Abstract
Previously (Shan et al, 2005), we reported that adenoviral vector-mediated transfer of the human aquaporin-1 (hAQP1) cDNA to minipig parotid glands following irradiation (IRti) transiently restored salivary flow to near normal levels. This study evaluated a serotype 2, adeno-associated viral (AAV2) vector for extended correction of IR (single dose; 20 Gy)-induced, parotid salivary hypofunction in minipigs. Sixteen weeks following IR, parotid salivary flow decreased by 85-90%. AAV2hAQP1 administration at week 17 transduced only duct cells and resulted in a dose-dependent increase in salivary flow to ∼35% of pre-IR levels (to ∼1ml/10min) after 8 weeks (peak response). Administration of a control AAV2 vector or saline, was without effect. Little change was observed in clinical chemistry and hematology values after AAV2hAQP1 delivery. Vector treated animals generated high anti-AAV2 neutralizing antibody titers by week 4 (∼1:1600) and significant elevations in salivary (∼15%), but not serum, GM-CSF levels. Following vector administration, salivary [Na+] was dramatically increased, from ∼10mM to ∼55 (at 4 weeks) and 39 mM (8 weeks). The findings demonstrate that localized delivery of AAV2hAQP1 to IR-damaged parotid glands leads to increased fluid secretion from surviving duct cells, and may be useful in providing extended relief of salivary hypofunction in previously irradiated patients.
Keywords: salivary gland, irradiation, aquaporin-1, AAV2, gene therapy
Introduction
Oral cavity and pharyngeal (oral) cancers are fairly common, and each year in the United States there are 35,000-40,000 new cases 1. The treatment of most patients with oral cancers includes radiation therapy, and recent data show a survival rate of ∼60% for patients with this type of neoplasm 1. Salivary glands in the radiation field suffer irreversible damage leading to a marked reduction in salivary flow and, as a consequence, xerostomia, dysphagia and oral infections 2,3. Importantly, this irradiation (IR)-induced salivary hypofunction results in a significant diminution in quality of life for surviving oral cancer patients 4,5.
Despite considerable effort to prevent or limit this problem (e.g., see 6-9), many surviving patients experience significant salivary hypofunction, i.e., in the Radiation Therapy Oncology Group's categories 2 and 3 10. There currently is no effective treatment available for these individuals. As a result, for many years we have tried to develop a novel, gene transfer-based corrective treatment for IR-induced salivary hypofunction11, using the human aquaporin-1 (hAQP1) cDNA 12 encoded within a serotype 5 adenoviral vector (AdhAQP1) 13,14. As a result of pre-clinical efficacy studies in rats and miniature pigs (minipigs) 13,14, and extensive safety studies 15, we received approval to conduct a phase I clinical study with AdhAQP1, treating a single parotid gland of previously irradiated patients, which is ongoing 16.
A drawback to the strategy of using AdhAQP1 is that any beneficial results experienced by patients would be relatively short lived due to the transient nature of adenoviral vector-mediated transduction of salivary glands 17. As a result, we have also developed a serotype 2 adeno-associated viral (AAV2) vector encoding hAQP1, AAV2hAQP1, which is capable of increasing net fluid secretion across polarized salivary epithelial cell layers in vitro 18. AAV2 vectors mediate long-term transgene expression in the salivary glands of mice (>1 year) and non-human primates (up to 6 months; longest time studied) 19,20.
In the present series of experiments, we used a single dose IR scheme (20 Gy) to reduce parotid salivary flow and test the hypothesis that AAV2hAQP1 could increase fluid secretion from irradiated parotid glands of minipigs for an extended time period. While patients typically receive fractionated IR dosing, our previous studies in mice have shown quite similar effects of single and fractionated IR dosing on salivary flow rates 21. Minipig parotid glands provide an excellent and readily affordable (e.g., much less expensive than non-human primates) model of IR-induced salivary hypofunction in large animal (∼25-30 kg) 22. Furthermore, minipig salivary duct cells, which survive IR, are readily transduced by AAV2 vectors, albeit effective gene transfer is not as long-lived as seen in other species, with peak transgene expression occurring at ∼8-10 weeks and returning to background levels by ∼32 weeks 23. The results reported herein support our hypothesis.
