Abstract
Context
Disturbances in markers of cortical GABA neurotransmission are a common finding in schizophrenia. The nature of GABA neurotransmission (hyperpolarizing or depolarizing) depends on the local intracellular Cl− concentration. In the central nervous system, the intracellular Cl− level is determined by the activity of two cation-chloride transporters, NKCC1 and KCC2. The activities of these transporters are in turn regulated by a network of serine-threonine kinases that includes OXSR1, STK39 and the WNK kinases WNK1, 3 and 4.
Objective
To compare the levels of NKCC1, KCC2, OXSR1, STK39, WNK1, WNK3 and WNK4 transcripts in prefrontal cortex area 9 between schizophrenia and normal comparison subjects.
Design
Real-time qPCR technique was used to measure transcript levels in prefrontal cortex.
Setting
Human brain specimens were obtained from autopsies conducted at the Allegheny County Medical Examiner’s Office, Pittsburgh, PA.
Participants
Postmortem brain specimens from 42 subjects with schizophrenia and 42 matched normal comparison subjects. Brain specimens from 18 macaque monkeys chronically exposed to haloperidol, olanzapine, or sham.
Main outcome measures
Relative expression levels for NKCC1, KCC2, OXSR1, STK39, WNK1, WNK3 and WNK4 transcripts compared to the mean expression level of three housekeeping transcripts.
Results
OXSR1 and WNK3 transcripts were substantially over-expressed in schizophrenia relative to comparison subjects. In contrast, NKCC1, KCC2, STK39, WNK1 and WNK4 transcript levels did not differ between subject groups. OXSR1 and WNK3 transcript expression levels were not changed in antipsychotic-exposed monkeys and were not affected by potential confounding factors in the subjects with schizophrenia.
Conclusions
In schizophrenia, increased expression levels, and possibly increased kinase activities, of OXSR1 and WNK3 may shift the balance of chloride transport by NKCC1 and KCC2 and alter the nature of GABA neurotransmission in the prefrontal cortex.
Keywords: GABA, inhibition, prefrontal cortex
Introduction
A core feature of schizophrenia is the impairment of certain cognitive functions, such as working memory1, that are dependent on the circuitry of the dorsolateral prefrontal cortex (DLPFC)2. These cognitive deficits in schizophrenia are associated with altered neural network synchrony3–4 that may be attributable, at least in part, to functional abnormalities in GABA neurotransmission in the DLPFC5.
Disturbed GABA neurotransmission in schizophrenia might arise from alterations in one or more of the following factors: 1) the strength of GABA neurotransmission, which depends on the amount of GABA available at the synapse; 2) the kinetics of GABA neurotransmission which is determined, in part, by the subunit composition of GABA-A receptors; and 3) the nature of GABA neurotransmission, which can be hyperpolarizing, depolarizing or shunting depending on the flow of Cl− ions when GABA-A receptors are activated6. Pre- and post-synaptic markers of the strength and kinetics of GABA neurotransmission are known to be altered in the DLPFC in subjects with schizophrenia7–8, but measures of the nature of GABA neurotransmission have not been evaluated.
The flow of Cl− ions through GABA-A receptors depends on intracellular levels of Cl− which are regulated by the relative activities of the sodium-potassium-chloride cotransporter 1 (NKCC1; SLC12A2), which mediates Cl− uptake, and the potassium-chloride cotransporter 2 (KCC2; SLC12A5) which mediates Cl− extrusion from the cell. In the central nervous system, NKCC1 is found in both neurons and glial cells, but KCC2 is strictly neuronal9–11. The activities of both transporters are sensitive to intracellular Cl− levels, and their activation depends upon their phosphorylation (NKCC1) or dephosphorylation (KCC2) status12–14 (for review see15). OXSR1 (oxidative stress response kinase; also known as OSR1) and STK39 (Ste-20-related, proline-alanine-rich kinase; also known as SPAK) are highly expressed in the brain16–19 and bind to NKCC1 where they phosphorylate residues present in the N-terminal domain, resulting in an increase of NKCC1 activity17, 20–23. In addition, NKCC1 can be phosphorylated by the co-expression of STK39 and WNK4 (with no K (lysine) protein kinase-4) kinases in vitro24. Conversely, STK39-mediated phosphorylation has a dominant-negative effect on KCC2 function24. Another WNK kinase, WNK1, activates both STK39 and OXSR1 via phosphorylation23, 25–27. Finally, a fifth kinase, WNK3 both activates NKCC1 and inhibits KCC2, regardless of cellular tonicity28–29. Consequently, alterations in the relative expression levels of NKCC1 and KCC2, or of their regulatory kinases, in the DLPFC of subjects with schizophrenia could, by shifting intracellular Cl− levels, alter the nature of GABA transmission and thereby contribute to impaired neural network synchrony and cognitive dysfunction in affected individuals.
In order to examine this possibility, we quantified mRNA expression levels of NKCC1 and KCC2 and their associated regulatory kinases STK39, OXSR1, WNK1, WNK3 and WNK4 in the DLPFC from subjects with schizophrenia and matched normal comparison subjects. In schizophrenia, OXSR1 and WNK3 transcripts were substantially over-expressed, relative to comparison subjects. In contrast, NKCC1, KCC2, STK39, WNK1 and WNK4 transcripts levels did not differ between subject groups. Thus, increased expression levels, and possibly increased kinase activities, of OXSR1 and WNK3 in schizophrenia, would be predicted to both increase NKCC1 and decrease KCC2 activities, producing greater intracellular Cl− concentration, and thus altering the nature of GABA neurotransmission in the DLPFC.
Methods
Subjects
Brain specimens (n = 84) were obtained during autopsies conducted at the Allegheny County Medical Examiner’s Office (Pittsburgh, PA) after consent was obtained from the next-of-kin. An independent committee of experienced research clinicians made consensus DSM-IV diagnoses for each subject on the basis of medical records and structured diagnostic interviews conducted with the decedent’s family members, as described previously30. In order to control for experimental variance and to reduce biological variance between subject groups, each subject with schizophrenia (n=42) was matched with one normal comparison subject for sex, and as closely as possible for age and postmortem interval (PMI); samples from a given subject pair were always processed together. The subject groups (Table 1; see supplemental Table 1 for details on individual subjects) did not significantly differ in mean age, PMI, brain pH, RNA integrity number (RIN), or tissue storage time at −80°C (all t82< 1.67; all p>0.1) or in race (Χ2= 1.587; p=0.207). All procedures were approved by the University of Pittsburgh’s Committee for the Oversight of Research Involving the Dead and Institutional Review Board for Biomedical Research.
Table 1.
