Abstract
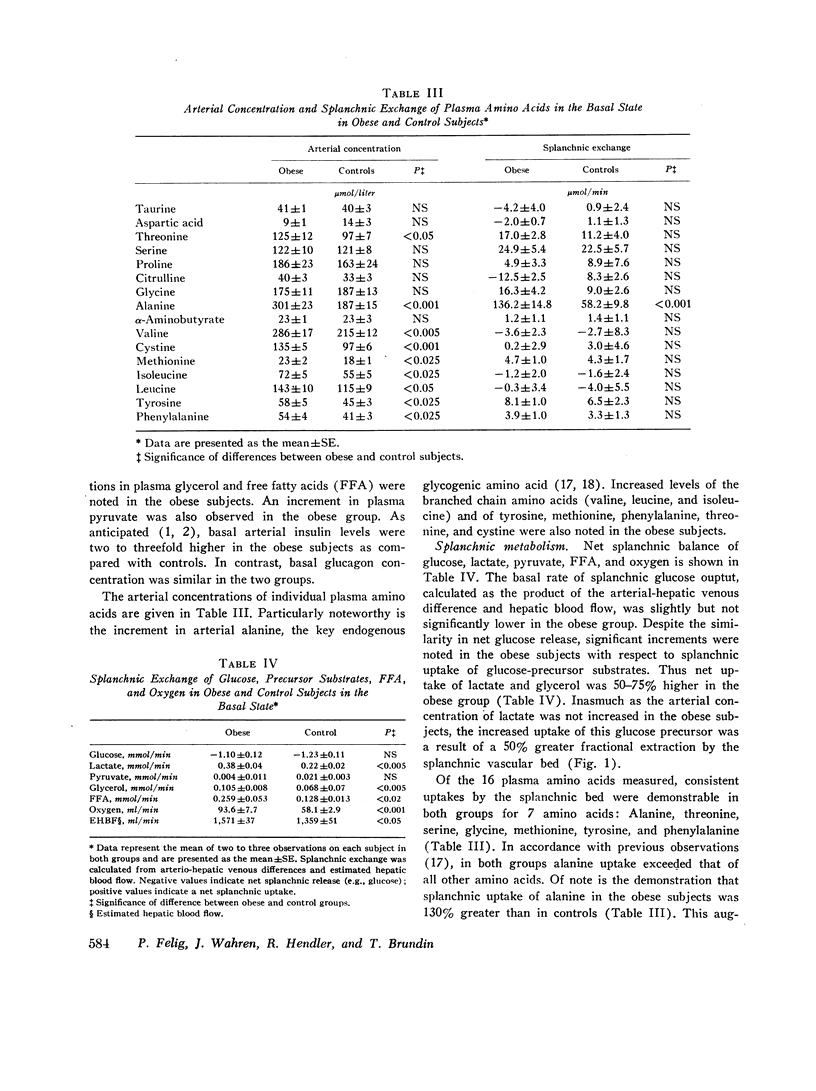
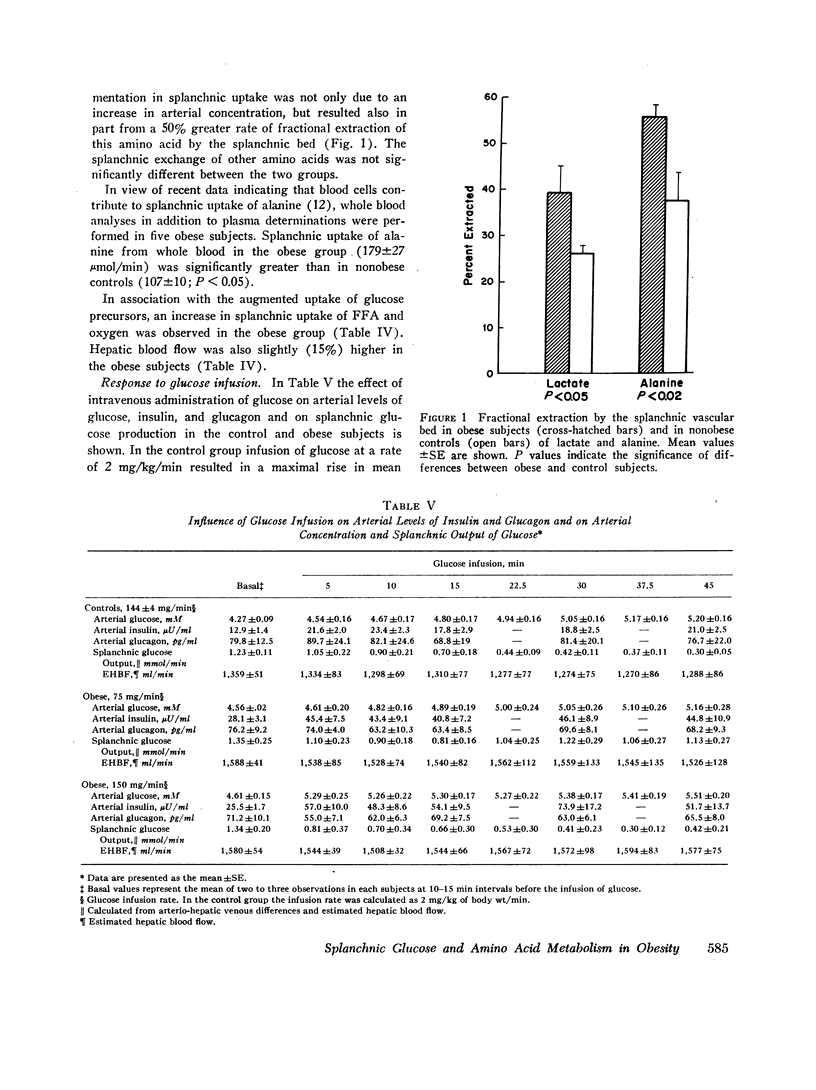
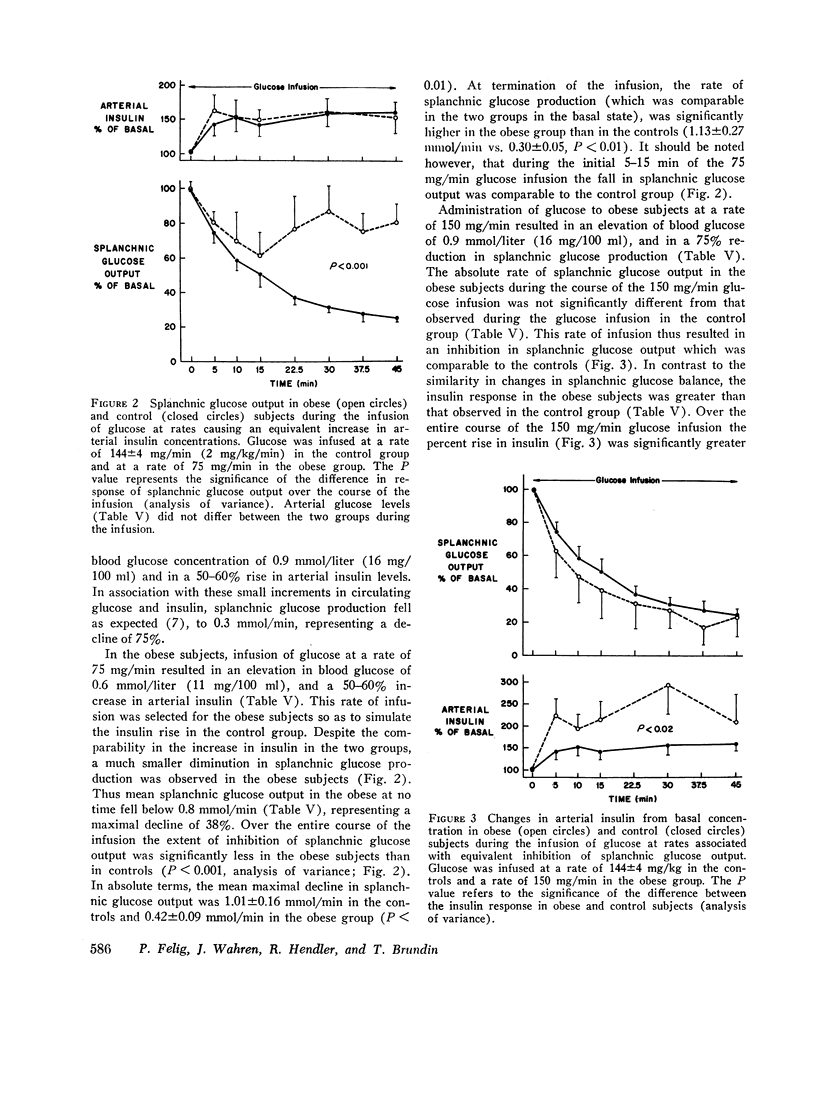
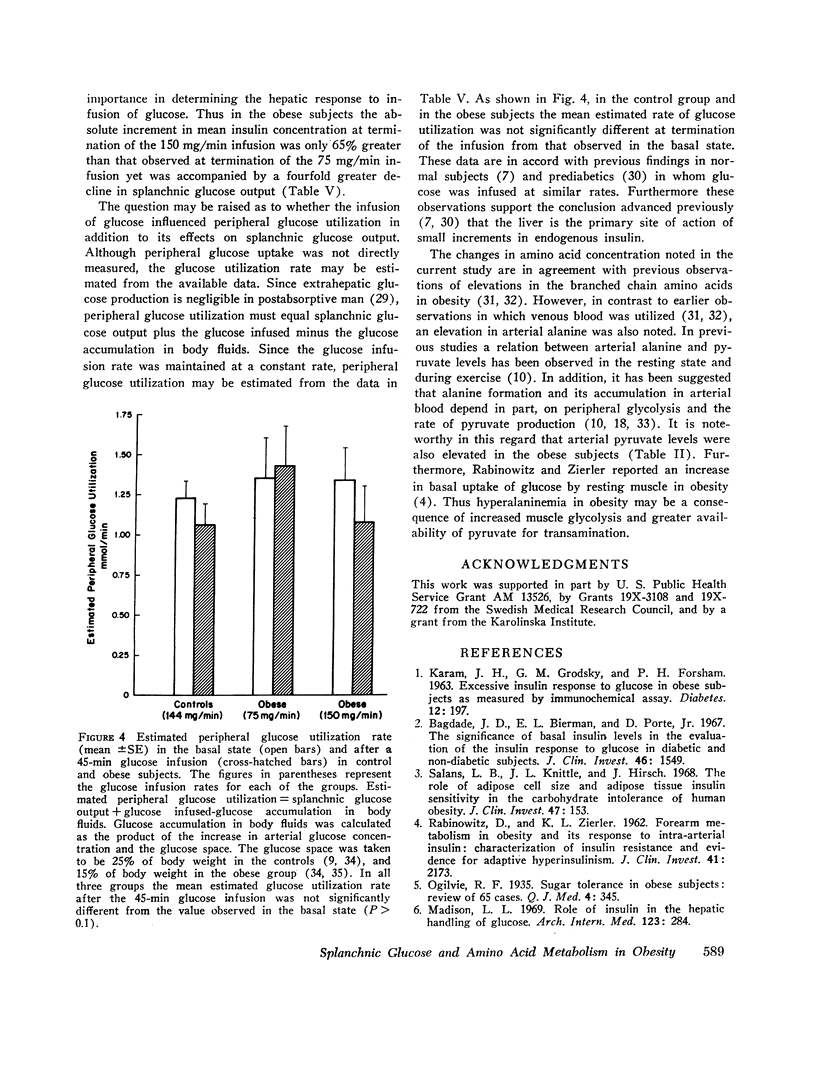
Arterial concentrations and splanchnic exchange of glucose, lactate, pyruvate, glycerol, free fatty acids, and individual acidic and neutral amino acids were determined in obese and nonobese control subjects in the basal state and during a 45 min infusion of glucose. Glucose was administered to the controls at a rate (2 mg/kg/min; 144 +/- 4 mg/min) known to inhibit splanchnic glucose output without influencing peripheral glucose utilization. The obese subjects received glucose at two dose levels (75 and 150 mg/min) which simulated either the rise in insulin or the inhibition in splanchnic glucose production observed in the controls. In the basal state splanchnic glucose production did not differ significantly between obese and control subjects. However splanchnic uptake of lactate, glycerol, alanine, free fatty acids, and oxygen was 50-160% greater in obese subjects. Splanchnic uptake of glucose precursors could account for 33% of hepatic glucose output in the obese group as compared to 19% in controls. The increase in alanine and lactate uptake was due in part, to a 50% increase in splanchnic fractional extraction. Administration of glucose to the control subjects 144 +/- 4 mg/min) resulted in a 50-60% increment in arterial insulin and a 75% reduction in splanchnic glucose output. In the obese group, infusion of glucose at a rate of 75 mg/min resulted in an equivalent rise in arterial insulin, but was accompanied by a less than 40% inhibition in splanchnic glucose output. Glucose infusion at a rate of 150 mg/min in the obese