Results and Discussion
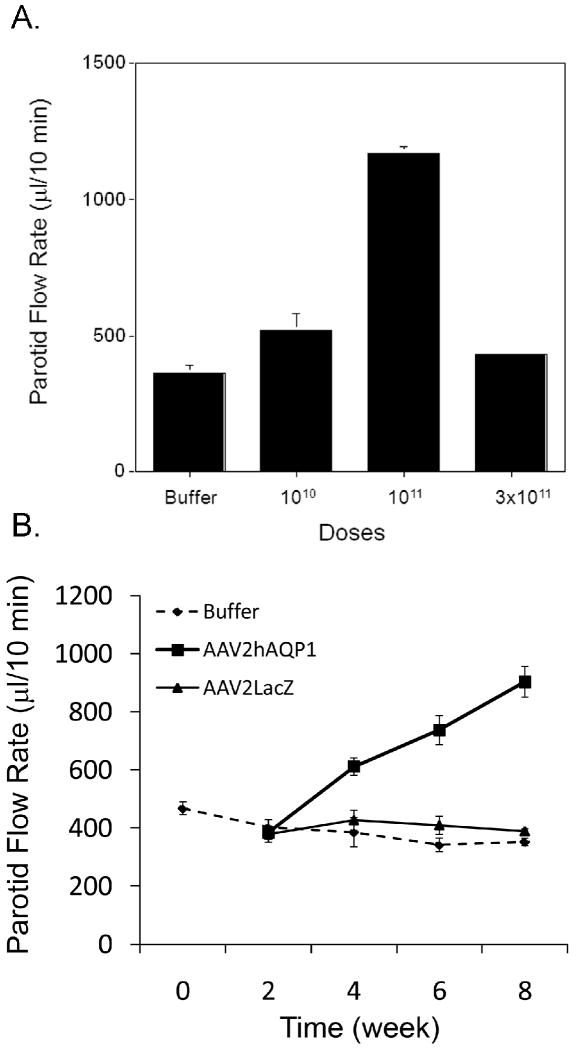
After a single radiation dose of 20 Gy to targeted parotid glands, there is a marked reduction in salivary flow rate (Figure 1). At 16 weeks post-IR, the average rate of saliva production from the targeted glands (n=9) had fallen to ∼15% of pre-IR levels. Preliminary experiments with separate cohorts of minipigs established both the optimal time to measure vector effects (∼8-10 weeks; three animals were followed for 24 weeks post-IR; see Supplementary Figure 1), as well as the optimal dose to administer (1011 vector particles, vp/gland; two animals/dose; Figure 2A). Both results were consistent with previous findings with minipig parotid glands using an AAV2 vector encoding erythropoietin 23. At week 17, the animals shown in Figure 1 were divided into three groups (n=3/group) and either administered the AAV2hAQP1 vector, a control vector (AAV2LacZ), both at 1011 vector particles (vp)/gland, or saline (the diluent buffer used) to the IR-damaged parotid gland. Thereafter, salivary flow rates were monitored every two weeks for 8 weeks as shown (Figure 2B). By the 8-week, post-vector administration time-point parotid salivary flow rates for animals treated with AAV2LacZ or saline buffer continued to diminish to 12% and 11%, respectively, of the pre-IR values. Conversely, in glands treated with the AAV2hAQP1 vector a steady increase in parotid salivary flow rates was seen, such that by week 8 they were on average ∼35% of pre-IR values, to nearly 1ml/10 min (Figure 2B).
Figure 1.
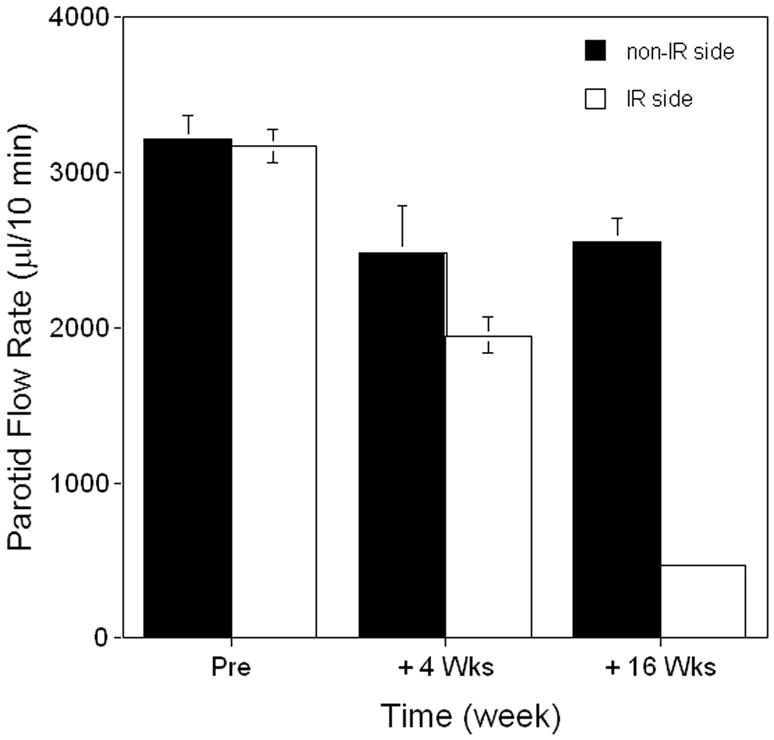
Effect of irradiation on parotid saliva flow rate in minipigs. One parotid gland of animals (n=9) was irradiated with 20 Gy, as described in the Materials and Methods. Parotid saliva flow rates were thereafter measured at weeks 4 and 16. Data shown are the mean±SD of salivary flow rates for the targeted glands (open bars; note that for the 16 week value the SD is too small to be seen) and the contralateral glands (black bars). Data were analyzed using a one-way analysis of variance (F=72.69, p<0.001), followed by the Tukey test. For the irradiated side, the values at +4 and +16 weeks are significantly different from that prior to irradiation (p<0.001, as well as different from each other, p<0.001). Similar differences exist for the values for the non-irradiated side (p<0.01), although there is no difference between the +4 and +16 week values. The parotid flow rates on the two sides are significantly different at +4 (p=0.031) and +16 weeks (p<0.001).
