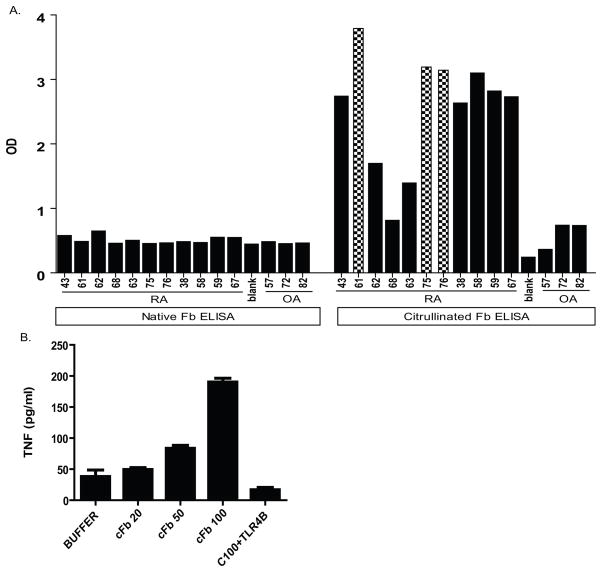
Figure 5.
cFb stimulate human macrophages to produce TNF production. A, Plasma samples from patients with ACPA-positive RA or from patients with osteoarthritis (OA) were assayed by ELISA for reactivity against native fibrinogen (nFb) and citrullinated fibrinogen (cFb). Values are O.D. for singlicate measures. B, Human monocyte-derived macrophages were stimulated with increasing concentrations of cFb in solution for 18 hours. For assessment of TLR4 dependence, cells were pretreated with TLR4-inhibitor CLI-095 (1.0 ug/ml) before adding cFb. Results are representative of experiments performed at least twice. Values are the mean and SEM of duplicate cultures.
Detection of antibodies to nFb or cFb
For the detection of antibodies against nFb or cFb, we used a direct ELISA that we developed previously (4). Briefly, microtiter plates (Nunc) were coated with 50μl of nFb or cFb in carbonate buffer, at a concentration of 20 μg/ml, and incubated at 4°C overnight. After being blocked in PBS containing 1% BSA, plasma samples were diluted 1:10 in dilution buffer (PBS containing 0.1% BSA and 0.1% Tween −20), added to the plate in duplicate at 100 μl/well, and incubated for 2 hours at room temperature. The samples were then incubated with a 1:5000 dilution of a monoclonal, HRP-labeled goat anti-human antibody for 1 hour at room temperature. The reaction was developed using 3,3′,5,5′-tetramethylbenzidinesubstrate (Sigma-Aldrich) for 15 minutes and stopped by addition of 50 μl of 2 N H2SO4. Relative quantification of antibodies against nFb or cFb was done by optical densitometry.
Statistical analysis
An unpaired Student’s t-test (GraphPad Prism Software) was used to compare cytokine production between groups. P values less than 0.05 were considered statistically significant.
RESULTS
cFb stimulates macrophage TNF production
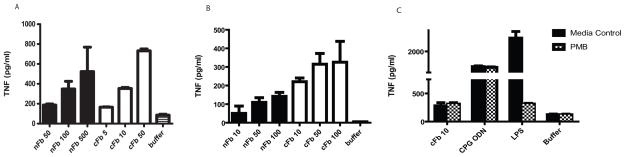
nFb has been shown to stimulate the production of cytokines by monocytes and macrophages (20, 21). To determine the effect of citrullination on fibrinogen’s proinflammatory properties, we compared the effect of nFb and cFb on macrophage TNF production. nFb dose dependently induced the production of TNF by RAW 274.6 macrophages (Figure 1A), in line with previous findings (20, 21). Compared to nFb, cFb was 10 times more potent an inducer of macrophage TNF production (Figure 1A). Similar results were obtained in primary murine PEM cells (Figure 1B). To rule out a significant contribution of endotoxin contaminant to the induction of TNF, we performed parallel stimulations in the presence of polymyxin B, an antibiotic that binds and inactivates endotoxin. Polymyxin B markedly suppressed TNF production induced by LPS but did not affect TNF production induced by nFb, cFb, or CpG ODN (Figure 1C), confirming that endotoxin contamination is not a significant confounding factor in these assays. These findings demonstrate that citrullination renders fibrinogen a more potent inducer of macrophage TNF production.