resulted in a 75% reduction in splanchnic glucose output which was equivalent to that observed in controls, but was accompanied by a significantly greater rise (100-200%) in arterial insulin. It is concluded that in obesity (a) despite basal hyperinsulinemia, splanchnic uptake of glucose precursors is increased, the relative contribution to total glucose release attributable to gluconeogenesis being 70% higher than in controls; (b) infusion of glucose at rates causing equivalent increases in arterial insulin induces a smaller inhibition in splanchnic glucose output than in controls; (c) infusion of glucose at rates causing comparable inhibition in splanchnic glucose output is accompanied by a disproportionately greater increase in endogenous insulin than in controls. These data are compatible with hepatic resistance to insulin in obesity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adibi S. A. Influence of dietary deprivations on plasma concentration of free amino acids of man. J Appl Physiol. 1968 Jul;25(1):52–57. doi: 10.1152/jappl.1968.25.1.52. [DOI] [PubMed] [Google Scholar]
- Arky R. A., Freinkel N. Alcohol hypoglycemia. V. Alcohol infusion to test gluconeogenesis in starvation, with special reference to obesity. N Engl J Med. 1966 Feb 24;274(8):426–433. doi: 10.1056/NEJM196602242740803. [DOI] [PubMed] [Google Scholar]
- Bagdade J. D., Bierman E. L., Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest. 1967 Oct;46(10):1549–1557. doi: 10.1172/JCI105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackard W. G., Nelson N. C. Portal and peripheral vein immunoreactive insulin concentrations before and after glucose infusion. Diabetes. 1970 May;19(5):302–306. doi: 10.2337/diab.19.5.302. [DOI] [PubMed] [Google Scholar]