Summary of demographic characteristics of subjects
|
qPCR |
||
|---|---|---|
| Comparison | Schizophrenia | |
| Number | 42 | 42 |
| Sex | 31 M, 11 F | 31 M, 11 F |
| Race | 34 W, 8 B | 29 W, 13 B |
| Age (years) | 48.5 (13.1) | 47.0 (12.8) |
| PMI (h) | 17.7 (5.7) | 18.7 (8.8) |
| Brain pH | 6.7 (0.20) | 6.6 (0.4) |
| RIN* | 8.2 (0.7) | 8.1 (0.8) |
| Storage time (months at −80°C) | 74.2 (42.9) | 78.5 (42.5) |
Values are Mean(SD)
RIN: RNA integrity number
Tissue preparation
The right hemisphere of each brain was blocked coronally, immediately frozen and stored at −80°C as described previously31. Area 9 of the DLPFC was identified cytoarchitectonically from Nissl-stained coronal sections31 spanning the rostrocaudal axis of the superior frontal sulcus. The cortical grey matter was dissected from cryostat sections (40 μm) in a manner that insured limited white matter contamination and excellent RNA preservation as described previously32. Total RNA was isolated from tissue homogenates using the TRIzol® protocol from Invitrogen (Invitrogen Corporation, Carlsbad, CA) and further purified using the RNeasy® kit (QIAGEN, Valencia, CA). RNA integrity was assessed by measuring the RNA integrity number (RIN) using the Agilent 2100 bioanalyzer (Agilent technologies, Santa Clara, CA).
Quantitative Real-Time Polymerase Chain Reaction
Total RNA was converted to complementary DNA (cDNA) using the High Capacity cDNA Archive Kit from Applied Biosystems (Foster City, CA). For each reaction, we used 50 ng of total RNA from each subject. Priming was performed with random hexamers according to the manufacturer’s recommendations. The efficiency for each primer set (Supplemental Table 2) was between 92% and 100%, and the amplified product resulted in a specific single product in dissociation curve analysis. Given the high level of homology between certain domains of OXSR1 and STK39, the qPCR primer sets for these two transcripts were designed within a unique sequence in the 3’ UTR region of each mRNA.
Samples from each matched pair of schizophrenia and comparison subjects were always assayed on the same plate. For each sample, amplified product differences for each transcript were measured with four replicates using SYBR Green chemistry-based detection33.
Beta-actin, cyclophillin A and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as endogenous reference genes. These three transcripts were selected based on their previously demonstrated stable expression across both schizophrenia and normal comparison subjects32. The qPCR reactions were carried out in an ABI Prism 7000 thermal cycler (Applied Biosystems, Foster City, CA) using the ABI Prism 7000 SDS software with the automatic baseline and threshold detection options selected. These data were exported to Microsoft Excel and delta cycle thresholds (dCTs) were calculated for each sample by using the geometric mean of the three endogenous reference genes as the normalization factor (i.e., CT for each transcript in a sample minus the geometric mean of Beta-actin, cyclophillinA and GAPDH CTs for the same sample)34. The expression level for each transcript was then calculated as the expression ratio value (ER, where ER= 2−dCTs), and all results are reported as ER.
Two isoforms of the WNK3 gene are expressed in the brain: isoform 1 which is brain-specific and isoform 2 which is ubiquitous. Isoform 1 differs from isoform 2 only by the presence of an additional stretch of 47 amino acids in the protein35. Given the differential expression pattern of both isoforms, we designed one primer set detecting WNK3 isoform 1 (primer set WNK3-1) and one primer set detecting both isoforms 1 and 2 (primer set WNK3-1&2). WNK3-1 was used on the 42 pairs of subjects, and WNK3-1&2 was used only on an arbitrary subset of 12 pairs of schizophrenia and normal comparison subjects (Supplementary Table 1A, pairs with *) that had been used in a previous RT-qPCR study32.
Hierarchical clustering
Two-way cluster analyses were performed using delta-delta CTs (ddCTs; schizophrenia subject dCT minus matched normal comparison subject dCT) across all 42 subject pairs for all transcripts using Pearson mean analysis in Genes@Work (IBM, Armonk, NY)36.
Antipsychotic-treated monkeys
The effect of long-term exposure to antipsychotic medication on the levels of transcripts showing altered expression in schizophrenia was examined using macaque monkeys exposed to haloperidol, olanzapine and placebo as described before37–38 (see Supplemental Methods for details). All housing and experimental procedures were conducted in accordance with NIH guidelines and with approval of the University of Pittsburgh’s Institutional Animal Care and Use Committee.
Immunocytochemistry
In order to assess the distribution and relative levels of OXSR1 protein, we conducted immunocytochemical experiments using immersion-fixed cryostat sections (40μm) of DLPFC area 9 from 6 white male human subjects (17–62 years of age; 3 subjects with a short PMI (5–8.2 hours) and 3 subjects with a long PMI (14.5–37 hours)]. Tissue sections were processed for OXSR1 immunoreactivity using a 1:200 dilution of a goat anti-OXSR1 (SC49473, Santa Cruz, Santa Cruz, CA) and the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) according to a previously described protocol39. The OXSR1 antibody was raised against the C-terminal portion (amino acids 370 to 420) of the human OXSR1 protein. Antibody specificity was demonstrated by the absence of labeling in the thymus40 and by the absence of specific labeling when the primary antibody was omitted.
Western blotting and SDS gel electrophoresis
Western blots were conducted using brain tissue lysate prepared as previously described41 (see Supplemental Methods for details). The specificity of the anti-OXSR1 antibody was confirmed by detection of a band of identical molecular weight (58kDa) compared to cloned human OXSR1 expressed in vitro and by lack of cross reactivity with a cell lysate of in vitro expressed STK39 (OriGene, Rockville, MD, data not shown).
To assess the effect of PMI on OXSR1 immunoreactivity, four adjacent coronal tissue blocks (2 to 3 mm thick) were prepared from the DLPFC of the same adult macaque monkey, stored in room temperature ACSF for 0, 6, 12 or 24 hours and then flash frozen42. The OXSR1 protein content was reported as percentage relative to the 0 hour PMI.
Laser microdissection analyses
In order to assess the laminar and cellular patterns of the expression differences in OXSR1 and WNK3-1&2, we used laser microdissection techniques to obtain mRNA samples from layer 3 tissue and from individually-dissected layer 3 pyramidal cells from a subset of 10 subjects pairs (Supplementary Table 1A and 1B, pairs with •). Using the results from qPCR experiments conducted on the entire area 9 gray matter for all 42 subject pairs, we selected the 10 pairs with mean expression differences between schizophrenia and control subjects that were closest to the mean of the entire group.
