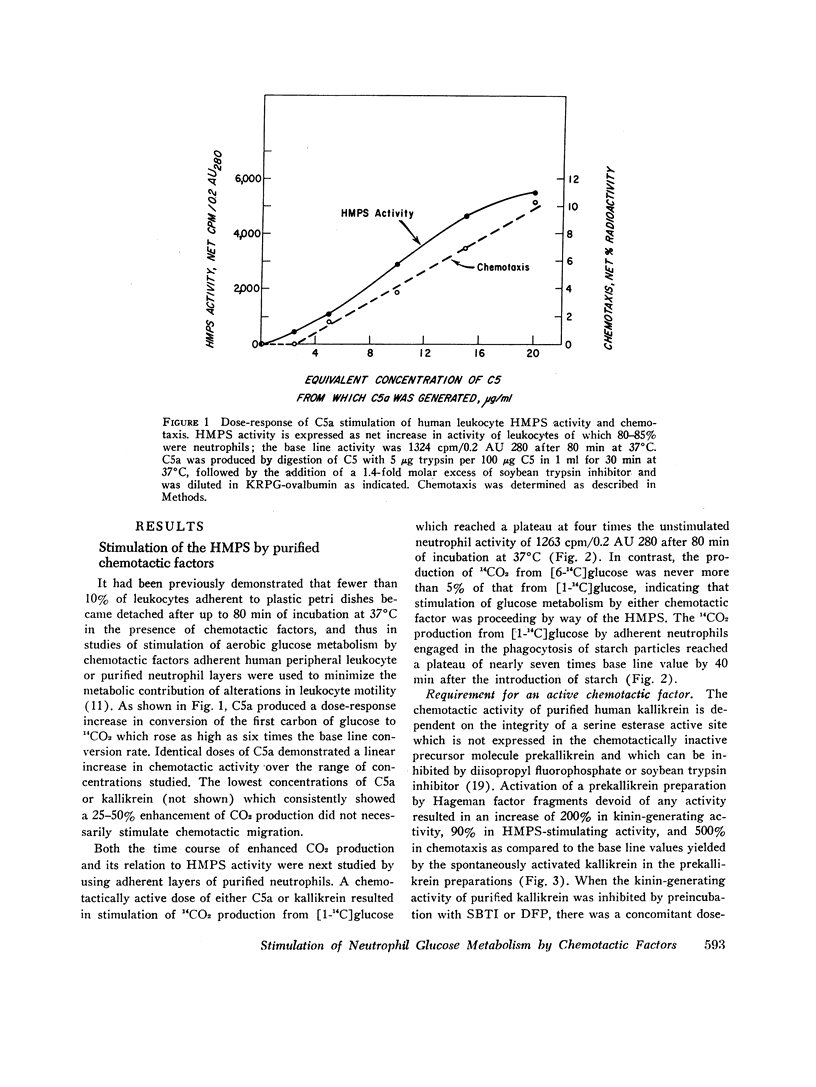
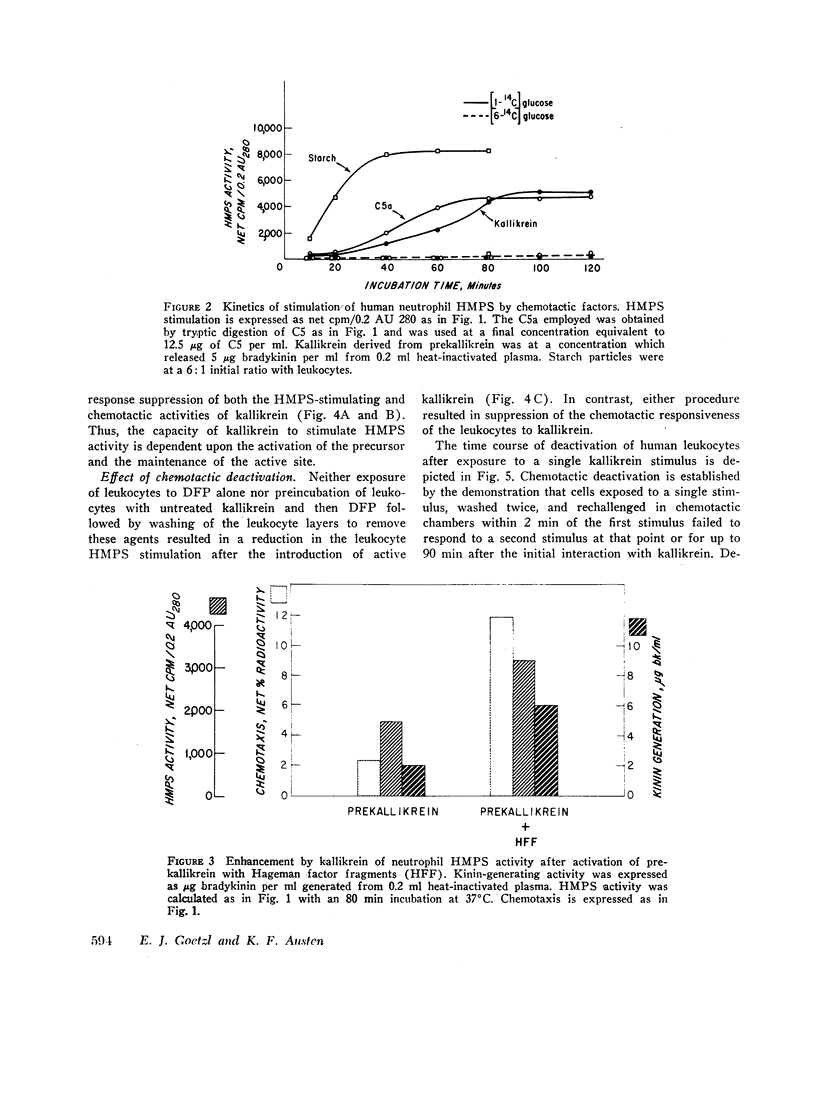
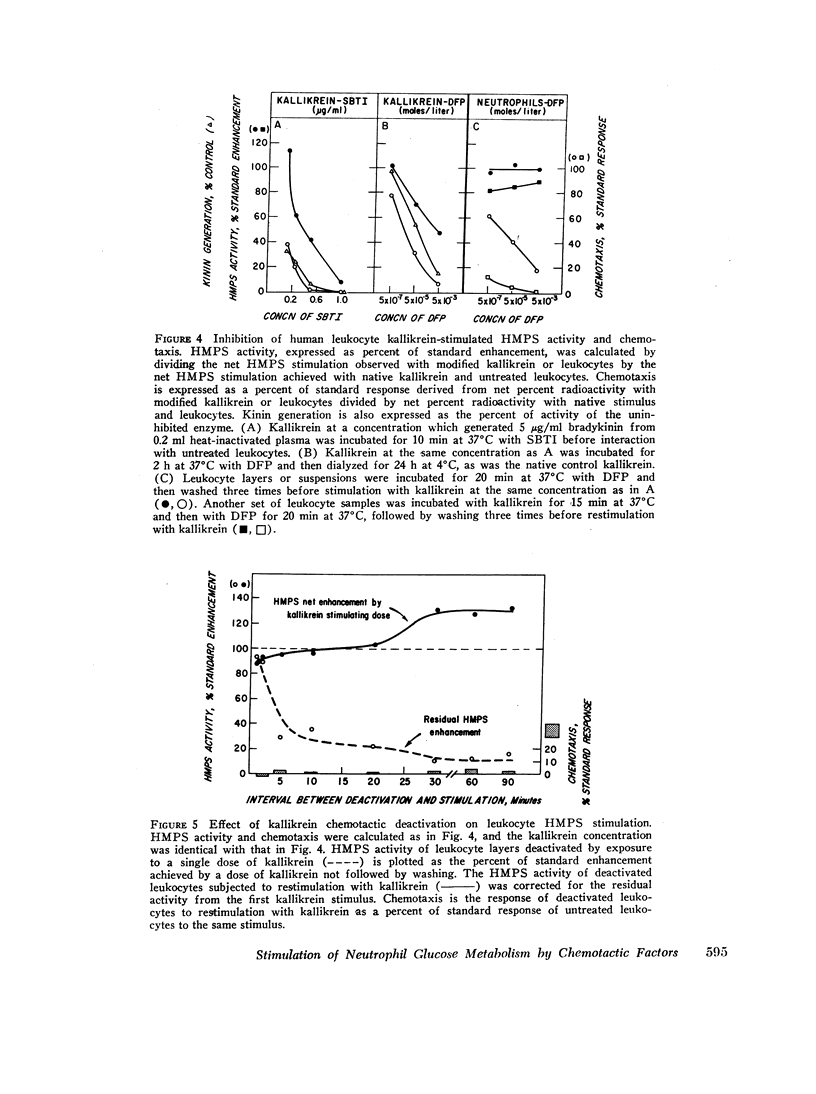
Abstract
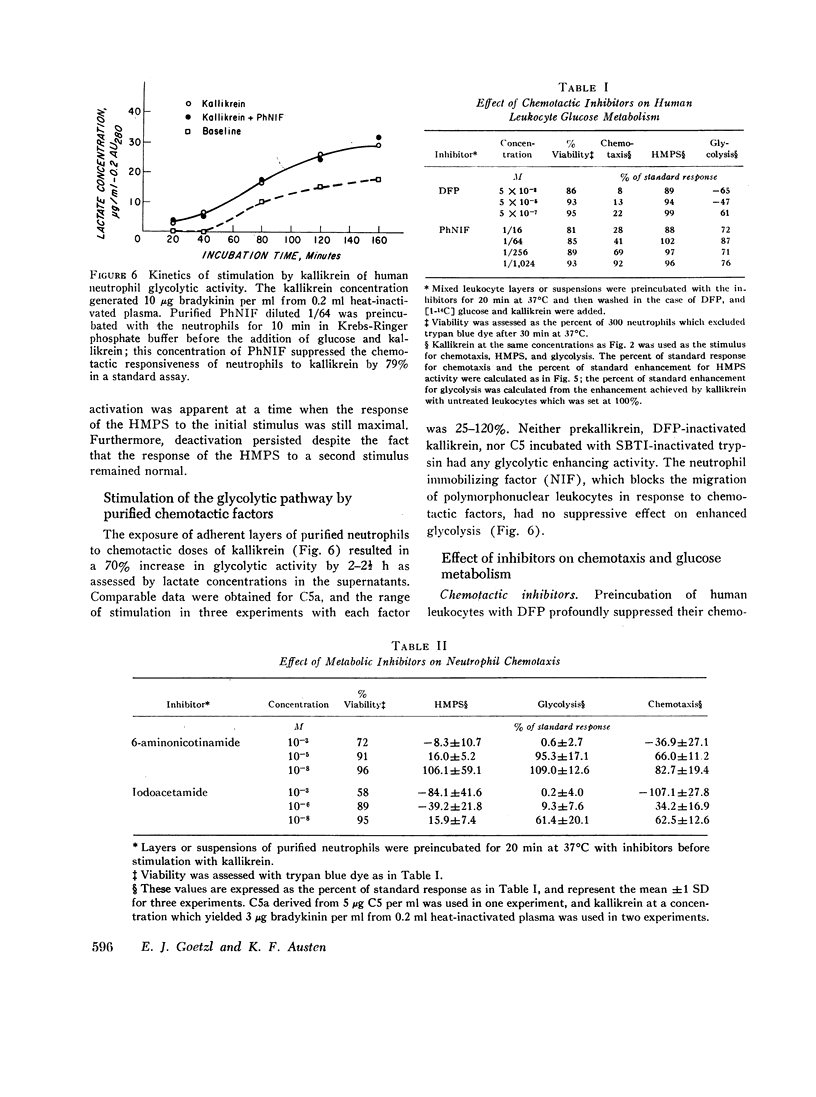
The interaction of human neutrophils adherent to plastic petri dishes with the purified chemotactic factors C5a and kallikrein increased their rate of aerobic glycolysis 25-120% and the activity of their hexose monophosphate shunt (HMPS) 100-600%, reaching a plateau after 2 hr at 37 degrees C. The stimulation of either pathway required a chemotactically active stimulus since neither C5 nor prekallikrein or inactivated kallikrein could enhance metabolic activity. Marked suppression of the neutrophil chemotactic response by preincubation with a chemotactic factor to achieve deactivation, 5 x 10(-7) M diisopropyl fluorophosphate, or the neutrophil immobilizing factor (NIF) did not prevent the stimulation of HMPS activity or glycolysis by chemotactic factors. The metabolic inhibitors iodoacetate and 6-aminonicotinamide at concentrations which blocked enhancement of glycolysis or HMPS activity, respectively, partially suppressed the chemotactic response of neutrophils to the chemotactic factors. The capacity of a chemotactic factor to stimulate glucose metabolism of human neutrophils is associated with a maximal chemotactic response, but this stimulation is not alone sufficient for chemotaxis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammon H. P., Steinke J. Effect of 6-aminonicotinamide on insulin release and C-14 glucose oxidation by isolated pancreatic rat islets: difference between glucose, tolbutamide and aminophylline. Endocrinology. 1972 Jul;91(1):33–38. doi: 10.1210/endo-91-1-33. [DOI] [PubMed] [Google Scholar]