Figure 1.
Citrullination renders fibrinogen a more potent inducer of macrophage TNF production. RAW 267.4 macrophages A, and murine peritoneal macrophages B, were stimulated with native fibrinogen (nFb) or citrullinated fibrinogen (cFb) at the indicated concentrations (μg/ml). C, RAW 267.4 macrophages were pretreated with the endotoxin inhibitor Polymyxin B (PMB; 10 μg/ml) for 30 minutes and then stimulated with cFb, CpG oligodeoxynucleotides (CPG ODN; 1 μg/ml), or lipopolysaccharide (LPS; 100 ng/ml). TNF levels in culture supernatants following 16–18 hours of stimulation were measured by ELISA. Results are representative of experiments performed at least twice. Values are the mean and SEM of triplicate cultures.
cFb induces TNF production via TLR4
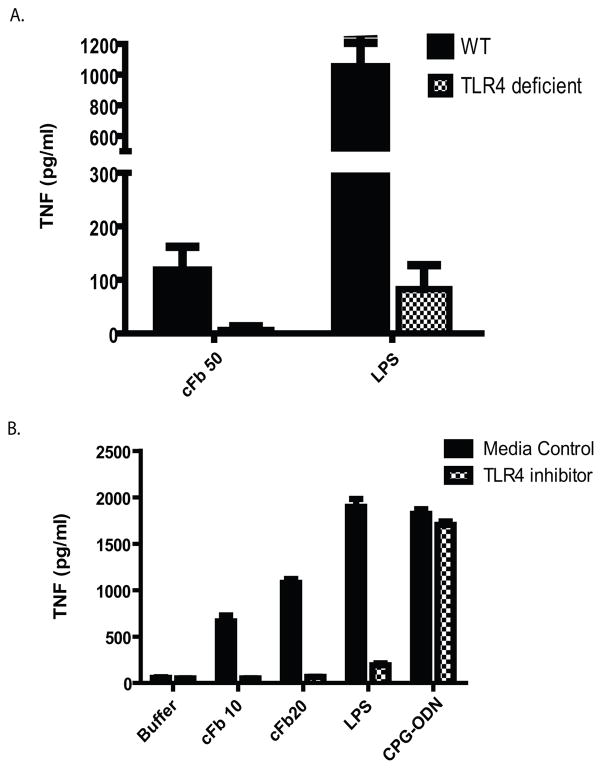
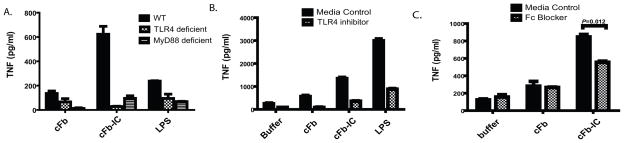
nFb has been shown to activate monocytes and macrophages via ligation of TLR4 (20, 21). To determine whether cFb activation of macrophages is also mediated by TLR4, we examined cFb-induced TNF production in PEM from wild-type mice and from TLR4-deficient mice. TLR4 deficiency abrogated TNF production in response to cFb (Figure 2A). Likewise, the TLR4-specfic inhibitor CLI-095 abrogated the production of TNF in response to cFb and LPS (Figure 2B), but it did not affect TNF production in response to the TLR9 agonist CpG ODN.
Figure 2.
Citrullinated fibrinogen induces macrophage TNF production via TLR4. A, Peritoneal macrophages from TLR4-deficient or wild-type (WT) B6 mice were stimulated with citrullinated fibrinogen (cFb; 50 μg/ml) or lipopolysaccharide (LPS; 100 ng/ml). B, RAW 267.4 macrophages were pretreated with the TLR4-inhibitor CLI-095 (0.5 μg/ml) or vehicle and then stimulated with increasing concentrations of cFb, with the TLR4 agonist LPS (100 ng/ml), or with the TLR9 agonist CpG oligodeoxynucleotides (CPG ODN; 1 μg/ml). TNF levels in culture supernatants following 16–18 hours of stimulation were measured by ELISA. Results are representative of experiments performed at least twice. Values are the mean and SEM of triplicate cultures.