Cryostat sections (12 μm) were cut and thaw-mounted onto glass PEN membranes slides (LEICA-Microsystems, Bannockburn, IL) which had been previously UV-treated at 254nm for 30 min, dried and stored at −80°C. On the day of the microdissection, slides were immersed in an ethanol-acetic acid fixation solution, stained with thionin, dehydrated through 100% ethanol and air-dried. Using a LEICA (LMD 6500) microdissection system, 2 independent samples of layer 3 pyramidal neurons (Supplemental Figure 3A) per subject from 2 different slides (40X objective, Power 15, Aperture 9, Speed 12, Balance 14, and Offset 120) or 3 strips of tissue from layer 3 as previously described43 (5X objective, Power 43, Aperture 18, Speed 13, Balance 25, offset 60) were obtained. An average of 50 single pyramidal cells or 4.1 mm2 of layer 3 tissue were collected per sample in 0.5 mL microtubes caps (Ambion/Applied Biosystems), and lyzed by vortexing for 30 seconds in 200μL RLT Buffer Plus (QIAGEN, Valencia, CA). The RNA was extracted and PCR analyses were conducted, and cell type specificity and absence of glial contamination of the single pyramidal cell samples were confirmed, as described in the Supplemental Methods. We elected to measure both isoforms of WNK3 using the WNK3-1&2 primer set because of the lower expression of WNK3-1, although the expression difference between schizophrenia and comparison samples using WNK3-1&2 was only half of the expression difference obtained using WNK3-1.
Statistical analyses
The effect of diagnosis and the influence of potential confounding variables on the transcript expression ratios were assessed using two analyses of covariance (ANCOVA) models. In the first model, transcript expression ratio was used as the dependent variable, diagnostic group as the main effect, and subject pair as a blocking factor. RIN, pH and freezer storage time were entered as covariates as they may affect RNA integrity. Subject pairing may be considered an attempt to control for experimental variance by the parallel processing of tissue samples from each subject pair, and not a true statistical pair design. Therefore, to validate the first model, a second unpaired ANCOVA model was performed, using diagnostic group as the main effect and with age, sex, PMI, RIN, pH, and freezer storage time as covariates. Because the 42 pairs of subjects were used in two successive qPCR runs of 24 and 18 pair cohorts (see Supplementary Table 1A) and 18 (see Supplementary Table 1B) that involved different lots of reagents, qPCR run was also entered as a covariate in both models. Tissue storage time did not have a significant effect in either model for any transcript and was excluded in the reported analyses. The reported p values for each model were corrected for multiple comparisons (n=21) using the Bonferroni procedure.
The influences of potential confounding variables on the expression ratio values in subjects with schizophrenia were assessed with ANCOVA models using each confounding variable as the main effect and sex, age, postmortem interval, pH, RIN and run as covariates. Pearson’s correlation was used to assess the relationships of the expression ratios for all pairs of transcripts; reported p values were also corrected for multiple comparisons (n=21) using the Bonferroni procedure.
A one-way analysis of variance (ANOVA) model with expression ratios as the dependent variable and treatment group as the main effect was used to compare transcript expression levels in the haloperidol-, olanzapine- and placebo-exposed monkeys.
Results
Transcript levels for chloride transporters and related transcripts
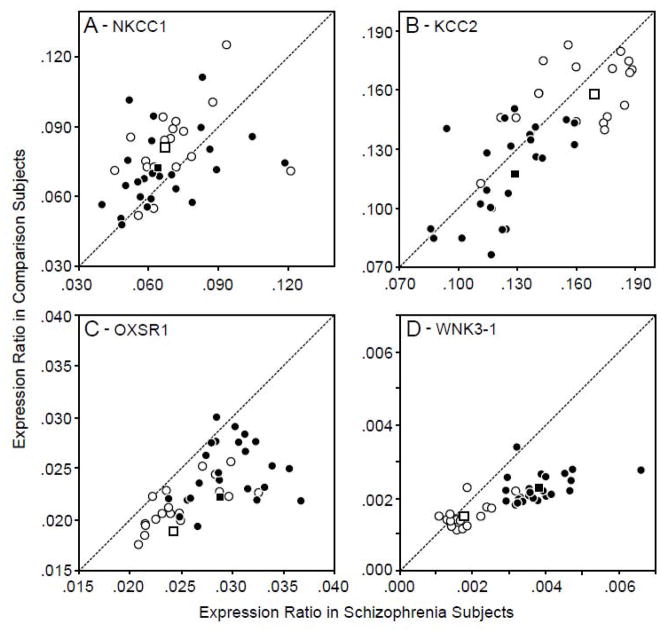
After corrections for multiple comparisons, neither the mean level of NKCC1 mRNA (Figure 1A) nor KCC2 mRNA (Figure 1B) was significantly altered in schizophrenia (paired ANCOVA, both F1,38< 3.8 and p> 0.4; unpaired ANCOVA, both F1,75< 4.1 and p> 0.3). For the chloride transporter-related kinases, group differences in gene expression levels were not significant for STK39, WNK1 or WNK4 (paired, all F1,38< 5.1, all p>0.2; unpaired, all F1,75< 4.11, all p>0.3). In contrast, the mean expression level of OXSR1 mRNA (Figure 1C) was significantly (paired, F1,38= 54.3, p<0.0001; unpaired, F1,75= 38.1, p<0.0001) 19.4% greater in the subjects with schizophrenia. In addition, mean WNK3-1 mRNA levels (Figure 1D) were significantly (paired, F1,38= 50.9, p<0.0001; unpaired, F1,75= 45.6, p<0.0001) 48.7% greater in the schizophrenia subjects. Relative to their matched normal comparison subjects, OXSR1 and WNK3-1 mRNA levels were higher in 41 and 37, respectively, of the 42 subjects with schizophrenia (Figure 1C, 1D). None of the covariates (age, sex, PMI, RIN, brain pH or freezer storage time) were significant in any of the analyses for OXSR1 and WNK3-1 mRNAs (paired, all F1,38< 2.69, all p>0.109; unpaired, all F1,75< 3.76, all p>0.06).
Figure 1.
NKCC1 (A), KCC2 (B), OXSR1 (C) and WNK3-1 (D) expression ratio (ER, where ER= 2−dCTs) values for CR and SZ subjects in the 24-pair (black circles) and 18-pair (white circles) cohorts. Data points below the diagonal unity line represent subject pairs in which the expression level for the gene of interest is greater in the schizophrenia subjects relative to the matched normal comparison subject.
WNK3 isoforms 1 and 2
Due to the low expression level of the brain-specific WNK3 isoform 1 mRNA (primer set WNK3-1) in the DLPFC, we designed a new set of primers binding to both isoforms 1 and 2 of WNK3 in human brain (primer set WNK3-1&2). This primer set produced lower CT values than the WNK3 isoform 1-specific primer set in the same subjects (n=12 pairs), reflecting the higher level of expression for both WNK3 isoforms combined than for isoform 1 alone. Consistent with the findings for isoform 1 alone, the combined expression of both WNK3 isoforms was also higher (+27.0%; paired, F1,8=3.97, p=0.082; unpaired, F1,19=7.1, p=0.01) in the schizophrenia subjects, and the expression levels from both primer sets were highly correlated (r = 0.561, p<0.01) across all subjects.
