Abstract
We examined horizontal directional hearing in patients with acquired severe unilateral conductive hearing loss (UCHL). All patients (n = 12) had been fitted with a bone conduction device (BCD) to restore bilateral hearing. The patients were tested in the unaided (monaural) and aided (binaural) hearing condition. Five listeners without hearing loss were tested as a control group while listening with a monaural plug and earmuff, or with both ears (binaural). We randomly varied stimulus presentation levels to assess whether listeners relied on the acoustic head-shadow effect (HSE) for horizontal (azimuth) localization. Moreover, to prevent sound localization on the basis of monaural spectral shape cues from head and pinna, subjects were exposed to narrow band (1/3 octave) noises. We demonstrate that the BCD significantly improved sound localization in 8/12 of the UCHL patients. Interestingly, under monaural hearing (BCD off), we observed fairly good unaided azimuth localization performance in 4/12 of the patients. Our multiple regression analysis shows that all patients relied on the ambiguous HSE for localization. In contrast, acutely plugged control listeners did not employ the HSE. Our data confirm and further extend results of recent studies on the use of sound localization cues in chronic and acute monaural listening.
Keywords: bone conduction, head movements, head-shadow effect, perceptual learning, sound localization
Introduction
Unilateral conductive hearing loss (UCHL) in patients with a contralateral normal hearing ear may involve the typical problems associated with unilateral hearing (i.e., poor sound localization abilities and poor speech recognition performance in noise). However, Colburn (1982, review) reported that several UCHL patients displayed near normal performance on an interaural intensity discrimination task. In addition, fairly good monaural sound localization abilities have been reported in congenital UCHL patients (Wilmington et al. 1994; Priwin et al. 2007a), in patients with acquired UCHL (Snik et al. 2002), and in patients with unilateral congenital deafness (Slattery and Middlebrooks 1994). The reported good monaural localization performance in these studies is surprising because sound localization in the horizontal plane (azimuth) is supposed to rely on the neural processing of binaural acoustic differences in sound level (for frequencies above 3 kHz) and phase (below about 1.5 kHz; Blauert 1997). Both cues are highly distorted in patients with UCHL. We hypothesized that the reported monaural azimuth localization performance could be due to the limited range of sound levels used in those studies. Under such listening conditions, the acoustic head-shadow effect (HSE) might serve as a valid azimuth cue because listeners can learn that sounds presented at the hearing side are perceived louder, as opposed to sounds at the deaf side (Perrett and Noble 1995; Van Wanrooij and Van Opstal 2004). Note, however, that the HSE provides an ambiguous localization cue under natural listening to unfamiliar sound sources and is therefore not beneficial for more general listening conditions.
It is unclear whether the effort to restore binaural hearing in UCHL patients with a conventional acoustic hearing aid successfully restores directional hearing (Markides 1977; Wazen et al., 2008). An additional problem concerns cases with a chronically diseased ear, or with aural atresia, for which fitting of a conventional hearing aid is either not possible or contraindicated (Snik et al. 2005). Often, for those patients, a bone conduction device (BCD) is the only established option, and near symmetric hearing thresholds can be obtained in these patients after BCD application (Snik et al. 2005).
The current study had two primary objectives. First, we investigated whether the BCD was indeed beneficial for sound localization in azimuth, and second, we studied whether UCHL patients were able to localize sounds in the azimuth direction when their BCD was turned off (i.e., monaural listening). Objective data about the improvement of sound localization are still scarce, and cross-hearing, which refers to the additional acoustic stimulation of the cochlea contralateral to the BCD, might harm the possible localization abilities of BCD recipients (Snik et al. 2002; Hol et al. 2005; Stenfelt 2005; Wazen et al. 2005; Priwin et al. 2007a, b). We selected patients with acquired UCHL, as it is unclear whether monaural localization ability develops differently in congenital vs. acquired UCHL patients. We further compared the patient results with monaural and binaural localization performance of normal hearing control listeners.
In the experiments, we varied several stimulus characteristics with the aim to quantify the potential contributions of different sound localization factors: (1) To investigate whether UCHL patients used interaural level differences (ILDs) and/or interaural timing differences (ITDs), we employed narrow band noises around either 500 Hz (ITD) or 3 kHz (ILD). (2) The use of narrow band noises minimized the possibility to use spectral shape cues provided by the pinna of the hearing ear as a potential monaural localization cue (Middlebrooks and Green, 1991; Middlebrooks 1992; Blauert 1997; Van Wanrooij and Van Opstal 2004, 2007). (3) To establish whether head movements affected sound localization because of the associated dynamic changes in localization cues, patients were exposed to stimuli with long (1,000 ms) and short (150 ms) durations (Wallach 1940; Vliegen et al. 2007). (4) Finally, to deny listeners the beneficial use of the HSE, which is in a range of ~10 dB for high-frequency (>3 kHz) stimuli across the azimuth domain (Van Wanrooij and Van Opstal 2004), we varied stimulus presentation levels over a broad range (40–70 dB SPL) and presented all stimuli randomly interleaved.
Methods
Patients
We report on the localization results of 12 UCHL patients who responded with a rapid head-orienting saccade toward narrow band noises presented at random locations within the horizontal plane. Patients were suffering from a chronic draining ear resistant to medical therapy for a period of at least 5 years or from another cause of maximal acquired UCHL without a surgical rehabilitation option. The patients had normal hearing in one ear (thresholds of 20 dB HL or better between 500 Hz and 8 kHz) and pure conductive hearing loss in the other ear. All patients experienced binaural hearing before they received a BCD (bone-anchored hearing aid, Cochlear®). Ten of the participants used their BCD 7 days a week for at least 8 h a day for more than 1 year. Two patients (P4 and P9) used the BCD only several hours a week. Audiometric characteristics of the patients and the type of BCD that the patients were using are presented in Table 1. Part of the measurements was carried out in a subgroup of five patients (P1–P5), aged 27–53 (mean 39.4 years), who had participated previously in a study performed by Hol et al. (2005). In a first experimental session, these five patients responded to stimuli of 1,000-ms duration (long) and, in a second session, to stimuli of 150-ms (short) duration. Patients P2 and P4 were excluded from the second recording session with short duration stimuli: P2 because of inconsistent baseline results and P4 because this patient stopped using the BCD. Seven other patients (P6–P12), aged 30–68 (mean 42.6 years), were only exposed to the short duration stimuli (150 ms).