- Bortz W. M., Paul P., Haff A. C., Holmes W. L. Glycerol turnover and oxidation in man. J Clin Invest. 1972 Jun;51(6):1537–1546. doi: 10.1172/JCI106950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCKSON J. R., OOMS H. A., BELLENS R., CONARD V., BASTENIE P. A. Physiologic significance of the intravenous glucose tolerance test. Metabolism. 1962 May;11:482–500. [PubMed] [Google Scholar]
- Felig P., Marliss E., Cahill G. F., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969 Oct 9;281(15):811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- Felig P., Owen O. E., Wahren J., Cahill G. F., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Pozefsky T., Marliss E., Cahill G. F., Jr Alanine: key role in gluconeogenesis. Science. 1970 Feb 13;167(3920):1003–1004. doi: 10.1126/science.167.3920.1003. [DOI] [PubMed] [Google Scholar]
- Felig P. The glucose-alanine cycle. Metabolism. 1973 Feb;22(2):179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Amino acid metabolism in exercising man. J Clin Invest. 1971 Dec;50(12):2703–2714. doi: 10.1172/JCI106771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Wahren J. Influence of endogenous insulin secretion on splanchnic glucose and amino acid metabolism in man. J Clin Invest. 1971 Aug;50(8):1702–1711. doi: 10.1172/JCI106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Wahren J., Räf L. Evidence of inter-organ amino-acid transport by blood cells in humans. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1775–1779. doi: 10.1073/pnas.70.6.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franckson J. R., Malaise W., Arnould Y., Rasio E., Ooms H. A., Balasse E., Conard V., Bastenie P. A. Glucose kinetics in human obesity. Diabetologia. 1966 Sep;2(2):96–103. doi: 10.1007/BF00423017. [DOI] [PubMed] [Google Scholar]