Analysis of potential antipsychotic medication effects
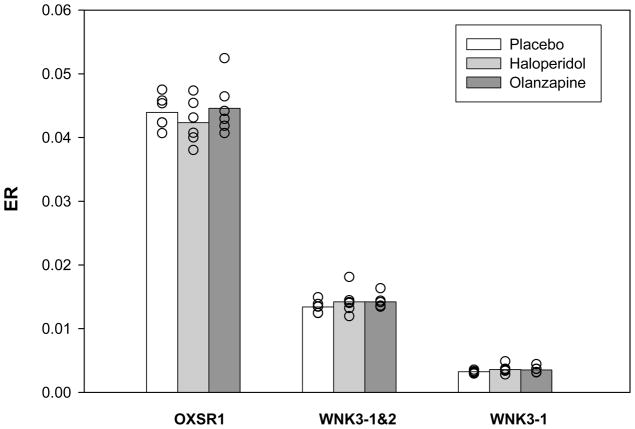
The expression ratios for OXSR1, WNK3-1 and WNK3-1&2 did not differ (all F2,15 < 0.92, all p >0.4) across the monkeys with long-term exposure to placebo, haloperidol or olanzapine (Figure 2).
Figure 2.
Effect of antipsychotic medication on the transcript expression for OXSR1, WNK3-1 and WNK3-1&2. Expression ratio values (ER) were calculated for each transcript for six triads of monkeys with long-term exposure to placebo (white bars), haloperidol (light grey bars) or olanzapine (dark grey bars).
Analysis of potential confounding factors
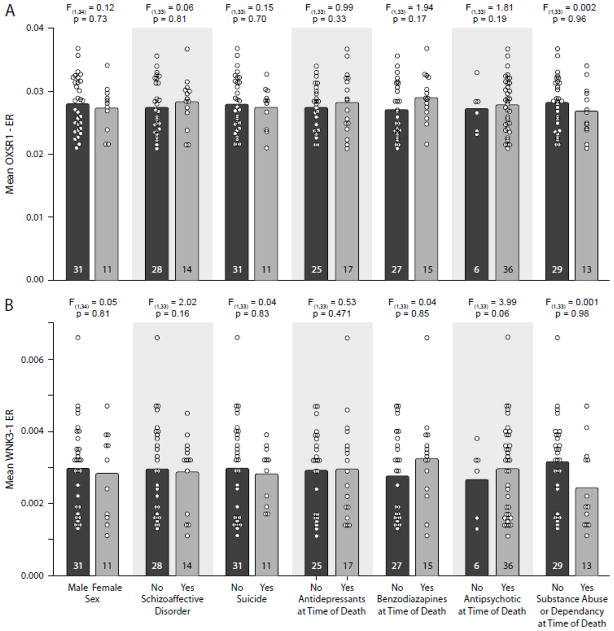
The mean expression ratio in the subjects with schizophrenia did not differ for OXSR1 (Figure 3A; all F< 1.941, all p> 0.188, uncorrected) or WNK3-1 (Figure 3B; all F < 3.99, all p> 0.054, uncorrected) as a function of sex; diagnosis of schizoaffective disorder; suicide; antidepressant medication, benzodiazepines or sodium valproate, or antipsychotic medication use at the time of death; or diagnosis of substance abuse/ dependence at time of death.
Figure 3.
Mean (bar) and individual (circle) expression ratio (ER) values for OXSR1 (A) and WNK3-1 (B) are shown for the subjects with schizophrenia grouped by potential confounding factors. Neither sex, diagnosis of schizoaffective disorder, suicide, antidepressant medication use at the time of death, use of benzodiazepines or sodium valproate at the time of death, antipsychotic medication use at the time of death, nor diagnosis of substance abuse or dependence at the time of death significantly affected OXSR1 (A) or WNK3-1 (B) transcript expression. Numbers in bars indicate the number of subjects with schizophrenia in each category.
Hierarchical clustering and correlations
A two-way cluster analysis performed on ddCTs values across the 42 pairs of matched subjects for all seven transcripts with Pearson mean distance analysis resulted in the separation of the transcript expression changes in two different clusters (Supplementary Figure 1). OXSR1 clustered in a pathway with WNK1, WNK4, NKCC1 and STK39, whereas WNK3-1 and KCC2 clustered in a distinct pathway.
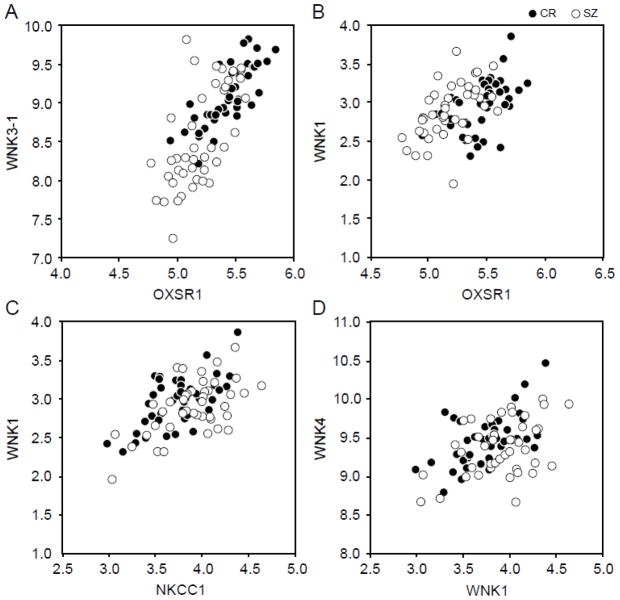
Pearson’s correlations were performed using the dCTs values obtained for each transcript for each of the 84 subjects (Figure 4; Supplementary Table 3); the reported p values were calculated using Bonferroni correction for multiple comparisons. Expression levels of OXSR1 were significantly positively correlated with those for WNK3-1 (r =0.744, p <10−7) and WNK1 (r = 0.478, p <10−4), and WNK3-1 expression was significantly correlated with WNK1 (r = 0.346, p =0.027). In contrast, WNK3-1 expression was negatively correlated with KCC2 (r =−0.356, p =0.019). NKCC1 expression was positively correlated with WNK1 (r =0.583, p <10−7), WNK4 (r = 0.425, p =0.001), and STK39 (r=0.371, p = .011).
Figure 4.
Four most significant Pearson correlations (p<10−3) between dCTs for 84 subjects (normal comparison subjects in black circles and subjects with schizophrenia in white circles).