cFb-containing immune complexes co-stimulate via TLR4 and FcγR
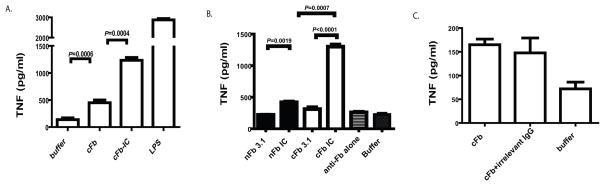
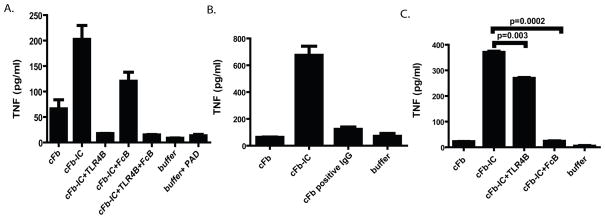
To examine whether incorporation of cFb into IC enhances its ability to stimulate macrophage TNF production, we stimulated macrophages with in vitro-generated cFb-IC or with cFb alone. Macrophages produced significantly greater amounts of TNF in response to cFb-IC than in response to cFb alone. cFb-IC induced significantly greater TNF production than that induced by nFb-IC (Figure 3B), suggesting that the proinflammatory response to IC is influenced by the specific autoantigen incorporated in the IC. Furthermore, IC containing sub-stimulatory concentrations of cFb (i.e., which do not themselves increase TNF production) were also able to induce substantial TNF production, whereas similar concentrations of nFb-IC induced only minimal TNF production (Figure 3B). Treatment with anti-Fb antibody alone did not significantly stimulate TNF production. Unlike nFb-IC or cFb-IC, a mixture of cFb and normal polyclonal rabbit IgG (which is unable to form IC) did not increase TNF production above that achieved with cFb alone (Figure 3C). Together, these findings suggest that incorporation of cFb into IC enhances its ability to induce macrophage TNF production. Interestingly, measurement of several other inflammatory cytokines including IL-1, IL-6, and IL-12 in the same culture supernatants did not demonstrate a similar pattern of cFb-IC induced co-stimulation (data not shown).
Figure 3.
Citrullinated-fibrinogen-containing immune complexes co-stimulate macrophage TNF production. A, RAW 267.4 macrophages were stimulated with citrullinated fibrinogen (cFb; 10μg/ml) alone or with cFb-containing immune complexes (cFb-IC) generated by incubating cFb (10μg/ml) with polyclonal anti-Fb antibody (50μg/ml). Citrullination buffer alone and lipopolysaccharide (LPS, 100 ng/ml) were used as negative and positive controls, respectively. B, RAW 267.4 macrophages were stimulated with Fb-IC generated using a concentration (3.12μg/ml) of native Fb (nFb) or cFb that alone elicits negligible macrophage TNF production, with nFb or cFb alone (3.12 μg/ml), or with anti-Fb antibody alone. C, RAW 267.4 cells were stimulated with cFb alone (10μg/ml) or cFb (10 μg/ml) incubated with normal polyclonal IgG (50μg/ml). TNF levels in culture supernatants following 16–18 hours of stimulation were measured by ELISA. Results are representative of experiments performed at least twice. Values are the mean and SEM of triplicate cultures.
To determine whether TLR4 is required for cFb-IC-mediated TNF production, we examined cFb-IC-induced TNF production in wild-type, TLR4-deficient, and MyD88-deficient PEM. TLR4 deficiency abrogated both cFb- and cFb-IC-induced TNF production (Figure 4A). Signaling downstream of TLR4 bifurcates into a TRIF-dependent pathway and an MyD88-dependent pathway (24). We found that cFb- and cFb-IC-induced TNF production was abrogated in MyD88-deficient PEM, indicating that cFb-induced TNF production is dependent on the TLR4-MyD88 signaling pathway. The small-molecule TLR4-inhibitor CLI-095 also abrogated the macrophage response to cFb-IC (Figure 4B), confirming the dependence of the response on the TLR4 signaling pathway.
Figure 4.