Figure 2.
Effect on saliva flow rates of AAV2hAQP1 administration to minipig parotid glands after irradiation. At week 17 following 20 Gy irradiation, either AAV2hAQP1 (A, B), AAV2LacZ (B) or saline buffer (B) were administered to a single parotid gland. Panel A shows the effect of different doses of AAV2hAQP1 on parotid saliva flow rates in irradiated glands (n=2/group; data are mean values ±SD obtained 12 weeks following vector administration; note that for the 3×1011 vp dose the SD is too small to be seen). The data in Panel A was not analyzed for statistical significance, because there were only two animals/group. Panel B shows salivary flow rates at different times following administration of AAV2hAQP1, AAV2LacZ or saline buffer to irradiated parotid glands (n=3/group; data are mean values ±SD). Time zero corresponds to week 17 following irradiation. Note that for the 8-week time-point with buffer and AAV2LacZ treatment the SDs are too small to be seen. Data were analyzed using a one-way analysis of variance (F=27.88, p<0.001), followed by the Tukey test. All of the parotid flow rates for the AAV2hAQP1-treated minipigs are significantly different from those of the buffer- and AAV2LacZ-treated animals at weeks 4 (p<0.01 vs buffer; p<0.05 vs AAV2LacZ), 6 (p<0.001) and 8 (p<0.001). There were no significant differences in parotid flow rates between the buffer- and AAV2LacZ-treated animals at every time-point.
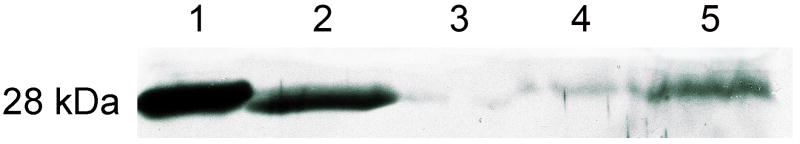
We next examined the expression of the transgenic hAQP1 in transduced glands. Initially, we used Western blotting to determine the expression of hAQP1 in crude parotid membranes (Figure 3). Eight weeks following treatment, membranes from minipigs administered AAV2LacZ or saline displayed low levels of AQP1 expression in the irradiated glands (lanes 3, 4 in Figure 3). Conversely, membranes obtained from the non-irradiated, contralateral gland showed a modest level of AQP1 present (lane 5). These three results are consistent with the known loss of parenchymal tissue that occurs following radiation damage to salivary glands and the presence of endogenous AQP1 in salivary microvascular endothelial cells8,14,24. In parotid membranes from irradiated glands of animals treated with AAV2hAQP1 there was a high level of AQP1 protein present (lane 2), indicating significant transduction of the targeted tissue occurred. We next examined parotid tissue by immunocytochemistry to determine the cell type transduced by the AAV2hAQP1 vector. The only parotid epithelial cells exhibiting AQP1 positive staining were duct cells transduced by AAV2hAQP1 (not shown). This immunostaining pattern is consistent with the known targeting of AAV2 vectors to minipig parotid duct cells, as assayed with a vector encoding E. coli β-galactosidase23.
Figure 3.