Immunolocalization of OXSR1
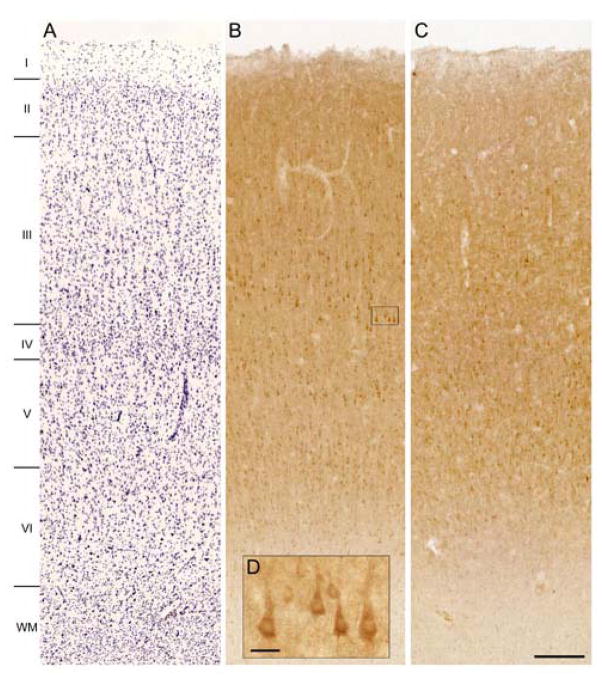
In human DLPFC from subjects with PMIs less than 6 hours, immunoreactivity for OXSR1 was highest in layers 3 and 5, intermediate in layers 2 and 6, and low in layers 1 and 4 (Figure 5A, 5B). OXSR1 immunoreactivity was mainly located in the perinuclear cytoplasmic compartment of neurons, most of which had the morphology of pyramidal cells. In contrast, OXSR1 labeling was severely reduced with PMIs longer than 12 hours (Figure 5C). This finding was confirmed by Western blotting in samples of monkey DLPFC with artificially-induced PMIs. At PMIs of 12 and 24 hours, levels of OXSR1 protein were only 60% and 40%, respectively, of that detected at 0 hours (Supplementary Figure 2). Thus, a quantitative study of OXSR1 protein levels in schizophrenia and control subjects could not be conducted.
Figure 5.
Brightfield photomicrographs showing Nissl staining (panel A) and laminar distribution pattern of OXSR1 immunoreactivity in human DLPFC area 9 for a subject with a short PMI (5.5 hours, panel B) and a long PMI (19.3 hours, panel C). The insert shows a magnification of OXSR1 immunoreactivity in layer 3.
Laminar and cellular assessments of OXSR1 and WNK3-1&2 mRNA expression
In order to determine if the greater expression of OXSR1 and WNK3-1&2 transcripts in the subjects with schizophrenia arose from the same cortical layer and neurons, we used laser microdissection to obtain samples of tissue restricted to layer 3 and samples of 50 pyramidal cells individually cut from layer 3. In the layer 3 tissue samples, the expression levels of OXSR1 and WNK3-1&2 mRNAs were significantly correlated (r=0.88, p< 0.0001) across all 20 subjects, and the within-pair expression differences for OXSR1 and WNK3-1&2 mRNAs were also significantly correlated (r=0.92, p=0.0001). Consistent with these findings, in the samples of layer 3 pyramidal cells, the expression levels of OXSR1 and WNK3-1&2 mRNAs were significantly correlated (r=0.56, p=0.001) across all 20 subjects, confirming that both transcripts are expressed in layer 3 pyramidal cells and suggesting that their expression is co-regulated. In addition, both transcripts showed greater expression in the subjects with schizophrenia (mean percentage increases: OXSR1, +12.0%; WNK3-1&2, +12.4%), although due to the smaller sample size (n= 10 pairs) these findings did not achieve statistical significance (t=−1.007, p=0.148 and t=−0.682, p=0.252 respectively). Together, these findings suggest an up-regulation of both transcripts in layer 3 pyramidal cells in the illness.
Discussion
In the DLPFC of subjects with schizophrenia, mRNA expression levels for the Cl− transporters NKCC1 and KCC2 were not significantly altered. Although it is possible that our measures of mRNA in total gray matter obscured schizophrenia-associated expression differences in neuronal subpopulation(s) in a different layer, we previously found that neither NKCC1 nor KCC2 mRNAs showed a preferential laminar pattern of expression in control human subjects43.
In contrast, the mRNA expression levels of two regulatory kinases, OXSR1 and WNK3, were markedly and consistently higher in the DLPFC of schizophrenia subjects. Although an increase in protein kinase activity may not always follow an increase in the corresponding mRNA transcript, previous reports have shown that increasing WNK3 mRNA or silencing OXSR1 expression is associated with alterations in NKCC1 transport activity28, 44. Thus, our results suggest that the increased expression of OXSR1 and WNK3 are likely to lead to shifts in the activity of the Cl− transporters that could substantially alter the nature of cortical GABA neurotransmission in schizophrenia. Indeed, the finding that both transcripts are up-regulated in the same layer 3 pyramidal neurons suggests that altered GABA signaling may be particularly prominent in these cells. The findings for OXSR1 and WNK3 are striking for several other reasons. First, the higher levels of these transcripts in subjects with schizophrenia contrast with the more commonly observed lower levels of GABA-related transcripts in the illness32. Second, the elevated transcript levels in schizophrenia indicate that the findings are not attributable to poorer RNA quality in these subjects, consistent with the excellent brain pH and RIN measures in all subjects used in this study and the absence of group differences in these variables. Third, relative to the matched normal comparison subjects, almost all of the 42 subjects with schizophrenia had higher levels of both OXSR1 and WNK3 mRNAs. The apparent consistency of these findings suggests that they are more likely to be related to a conserved downstream aspect of the disease process (e.g., impaired GABA neurotransmission) than to reflect the etiological complexity of schizophrenia8. Fourth, consistent with the findings that these transcripts are not altered in monkeys chronically exposed to antipsychotic medications, the conservation of these alterations across almost all subjects examined strongly suggests that they are not attributable to other factors (e.g., substance use, suicide, mood symptoms, etc) that were present in some of the schizophrenia subjects, an interpretation confirmed by the direct assessment of these factors (Figure 3).
A conserved OXSR1 binding motif present on NKCC117 has also been detected on WNK1 and WNK445 and the activation of NKCC1 by OXSR1 is regulated through upstream interactions with WNK1 and WNK423, 25–27, 46. Consistent with these observations, we found strong correlations across all subjects between OXSR1 and WNK1 (r=0.478, p< 0.01) and between WNK1 and WNK4 (r = 0.516, p < 0.0001) mRNA levels. The expression differences between schizophrenia and control subjects for WNK1 and WNK4 kinases clustered together, consistent with the idea that WNK1 and WNK4 regulate each other’s activity47. Nevertheless, neither WNK1 nor WNK4 transcript expression level was significantly altered in schizophrenia despite suggestions of overexpression (+7%, p=0.357 and + 12.6, p=0.174 respectively), indicating that the overexpression of the OXSR1 transcript could be primarily responsible for an increase in NKCC1 transporter activity.