Co-stimulation of macrophage TNF production by citrullinated-fibrinogen-containing immune complexes is dependent on TLR4 and FcγR. A, Peritoneal macrophages from wild-type (WT), TLR4-deficient, or MyD88-deficient mice were stimulated with citrullinated fibrinogen (cFb) alone (50 μg/ml) or cFb-containing immune complexes (cFb-IC; 50 μg/ml of cFb incubated with 75 μg/ml of polyclonal anti-Fb antibody). The TLR4-agonist lipopolysaccharide (LPS, 100 ng/ml) was used as a positive control B, RAW 267.4 macrophages were pretreated for 30 minutes with the TLR4-inhibitor CLI-095 (0.5μg/ml) or media control and then stimulated with cFb alone (10μg/ml) or cFb-IC (10 μg/ml of cFb incubated with 50 μg/ml of polyclonal anti-Fb antibody). C, RAW 267.4 macrophages were pretreated for 30 minutes with FcγR-blocking antibody (10μg/ml), and then both pretreated and untreated, control wells were washed twice with serum-free media and stimulated with cFb alone (10μg/ml) or cFb-IC (10μg/ml of cFb incubated with 50 μg/ml of polyclonal anti-Fb antibody). TNF levels in culture supernatants following 16–18 hours of stimulation were measured by ELISA. Results are representative of experiments performed at least twice. Values are the mean and SEM of triplicate cultures.
IC activate cell signaling by binding to FcγR on the surface of innate immune effector cells (25). To determine whether cFb-IC stimulate macrophages through co-stimulation of both FcγR and TLR4, we pretreated macrophages with an FcγR-blocking antibody. Blockade of FcγR significantly reduced TNF production in response to cFb-IC (Figure 4C). As expected, FcγR blockade had no effect on TNF production induced by cFb alone. Together, these results suggest that cFb-IC serve as potent inducers of macrophage TNF production by co-ligating TLR4 and the FcγR.
cFb, but not nFb, is targeted by autoantibodies in most ACPA-positive RA patients
cFb has been described as a target of ACPA (26), and we previously detected the presence of cFb-containing IC both in the circulation and in the synovium of patients with ACPA-positive RA (4). To confirm this finding in an additional cohort, and to obtain purified IgG for further experimentation, we evaluated the levels of antibodies against nFb or cFb in plasma derived from 11 of patients with ACPA-positive RA and 3 patients with OA. A majority (73%) of the ACPA-positive RA patients tested were found to possess antibodies targeting cFb but not nFb (Figure 5A). In contrast, control sera from OA patients targeted neither nFb nor cFb consistent with prior observations (26).
RA patient-derived IgG can form cFb-IC and co-stimulate TNF production from human monocyte-derived macrophage
To determine the relevance of our finding in a human system, we evaluated the ability of cFb to stimulate TNF production from human monocyte-derived macrophages. We demonstrate a clear dose response to cFb (though at concentrations slightly higher than that needed to stimulate murine RAW cells) and that this effect is TLR4 dependent (Figure 5B). PAD enzyme at a concentration identical to that obtained with cFb 100ug/ml displayed negligible stimulatory capacity for macrophage TNF release (Figure 6A). We then used in vitro generated cFb-IC formed with polyclonal anti-fibrinogen antibody (as above) to again demonstrate a TLR4 and FcγR dependent co-stimulation of human macrophages (Figures 6A). To further demonstrate the potential importance of cFb-IC-mediated macrophage co-stimulation in human RA, we stimulated human monocyte-derived macrophages with cFb-IC generated by incubating plate-bound cFb with pooled IgG derived from three ACPA-positive RA patients (patients 61, 75, and 76 in Figure 5A). Plate-bound cFb showed a non-significant trend toward stimulation of TNF production. cFb-IC induced significantly higher levels of TNF production than cFb alone and plates coated with nFb incubated with an identical concentration of IgG containing anti-cFb antibodies showed no stimulation of macrophage TNF production (Figure 6B) ruling out endotoxin contamination from human IgG. Interestingly, though both TLR4 and FcγR inhibition abrogated macrophage TNF production, the role of FcγR seems more significant and TLR4 less significant when stimulated with plate bound cFb-IC (Figure 6C). TLR4 blockade alone resulted in nearly complete abrogation of macrophage TNF release in response to soluble IC while FcγR blockade resulted in nearly complete abrogation in response to platebound IC. Thus, blockade of both TLR4 and FcγR resulted in minimal additional abrogation of TNF release in our assay (Figure 6A).