- Genuth S. M. Metabolic clearance of insulin in man. Diabetes. 1972 Oct;21(10):1003–1012. doi: 10.2337/diab.21.10.1003. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L. A gas chromatographic method for the determination of individual free fatty acids in plasma. Clin Chim Acta. 1966 Feb;13(2):266–268. doi: 10.1016/0009-8981(66)90304-4. [DOI] [PubMed] [Google Scholar]
- KARAM J. H., GRODSKY G. M., FORSHAM P. H. Excessive insulin response to glucose in obese subjects as measured by immunochemical assay. Diabetes. 1963 May-Jun;12:197–204. doi: 10.2337/diab.12.3.197. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Neville D. M., Jr, Roth J. Insulin-receptor interaction in the obese-hyperglycemic mouse. A model of insulin resistance. J Biol Chem. 1973 Jan 10;248(1):244–250. [PubMed] [Google Scholar]
- Kreisberg R. A. Glucose metabolism in normal and obese subjects. Effect of phenformin. Diabetes. 1968 Aug;17(8):481–488. doi: 10.2337/diab.17.8.481. [DOI] [PubMed] [Google Scholar]
- Kreisberg R. A. Kinetics of glucose utilization in obesity: the effect of phenformin. Ann N Y Acad Sci. 1968 Mar 26;148(3):743–755. doi: 10.1111/j.1749-6632.1968.tb27746.x. [DOI] [PubMed] [Google Scholar]
- Madison L. L. Role of insulin in the hepatic handling of glucose. Arch Intern Med. 1969 Mar;123(3):284–292. [PubMed] [Google Scholar]
- OPIE L. H., WALFISH P. G. Plasma free fatty acid concentrations in obesity. N Engl J Med. 1963 Apr 4;268:757–760. doi: 10.1056/NEJM196304042681404. [DOI] [PubMed] [Google Scholar]