WNK3 is a potent activator of NKCC128–29 and they are co-localized in neurons28. Furthermore, WNK3 possesses an OXSR1/STK39 consensus binding motif45 suggesting that an increase in WNK3 expression in schizophrenia could lead to increased NKCC1 transporter activity through OXSR1. Consistent with this hypothesis, we found very strong correlations (r = 0.744, p < 10−7) between OXSR1 and WNK3 mRNA levels across all subjects as well as within-pair expression differences in layer 3 samples (r=0.92, p=0.0001) suggesting that their effects on chloride transporter activity are synergistic.
WNK3 can inhibit KCC2 activity regardless of cellular tonicity28–29, suggesting that the elevated WNK3 expression in subjects with schizophrenia could be associated with lower KCC2 activity. Consistent with this interpretation, KCC2 and WNK3 expression levels were inversely correlated (r= −0.356, p=0.019) across all 84 subjects. Additionally, in our dataset, both KCC2 and WNK3 clustered together, suggesting that indeed both KCC2 and WNK3 belong to the same regulatory pathway. Consistent with this hypothesis, KCC2 and WNK3 share the same developmental trajectory 28, 48.
As kinase-dead WNK3 activates KCC2 28–29, KCC2 inhibition triggered by the increase in WNK3 could be indirect, through the potential inhibition of a yet to be identified phosphatase or the activation of another kinase. Co-immunoprecipitation studies have shown than WNK3 can interact with WNK4 via their carboxy termini49 and it has been suggested that WNK4 could act as a regulator of WNK3 activity and therefore KCC2/NKCC1 activity50. In our dataset, WNK4 gene expression did display an upregulated trend (+12.6%, p=0.17) but its expression level in all 84 subjects was correlated with NKCC1 and not with WNK3. This suggests that NKCC1 and KCC2 regulation by WNK3 may happen through different regulatory pathways. Finally, the strong Pearson’s correlation between OXSR1 and WNK3 (0.744, p<0.01) suggest that the regulation of both NKCC1 and KCC2 activities could be linked through the interaction of these two kinases.
Synergistic effects of altered kinase levels on Cl− transport in schizophrenia
The combined and coordinated greater expression of OXSR1 and WNK3 in schizophrenia would be expected to increase both NKCC1 and KCC2 phosphorylation levels. Because NKCC1 and KCC2 are activated through phosphorylation and dephosphorylation, respectively24, 51–52, our findings suggest that schizophrenia is associated with both increased NKCC1 activity and decreased KCC2 activity. Interestingly, the latter finding may be consistent with preliminary reports of decreased levels of KCC2 mRNA in the hippocampus of patients with schizophrenia53–54.This combined shift in transporter activities would be expected to increase intracellular Cl− levels, reducing or reversing the net inward flow of Cl− ions when GABA-A receptors are activated, and thus altering the nature of synaptic GABA neurotransmission.
Early in development, the ratio of NKCC1 to KCC2 is high, resulting in high intracellular Cl− content and a depolarizing excitatory response upon GABA-A receptor activation. Later, as the expression of NKCC1 declines and KCC2 increases the level of intracellular levels of Cl− decreases55 and GABA-A receptor activation triggers Cl− influx, hyperpolarization and inhibition. Interestingly, an increase in OXSR1 and WNK3 kinase activity could promote a change of transporter activities (i.e., increased NKCC1 to KCC2 ratio) and a shift in the nature of GABA neurotransmission to be more similar to that observed earlier in development. Specifically, our findings suggest that schizophrenia is associated with a tendency for GABA neurotransmission to be less strongly inhibitory in the DLPFC, at least in the neurons that have elevated levels of OXSR1 and WNK3 expression.
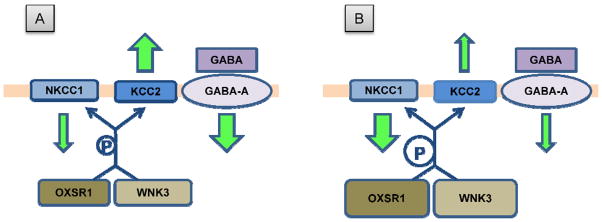
A provisional model summarizing these potential interactions between NKCC1 and KCC2 chloride transporters and OXSR1 and WNK3 kinases is shown in Figure 6. In normal adult neurons (Figure 6A), NKCC1 levels are low and KCC2 levels are high resulting in a low level of intracellular Cl−. GABA binding to GABA-A receptors triggers an inward flow of Cl− leading to hyperpolarization. In schizophrenia (Figure 6B), greater levels of OXSR1 and WNK3 result in greater phosphorylation of both chloride transporters, and a consequent increase in NKCC1 and decrease in KCC2 activities, leading to a higher level of intracellular Cl−. This change in intracellular Cl− concentration has the potential to reduce the hyperpolarizing effect triggered by GABA binding and therefore to substantially change the nature of GABA neurotransmission.
Figure 6.
Putative interaction model between NKCC1 and KCC2 and OXSR1 and WNK3-1 kinases. In both panels, the orange bar represents the cell membrane, with the extracellular domain above and the intracellular domain below the bar. The size and orientation of the green arrows indicates the magnitude and direction of Cl− ion flow mediated by NKCC1, KCC2 and GABA-A receptor Cl− channels. (A) In normal adult neurons, intracellular Cl−concentration is low due to low levels of NKCC1 and high levels of KCC2. The binding of GABA to GABA-A receptors triggers Cl− entry (Cl− flow is represented by green arrows) and hyperpolarization. (B) In schizophrenia, increased OXSR1 and WNK3-1 kinase levels lead to increased phosphorylation (blue P) and consequently increased NKCC1 activity and decreased KCC2 activity, producing a greater intracellular Cl− concentration. Thus, when GABA-A receptors are activated Cl− influx is reduced (or perhaps reversed) and the nature of GABA neurotransmission is altered.
The affected cortical neurons are likely to include pyramidal cells, given the immunocytochemical evidence that OXSR1 is preferentially localized in pyramidal neurons in layers 3 and 5 and the correlated expression of OXSR1 and WNK3-1&2 mRNAs in layer 3 pyramidal neurons. Effective synchronization of networks of pyramidal neurons at the gamma band frequencies associated with working memory requires strongly hyperpolarizing inputs to a network of pyramidal cells so that the postsynaptic pyramidal neurons then escape from inhibition simultaneously, and fire subsequent action potentials in unison, producing synchronized activity56–58. Thus, a shift to increased NKCC1 and decreased KCC2 activity in pyramidal neurons in schizophrenia could alter the reversal potential of the GABA-A receptor current to be closer to the resting membrane potential, reducing the strong inhibition required for gamma oscillations6, and thus contributing to the alterations in the prefrontal gamma oscillations associated with impaired working memory in schizophrenia4.