Figure 6.
cFb immune complexes formed with polyclonal anti-Fb antibodies or RA patients’ IgG co-stimulate human macrophages to produce TNF. A, Human monocyte-derived macrophages were pretreated for 30 minutes with the TLR4-inhibitor CLI-095 (1.0 ug/ml), FcγRIIa blocking antibody (10 ug/ml), both inhibitors, or media alone for 30 minutes and then stimulated with cFb (50 ug/ml) or cFb-IC (cFb-IC; 50 ug/ml of cFb incubated with 75 ug/ml of polyclonal anti-Fb antibody). B, Human monocyte-derived macrophages were added to plates pre-coated with citrullinated-fibrinogen immune complexes (cFb-IC) generated using pooled IgG from three RA plasma specimens with high levels of anti-cFb antibodies (Figure 5A, crosshatched bars). Wells coated with nFb incubated with IgG containing anti-cFb antibodies, and plates coated with buffer alone were used as negative controls. C, For assessment of TLR4 dependence and/or FcγR dependence, an identical aliquot of monocyte-derived macrophages was preincubated with the CLI-095 (1.0 ug/ml) or FcγRIIa blocking antibody before being added to the cFb-IC-coated plates. Results are representative of experiments performed at least twice. Values are the mean and SEM of duplicate cultures.
DISCUSSION
Neither the mechanisms contributing to the persistent activation of macrophages in the RA synovium nor the role of ACPAs in contributing to RA pathogenesis have been fully elucidated. Here we show that two interlinked processes can augment Fb’s proinflammatory properties: (i) citrullination of Fb increases its potency as a TLR4 agonist and hence inducer of TNF production, and (ii) incorporation of cFb into IC further increases its potency as an inducer of TNF production by enabling co-stimulation of macrophages via simultaneous ligation of TLR4 and FcγR. Thus, a scenario can be envisioned in which local inflammation in the RA synovium induces citrullination of Fb, resulting in the generation of cFb-specific ACPA and the consequent formation of cFb-IC, which in turn amplify local inflammation and lead to further citrullination of Fb. In this way, cFb-IC could potentially participate in a feed-forward amplification loop that propagates the synovial inflammation characteristic of RA.
In addition to Fb, several other innate immune ligands of TLR4 have been identified, including heat shock protein (HSP) 22 (27), HSP60 (28), HSP70 (29), biglycan (30), fibronectin (31), (28–32) and, in some cases, implicated in the pathogenesis of RA (18, 19). Notably, Tenascin-C was shown to induce TLR4-mediated inflammation in synovial joints via its fibrinogen globe region (19), a region with significant homology to the globular domain of the β- and γ-chains of fibrinogen (33)Several lines of evidence suggest that, among the many targets of the ACPA immune response, cFb is an important contributor to RA pathogenesis: Immunization of mice with citrulline-containing Fb can induce murine arthritis (34); transfer of anti-cFb antibodies can exacerbate murine arthritis (5); and the majority of patients with ACPA-positive RA have cFb-IC (4). Our current observations provide mechanistic evidence in support of a key role for cFb-IC. Nevertheless, not all ACPA-positive RA patients have cFb-IC. It is therefore notable that several other DAMPs have also been identified as targets of the RA-associated autoantibody response (35, 36). Thus the paradigm of autoantigen-IC-mediated inflammation via innate immune receptors may extend beyond cFb to other targets of the immune response in RA.
Our findings present the first example of an inflammation-associated post-translational modification directly increasing the potency of the DAMP-induced inflammatory response. Because citrullination is a widespread phenomenon during inflammation, its ability to modify local DAMPs could have broad-reaching implications for the innate immune response in a variety of inflammatory disorders. cFb itself could also potentially play a role in a variety of inflammatory disorders. Although normally confined to the vascular space, Fb typically exudes into extra-vascular tissue during inflammation—the same sites of inflammation to which neutrophils and monocytes migrate and release the PAD enzymes that mediate protein citrullination (37).
DNA-containing IC have been shown to co-stimulate TLR9 and the B-cell receptor (38) or TLR9 and FcγR (39, 40), and are thought to play an important role in the pathogenesis of systemic lupus erythematosus (41). We show that, in an analogous manner, an RA-associated IC can co-ligate TLR4 and FcγR, and that such an IC can elicit a potent inflammatory response from the macrophage through the synergistic action of an innate immune receptor and an adaptive immune receptor.