AAV2-mediated transgene expression in minipig parotid glands. Representative results of Western blot analyses are shown. 10 ug of crude membrane protein was subjected to SDS–gel electrophoresis and Western blotting, as described in Materials and Methods. Lane 1, positive control membranes from rat kidney; lane 2, membranes from an AAV2hAQP1-transduced, irradiated minipig parotid gland (8 weeks after 1×1011 vp was delivered) showing AQP1 expression; lane 3, membranes from an AAV2LacZ-transduced, irradiated minipig parotid gland (8 weeks after 1×1011 vp was delivered) showing little AQP1 expression; lane 4, membranes from saline-infused, irradiated minipig parotid gland, also showing little AQP1expression; lane 5, membranes from the non-irradiated, contralateral side, showing AQP1 expression normally present in minipig parotid glands, which is derived from microvascular endothelial cells8,14,24. The migration position of all immunopositive protein bands represents the nonglycosylated AQP1 monomer (28 kDa).
As noted, similar to our previous report23, peak parotid salivary flow rates occurred between weeks 6-10 and declined thereafter, decreasing by ∼20% from week 10 to week 12 (Supplementary Figure 1). We hypothesized that this was likely due to a cellular immune response and examined serum and saliva samples for the presence of several cytokines. Specifically, we measured the levels of granulocyte macrophage colony-stimulating factor (GM-CSF), interleukin-2, interleukin-6, interferon γ and macrophage inflammatory protein-1β in both fluids. When compared with observations following saline administration, eight weeks after administration of 1011 AAV2hAQP1 vp no significant changes were seen in the levels of all five cytokines measured in serum (not shown). Similar results were found for all cytokines in saliva, except for salivary GM-CSF levels, which was elevated significantly (∼ 15%; average value 509 versus 444 pg/ml), a finding consistent with our hypothesis. Clearly more study is required to understand these events25,26. An enhanced cellular immune response may also have occurred when glands were transduced with 3×1011 vp/gland (Figure 2A).
The levels of serum anti-AAV2 neutralizing antibodies, although unlikely related to the decreasing fluid secretion seen after 8 weeks in the transduced animals, were also measured. Saline-administered minipigs had essentially no neutralizing antibodies directed at AAV2 at either week 4 or week 8 (not shown). Conversely, animals treated with 1×1011 AAV2hAQP1 vp had on average a neutralizing antibody titer of ∼1:1600 at week 4 and 1:6400 at week 8.
To assess overall vector safety following administration of the AAV2hAQP1vector, we examined multiple clinical chemistry and hematology parameters, as we have previously done in a GLP-level study conducted with the AdhAQP1 vector15. Vector treatment had little effect on animal general well being (see Supplementary Table 1). The only significant hematological change was a slight elevation in hematocrit values at week 8 after vector delivery. There were no significant alterations in serum chemistry values. Furthermore, all treated animals appeared to eat normally, gain weight and thrive.
Finally, we examined the composition of saliva obtained from the targeted parotid glands (Table 1).Several changes were found following IR, as well as following AAV2hAQP1vector administration, particularly in electrolytes. Most notably, [Na+] was reduced (∼30%; not significant) at 16 weeks after IR and then, following hAQP1 gene transfer, there was a marked elevation in [Na+] at both 4 and 8 weeks (4-5 fold). Total [Ca2+] was dramatically reduced by IR (∼50%), and remained low after gene transfer. There was also an elevation in [K+] after IR, which returned to pre-IR levels following gene transfer. Finally, [Cl-] in parotid saliva was reduced following vector administration. These results, with the particular exception of those for Na+, are reasonably similar to our observations three days following AdhAQP1-mediated gene transfer to irradiated minipig parotid glands 14. The Na+ values following gene transfer, however, are quite different and likely are a result of (i) the longer time points studied here and (ii) a probably much greater role for Na+ in facilitating fluid movement across duct cells than we had previously hypothesized 13,14. Additional physiological and molecular studies 27,28 are needed to understand the specific mechanism(s) involved in fluid secretion from the transduced duct cells.
Table 1. Representative parotid saliva chemistry values in irradiated minipigs after AAV2hAQP1 vector treatment*.