Other potential effects of altered OXSR1 and WNK3 expression in schizophrenia
The increased expression of OXSR1 and WNK3 could also contribute to other circuitry disturbances in the DLPFC. For example, vesicular glutamate transport appears to be highly dependent on the Cl− content outside of synaptic vesicles59. Thus, the change in intracellular Cl− concentration predicted by our findings could also alter vesicular glutamate transporter activity and contribute to alterations in glutamate transmission and synaptic plasticity reported in schizophrenia (for review see60),
Recent work also indicates that KCC2, through its interaction with the dendritic cytoskeleton, regulates spine maturation61. Thus, a decrease in KCC2 activity, secondary to increased OXSR1 and WNK3 expression, could contribute to impaired spine maturation and thus to lower dendritic spine density in the DLPFC30. Consistent with this interpretation, we observed higher OXSR1 and WNK3 expression in layer 3 pyramidal cells where the spine deficit in schizophrenia is most pronounced62.
Conclusion
In summary, we report substantial increases in the expression of the regulatory kinases OXSR1 and WNK3 in the DLPFC that appear to be highly conserved across subjects with schizophrenia. This combination is likely to alter the relative activities of the chloride transporters NKCC1 and KCC2, producing a higher than normal intracellular Cl− concentration, such that activation of GABA-A receptors results in less Cl− influx and a smaller increase in hyperpolarization, or depending on the Cl− reversal potential, could even lead to an outflow of Cl− ions, and some degree of membrane depolarization of the postsynaptic cell.
Supplementary Material
Acknowledgments
The authors thank Mary Brady for assistance with the graphics and Kelly Rogers and Anthony Cipriano for assistance with the immunocytochemistry runs. We also wish to express our deep gratitude to the families of the brain donors who made this research possible. Dr. Lewis had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported by grants from the National Institutes of Health (MH-084053 and MH-043784). David A. Lewis currently receives investigator-initiated research support from the BMS Foundation, Bristol-Myers Squibb, Curridium Ltd and Pfizer and in 2007–2009 served as a consultant in the areas of target identification and validation and new compound development to AstraZeneca, BioLine RX, Bristol-Myers Squibb, Hoffman-Roche, Lilly, Merck, Neurogen and SK Life Science.
Funding support:
National Institutes of Health grants MH-084053 and MH-043784
Contributor Information
Dominique Arion, Department of Psychiatry, Translational Neuroscience Program, University of Pittsburgh, Pittsburgh, PA, 15213.
David A. Lewis, Departments of Psychiatry and Neuroscience, Translational Neuroscience Program, University of Pittsburgh, Pittsburgh, PA, 15213.
References
- 1.Elvevag B, Goldberg TE. Cognitive impairment in schizophrenia is the core of the disorder. Crit Rev Neurobiol. 2000;14(1):1–21. [PubMed] [Google Scholar]
- 2.Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133(1):23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- 3.Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The Role of Oscillations and Synchrony in Cortical Networks and Their Putative Relevance for the Pathophysiology of Schizophrenia. Schizophr Bull. 2008;34(5):927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103(52):19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez-Burgos G, Lewis DA. GABA Neurons and the Mechanisms of Network Oscillations: Implications for Understanding Cortical Dysfunction in Schizophrenia. Schizophr Bull. 2008;34(5):944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8(1):45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 7.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 8.Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest Apr. 2009;119(4):706–716. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative K-Cl cotransporter in rat brain. A neuronal-specific isoform. J Biol Chem. 1996;271(27):16245–16252. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- 10.Plotkin MD, Kaplan MR, Peterson LN, Gullans SR, Hebert SC, Delpire E. Expression of the Na(+)-K(+)-2Cl− cotransporter BSC2 in the nervous system. Am J Physiol. 1997;272(1 Pt 1):C173–183. doi: 10.1152/ajpcell.1997.272.1.C173. [DOI] [PubMed] [Google Scholar]
- 11.Kanaka C, Ohno K, Okabe A, et al. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience. 2001;104(4):933–946. doi: 10.1016/s0306-4522(01)00149-x. [DOI] [PubMed] [Google Scholar]
- 12.Lauf PK, Adragna NC. K-Cl cotransport: properties and molecular mechanism. Cell Physiol Biochem. 2000;10(5–6):341–354. doi: 10.1159/000016357. [DOI] [PubMed] [Google Scholar]
- 13.Lauf PK, Bauer J, Adragna NC, et al. Erythrocyte K-Cl cotransport: properties and regulation. Am J Physiol. 992;263(5 Pt 1):C917–932. doi: 10.1152/ajpcell.1992.263.5.C917. [DOI] [PubMed] [Google Scholar]
- 14.Flatman PW. Regulation of Na-K-2Cl cotransport by phosphorylation and protein-protein interactions. Biochim Biophys Acta. 2002;1566(1–2):140–151. doi: 10.1016/s0005-2736(02)00586-2. [DOI] [PubMed] [Google Scholar]
- 15.Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26(4):199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 16.Ushiro H, Tsutsumi T, Suzuki K, Kayahara T, Nakano K. Molecular cloning and characterization of a novel Ste20-related protein kinase enriched in neurons and transporting epithelia. Arch Biochem Biophys. 1998;355(2):233–240. doi: 10.1006/abbi.1998.0736. [DOI] [PubMed] [Google Scholar]
- 17.Piechotta K, Lu J, Delpire E. Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1) J Biol Chem. 2002;277(52):50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- 18.Piechotta K, Garbarini N, England R, Delpire E. Characterization of the interaction of the stress kinase SPAK with the Na+-K+-2Cl− cotransporter in the nervous system: evidence for a scaffolding role of the kinase. J Biol Chem. 2003;278(52):52848–52856. doi: 10.1074/jbc.M309436200. [DOI] [PubMed] [Google Scholar]
- 19.Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J. 2008;409(2):321–331. doi: 10.1042/BJ20071324. [DOI] [PubMed] [Google Scholar]
- 20.Flemmer AW, Gimenez I, Dowd BF, Darman RB, Forbush B. Activation of the Na-K-Cl otransporter NKCC1 detected with a phospho-specific antibody. J Biol Chem. 2002;277(40):37551–37558. doi: 10.1074/jbc.M206294200. [DOI] [PubMed] [Google Scholar]
- 21.Darman RB, Forbush B. A regulatory locus of phosphorylation in the N terminus of the Na-K-Cl cotransporter, NKCC1. J Biol Chem. 2002;277(40):37542–37550. doi: 10.1074/jbc.M206293200. [DOI] [PubMed] [Google Scholar]
- 22.Dowd BF, Forbush B. PASK (proline-alanine-rich STE20-related kinase), a regulatory kinase of the Na-K-Cl cotransporter (NKCC1) J Biol Chem. 2003;278(30):27347–27353. doi: 10.1074/jbc.M301899200. [DOI] [PubMed] [Google Scholar]