TABLE 1.
Audiometric characteristics of the patients
| Patients | Age (years) | Threshold dB HL | PTA dB HL | Type BCD | ||
|---|---|---|---|---|---|---|
| 500 Hz | 3,000 Hz | BC | AC | |||
| P1 | 53 | 55 | 65 | 10 | 57 | Baha-Compact |
| P2 | 37 | 65 | 60 | 25 | 63 | Baha-Divino |
| P3 | 46 | 35 | 60 | 12 | 57 | Baha-Compact |
| P4 | 27 | 50 | 50 | 8 | 55 | Baha-Compact |
| P5 | 34 | 60 | 65 | 17 | 62 | Baha-Compact |
| P6 | 47 | 90 | 62 | 33 | 72 | Baha-Divino |
| P7 | 31 | 50 | 42 | 13 | 43 | Baha-Divino |
| P8 | 40 | 80 | 75 | 22 | 75 | Baha-Divino |
| P9 | 30 | 60 | 45 | 10 | 52 | Baha-Compact |
| P10 | 68 | 50 | 48 | 15 | 48 | Baha-Compact |
| P11 | 51 | 45 | 82 | 22 | 75 | Baha-Compact |
| P12 | 30 | 85 | 65 | 25 | 70 | Baha-Compact |
PTA Pure tone audiometry (1, 2, and 4 kHz), BC bone conduction, AC air conduction, BCD bone conduction device, Baha Bone-anchored hearing aid
Control listeners
For comparative purposes, we recruited a reference group of five control listeners (C1–C5), aged 27–62 (mean 43.2 years), without hearing loss. These listeners had thresholds of 20 dB HL or less between 500 Hz and 8 kHz in both ears. One control listener (C4) is the author of this paper; the other control listeners were naive about the purpose of the study. Measurements were performed after altering binaural hearing by plugging and covering the left or right ear with an earmuff (monaural sound localization) and in the normal binaural listening condition (unplugged). The plugs were fabricated by filling the ear canal and pinna with rubber casting material (Otoform Otoplastik –K/c; Dreve, Unna, Germany). After plugging, the ear was additionally covered with an earmuff (E.A.R. Muff Model 4000 conforms to EN352-1: 2001 specifications). The hearing thresholds for the plugged ears were ~30 dB HL for low frequencies (<1 kHz) and ~50 dB HL for high frequencies (>3 kHz).
Stimuli
The directional hearing experiments were carried out with low-frequency (500 Hz) and high-frequency (3 kHz) narrow band (1/3 octave) noises. Stimuli with a 1,000-ms (long) duration and 5-ms sine-squared on- and offset ramps had levels of 40, 50, 60, and 70 dB SPL and were randomly interleaved during the experiments. All stimuli with a 150-ms (short) duration were presented at a fixed sound level of 55 dB SPL, but these stimuli were randomly interleaved within a series of broadband stimuli that had randomly selected sound levels in the 45–65-dB SPL range. Sounds were digitally generated in Matlab (The Mathworks 7.4) at a sampling rate of 50 kHz. Stimuli were delivered through a broad range speaker at a distance of 0.85 m from the subjects that was moved by a computer-controlled motorized system (Hofman and Van Opstal 1998) to either one of seven different azimuth positions, ranging from −90° (far left) to +90° (far right) at 30° intervals in the first experimental session, and to randomly selected locations in the ±70° range in the second session. To force subjects to make a head movement response, even for sounds presented at straight ahead, the straight-ahead sound location was positioned at 4° right from the initial fixation point.
Setup
To ensure that subjects could only use acoustic information to localize sounds, directional hearing was tested in a completely dark, sound-attenuated room (3.2 × 3.2 × 3.5 m). Walls, ceiling, floor, and every large object present were covered with sound-attenuating foam (50-mm thick with 30 mm pyramids, AX2250, Uxem b.v., Lelystad, The Netherlands). Through acoustic measurements (Brüel & Kjær BK2610 sound amplifier and Brüel & Kjær BK4144 microphone) at different positions in the room, we observed slight reverberations only for low frequencies (around 500 Hz) near the walls of the room, and we verified that no discernable room reverberations occurred at the position of the subject’s head in the center of the room at a minimum distance of 1.6 m from the walls. We also verified that the subject’s ears were within the room’s reverberation radius for the low-frequency stimuli (approximately 1.1 m at a T 60 = 0.09 s, given that the absorption coefficient of the walls for 500 Hz sounds was about 0.7; manufacturer’s data sheet). From this, we conclude that listeners were exposed to the speaker’s direct sound field only. The room had an ambient background noise level of 30 dB SPL. Horizontal and vertical head movement components were recorded with the magnetic search coil induction technique (Robinson 1963; Hofman and Van Opstal 1998). To that end, the listener was seated comfortably in a chair in the center of the room and wore a lightweight spectacle frame that was fastened with Velcro. On the frame, a small coil was attached. Three orthogonal pairs of square coils (6 mm2 wires, 3 m × 3 m) were attached to the rooms’ edges to generate the horizontal (80 kHz), vertical (60 kHz), and frontal (48 kHz) magnetic fields, respectively. The head coil signal was amplified and demodulated, low-pass-filtered at 150 Hz, and digitized at 500 Hz (Hofman and Van Opstal 1998).
A head-fixed laser pointer projected onto a small (1 cm2) plastic plate that was attached at the end of a 40-cm-long thin aluminum rod that was attached to the subject’s head and positioned in front of the subjects’ eyes (Van Wanrooij and Van Opstal 2004). Subjects were asked to point the laser dot as fast and as accurately as possible in the perceived sound direction after stimulus offset. This procedure ensured that the eyes remained centered in the head, and subjects pointed with their head, rather than with their eyes to the perceived location.
