Abstract
Voltage dependent calcium channels (VDCC) participate in regulation of neuronal Ca2+. The Rolling mouse Nagoya (Cacna1atg-rol) is a spontaneous P/Q type VDCC mutant, which has been suggested as an animal model for some human neurological diseases such as autosomal dominant cerebellar ataxia (SCA6), familial hemiplegic migraine and episodic ataxia type-2. Morphology of Purkinje cell (PC) dendritic spine is suggested to be regulated by signal molecules such as Ca2+ and by interactions with afferent inputs. The amplitude of excitatory postsynaptic current was decreased in parallel fiber (PF) to PC synapses, whereas apparently increased in climbing fiber (CF) to PC synapses in rolling mice Nagoya. We have studied synaptic morphology changes in cerebella of this mutant strain. We previously found altered synapses between PF varicosity and PC dendritic spines. To study dendritic spine plasticity of PC in the condition of insufficient P/Q type VDCC function, we used high voltage electron microscopy (HVEM). We measured the density and length of PC dendritic spines at tertiary braches. We observed statistically a significant decrease in spine density as well as shorter spine length in rolling mice compared to wild type mice at tertiary dendritic braches. In proximal PC dendrites, however, there were more numerous dendritic spines in rolling mice Nagoya. The differential regulation of rolling PC spines at tertiary and proximal dendrites in rolling mice Nagoya suggests that two major excitatory afferent systems may be regulated reciprocally in the cerebellum of rolling mouse Nagoya.
Keywords: Ataxia, Dendritic spine, High voltage electron microscope, Purkinje cell, Voltage dependent calcium channel
Introduction
Ca2+ is involved in various neuronal functions, including excitability, neurotransmitter release, gene expression, neuronal proliferation and maturation (Llinás 1988; Tsien & Tsien, 1990; Wheeler et al., 1994; Ghosh & Greenberg, 1995). Neuronal Ca2+ is regulated by calcium-permeable channels and calcium binding proteins. Voltage dependent calcium channels (VDCC) are one of the strong regulators of neuronal Ca2+. VDCCs are composed of a pore-forming and voltage-sensitive α1 subunit and several auxiliary subunits, including α2, δ, β and γ subunits (Pietrobon 2002). Although L, N, P/Q, R, and T type calcium channels were named according to their pharmacological and electrophysiological characteristics of the α1 subunits, new nomenclature has been recently proposed based on homology of the genes and order of discovery (Ertel et al., 2000).
The P/Q type (α1A Cav 2.1) calcium channels are diffusely expressed in the brain, especially strongly in Purkinje cells and granule cells of the cerebellum (Westenbroek et al., 1995). Mutations of the P/Q type calcium channel have been linked to several human neurological disorders such as spinocerebellar ataxia type-6 (SCA-6), familiar hemiplegic migraine (FHM), and episodic ataxia type-2 (EA-2) (Ophoff et al., 1996; Zhuchenko et al., 1997). Rolling mouse Nagoya (RMN: Cacna1atg-rol) is a spontaneous ataxic mutant mouse (Oda 1973), which carries a point mutation at the voltage-sensing S4 segment of the third repeat of α1A subunit (Mori et al., 2000). There are additional VDCC α1A subunit mutants called tottering locus mutants; tottering, leaner and rocker (Zwingman et al., 2001). They show variable symptoms and pathological changes depending on their mutation points (Austin et al., 1992; Isaacs & Abbott, 1995; Rhyu et al., 1999a, 1999c; Cicale et al., 2002; Suh et al., 2002; Rhyu et al., 2003); they thus serve useful biological model for human calcium channelopathies (Rhyu et al., 1999a; Zwingman et al., 2001; Pietrobon 2002).
Recent electrophysiological studies reported a decreased amplitude of evoked postsynaptic current (EPSC) of the parallel fiber-Purkinje cell (PF-PC) synapses but an increased EPSC amplitude of climbing fiber-Purkinje cell (CF-PC) synapses (Matsushita et al., 2002). This implies that two major synapses of Purkinje cells might be differentially regulated by each afferent input. Morphological investigation revealed characteristic synaptic alteration between parallel fiber varicosity (PFV) and Purkinje cell dendritic spines; Synapses with a parallel fiber varicosity contacting more than 3 spines of the Purkinje cell dendrite were frequently observed in rolling mouse Nagoya (Rhyu et al., 1999c).