A limitation of this study is the use of a commercial polyclonal anti-Fb antibody for the generation of IC. We screened several polyclonal and monoclonal antibodies and all of them demonstrated some preference for cFb or nFb, as assessed by dot-blot analysis (data not shown). The antibody used in this study was chosen on the basis of its ability to bind to nFb and cFb with equal affinity, thereby enabling us to demonstrate the importance of the cFb antigen in the synergistic cFB-IC-induced stimulation of macrophage TNF production. We further demonstrate similar co-stimulation by cFb-IC derived from ACPA-positive IgG thus reinforcing the validity of results obtained using the polyclonal anti-Fb antibody. Antibodies isolated from ACPA-positive RA patients have been shown to bind cFb and stimulate macrophage TNF production (42) in a study that was limited to the effects mediated through FcγRIIa. Although TNF production in response to cFb alone was not detected in that study, only platebound cFb was examined. We too found that, whereas cFb added to the culture media could induce TNF production, plate-bound cFb alone could not. This could be due to relatively low concentration of bound cFb remaining after washing or, alternatively, to constrained structure of plate-bound cFb, such that cFb can no longer bind TLR4 or accessory receptors. This may additionally explain why FcγR blockade was proportionally more inhibitory in the setting of platebound IC, while TLR4 blockade was more inhibitory in the setting of soluble IC. Which formation is more relevant in vivo remains unclear. However, previous work in our laboratory clearly demonstrated the presence of cFb-IC both circulating in blood and in RA pannus tissue (4). Given that citrullination of Fb has been demonstrated to inhibit fibrin polymerization (43), it is possible that citrullination of Fb may contribute to it’s ability to remain soluble and thus available for both innate immune signaling and IC formation. Finally, as with most studies on citrullination and the ACPA immune response, in vitro citrullination of an antigen may not accurately reflect the true physiologic process that occurs in vivo.
Our data support a role for ACPA, and specifically cFb-IC, in the pathogenesis of RA. Not only is cFb capable of inducing robust cytokine production by macrophages, but also cFb-IC can further enhance macrophage TNF production by co-stimulating TLR4 and FcγR. Exudation and citrullination of Fb at sites of inflammation (such as the RA joint) may provide a potent inflammatory signal for macrophage cytokine production, and the ability of cFb-IC to co-stimulate macrophage TNF production suggests that citrullination of autoantigens, which are themselves self adjuvants, may provide a mechanism for the amplification and perpetuation of the inflammatory response. TLR4 activation is increased in RA synovial fluid macrophages (12), and findings from both murine models (13, 14) and early-phase clinical trials (44) suggest that TLR4 blockade may have potential as a therapeutic approach in RA. These observations suggest several potential therapeutic targets, including the citrullination of innate inflammatory proteins, the production of anti-citrulline protein antibodies, and the activation of the TLR4 pathway.
Acknowledgments
This work was supported by NIH NIAMS RC1 AR058713, NIAMS R01 AR-054822, an American College of Rheumatology Research and Education Foundation Within-Our-Reach Award, and Veterans Affairs Health Care System funding to W.H.R. J.S. received salary support from an American College of Rheumatology Research and Education Foundation Physician Scientist Development Award.
We thank Drs. Peggy Ho and Lawrence Steinman for providing TLR4 and MyD88 deficient mice. We thank the members of the Robinson laboratory for their insight into this study and Dr. Tamsin Lindstrom for her critical review of this manuscript. This work was supported by NIH NIAMS RC1 AR058713, NIAMS R01 AR-054822, an American College of Rheumatology Research and Education Foundation Within-Our-Reach Award, and Veterans Affairs Health Care System funding to W.H.R. J.S. received salary support provided by the American College of Rheumatology Research and Education Foundation Physician Scientist Development Award.