Paradigm
All measurements were carried out in a monaural and a binaural condition, measured in separate stimulus sequences within the same session. This meant that the patients were tested in the unaided (monaural, BCD-off) and aided (binaural, BCD-on) conditions and the control listeners under normal (two ears) and monaural (one ear plugged and covered with an earmuff) hearing conditions. Whether a subject was first tested in the binaural or monaural condition was randomized. Subjects did not receive any explicit feedback about their performance during the experiments.
The experimental session always started with a brief calibration experiment needed to establish the off-line mapping of the coil signals onto known target locations. After the calibration experiment, listeners performed three brief practice sessions (totalling 40 trials). In these practice sessions, the subject became familiar with the sounds, with hearing in the monaural condition, and with the open-loop head movement response procedure.
During the sound localization experiments, the listener first fixated the straight-ahead LED and triggered the trial by pressing a button. Within 150 ms, the LED disappeared and the sound stimulus was presented. After stimulus exposure, the subject had to redirect the head toward the apparent sound direction. Subjects were observed continuously by the experimenter with an infrared camera while they performed the task.
Data analysis
We analyzed all responses separately for each stimulus condition (500 Hz and 3 kHz; 1,000 ms and 150 ms) and for each listener. We determined the best linear fit (in the mean-squared error sense) of the stimulus–response relationship on the azimuth data for the different stimulus conditions (pooled across presentation levels):
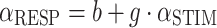 |
1 |
in which α is the azimuth angle (in degree), b is the response bias (in degree), and g the response gain (dimensionless). We also computed Pearson’s correlation coefficient between fit and data, as well as the mean absolute error (in degree). To differentiate the potential contribution of the HSE from the actual stimulus location, we incorporated the proximal sound level to the subject’s responses in a normalized multiple linear regression analysis:
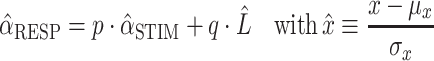 |
2 |
in which variables α RESP, α STIM, and L were transformed into their (dimensionless) z-scores, by subtracting their mean and dividing them by their standard deviation. L is the proximal loudness level at the hearing ear. We determined L by correcting the free-field presentation levels of the stimuli with the frequency- and azimuth-dependent attenuation of the head, owing to the HSE (Van Wanrooij and Van Opstal 2004, for details). Although for 500 Hz the HSE is quite modest, for 3 kHz the effect is appreciable (between −5 and +5 dB over the entire azimuth range). Regression parameter p is the partial correlation coefficient for azimuth, and q the proximal sound level coefficient, and each determines to what extent sound source azimuth and/or proximal sound level explains the observed responses.
Results
Sound localization in azimuth
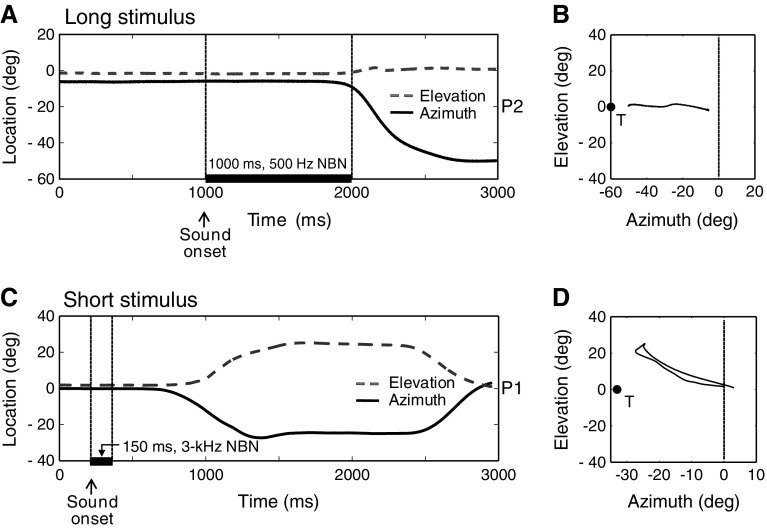
Figure 1 shows two typical single trials of head saccades and the corresponding spatial trajectories of a localization response to a long 500-Hz narrow band noise stimulus of 70 dB SPL (A, B), and a short 3-kHz narrow band noise stimulus of 55 dB SPL (C, D). Both stimuli were presented in the aided (BCD-on) condition. The lines in Figure 1A and C show both the horizontal (bold line) and vertical (gray dashed line) head position traces as a function of time, relative to trial onset. Although we also recorded vertical head movement components, they were not further analyzed, as stimulus locations did not vary in elevation. The stimulus (indicated by the bold black line) was presented in the horizontal plane at α STIM = −60° for patient P2 (which was at the patient’s hearing side) and at α STIM = −33° for patient P1 (which was at the patient’s BCD side). Note that the depicted localization response for the long stimulus (Fig. 1A) started just before stimulus offset. Although subjects were instructed to start the localization response after stimulus offset, many localization responses to these long duration stimuli started during stimulus presentation. All localization responses to short duration stimuli started well after stimulus offset, as illustrated by the representative example in Figure 1C.
FIG. 1.
Head movement localization responses to narrow band noises (NBN) in the azimuth and elevation directions. A Typical example of a head saccade to a long (1,000 ms) stimulus (500 Hz, 70 dB SPL at location Azi = −60°, Ele = 0°) from patient P2. Azimuth (black line) and elevation (gray line) are plotted as a function of time. The stimulus event (1,000 ms) is indicated by the bold black line. B Two-dimensional trajectory of the same head movement. C Example of a head saccade to a short (150 ms) stimulus (3 kHz, 55 dB SPL at location Azi = −33°, Ele = 0°) from patient P1. D Two-dimensional trajectory.