The morphology of dendritic spines changes in response to various physiological and pathological states. The length and density of dendritic spine are thought to be controlled by intracellular calcium concentration in neurons (Segal et al., 2000) and balance of the two major excitatory inputs (Strata et al., 2000). The dendritic spine shape of Purkinje cells is also regulated by intracellular calcium changes (Vecellio et al., 2000).
Although dendritic spine plasticity would be expected in rolling mouse Nagoya based on previous studies, no detailed analysis of the PC spines has been reported yet. To understand the response of the dendritic spines under the insufficient VDCC function, we investigated PC dendritic spines of Rolling mouse Nagoya using high-voltage electron microscopy (HVEM) following rapid Golgi impregnation.
Materials and Methods
Animal
Mice with either the control genotype PROD: +/+ (wild type) or mutant phenotypes PROD: tgrol/+ (heterozygous) or PROD: tgrol/tgrol (homozygous rolling) were bred at Nagoya University and housed at Korea University at a constant room temperature with a 12 hour light/dark cycle and allowed access to food and water ad libitum. Male 60-day-old wild type (N=7) and rolling (N=7) mice were used in these studies. All experimental procedures were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.
Golgi stain and electron microscopic observation
In the preparation for HVEM, we adopted a rapid Golgi impregnation method of Hama et al. (Hama et al., 1989) with slight modifications (Lee et al., 2004). Briefly, the mice were anesthetized with sodium pentobarbital (50 mg/kg) and transcardially perfused with 4% paraformaldehyde in 0.1 M Millonig's buffer (pH 7.4). Sagittal sections (3~4 mm thick) were dissected from each cerebellum and stored in the same fixative for overnight at 4℃. The sections were washed with 0.1 M cacodylate buffer and immersed in a mixture of 2.25% potassium dichromate and 0.4% osmium tetroxide for 4 days at 20℃ in the dark, and stored in 0.75% silver nitrate solution for 3 days at 20℃ in the dark. The blocks were dehydrated through an ascending series of ethanol, and propylene oxide, and then embedded in Epon Araldite mixture. The sagittal sections (75 µm thick) from embedded samples were made with a sliding microtome and they were re-embedded on glass slides. The adequately stained cells were selected under the standard light microscope, and attached on blank Epon-Araldite blocks. After trimming, 3~4 µm thick sections (Ultratome Ultracut-E, Reichert-Jung) were made, and mounted on 75 mesh double copper grids.
The grids were observed with Hitachi H-1250M HVEM (NIPS, Okazaki, Japan) at the accelerating voltage of 1,000 kV. Purkinje cell dendrites (5 cells/animal) were randomly photographed at a magnification of 2,000. The density and length of the dendrite were measured at tertiary dendritic branches with NIH image after capturing the films (Rhyu et al., 1999b). At first, we set the scale correlated with pixel size and magnification of original film and center line of each dendrite was traced with Segmented Line Tool and measured the length. After that we have measured length of dendritic spines in the measured dendritic segment and counted the number of dendritic spines, too. Student-t test (SPSS 12.0 K for Windows) was used for statistical evaluation for the dendrtic spine density and length between wild type mice and rolling mouse Nagoya.
Some coronal sections of the cerebellum blocks were prepared for brief observation of PF varicosity. In addition to HVEM observation, we also employed conventional transmission electron microscope to reveal fine structures that contact with spines of proximal dendrites of rolling PC. The thin sections were made from the Purkinje cells showing many dendritic spines under a light microscope and a Hitachi H-600 electron microscope.