- BOYDEN S. The chemotactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J Exp Med. 1962 Mar 1;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehner R. L., Gilman N., Karnovsky M. L. Respiration and glucose oxidation in human and guinea pig leukocytes: comparative studies. J Clin Invest. 1970 Apr;49(4):692–700. doi: 10.1172/JCI106281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Davis A. T., Estensen R. D., Quie P. G. Cytochalasin B. IV. Inhibition and stimulation of chemotaxis of rabbit and human polymorphonuclear leukocytes. J Immunol. 1972 Feb;108(2):396–402. [PubMed] [Google Scholar]
- Becker E. L. The relationship of the chemotactic behavior of the complement-derived factors, C3a, C5a, and C567, and a bacterial chemotactic factor to their ability to activate the proesterase 1 of rabbit polymorphonuclear leukocytes. J Exp Med. 1972 Feb 1;135(2):376–387. doi: 10.1084/jem.135.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, dextran, and ficoll as erythrocyteaggregating agents. Scand J Clin Lab Invest Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- Carruthers B. M. Leukocyte motility. I. Method of study, normal variation, effect of physical alterations in environment, and effect of iodoacetate. Can J Physiol Pharmacol. 1966 May;44(3):475–485. doi: 10.1139/y66-056. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. A method for assessing the in vitro chemotactic response of neutrophils utilizing 51cr-labeled human leukocytes. Immunol Commun. 