Benefit of the BCD
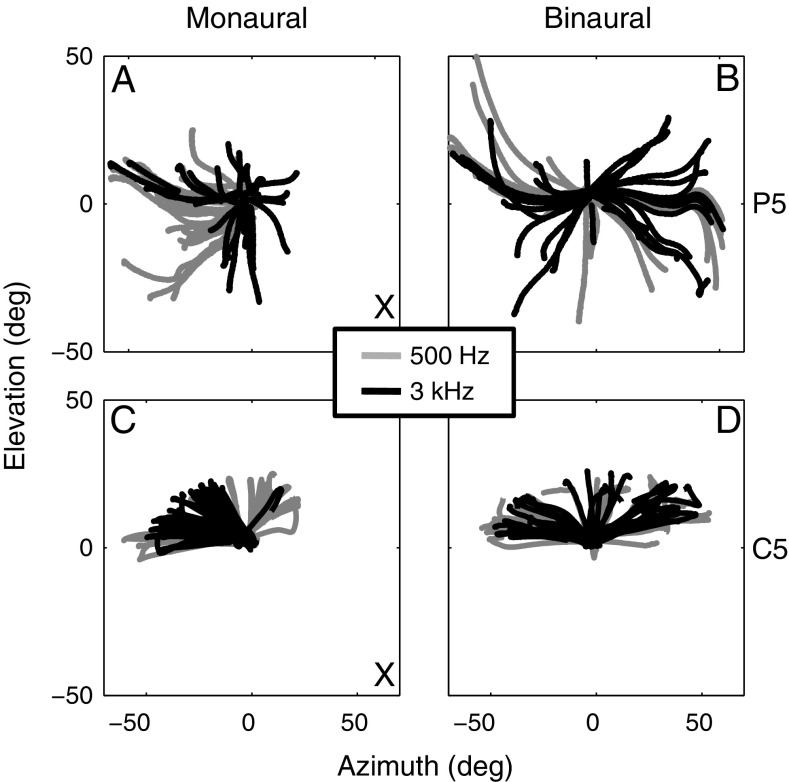
Panels A and B of Figure 2 show the spatial trajectories obtained for the 500-Hz (gray) and 3-kHz (black) frequencies (long stimuli) of patient P5, listening only with his normal left ear (A), or with the BCD on (B). Figure 2C plots the trajectories of a control listener (C5) with a plug in the right ear and under normal binaural listening in Figure 2D. The cross in the subfigures indicates the hearing-impaired side (Fig. 2A) and plugged side (Fig. 2C). These plots demonstrate that listeners made head movements in both the horizontal and vertical direction although all stimuli had an elevation angle of 0°. This obvious error in the elevation direction is typical for narrow band (and pure tone) sound localization and has been reported to depend in an idiosyncratic way on stimulus frequency (Middlebrooks 1992; Goossens and Van Opstal 1999). The plots illustrate that in a monaural listening condition (Fig. 2A, C) most head saccades were strongly biased towards the hearing (left) side. Such a pronounced lateral bias is often observed in ear-plugged control listeners (Slattery and Middlebrooks 1994; Van Wanrooij and Van Opstal 2007). Figure 2C shows that for the control listener the responses to 3-kHz stimuli shifted more towards the hearing side than the responses to the 500-Hz stimuli. In the binaural listening mode (Fig. 2B, D), responses were more symmetric for both listeners. This suggests that the patient may have learned to use the BCD for localizing sounds in the horizontal plane.
FIG. 2.
Spatial trajectories of patient P5, listening with his intact left ear only (A) or with the BCD on (B), and of control listener C5, listening with the right ear plugged (C) or with both ears (D). Note that for both listeners monaural listening (A and C) shifts responses towards the hearing side, while BCD and binaural listening yields more symmetric response distributions in the horizontal plane (B and D). Stimuli (1,000 ms, four per location) were presented at seven different azimuth positions ranging from −90° (far left) to +90° (far right) at 30° intervals. X indicates the hearing-impaired (A) and plugged (C) sides.
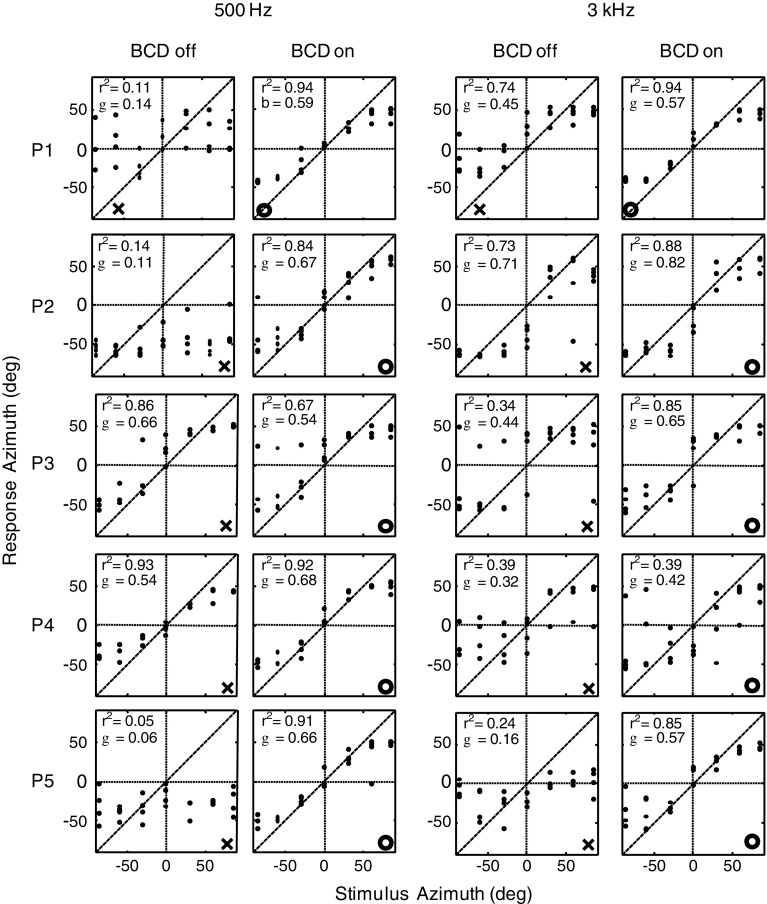
Figure 3 shows the stimulus–response relations and the fitted linear regression lines (Eq. 1) on the azimuth response components of patients P1–P5 to 1,000 ms stimuli (pooled for the different presentation levels). In each subfigure, the impaired side is indicated with a cross (BCD off) or with a circle (BCD on). Three patients (P1, P2, and P5) perceived the 500-Hz stimuli mainly at the hearing side for the unaided condition and demonstrated low coefficients of determination (r 2 < 0.15) (cf. Fig. 2A). These patients had an obvious benefit from the BCD. The r 2 values and the response gains (g in Eq. 1) for these patients were higher in the aided condition than in the unaided condition for both 500-Hz and 3-kHz stimuli. The other two patients (P3 and P4) demonstrated good localization performance of 500-Hz stimuli in the unaided condition (r 2 > 0.86), and their localization ability of these stimuli did not further improve with the BCD turned on. Patients P1 and P2 yielded good unaided localization of the 3-kHz stimuli (r 2 > 0.73). Localization of the 3-kHz stimuli improved with the BCD on in all subjects, except patient P4. Interestingly, most patients did not demonstrate an appreciable localization bias toward the hearing side for the unaided, nor for the aided condition.