Results
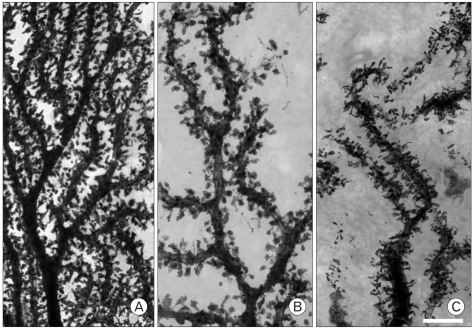
High voltage electron microscope prepared clear images of PC dendritic spines for quantitative analysis (Fig. 1). The emanating pattern of the spines in mutants was irregular; the spines are tangled and intermingled with adjacent spines (Fig. 1C).
Fig. 1.
The dendritic spines at tertiary dendritic tree of a Purkinje cell. Decreased density and length of spines are observed in rolling mouse Nagoya (B) compared to wild type mouse (A). Some spines of rolling dendritic tree projected to irregular direction and intermingled adjacent spines (C) (Scale bar: 5 µm).
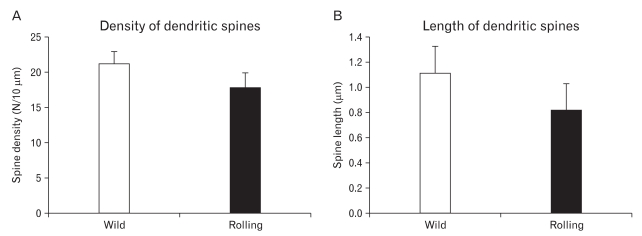
The average spine density at tertiary dendrite branches was decreased in rolling PC compared to wild type (Fig. 1B, C). The average spine density was 17.8±2.2/10 µm in rolling and 21.2±1.8/10 µm in wild type mice (Fig. 2A). The spine length of rolling PC was shorter than that of wild type (Fig. 2B). The average spine length was 0.82±0.21 µm in rolling and 1.12±0.21 µm in wild. Both density and length of the spines were significantly decreased in rolling PC at tertiary dendrite branches (P<0.01, Fig. 2).
Fig. 2.
Quantitative analyses of the dendritic spines with NIH image. The density (A) and length (B) of spines have been decreased significantly in rolling mice compared to wild type (Student t-test, P<0.05).
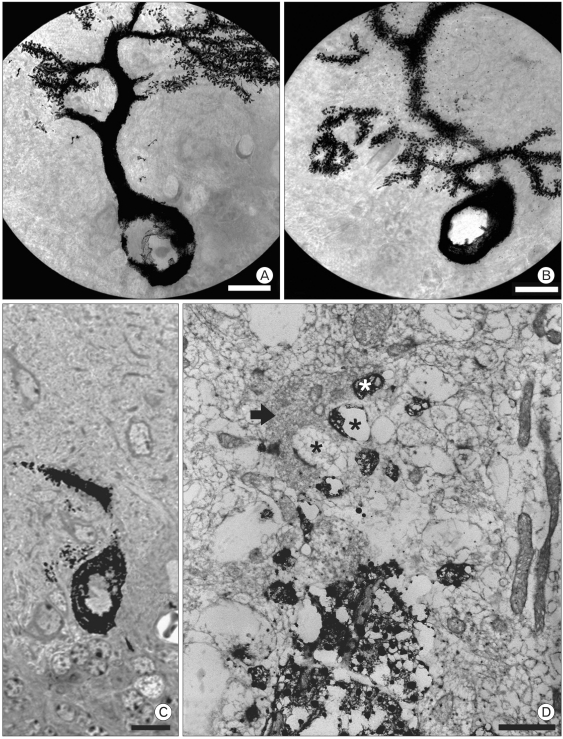
Numerous spines from proximal dendrites were observed in rolling mice, whereas only few were seen in wild type. Many ectopic spines from proximal dendrites of rolling mice Nagoya Purkinje cells were observed under the high voltage electron microscope (Fig. 3B) and light microscope (Fig. 3C). Alternative observation of semithin and thin section of the ectopic dendritic spine region comparable to finding of HVEM revealed that numerous proximal dendritic spines made synapses with climbing fibers (Fig. 3D). There were also synapses between spines from proximal dendrites and PFV (data not shown).
Fig. 3.