| Pre | IR-16 weeks | Week 4 | Week 8 | |
|---|---|---|---|---|
| Total protein | 1.2 ± 0.4 |
1.3 ± 0.3 |
1.6 ± 0.1 |
1.0 ± 0.1 |
| Amylase | 1122 ± 147 b |
734 ± 166 a |
1986 ± 371 a,b |
1139 ± 84 b |
| Na+ | 14.3 a,
d ± 1.5 |
10.3 a,
d ± 1.5 |
55.7 a ± 2.5 |
39.0 a ± 2.0 |
| Cl- | 17.1 ± 1.0 |
20.0 c ± 2.7 |
12.3 c,d ± 0.6 |
13.7 c,d ± 3.5 |
| K+ | 23.6 a,d ± 1.7 |
43.1 a ± 4.4 |
23.7 a,d ± 0.8 |
21.7 a,d ± 0.5 |
| Ca2+ | 3.0 a ± 0.1 |
1.6 a,c ± 0.2 |
1.1 a,c,d ± 0.2 |
0.7 a,d ± 0.1 |
Data shown are the mean ± SD for minipigs treated with AAV2hAQP1 (n=3; 1011 particles directly administered to one parotid gland). Total protein, g/dl; Amylase, IU/l; all electrolytes are as mmol/l. Data were analyzed using a one-way analysis of variance (ANOVA), followed by the Tukey test. Superscript letters indicate values significantly different, or not, from each other by the Tukey test for each measured salivary constituent. The ANOVA for the total protein data was non-significant, while those for all other salivary constituents were significant. For amylase F=17.29 (p<0.001). For Na+ F=365.84 (p<0.001). For Cl- F=35.64 (p=0.013). For K+ F=52.93 (p<0.001). For Ca2+ F=104.17 (p<0.001).
p<0.001
p<0.01
p<0.05
NS, non-significant
The protein composition of saliva showed more modest changes, both in total values, as well as specifically for amylase (Table 1). The amylase values reported by us previously 16 weeks after IR and three days following AdhAQP1 administration were similar to those observed herein (total protein was not studied previously) 14. Additionally, we did not see any consistent, significant effect of hAQP1 gene transfer on parotid salivary protein composition, when analyzed by SDS gel electrophoresis and silver staining, eight weeks after administration (see Supplementary Figure 2).
The findings in the present study are important for the continued development of a long-term gene therapy approach for the treatment of patients with existing parotid hypofunction after IR. We show, first, that hAQP1 cDNA transfer to parotid duct cells unequivocally leads to fluid secretion from these normally non-fluid secreting cells, as AAV2 vectors only transduce minipig parotid duct cells. Second, we show the originally hypothesized osmotic gradient operative for this duct cell-derived fluid secretion, based on rat experiments (i.e., KHCO3 -driven), is unlikely correct in minipigs, because of the high [Na+] measured in parotid saliva following AAV2hAQP1 transduction. Finally, we show AAV2hAQP1 transduction of minipig parotid glands is safe and without any adverse effect, consistent with a previous GLP-level AAV2 toxicology study in murine salivary glands29.
Although AAV2-mediated transgene expression in minipig parotid glands is transient, AAV2 transduction of rhesus macaque parotid glands is significantly more stable (up to six months following peak expression levels; length of experiment 20). Nonetheless, in the event human parotid glands exhibit similar AAV2-mediated expression patterns as those observed in minipigs, the delivery of alternative AAV serotypes likely can be used to extend transgene expression30.
In conclusion, the studies presented herein support the hypothesis that the AAV2hAQP1 vector would be useful for targeting IR-surviving duct cells in previously irradiated head and neck cancer patients and provide them with a more extended (months) means of increasing salivary flow versus the AdhAQP1 vector (days).
Materials and Methods
Animals
Healthy male minipigs (total studied, n = 20), ∼8 months old, weighing 25–30 kg were obtained from the Institute of Animal Science of the Chinese Agriculture University. Animals were kept under conventional conditions with free access to water and food. Food stock (200-250g, mixed with water) was provided twice daily, at 08:30 and 17:00 hours. This study was reviewed and approved by the Animal Care and Use Committee of Capital Medical University.
Irradiation of parotid glands
All minipigs were subjected to a single IR dose of 20 Gy directed at one parotid gland using a three-dimensional conformal technique 14, 22. Three separate cohorts of animals were studied, using the same general experimental design. Animals were first anesthetized with a combination of ketamine chloride (6 mg/kg) and xylazine (0.6mg/kg) injected intramuscularly. A radiation field of 12×10 cm2, and a 95 cm source-to-skin distance, including one parotid gland, was defined using an X-ray simulator for each minipig and the margins tattooed on the skin for the subsequent exposures. Thereafter, animals were placed on their side and 20 Gy was directed to the targeted parotid gland (randomly selected right or left glands) using a Philips linear accelerator (SL 7520 Philips Medical Systems, Inc., Bothell, WA, USA) with 6 mV of photon energy at 3.2 Gy/min. The contralateral gland was outside of the radiation field and received <1Gy.