FIG. 3.
Sound localization responses for patients P1–P5. Responses are plotted for the 500-Hz and 3-kHz stimuli in the unaided (BCD-off) and aided (BCD-on) conditions. Note that two patients (P3 and P4) did not benefit from the BCD system when they had to localize 500-Hz stimuli, as they had already fairly good localization of 500-Hz stimuli in the unaided condition. Two other patients (P1 and P2) demonstrated a surprisingly good unaided localization of 3-kHz stimuli. r 2 = coefficient of determination, b = response gain. X indicates the hearing-impaired side. Empty circle indicates the side of the BCD.
Figure 4 shows, for comparison, the results of linear regression for a typical control listener (C2; responses pooled across sound presentation levels). Panels A and C of Figure 4 plot the responses for the plugged condition (plugged side indicated with a cross), whereas panels B and D of Figure 4 show the responses for the normal binaural hearing condition. These data show that the acutely plugged control listener was able, to some extent, to still localize the azimuth location of 500-Hz sounds. This was a consistent finding for all control listeners. The reason for this reasonable localization behavior is that the attenuation of the plug for low frequencies (~30 dB) was considerably less than for high frequencies (~50 dB). Furthermore, for 500-Hz stimuli, normal hearing listeners rely on ITDs, rather than on ILDs. Thus, although the plug and muff attenuated the sound levels, interaural time differences were much less affected and apparently still adequately processed. Thus, the auditory system seems to largely ignore inconsistent level differences for low-frequency sounds.
FIG. 4.
Sound localization responses for a representative control listener (C2) with the left ear plugged. A, C Responses toward 500 Hz were less affected by the earplug than responses towards the 3-kHz noise, as indicated by the higher r 2 value. All 3-kHz noises (C) were perceived at the hearing side (large positive bias +49° vs. +14° for 500 Hz). B, D Binaural hearing performance is good for both stimulus types (r 2 > 0.9, b small). Data pooled across presentation levels. The dashed gray line denotes the linear regression fit. r 2 coefficient of determination, g response gain, b bias, X indicates the plugged side.
For the high-frequency stimuli, however, the plugged responses (see Fig. 4) were significantly worse (r 2 = 0.03) than for low-frequency stimuli (r 2 = 0.65). Listeners perceived the stimulus always at the hearing (unplugged) side, which resulted in a strong rightward bias (b = +49°). The mean bias for the five control listeners was 44.6° contralateral to the plug. This result is consistent with a previous study of Van Wanrooij and Van Opstal (2007), who tested plugged listeners to broadband-filtered noises. In conclusion, acutely plugged control listeners were unable to localize the azimuth of high-frequency sounds because of the severely altered binaural level difference cues, while the plug had a modest effect on the localization of low-frequency stimuli. In the normal hearing condition, the localization behavior for both the 500-Hz (Fig. 4B) and 3-kHz (Fig. 4D) stimuli was accurate (high gain and high r 2).
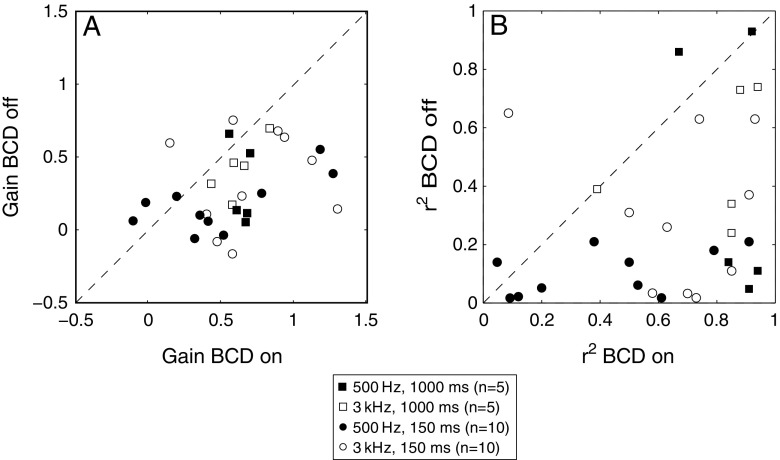
Figure 5 compares the response gain (Fig. 5A, g in Eq. 1) and r 2 values (Fig. 5B) of the unaided condition (BCD off) against the aided condition (BCD on) for responses to 500 Hz (black symbols) and 3 kHz (open symbols) for all 12 patients (P1–P12) for long (1,000 ms, squares, P1–P5) and short (150 ms, circles, P1, P3, P5–P12) stimuli (pooled for sound levels). Analysis of the data set demonstrates that the response gains (Fig. 5A) increased significantly for the binaural hearing (BCD-on) condition for responses to 500-Hz (binomial test; p < 0.05) and for the 3-kHz stimuli (binomial test, p < 0.01). This means that, on average, the sensitivity of the auditory system to changes in stimulus location, and hence binaural hearing, increased with the BCD on. The r 2 values were also significantly higher for the BCD-on condition than for the BCD-off condition for the two stimulus types (Fig. 5B; p < 0.01 for both stimuli), which indicates a decrease of response variability. These results therefore demonstrate that, on average, localization improved when the BCD was turned on, as the majority of data points fall below the main diagonal of no improvement. For a small group of patients (4/12), however, this improvement was small and insignificant, as their data scattered around the diagonal for either stimulus. In the “Discussion”, we will elaborate on potentially underlying causes for this result.
FIG. 5.