Ectopic spines in proximal dendrites of rolling mouse Nagoya. High voltage electron microscope (HVEM) photomicrographs of wild type (A) and rolling (B) Purkinje cells. Numerous spines from proximal dendrtie of rolling mouse Purkinje cells are observed in both HVEM and semithin section (B, C). Spines (black or white stars) from proximal dendrite of rolling Purkinje cell make synapses with climbing fiber (Arrow, D). (Scale bar=10 µm in A, B and C; 1 µm in D).
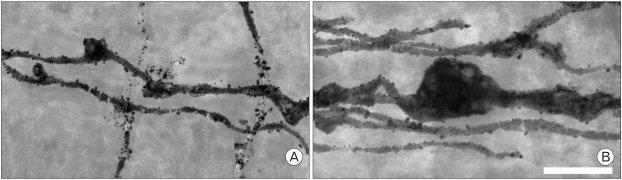
We could also observe silver impregnated PF varicosities very clearly. Many hypertrophied PFVs with multiple pale spots were frequently observed in rolling mouse Nagoya (Fig. 4), which was well matched with previous TEM observation (Rhyu et al., 1999c).
Fig. 4.
Parallel fiber varicosities images taken by HVEM (×8,000). Each of the varicosities was the largest varicosity observed in rolling (B) and wild type mice (A) under the same magnification observation. The varicosity of rolling was large and had multiple pale spots, considered synaptic points (Scale bar: 5 µm).
Discussion
We investigated the morphology of Purkinje cell dendritic spines of rolling mice and wild type with HVEM. The HVEM is one of the effective tools for large scale analysis of dendritic spines. The size of the dendritic spine of PC is between 0.5~2.0 µm (Hama & Kosaka, 1979), which is not easy to analyze at quantitative level with conventional light microscope. The dimension of the spine is too thick to observe in a single thin section with transmission electron microscopy (TEM). Three dimensional reconstruction based serial TEM images provides detailed information of the spines, but it requires extensive work (Harris & Stevens, 1988).
In addition to HVEM observation, we observed rapid Golgi block with conventional TEM. Because HVEM images provide information on spines but not their connections, we performed conventional TEM observation to get information of their connections. Although the latter images are not optimal, due to use of rapid Golgi prepared block for HVEM, still they provide important information on spine connections and cellular context.
We could observe silver impregnated spines and their neighbor structures, although quality of images was not good compared to routinely prepared EM blocks.
One of the important factors modulating spines morphology is intracellular Ca2+. Segal et al., proposed a unifying hypothesis relating intracellular calcium concentration with spine plasticity: moderate increase of Ca2+ would increase size and number of spines; low or high concentration of Ca2+ would lead to shrinkage and repression (Segal et al., 2000). Reduced voltage sensitivity and amplitude of EPSC of PF-PC synapse in rolling mouse Nagoya were reported recently (Mori et al., 2000; Matsushita et al., 2002). Based on these morphometric results and the above-mentioned hypothesis, the concentration of Ca2+ in the tertiary dendritic spines seems decreased in rolling mouse Nagoya (Mori et al., 2000; Segal et al., 2000). In contrast to the decreased density of spine at the tertiary dendrite, numerous ectopic spines from the proximal dendrite of the PC were observed in this mutant. Matsushita et al. (Matsushita et al., 2002) described an increased EPSC amplitude in PC climbing fibers in rolling mouse Nagoya. This suggests some alterations of the postsynaptic structure or of the receptor sensitivity in this mutant. The increased spine number shown here might provide a structural basis of increased EPSC amplitued in PC climbing fiber.
The altered Ca2+ homeostasis in PC caused by P/Q-calcium channel mutations could be compensated by various ways; increased activities of N and L type calcium channels (Jun et al., 1999), modulation of calcium binding proteins, and down regulation of intracellular calcium channels such as ryanodine receptor 1(RyR1) (Cicale et al., 2002).
In addition to intracellular efforts to keep Ca2+ homeostasis at PC, we could expect some contribution through morphological and/or electrophysiological changes of the synapses as a whole neuronal circuit (Rhyu et al., 1999c; Matsushita et al., 2002). The decreased EPSC amplitude of PF-PC might be compensated by increased synaptic contacts observed in this mutant. The numerous spines at proximal PC could be related with increased EPSC amplitude of PF-CF.