1972;1(5):421–430. doi: 10.3109/08820137209022954. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Austen K. F. A neutrophil-immobilizing factor derived from human leukocytes. I. Generation and partial characterization. J Exp Med. 1972 Dec 1;136(6):1564–1580. doi: 10.1084/jem.136.6.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Gigli I., Wasserman S., Austen K. F. A neutrophil immobilizing factor derived from human leukocytes. II. Specificity of action on polymorphonuclear leukocyte mobility. J Immunol. 1973 Sep;111(3):938–945. [PubMed] [Google Scholar]
- Goldfinger S. E., Howell R. R., Seegmiller J. E. Suppression of metabolic accompaniments of phagocytosis by colchicine. Arthritis Rheum. 1965 Dec;8(6):1112–1122. doi: 10.1002/art.1780080610. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Enhancement of immunologically induced granule exocytosis from neutrophils by cytochalasin B. J Immunol. 1973 Jan;110(1):290–293. [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- Kaplan A. P., Austen K. F. A pre-albumin activator of prekallikrein. J Immunol. 1970 Oct;105(4):802–811. [PubMed] [Google Scholar]
- Kaplan A. P., Kay A. B., Austen K. F. A prealbumin activator of prekallikrein. 3. Appearance of chemotactic activity for human neutrophils by the conversion of human prekallikrein to kallikrein. J Exp Med. 1972 Jan;135(1):81–97. doi: 10.1084/jem.135.