AAV2 vector preparation and administration
Generation of AAV2 vectors was performed under helper-free conditions using established methods, as previously described 18, 23. The cytomegalovirus (CMV) promoter was used to drive transgene expression. Crude viral lysates (CVLs) obtained after three freeze–thaw cycles from co-transfected 293T cells were treated with benzonase (100 units/mL CVL; 37°C for 45 min), adjusted to a refractive index of 1.372 by addition of CsCl, and centrifuged (SW41 rotor) at 38,000 rpm for 65 h at 20°C. Equilibrium density gradients were fractionated, fractions with a refractive index of 1.369–1.375 were collected, vector presence was determined by the quantitative Polymerase Chain Reaction (QPCR).After pooling appropriate fractions, vector titers were determined by QPCR. The sequences used for the forward primer, reverse primer, and probe were selected using Primer Express™ Primer Design software (PE Applied Biosystems; Foster City, CA) based on the CMV promoter. Vector titers were ∼1012 vp/mL. Viral fractions were dialyzed against 0.9% NaCl immediately before experiments.
Gene transfer procedures were performed as described 23, 31. In brief, minipigs were fasted (i.e., water available, but solid food withheld) for 12 hours prior to performing the gene transfer. Animals were anesthetized, as above, placed on their side and polyethylene tubing (Intramedic, PE10, Becton-Dickinson, Sparks, MD) used to cannulate the anterior part of the parotid (Stensen's) duct. Atropine (0.02mg/kg) was injected intramuscularly 30 minutes before cannulation. Cannulae were inserted approximately 3 cm into the orifice of the duct. Vectors were suspended in 0.9% NaCl, pH 7.4, and delivered to the targeted parotid glands by retrograde infusion. Glands received from 1010-3×1011 vp (see figure legends for Figures 2 and 3, and related text, for details) in an infusion volume of 4 mL.
Histological and immunocytochemical analyses
Following sacrifice, parotid glands were obtained from animals. Tissues were quickly removed, cleaned of extraneous tissue, cut into multiple pieces (∼5×5×5 mm), fixed in 10% formalin, embedded in paraffin, and then sectioned at 3-4 μm. The sections were either (i) stained with hematoxylin and eosin, and examined for evidence of pathological changes (there were none observed) or (ii) processed for evidence of AQP1 expression exactly as described previously 14.
Collection of blood and saliva
Parotid saliva was collected, and salivary flow rates determined, using a modified Carlson-Cittenden cup 14 on anesthetized animals following an intramuscular injection of pilocarpine (0.1mg/kg). The collection device was identical to that used clinically in collecting parotid saliva from children and adolescents (9mm inner ring diameter; 16 mm outer ring diameter). Parotid saliva was collected from each parotid gland of all animals for ∼10 min on the days indicated in the Results section and figures. Blood was obtained from the precaval vein at the same time points.
Western blot analyses
Western blots for hAQP1 expression were performed as previously described 14. In brief, frozen tissue samples were thawed on wet ice, placed in ice-cold homogenization buffer (TE buffer, 10mM Tris-HCl, pH 7.4), minced with scissors, and homogenized in a Polytron homogenizer (twice, in 10 sec bursts at setting 5). After centrifugation at 1,000g for 10 min, supernatants were collected and re-centrifuged at 17,000g for 30 min. The resulting supernatant was discarded and the final pellet re-suspended in 20 μL loading buffer. A protein assay was performed and 10 μg of protein was loaded per lane for gel electrophoresis and Western blotting. Samples were electrophoresed in 12% SDS polyacrylamide gels, and incubated with anti-sera directed at the C-terminus of hAQP1, as previously reported 14.
Clinical laboratory analyses with blood and saliva samples
As previously reported 14, collected saliva and blood samples were analyzed by standard clinical chemistry and, for blood, hematology procedures. Serum analyses performed included Na+, K+, Cl-, Ca2+, phosphorous, glucose, total protein, albumin, globulin, aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, blood urea nitrogen, alkaline phosphatase, amylase, and creatinine. Hematology analyses included the number of white blood cells, red blood cells, platelets, hematocrit, concentration of hemoglobin, mean corpuscular volume, % lymphocytes, % monocytes, and % granulocytes. Parotid saliva samples were analyzed for total protein, albumin, amylase, Na+, K+, Cl-, Ca2+, and PO4-. Data at different time points were analyzed using a one-way analysis of variance followed by the Tukey test, and representative results are shown in Table 1 and Supplementary Table 1. For analyses of salivary protein composition (Supplementary Figure 2), 10 μL of each parotid saliva sample was electrophoresed in 12% SDS polyacrylamide gels, as described above, and silver stained as reported 32.