Comparison of the response gain (A) and r 2 values (B) of the unaided condition (BCD off) against the aided condition (BCD on). Filled square indicates response gains and r 2 for responses to long 500-Hz narrow band noise stimuli. Empty square indicates response gains and r 2 for the response to long 3-kHz narrow band noise stimuli. Filled circle indicates response gains and r 2 for responses to short 500-Hz narrow band noise stimuli. Empty circle indicates response gains and r 2 for the response to short 3-kHz narrow band noise stimuli.
Head-shadow effect
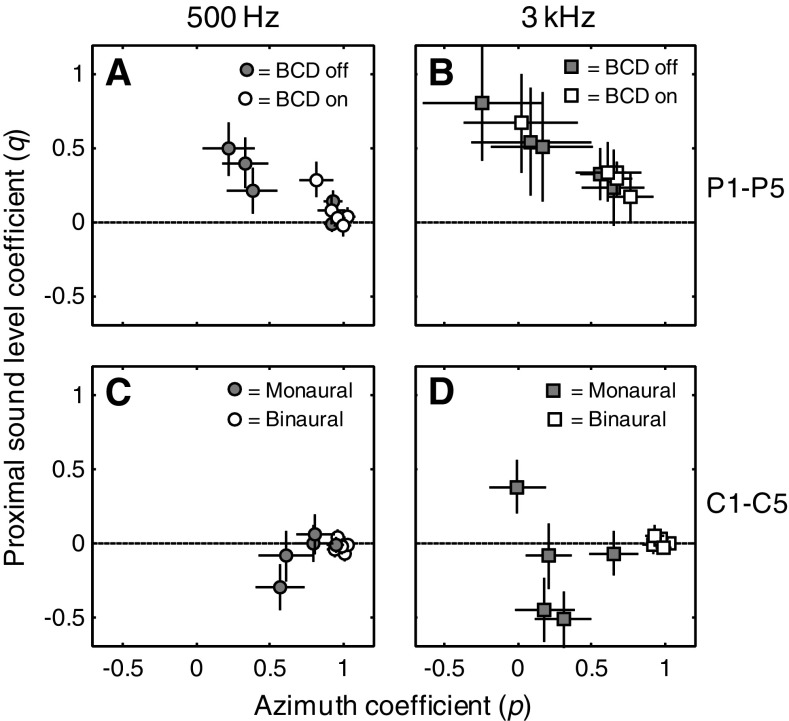
To assess the relative contributions of target azimuth and proximal sound level to the azimuth localization responses of patients P1–P5 and the control listeners (who were all exposed to the randomly interleaved sound presentation levels; see “Methods”), we also performed multiple linear regression on the data (Eq. 2). Figure 6 shows the partial correlation coefficients for azimuth (abscissa) and proximal sound level (ordinate) for the 500-Hz (left) and 3-kHz (right) stimuli, for patients (top) and control listeners (bottom). Filled symbols correspond to monaural hearing and open symbols to aided/binaural hearing.
FIG. 6.
Multiple linear regression analysis of monaural and binaural azimuth localization performance for 500-Hz (circles, left) and 3-kHz (squares, right) noises of patients P1–P5 and control listeners. The coefficients for proximal sound level (q in Eq. 2) and azimuth (p in Eq. 2) are plotted against one another for each patient (A and B) and control listener (C and D). For clarity, data points with a proximal sound level coefficient of zero are slightly shifted. Note that most patients have an azimuth coefficient close to one in the aided condition, except patient P4 who demonstrates a high sound level coefficient for 3-kHz stimuli.
The figure immediately points to clear behavioral differences for patients vs. acute controls in monaural listening and to differences for the low-frequency vs. high-frequency stimuli. First, binaural responses of the control listeners were near ideal, as azimuth coefficients (p in Eq. 2) were close to one, and proximal sound level coefficients (q in Eq. 2) were close to zero for either stimulus type (open symbols in panels C and D of Figure 6). Although in the plugged hearing condition the azimuth coefficients declined, the effect of stimulus presentation level was not systematic (filled symbols). Thus, the group of control listeners did not rely in a systematic way on the HSE. Furthermore, the control listeners demonstrated a smaller shift of the azimuth coefficient (away from the ideal value of one) for 500-Hz stimuli than for 3-kHz stimuli. This effect might be due to the fact that the plug/earmuff attenuated the 500-Hz stimuli less effectively than the 3-kHz stimuli.
In contrast, the proximal sound level coefficients for the patient group were always positive, indicating that their responses were partly due to the use of the HSE. In other words, louder sounds tended to be perceived in the direction of the hearing side, regardless of their actual location. Interestingly, this effect was strongest for the high-frequency sounds, for which the HSE is also much larger. Importantly, however, use of the HSE immediately diminished in most patients when the BCD was turned on (open symbols). In this binaural listening condition, the azimuth coefficient was closer to the ideal value of one, and the proximal sound level coefficient decreased. This finding further supports our observation of Figure 5 that the BCD was beneficial for localization of both 500-Hz (circles) and 3-kHz (squares) noises. Note that use of the HSE was not beneficial for localization because stimulus presentation levels were randomly interleaved. Therefore, the localization errors in the monaural hearing condition did not decrease as a result of using the HSE (see also Van Wanrooij and Van Opstal 2004, their Fig. 3).
Discussion
Improved sound localization with the BCD
The present data demonstrate that most patients with acquired UCHL fitted with a bone conduction device (BCD) on their impaired side localized sounds better when their BCD was turned on (i.e., in the aided condition) than when their BCD was off (unaided condition; Figs. 3 and 5). In particular, patients with poor unaided scores (e.g., P2 and P5) demonstrated a clear improvement in their sound localization performance with the BCD on (Figs. 3 and 5). This is an important finding since objective data about the improvement of sound localization after fitting a BCD in unilateral hearing-impaired listeners are still scarce (Snik et al. 2002; Hol et al. 2005; Wazen et al. 2005; Priwin et al. 2007a, b).
So far, most studies that reported improved measures of sound localization with a BCD used auditory stimuli of a fixed sound level (Snik et al. 2002; Hol et al. 2005; Priwin et al. 2007a). In this situation, participants could have benefitted from the HSE as a monaural localization cue. Our data demonstrate that BCD users with UCHL were able to localize sounds on the basis of restored binaural hearing (i.e., use of ILDs and ITDs; Figs. 5 and 6).