- Paul P., Bortz W. M. Turnover and oxidation of plasma glucose in lean and obese humans. Metabolism. 1969 Jul;18(7):570–584. doi: 10.1016/0026-0495(69)90091-2. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ D., ZIERLER K. L. Forearm metabolism in obesity and its response to intra-arterial insulin. Characterization of insulin resistance and evidence for adaptive hyperinsulinism. J Clin Invest. 1962 Dec;41:2173–2181. doi: 10.1172/JCI104676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salans L. B., Knittle J. L., Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J Clin Invest. 1968 Jan;47(1):153–165. doi: 10.1172/JCI105705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeta Y., Oji N., Hoshi M., Kang M. Fatty acid synthesis and gluconeogenesis in rats fed a high caloric diet. Metabolism. 1966 Aug;15(8):761–766. doi: 10.1016/s0026-0495(66)80012-4. [DOI] [PubMed] [Google Scholar]
- Shreeve W. W., Hoshi M., Oji N., Shigeta Y., Abe H. Insulin and the utilization of carbohydrates in obesity. Am J Clin Nutr. 1968 Dec;21(12):1404–1418. doi: 10.1093/ajcn/21.12.1404. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P., Ahlborg G., Jorfeldt L. Glucose metabolism during leg exercise in man. J Clin Invest. 1971 Dec;50(12):2715–2725. doi: 10.1172/JCI106772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Felig P., Cerasi E., Luft R., Hendler R. Splanchic glucose production and its regulation in healthy monozygotic twins of diabetics. Clin Sci. 1973 May;44(5):493–504. doi: 10.1042/cs0440493. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P., Cerasi E., Luft R. Splanchnic and peripheral glucose and amino acid metabolism in diabetes mellitus. J Clin Invest. 1972 Jul;51(7):1870–1878. doi: 10.1172/JCI106989. [DOI] [PMC free article] [PubMed] [Google Scholar]