Assessment of AAV2 serum neutralizing antibodies
To determine whether neutralizing antibodies to AAV2 were present in serum, we evaluated all serum samples using an assay similar to one previously described by Katano et al 33. Titers are reported as that dilution of serum that resulted in a 50% inhibition in measuring transduction of 293 cells with an AAV2 vector expressing green fluorescence protein.
Assessment of serum and salivary cytokine levels
Serum and saliva samples were examined by enzyme-linked immunoabsorbance assays (ELISAs; all from R&D Systems; Minneapolis, MN) for the presence of several cytokines: GM-CSF, interleukin-2, interleukin-6, interferon γ, and macrophage inhibitory protein-1β. Prior to measuring actual experimental samples, randomly chosen serum and saliva samples were tested for ELISA interference using spiking assays with standard proteins obtained with the ELISA kits. None of the samples showed evidence for assay inhibition or enhancement. All samples used for final data determination were assayed in duplicate.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (2007CB947304 and 2010CB944801), the Funding Project for Academic Human Resources Development in Institutions of Higher Learning under the jurisdiction of Beijing Municipality (PHR20090510, 2006 ID 0301200094, KM200510025002), National Excellent PhD Theses Award grant from Ministry of Education, China (200778), and the Intramural Research Program of the National Institute of Dental and Craniofacial Research.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- 3.Vissink A, Burlage FR, Spijkervet FK, Jansma J, Coppes RP. Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:213–225. doi: 10.1177/154411130301400306. [DOI] [PubMed] [Google Scholar]
- 4.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–3776. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 5.Ho KF, Farnell DJ, Routledge JA, Burns MP, Sykes AJ, Slevin NJ, et al. Developing a CTCAEs patient questionnaire for late toxicity after head and neck radiotherapy. Eur J Cancer. 2009;45:1992–1998. doi: 10.1016/j.ejca.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Brizel DM, Overgaard J. Does amifostine have a role in chemoradiation treatment? Lancet Oncol. 2003;4:378–381. doi: 10.1016/s1470-2045(03)01132-x. [DOI] [PubMed] [Google Scholar]
- 7.Vergeer MR, Doornaert P, Reitveld DH, Leemans CR, Slotman BJ, Langendijk JA. Intensity-modulated radiotherapy reduces radiation-induced morbidity and improves health-related quality of life: results of a non-randomized prospective study using a standardized follow-up program. Int J Radiat Oncol Biol Phys. 2009;74:1–8. doi: 10.1016/j.ijrobp.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 8.Cotrim AP, Sowers A, Mitchell JB, Baum BJ. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther. 2007;15:2101–2106. doi: 10.1038/sj.mt.6300296. [DOI] [PubMed] [Google Scholar]
- 9.Epperly MW, Melendez JA, Zhang X, Nie S, Pearce L, Peterson J, et al. Mitochondrial targeting of a catalase transgene product by plasmid liposomes increases radioresistance in vitro and in vivo. Radiat Res. 2009;171:588–595. doi: 10.1667/RR1424.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox JD, Steitz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for research and treatment of cancer. Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 11.Baum BJ, Zheng C, Cotrim AP, McCullagh L, Goldsmith CM, Brahim JS, et al. Aquaporin-1 gene transfer to correct radiation-induced salivary hypofunction. In: Beitz E, editor. Aquaporins Handbook of Experimental Pharmacology. Vol. 190. Berlin Heidelberg: Springer-Verlag; 2009. pp. 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preston GM, Agre P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: member of an ancient channel family. Proc Natl Acad Sci U S A. 1991;88:11110–11114. doi: 10.1073/pnas.88.24.11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delporte C, O'Connell BC, He X, Lancaster HE, O'Connell AC, Agre P, et al. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci USA. 1997;94:3268–3273. doi: 10.1073/pnas.94.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shan Z, Li J, Zheng C, Liu X, Fan Z, Zhang C, et al. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther. 2005;11:444–451. doi: 10.1016/j.ymthe.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Zheng C, Goldsmith CM, Mineshiba F, Chiorini JA, Kerr A, Wenk ML, et al. Toxicity and biodistribution of a first-generation recombinant adenoviral vector, encoding aquaporin-1, after retroductal delivery to a single rat submandibular gland. Hum Gene Ther. 2006;17:1122–1133. doi: 10.1089/hum.2006.17.1122. [DOI] [PubMed] [Google Scholar]