Note that in daily life sound source levels are unknown and may vary widely, making the HSE an ambiguous localization cue and therefore not useful (Van Wanrooij and Van Opstal 2004). Nevertheless, the data in Figure 6 indicate that the unilateral deaf do rely on the HSE in their localization responses when the BCD is turned off. Apparently, patients have learned that under certain listening conditions the HSE may be beneficial for localization, e.g., in familiar acoustic environments. That the HSE might serve as a valid azimuth cue when stimuli have a fixed sound level has been supported by the observation that unilateral deaf listeners rapidly improve azimuth localization performance when being explicitly told that the stimulus level is fixed and when provided with additional visual feedback during the training phase of the localization task (Van Wanrooij and Van Opstal 2004).
The multiple linear regression results of Figure 6A and B clearly indicate that, especially for the high-frequency stimulus, most UCHL patients yielded considerable proximal sound level coefficients under unaided hearing. Because the sound levels were roved, this resulted in poor localization performance (low azimuth coefficients, large localization errors; Figs. 3 and 6). Interestingly, with the BCD turned on, these patients had learned to use the restored binaural difference cues to better localize across the different presentation levels, as the proximal sound level coefficient dropped significantly, with a concomitant increase in the azimuth coefficient. This rapid switching of cue contributions to localization performance is a clear demonstration of perceptual learning in the auditory system of these patients. In contrast to this result, and in line with the concept of perceptual learning, the acutely plugged control listeners did not employ the HSE (Van Wanrooij and Van Opstal 2007).
Monaural sound localization
In agreement with earlier studies (Wilmington et al. 1994; Snik et al. 2002; Priwin et al. 2007a), some patients had fairly good directional hearing abilities in the unaided (BCD-off) condition for at least one of the test frequencies (Figs. 3 and 5). These patients had apparently learned to successfully deal with monaural sound localization conditions. It has been suggested that the patients’ ability to monaurally localize in the horizontal plane is related to spectral shape discrimination (Batteau 1967; Shub et al. 2008). The spectral shape of a sound arises from direction-dependent reflections by the head, pinna, and torso, and subjects are able to localize broadband stimuli monaurally in the vertical plane by using those spectral shape cues. In the present study, however, such spectral effects could not have played a role as we deliberately employed narrow band noises that did not provide sufficient spectral shape information to be used as a monaural localization cue (Blauert 1997; Middlebrooks 1992). For that reason, other factors should be considered to explain the unaided hearing results.
For example, during the 1,000-ms sound presentations, listeners initiated many sound-evoked head saccades already during the stimulus so that in principle head movement-related changes in the acoustic input could have contributed to their monaural directional hearing. However, we demonstrated that patients could also localize the much shorter stimuli of 150-ms duration. These stimuli were always finished well before the start of any head movement (Fig. 1C). We also found that localization of long duration stimuli was not systematically better than of shorter sounds (Fig. 5). Hence, a substantial enhancement of monaural sound localization performance through head movement-related changes in the acoustic cues is not likely.
An alternative explanation for fairly good directional hearing abilities in the unaided condition might be the use of strongly perturbed, but perhaps still present, binaural difference cues. When sound presentation levels (e.g., 70 dB SPL) exceed the patient’s hearing loss (at about 55 dB), the stimulus would still acoustically activate the impaired ear, and patients might have learned to use this remnant binaural information to localize loud sounds. To study this potential mechanism, patients should be tested under a variety of sound levels, using broadband, rather than narrow band sounds, to allow them the use of all potential acoustic cues across the auditory spectrum. This topic will be further explored in a follow-up study.
Factors that might negatively influence directional hearing with a BCD
A factor that might influence the benefit of a BCD for directional hearing is the decrease of acquired monaural hearing skills. It has been reported that these skills could deteriorate after the introduction of potent binaural cues provided by the BCD. As an example, Nava et al. (2009) reported that recovery of binaural spatial hearing in bilateral cochlear implant recipients resulted in a loss of their monaural localization abilities. In the present paper, we have shown that patients with poor unaided scores demonstrated an obvious improvement of sound localization with their BCD on (Figs. 3, 5, and 6). On the other hand, all patients still relied on the use of the monaural HSE with the BCD turned off (Fig. 6), even after years of wearing the BCD. An interesting question is whether patients would also have demonstrated poor monaural localization scores before their BCD was implanted, or whether the use of the HSE perhaps decreases over time. We would therefore suggest testing the monaural localization abilities of potential future BCD recipients prior to implantation as a possible indicator for binaural success (Fig. 6).
Another factor that might have negatively influenced directional hearing in BCD listeners is the so-called cross-hearing (Stenfelt 2005), which refers to additional acoustic stimulation of the cochlea contralateral to the BCD side (through bone conduction at the intact ear). Cross-stimulation is possible because of the limited transcranial attenuation of sound vibrations in the skull (Von Békésy 1948). This transcranial attenuation is in the order of 5–15 dB (Stenfelt and Goode 2005) and a BCD stimulates both cochleae (Stenfelt 2005). The propagation velocity of bone conduction is faster than the velocity of sound in air. Consequently, the contralateral cochlea could be stimulated through bone conduction prior to stimulation via the normal pathway. Cross-hearing might therefore particularly affect directional hearing in the horizontal plane, which not only relies on ITDs and ILDs, but which is also known to become more insensitive to sound that arrives after a brief (few milliseconds) delay, e.g., echos (the so-called precedence effect; Litovsky et al. 1999).
To what extent each of these factors contributes to the sound localization abilities of patients fitted with a BCD will require further study.