References
- 1.Makrygiannakis D, af Klint E, Lundberg IE, Lofberg R, Ulfgren AK, Klareskog L, et al. Citrullination is an inflammation-dependent process. Ann Rheum Dis. 2006;65(9):1219–22. doi: 10.1136/ard.2005.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vossenaar ER, Smeets TJ, Kraan MC, Raats JM, van Venrooij WJ, Tak PP. The presence of citrullinated proteins is not specific for rheumatoid synovial tissue. Arthritis Rheum. 2004;50(11):3485–94. doi: 10.1002/art.20584. [DOI] [PubMed] [Google Scholar]
- 3.Hueber W, Kidd BA, Tomooka BH, Lee BJ, Bruce B, Fries JF, et al. Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2005;52(9):2645–55. doi: 10.1002/art.21269. [DOI] [PubMed] [Google Scholar]
- 4.Zhao X, Okeke NL, Sharpe O, Batliwalla FM, Lee AT, Ho PP, et al. Circulating immune complexes contain citrullinated fibrinogen in rheumatoid arthritis. Arthritis Res Ther. 2008;10(4):R94. doi: 10.1186/ar2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116(4):961–73. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flick MJ, LaJeunesse CM, Talmage KE, Witte DP, Palumbo JS, Pinkerton MD, et al. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin alphaMbeta2 binding motif. J Clin Invest. 2007;117(11):3224–35. doi: 10.1172/JCI30134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busso N, Peclat V, Van Ness K, Kolodziesczyk E, Degen J, Bugge T, et al. Exacerbation of antigen-induced arthritis in urokinase-deficient mice. J Clin Invest. 1998;102(1):41–50. doi: 10.1172/JCI2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drexler SK, Kong PL, Wales J, Foxwell BM. Cell signalling in macrophages, the principal innate immune effector cells of rheumatoid arthritis. Arthritis Res Ther. 2008;10(5):216. doi: 10.1186/ar2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmann M, Brennan FM, Williams RO, Woody JN, Maini RN. The transfer of a laboratory based hypothesis to a clinically useful therapy: the development of anti-TNF therapy of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2004;18(1):59–80. doi: 10.1016/j.berh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Iwahashi M, Yamamura M, Aita T, Okamoto A, Ueno A, Ogawa N, et al. Expression of Toll-like receptor2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2004;50(5):1457–67. doi: 10.1002/art.20219. [DOI] [PubMed] [Google Scholar]
- 11.Radstake TR, Roelofs MF, Jenniskens YM, Oppers-Walgreen B, van Riel PL, Barrera P, et al. Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis Rheum. 2004;50(12):3856–65. doi: 10.1002/art.20678. [DOI] [PubMed] [Google Scholar]
- 12.Huang Q, Ma Y, Adebayo A, Pope RM. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56(7):2192–201. doi: 10.1002/art.22707. [DOI] [PubMed] [Google Scholar]
- 13.Abdollahi-Roodsaz S, Joosten LA, Roelofs MF, Radstake TR, Matera G, Popa C, et al. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 2007;56(9):2957–67. doi: 10.1002/art.22848. [DOI] [PubMed] [Google Scholar]
- 14.Choe JY, Crain B, Wu SR, Corr M. Interleukin 1 receptor dependence of serum transferred arthritis can be circumvented by toll-like receptor 4 signaling. J Exp Med. 2003;197(4):537–42. doi: 10.1084/jem.20021850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee EK, Kang SM, Paik DJ, Kim JM, Youn J. Essential roles of Toll-like receptor-4 signaling in arthritis induced by type II collagen antibody and LPS. Int Immunol. 2005;17(3):325–33. doi: 10.1093/intimm/dxh212. [DOI] [PubMed] [Google Scholar]
- 16.Schrijver IA, Melief MJ, Tak PP, Hazenberg MP, Laman JD. Antigen-presenting cells containing bacterial peptidoglycan in synovial tissues of rheumatoid arthritis patients coexpress costimulatory molecules and cytokines. Arthritis Rheum. 2000;43(10):2160–8. doi: 10.1002/1529-0131(200010)43:10<2160::AID-ANR3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 17.van der Heijden IM, Wilbrink B, Tchetverikov I, Schrijver IA, Schouls LM, Hazenberg MP, et al. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. 2000;43(3):593–8. doi: 10.1002/1529-0131(200003)43:3<593::AID-ANR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 18.Huang QQ, Sobkoviak R, Jockheck-Clark AR, Shi B, Mandelin AM, 2nd, Tak PP, et al. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J Immunol. 2009;182(8):4965–73. doi: 10.4049/jimmunol.0801563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15(7):774–80. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 20.Kuhns DB, Priel DA, Gallin JI. Induction of human monocyte interleukin (IL)-8 by fibrinogen through the toll-like receptor pathway. Inflammation. 2007;30(5):178–88. doi: 10.1007/s10753-007-9035-1. [DOI] [PubMed] [Google Scholar]