Large PFVs with multiple pale spots were frequently observed in rolling mouse Nagoya. This might correspond to characteristic multiple synaptic contacts between PC spines and PFV in rolling mouse Nagoya, if pale spots are considered as synaptic contacts without silver impregnation due to tight synaptic contacts (Palay & Chan-Palay, 1974).
To construct characteristic multiple synaptic contacts observed in rolling mouse Nagoya, two simple hypotheses might explain the observed increase in multiple synaptic contacts: (1) more spines, or (2) fewer spines but enlarged varicosities. Our observations do not support hypothesis (1). We have frequently observed hypertrophied PFV in rolling mouse Nagoya; but proof hypothesis (2) will require detailed morphometric analysis (Huang et al., 1999) of PFV. It should be noted that the irregular spine orientation we observed might also contribute to formation of multiple synaptic contacts. Thus, multiple synaptic contacts observed in this mutant might come from the combination of changes in morphology of PFV and irregular orientations of the spines.
The differential regulation of rolling PC spines at tertiary and proximal dendrite of rolling mouse Nagoya suggests two major excitatory afferent systems are regulated reciprocally in the cerebellum of this mutnat.
References
- 1.Austin MC, Schultzberg M, Abbott LC, et al. Expression of tyrosine hydroxylase in cerebellar Purkinje neurons of the mutant tottering and leaner mouse. Brain Res Mol Brain Res. 1992;15:227–240. doi: 10.1016/0169-328x(92)90113-p. [DOI] [PubMed] [Google Scholar]
- 2.Cicale M, Ambesi-Impiombato A, Cimini V, et al. Decreased gene expression of calretinin and ryanodine receptor type 1 in tottering mice. Brain Res Bull. 2002;59:53–58. doi: 10.1016/s0361-9230(02)00841-9. [DOI] [PubMed] [Google Scholar]
- 3.Ertel EA, Campbell KP, Harpold MM, et al. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh A, Greenberg ME. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995;268:239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- 5.Hama K, Arii T, Kosaka T. Three-dimensional morphometrical study of dendritic spines of the granule cell in the rat dentate gyrus with HVEM stereo images. J Electron Microsc Tech. 1989;12:80–87. doi: 10.1002/jemt.1060120203. [DOI] [PubMed] [Google Scholar]
- 6.Hama K, Kosaka T. Purkinje cell and related neurons and glial cells under high voltage electron microscopy. In: Zimmerman HM, editor. Progress in neuropathology. New York: Raven Press; 1979. pp. 61–77. [Google Scholar]
- 7.Harris KM, Stevens JK. Dendritic spines of rat cerebellar Purkinje cells: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1988;8:4455–4469. doi: 10.1523/JNEUROSCI.08-12-04455.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CM, Brown N, Huang RH. Age-related changes in the cerebellum: parallel fibers. Brain Res. 1999;840:148–152. doi: 10.1016/s0006-8993(99)01707-2. [DOI] [PubMed] [Google Scholar]
- 9.Isaacs KR, Abbott LC. Cerebellar volume decreases in the tottering mouse are specific to the molecular layer. Brain Res Bull. 1995;36:309–314. doi: 10.1016/0361-9230(94)00207-h. [DOI] [PubMed] [Google Scholar]