Acknowledgments
We thank Arjan Bosman, Chris-Jan Beerendonk, and Lars van Bolderen for their technical support. This research was funded by the Dutch Organization for Scientific Research, through a VICI grant within Earth and Life Sciences of NWO (project grant # ALW/VICI 865.05.003; AJVO, MMVW), the Radboud University Nijmegen (AJVO), the Donders Centre for Neuroscience (MJHA), and the Department of Otorhinolaryngology at the Radboud University Medical Centre Nijmegen (AFJS, TEMVE, MKSH, CWRJC).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Batteau DW. The role of the pinna in human localization. Proc R Soc Lond B Biol Sci. 1967;168:158–180. doi: 10.1098/rspb.1967.0058. [DOI] [PubMed] [Google Scholar]
- Blauert J. Spatial hearing. The psychophysics of human sound localization. Cambridge: MIT; 1997. [Google Scholar]
- Colburn HS. Binaural interaction and localization with various hearing impairments. Scand Audiol Suppl. 1982;15:27–45. [PubMed] [Google Scholar]
- Goossens HH, van Opstal AJ. Influence of head position on the spatial representation of acoustic targets. J Neurophysiol. 1999;81:2720–2736. doi: 10.1152/jn.1999.81.6.2720. [DOI] [PubMed] [Google Scholar]
- Hofman PM, Van Opstal AJ. Spectro-temporal factors in two-dimensional human sound localization. J Acoust Soc Am. 1998;103:2634–2648. doi: 10.1121/1.422784. [DOI] [PubMed] [Google Scholar]
- Hol MKS, Snik AFM, Mylanus EA, Cremers CW. Does the bone-anchored hearing aid have a complementary effect on audiological and subjective outcomes in patients with unilateral conductive hearing loss? Audiol Neurootol. 2005;10:159–168. doi: 10.1159/000084026. [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Colburn HS, Yost WA, Guzman SJ. The precedence effect. J Acoust Soc Am. 1999;106:1633–1654. doi: 10.1121/1.427914. [DOI] [PubMed] [Google Scholar]
- Markides A. Binaural hearing aids. London: Academic; 1977. [Google Scholar]
- Middlebrooks JC. Narrow-band sound localization related to external ear acoustics. J Acoust Soc Am. 1992;92:2607–2624. doi: 10.1121/1.404400. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Green DM. Sound localization by human listeners. Annu Rev Psychol. 1991;42:135–159. doi: 10.1146/annurev.ps.42.020191.001031. [DOI] [PubMed] [Google Scholar]
- Nava E, Bottari D, Portioli G, Bonfioli F, Beltrame MA, Formigoni P, Pavani F. Hearing again with two ears: recovery of spatial hearing after bilateral cochlear implantation. Neuropsychologia. 2009;47:928–932. doi: 10.1016/j.neuropsychologia.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Perrett S, Noble W. Available response choices affect localization of sound. Percept Psychophys. 1995;57:150–158. doi: 10.3758/bf03206501. [DOI] [PubMed] [Google Scholar]
- Priwin C, Jonsson R, Hultcrantz M, Granström G. BCD in children and adolescents with unilateral or bilateral conductive hearing loss: a study of outcome. Int J Pediatr Otorhinolaryngol. 2007;71:135–145. doi: 10.1016/j.ijporl.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Priwin C, Jonsson R, Magnusson L, Hultcrantz M, Granström G. Audiological evaluation and self-assessed hearing problems in subjects with single-sided congenital external ear malformations and associated conductive hearing loss. Int J Audiol. 2007;46:162–171. doi: 10.1080/14992020601077984. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movements using a sclera search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Shub DE, Carr SP, Kong Y, Colburn HS. Discrimination and identification of azimuth using spectral shape. J Acoust Soc Am. 2008;124:3132–3141. doi: 10.1121/1.2981634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery WH, III, Middlebrooks JC. Monaural sound localization: acute versus chronic unilateral impairment. Hear Res. 1994;75:38–46. doi: 10.1016/0378-5955(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Snik AFM, Mylanus EAM, Cremers CW. The bone-anchored hearing aid in patients with a unilateral air-bone gap. Otol Neurotol. 2002;23:61–66. doi: 10.1097/00129492-200201000-00015. [DOI] [PubMed] [Google Scholar]
- Snik AF, Mylanus EA, Proops DW, Wolfaardt JF, Hodgetts WE, Somers T, Niparko JK, Wazen JJ, Sterkers O, Cremers CW, Tjellström A. Consensus statements on the BCD system: where do we stand at present? Ann Otol Rhinol Laryngol Suppl. 2005;195:2–12. doi: 10.1177/0003489405114s1201. [DOI] [PubMed] [Google Scholar]
- Stenfelt S. Bilateral fitting of BCDs and BCD fitted in unilateral deaf persons. Acoustical aspects. Int J Audiol. 2005;44:178–189. doi: 10.1080/14992020500031561. [DOI] [PubMed] [Google Scholar]
- Stenfelt S, Goode RL. Transmission properties of bone conducted sound: measurements in cadaver heads. J Acoust Soc Am. 2005;118:2373–2391. doi: 10.1121/1.2005847. [DOI] [PubMed] [Google Scholar]
- Van Wanrooij MM, Van Opstal AJ. Contribution of head shadow and pinna cues to chronic monaural sound localization. J Neurosci. 2004;24:4163–4171. doi: 10.1523/JNEUROSCI.0048-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wanrooij MM, Van Opstal AJ. Sound localization under perturbed binaural hearing. J Neurophysiol. 2007;97:715–726. doi: 10.1152/jn.00260.2006. [DOI] [PubMed] [Google Scholar]
- Vliegen J, Van Grootel TJ, Van Opstal AJ. Dynamic sound localization during rapid eye-head gaze shifts. J Neurosci. 2007;24:9291–9302. doi: 10.1523/JNEUROSCI.2671-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Békésy G. Vibration of the head in a sound field and its role in hearing by bone conduction. J Acoust Soc Am. 1948;20:749–760. doi: 10.1121/1.1906433. [DOI] [Google Scholar]
- Wallach H. The role of head movements and vestibular and visual cues in sound localization. J Exp Psychol. 1940;27:339–368. doi: 10.1037/h0054629. [DOI] [Google Scholar]
- Wazen JJ, Ghossaini SN, Spitzer JB, Kuller M. Localization by unilateral BCD users. Otolaryngol Head Neck Surg. 2005;132:928–932. doi: 10.1016/j.otohns.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Wilmington D, Gray L, Jahrsdoerfer R. Binaural processing after corrected congenital unilateral conductive hearing loss. Hear Res. 1994;74:99–114. doi: 10.1016/0378-5955(94)90179-1. [DOI] [PubMed] [Google Scholar]