- 16.http://www.clinicaltrials.gov/ct/show/NCT00372320?order=
- 17.Kagami H, Atkinson JC, Michalek SM, Handelman B, Yu S, Baum BJ, et al. Repetitive adenovirus administration to the parotid gland: role of immunological barriers and induction of oral tolerance. Hum Gene Ther. 1998;9:305–313. doi: 10.1089/hum.1998.9.3-305. [DOI] [PubMed] [Google Scholar]
- 18.Braddon VR, Chiorini JA, Wang S, Kotin RM, Baum BJ. Adenoassociated virus-mediated transfer of a functional water channel into salivary epithelial cells in vitro and in vivo. Hum Gene Ther. 1998;9:2777–2785. doi: 10.1089/hum.1998.9.18-2777. [DOI] [PubMed] [Google Scholar]
- 19.Voutetakis A, Kok MR, Zheng C, Bossis I, Wang J, Cotrim AP, et al. Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics. Proc Natl Acad Sci U S A. 2004;101:3053–3058. doi: 10.1073/pnas.0400136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voutetakis A, Zheng C, Mineshiba F, Cotrim AP, Goldsmith CM, Schmidt M, et al. Adeno-associated virus serotype 2-mediated gene transfer to the parotid glands of nonhuman primates. Hum Gene Ther. 2007;18:142–150. doi: 10.1089/hum.2006.154. [DOI] [PubMed] [Google Scholar]
- 21.Cotrim AP, Hyodo F, Matsumoto KI, Sowers AL, Cook JA, Baum BJ, et al. Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of Tempol by magnetic resonance imaging. Clin Cancer Res. 2007;13:4928–4933. doi: 10.1158/1078-0432.CCR-07-0662. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Shan Z, Ou G, Liu X, Zhang C, Baum BJ, et al. Structural and functional characteristics of irradiation damage to parotid glands in the miniature pig. Int J Radiat Oncol Biol Phys. 2005;62:1510–1516. doi: 10.1016/j.ijrobp.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Hai B, Yan X, Voutetakis A, Zheng C, Cotrim AP, Shan Z, et al. Long-term transduction of miniature pig parotid glands using serotype 2 adeno-associated viral vectors. J Gene Med. 2009;11:506–514. doi: 10.1002/jgm.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Nielsen S, Dai Y, Lazowski KW, Christensen EI, Tabak LA, et al. Examination of rat salivary glands for the presence of the aquaporin CHIP. Pflugers Arch. 1994;428:455–460. doi: 10.1007/BF00374565. [DOI] [PubMed] [Google Scholar]
- 25.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 26.Voutetakis A, Zheng C, Cotrim AP, Mineshiba F, Afione S, Roescher N, et al. AAV5-mediated gene transfer to the parotid glands of non-human primates. Gene Ther. 2010;17:50–60. doi: 10.1038/gt.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamoto T, Srivastava A, Romanenko VG, Ovitt CE, Perez-Cornejo P, Arreola J, et al. Functional and molecular characterization of the fluid secretion mechanism in human parotid acinar cells. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2380–R2390. doi: 10.1152/ajpregu.00591.2006. [DOI] [PubMed] [Google Scholar]
- 28.Catalan MA, Nakamoto T, Melvin JE. The salivary gland fluid secretion mechanism. J Med Invest. 2009;56(Suppl):192–196. doi: 10.2152/jmi.56.192. [DOI] [PubMed] [Google Scholar]
- 29.Voutetakis A, Zheng C, Wang J, Goldsmith CM, Afione S, Chiorini JA, et al. Gender differences in serotype 2 adeno-associated virus biodistribution after administration to rodent salivary glands. Hum Gene Ther. 2007;18:1109–1118. doi: 10.1089/hum.2007.072. [DOI] [PubMed] [Google Scholar]
- 30.Kok MR, Voutetakis A, Yamano S, Wang J, Cotrim A, Katano H, et al. Immune responses following salivary gland administration of recombinant adeno-associated virus serotype 2 vectors. J Gene Med. 2005;7:432–441. doi: 10.1002/jgm.678. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Zheng C, Zhang X, Liu X, Zhang C, Goldsmith CM, et al. Developing a convenient large animal model for gene transfer to salivary glands in vivo. J Gene Med. 2004;6:55–63. doi: 10.1002/jgm.476. [DOI] [PubMed] [Google Scholar]
- 32.Yan JX, Wait R, Berkelman T, Harry RA, Westbrook JA, Wheeler CH, Dunn MJ. A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionization-mass spectrometry. Electrophoresis. 2000;21:3666–3672. doi: 10.1002/1522-2683(200011)21:17<3666::AID-ELPS3666>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Katano H, Kok MR, Cotrim AP, Yamano S, Schmidt M, Afione S, et al. Enhanced transduction of mouse salivary glands with AAV5-based vectors. Gene Ther. 2006;13:594–601. doi: 10.1038/sj.gt.3302691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.