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbach E. P., Weil M. H. Rapid enzymatic measurement of blood lactate and pyruvate. Use and significance of metaphosphoric acid as a common precipitant. Clin Chem. 1967 Apr;13(4):314–325. [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACKER J. T., GREENDYKE R. M., SWISHER S. N. The inhibition of erythrophagocytosis in vitro by corticosteroids. Trans Assoc Am Physicians. 1960;73:93–102. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- Simberkoff M. S., Elsbach P. The interaction in vitro between polymorphonuclear leukocytes and mycoplasma. J Exp Med. 1971 Dec 1;134(6):1417–1430. doi: 10.1084/jem.134.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Hartwig J., Vaughan M. Quantitative studies of phagocytosis by polymorphonuclear leukocytes: use of emulsions to measure the initial rate of phagocytosis. J Clin Invest. 1972 Mar;51(3):615–624. doi: 10.1172/JCI106851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD H. G., KATZ J., LANDAU B. R. ESTIMATION OF PATHWAYS OF CARBOHYDRATE METABOLISM. Biochem Z. 1963;338:809–847. [PubMed] [Google Scholar]
- Ward P. A., Becker E. L. The deactivation of rabbit neutrophils by chemotactic factor and the nature of the activatable esterase. J Exp Med. 1968 Apr 1;127(4):693–709. doi: 10.1084/jem.127.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Newman L. J. A neutrophil chemotactic factor from human C'5. J Immunol. 1969 Jan;102(1):93–99. [PubMed] [Google Scholar]
- Ward P. A. The chemosuppression of chemotaxis. J Exp Med. 1966 Aug 1;124(2):209–226. doi: 10.1084/jem.124.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]