- 23.Vitari AC, Thastrup J, Rafiqi FH, et al. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J. 2006;397(1):223–231. doi: 10.1042/BJ20060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagnon KB, England R, Delpire E. Volume sensitivity of cation-Cl− cotransporters is modulated by the interaction of two kinases: Ste20-related proline-alanine-rich kinase and WNK4. Am J Physiol Cell Physiol. 2006;290(1):C134–142. doi: 10.1152/ajpcell.00037.2005. [DOI] [PubMed] [Google Scholar]
- 25.Moriguchi T, Urushiyama S, Hisamoto N, et al. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2005;280(52):42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- 26.Anselmo AN, Earnest S, Chen W, et al. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci U S A. 2006;103(29):10883–10888. doi: 10.1073/pnas.0604607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitari AC, Deak M, Morrice NA, Alessi DR. The WNK1 and WNK4 protein kinases that are mutated in Gordon's hypertension syndrome phosphorylate and activate SPAK and OSR1 protein kinases. Biochem J. 2005;391(Pt 1):17–24. doi: 10.1042/BJ20051180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahle KT, Rinehart J, de Los Heros P, et al. WNK3 modulates transport of Cl− in and out of cells: implications for control of cell volume and neuronal excitability. Proc Natl Acad Sci U S A. 2005;102(46):16783–16788. doi: 10.1073/pnas.0508307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Los Heros P, Kahle KT, Rinehart J, et al. WNK3 bypasses the tonicity requirement for K-Cl cotransporter activation via a phosphatase-dependent pathway. Proc Natl Acad Sci U S A. 2006;103(6):1976–1981. doi: 10.1073/pnas.0510947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 31.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57(3):237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165(4):479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mimmack ML, Brooking J, Bahn S. Quantitative polymerase chain reaction: validation of microarray results from postmortem brain studies. Biol Psychiatry. 2004;55(4):337–345. doi: 10.1016/j.biopsych.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):1–12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holden S, Cox J, Raymond FL. Cloning, genomic organization, alternative splicing and expression analysis of the human gene WNK3 (PRKWNK3) Gene. 2004;335:109–119. doi: 10.1016/j.gene.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Lepre J, Rice JJ, Tu Y, Stolovitzky G. Genes@Work: an efficient algorithm for pattern discovery and multivariate feature selection in gene expression data. Bioinformatics. 2004;20(7):1033–1044. doi: 10.1093/bioinformatics/bth035. [DOI] [PubMed] [Google Scholar]
- 37.Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto T, Arion D, Unger T, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13(2):147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17(1):175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- 40.Chen W, Yazicioglu M, Cobb MH. Characterization of OSR1, a member of the mammalian Ste20p/germinal center kinase subfamily. J Biol Chem. 2004;279(12):11129–11136. doi: 10.1074/jbc.M313562200. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto T, Nguyen QL, Rotaru D, et al. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry. 2009;65(12):1015–1023. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayes TL, Cameron JL, Fernstrom JD, Lewis DA. A comparative analysis of the distribution of prosomatostatin-derived peptides in human and monkey neocortex. J Comp Neurol. 1991;303(4):584–599. doi: 10.1002/cne.903030406. [DOI] [PubMed] [Google Scholar]
- 43.Arion D, Unger T, Lewis DA, Mirnics K. Molecular markers distinguishing supragranular and infragranular layers in the human prefrontal cortex. Eur J Neurosci. 2007;25(6):1843–1854. doi: 10.1111/j.1460-9568.2007.05396.x. [DOI] [PubMed] [Google Scholar]
- 44.Geng Y, Hoke A, Delpire E. The STE20 kinases SPAK and OSR1 regulate NKCC1 function in sensory neurons. J Biol Chem. 2009;139(7):1353–1365. doi: 10.1074/jbc.M900142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delpire E, Gagnon KB. Genome-wide analysis of SPAK/OSR1 binding motifs. Physiol Genomics. 2007;28(2):223–231. doi: 10.1152/physiolgenomics.00173.2006. [DOI] [PubMed] [Google Scholar]
- 46.Richardson C, Rafiqi FH, Karlsson HK, et al. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121(Pt 5):675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- 47.Yang CL, Zhu X, Wang Z, Subramanya AR, Ellison DH. Mechanisms of WNK1 and WNK4 interaction in the regulation of thiazide-sensitive NaCl cotransport. J Clin Invest. 2005;115(5):1379–1387. doi: 10.1172/JCI22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu J, Karadsheh M, Delpire E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J Neurobiol. 1999;39(4):558–568. [PubMed] [Google Scholar]
- 49.Yang CL, Zhu X, Ellison DH. The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Invest. 2007;117(11):3403–3411. doi: 10.1172/JCI32033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCormick JA, Yang CL, Ellison DH. WNK kinases and renal sodium transport in health and disease: an integrated view. Hypertension. 2008;51(3):588–596. doi: 10.1161/HYPERTENSIONAHA.107.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kahle KT, Rinehart J, Ring A, et al. WNK protein kinases modulate cellular Cl− flux by altering the phosphorylation state of the Na-K-Cl and K-Cl cotransporters. Physiology (Bethesda) 2006;21:326–335. doi: 10.1152/physiol.00015.2006. [DOI] [PubMed] [Google Scholar]
- 52.Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80(1):211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- 53.Hyde TM. Cation Chloride Co-Transoprters in Human Brain Developement and Schizophrenia: NKCC1 and KCC2. Biological Psychiatry. 2008;63:47S. [Google Scholar]
- 54.Hyde TM. The Cation Chloride Co-Transporters KCC2 and NKCC1 and Schizophrenia. Biological Psychiatry. 2009;64:170S. [Google Scholar]
- 55.Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated 'menage a trois'. Trends Neurosci. 1997;20(11):523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- 56.Wang XJ, Buzsaki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16(20):6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30(7):343–349. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Mann EO, Kohl MM, Paulsen O. Distinct roles of GABA(A) and GABA(B) receptors in balancing and terminating persistent cortical activity. J Neurosci. 2009;29(23):7513–7518. doi: 10.1523/JNEUROSCI.6162-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schenck S, Wojcik SM, Brose N, Takamori S. A chloride conductance in VGLUT1 underlies maximal glutamate loading into synaptic vesicles. Nat Neurosci. 2009;12(2):156–162. doi: 10.1038/nn.2248. [DOI] [PubMed] [Google Scholar]
- 60.Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia--a synthesis and selective review. J Psychopharmacol. 2007;21(4):440–452. doi: 10.1177/0269881106073126. [DOI] [PubMed] [Google Scholar]
- 61.Li H, Khirug S, Cai C, et al. KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron. 2007;56(6):1019–1033. doi: 10.1016/j.neuron.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 62.Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162(6):1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.