- 10.Jun K, Piedras-Rentería ES, Smith SM, et al. Ablation of P/Q-type Ca(2+) channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proc Natl Acad Sci USA. 1999;96:15245–15250. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KJ, Kim H, Kim TS, Park SH, Rhyu IJ. Morphological analysis of spine shapes of Purkinje cell dendrites in the rat cerebellum using high-voltage electron microscopy. Neurosci Lett. 2004;359:21–24. doi: 10.1016/j.neulet.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 12.Llinás RR. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- 13.Matsushita K, Wakamori M, Rhyu IJ, et al. Bidirectional alterations in cerebellar synaptic transmission of tottering and rolling Ca2+ channel mutant mice. J Neurosci. 2002;22:4388–4398. doi: 10.1523/JNEUROSCI.22-11-04388.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori Y, Wakamori M, Oda S, et al. Reduced voltage sensitivity of activation of P/Q-type Ca2+ channels is associated with the ataxic mouse mutation rolling Nagoya (tg(rol)) J Neurosci. 2000;20:5654–5662. doi: 10.1523/JNEUROSCI.20-15-05654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oda S. The observation of rolling mouse Nagoya (rol), a new neurological mutant, and its maintenance (author's transl) Jikken Dobutsu. 1973;22:281–288. doi: 10.1538/expanim1957.22.4_281. [DOI] [PubMed] [Google Scholar]
- 16.Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- 17.Palay SL, Chan-Palay V. Cerebellar cortex - cytology and organization. New York, Berlin: Springer-Verlag; 1974. [Google Scholar]
- 18.Pietrobon D. Calcium channels and channelopathies of the central nervous system. Mol Neurobiol. 2002;25:31–50. doi: 10.1385/MN:25:1:031. [DOI] [PubMed] [Google Scholar]
- 19.Rhyu IJ, Abbott LC, Walker DB, Sotelo C. An ultrastructural study of granule cell/Purkinje cell synapses in tottering (tg/tg), leaner (tg(la)/tg(la)) and compound heterozygous tottering/leaner (tg/tg(la)) mice. Neuroscience. 1999a;90:717–728. doi: 10.1016/s0306-4522(98)00518-1. [DOI] [PubMed] [Google Scholar]
- 20.Rhyu IJ, Cho TH, Lee NJ, Uhm CS, Kim H, Suh YS. Magnetic resonance image-based cerebellar volumetry in healthy Korean adults. Neurosci Lett. 1999b;270:149–152. doi: 10.1016/s0304-3940(99)00487-5. [DOI] [PubMed] [Google Scholar]
- 21.Rhyu IJ, Nahm SS, Hwang SJ, et al. Altered neuronal nitric oxide synthase expression in the cerebellum of calcium channel mutant mice. Brain Res. 2003;977:129–140. doi: 10.1016/s0006-8993(03)02403-x. [DOI] [PubMed] [Google Scholar]
- 22.Rhyu IJ, Oda S, Uhm CS, Kim H, Suh YS, Abbott LC. Morphologic investigation of rolling mouse Nagoya (tg(rol)/tg(rol)) cerebellar Purkinje cells: an ataxic mutant, revisited. Neurosci Lett. 1999c;266:49–52. doi: 10.1016/s0304-3940(99)00254-2. [DOI] [PubMed] [Google Scholar]
- 23.Segal I, Korkotian I, Murphy DD. Dendritic spine formation and pruning: common cellular mechanisms? Trends Neurosci. 2000;23:53–57. doi: 10.1016/s0166-2236(99)01499-x. [DOI] [PubMed] [Google Scholar]
- 24.Strata P, Morando L, Bravin M, Rossi F. Dendritic spine density in Purkinje cells. Trends Neurosci. 2000;23:198. doi: 10.1016/s0166-2236(00)01571-x. [DOI] [PubMed] [Google Scholar]
- 25.Suh YS, Oda S, Kang YH, Kim H, Rhyu IJ. Apoptotic cell death of cerebellar granule cells in rolling mouse Nagoya. Neurosci Lett. 2002;325:1–4. doi: 10.1016/s0304-3940(02)00216-1. [DOI] [PubMed] [Google Scholar]
- 26.Tsien RW, Tsien RY. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- 27.Vecellio M, Schwaller B, Meyer M, Hunziker W, Celio MR. Alterations in Purkinje cell spines of calbindin D-28 k and parvalbumin knock-out mice. Eur J Neurosci. 2000;12:945–954. doi: 10.1046/j.1460-9568.2000.00986.x. [DOI] [PubMed] [Google Scholar]
- 28.Westenbroek RE, Sakurai T, Elliott EM, et al. Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. J Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 30.Zhuchenko O, Bailey J, Bonnen P, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 1997;15:62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- 31.Zwingman TA, Neumann PE, Noebels JL, Herrup K. Rocker is a new variant of the voltage-dependent calcium channel gene Cacna1a. J Neurosci. 2001;21:1169–1178. doi: 10.1523/JNEUROSCI.21-04-01169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]