- 21.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167(5):2887–94. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 22.Clemmensen I, Andersen RB. Properties of fibrinogen-antigenic material on the rheumatoid synovial membrane and in the rheumatoid synovial fluid. J Lab Clin Med. 1978;92(5):678–89. [PubMed] [Google Scholar]
- 23.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 24.Akira S, Hoshino K, Kaisho T. The role of Toll-like receptors and MyD88 in innate immune responses. J Endotoxin Res. 2000;6(5):383–7. [PubMed] [Google Scholar]
- 25.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 26.Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, Senshu T, et al. The major synovial targets of the rheumatoid arthritis-specific antifilaggrin autoantibodies are deiminated forms of the alpha-and beta-chains of fibrin. J Immunol. 2001;166(6):4177–84. doi: 10.4049/jimmunol.166.6.4177. [DOI] [PubMed] [Google Scholar]
- 27.Roelofs MF, Boelens WC, Joosten LA, Abdollahi-Roodsaz S, Geurts J, Wunderink LU, et al. Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol. 2006;176(11):7021–7. doi: 10.4049/jimmunol.176.11.7021. [DOI] [PubMed] [Google Scholar]
- 28.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164(2):558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 29.Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, et al. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277(17):15028–34. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- 30.Schaefer L, Babelova A, Kiss E, Hausser HJ, Baliova M, Krzyzankova M, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115(8):2223–33. doi: 10.1172/JCI23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276(13):10229–33. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 32.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195(1):99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gulcher JR, Nies DE, Alexakos MJ, Ravikant NA, Sturgill ME, Marton LS, et al. Structure of the human hexabrachion (tenascin) gene. Proc Natl Acad Sci U S A. 1991;88(21):9438–42. doi: 10.1073/pnas.88.21.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho PP, Lee LY, Zhao X, Tomooka BH, Paniagua RT, Sharpe O, et al. Autoimmunity against fibrinogen mediates inflammatory arthritis in mice. J Immunol. 184(1):379–90. doi: 10.4049/jimmunol.0901639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tishler M, Shoenfeld Y. Anti-heat-shock protein antibodies in rheumatic and autoimmune diseases. Semin Arthritis Rheum. 1996;26(2):558–63. doi: 10.1016/s0049-0172(96)80043-6. [DOI] [PubMed] [Google Scholar]
- 36.Monach PA, Hueber W, Kessler B, Tomooka BH, BenBarak M, Simmons BP, et al. A broad screen for targets of immune complexes decorating arthritic joints highlights deposition of nucleosomes in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106(37):15867–72. doi: 10.1073/pnas.0908032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vossenaar ER, van Venrooij WJ. Citrullinated proteins: sparks that may ignite the fire in rheumatoid arthritis. Arthritis Res Ther. 2004;6(3):107–11. doi: 10.1186/ar1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416(6881):603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 39.Boule MW, Broughton C, Mackay F, Akira S, Marshak-Rothstein A, Rifkin IR. Toll-like receptor 9-dependent and -independent dendritic cell activation by chromatin-immunoglobulin G complexes. J Exp Med. 2004;199(12):1631–40. doi: 10.1084/jem.20031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest. 2005;115(2):407–17. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–41. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 42.Clavel C, Nogueira L, Laurent L, Iobagiu C, Vincent C, Sebbag M, et al. Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008;58(3):678–88. doi: 10.1002/art.23284. [DOI] [PubMed] [Google Scholar]
- 43.Okumura N, Haneishi A, Terawasa F. Citrullinated fibrinogen shows defects in FPA and FPB release and nd fibrin polymerization catalyzed by thrombin. Clin Chim Acta. 2009;401(1–2):119–23. doi: 10.1016/j.cca.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 44.Vanags D, Williams B, Johnson B, Hall S, Nash P, Taylor A, et al. Therapeutic efficacy and safety of chaperonin 10 in patients with rheumatoid arthritis: a double-blind randomised trial. Lancet. 2006;368(9538):855–63. doi: 10.1016/S0140-6736(06)69210-6. [DOI] [PubMed] [Google Scholar]