Abstract
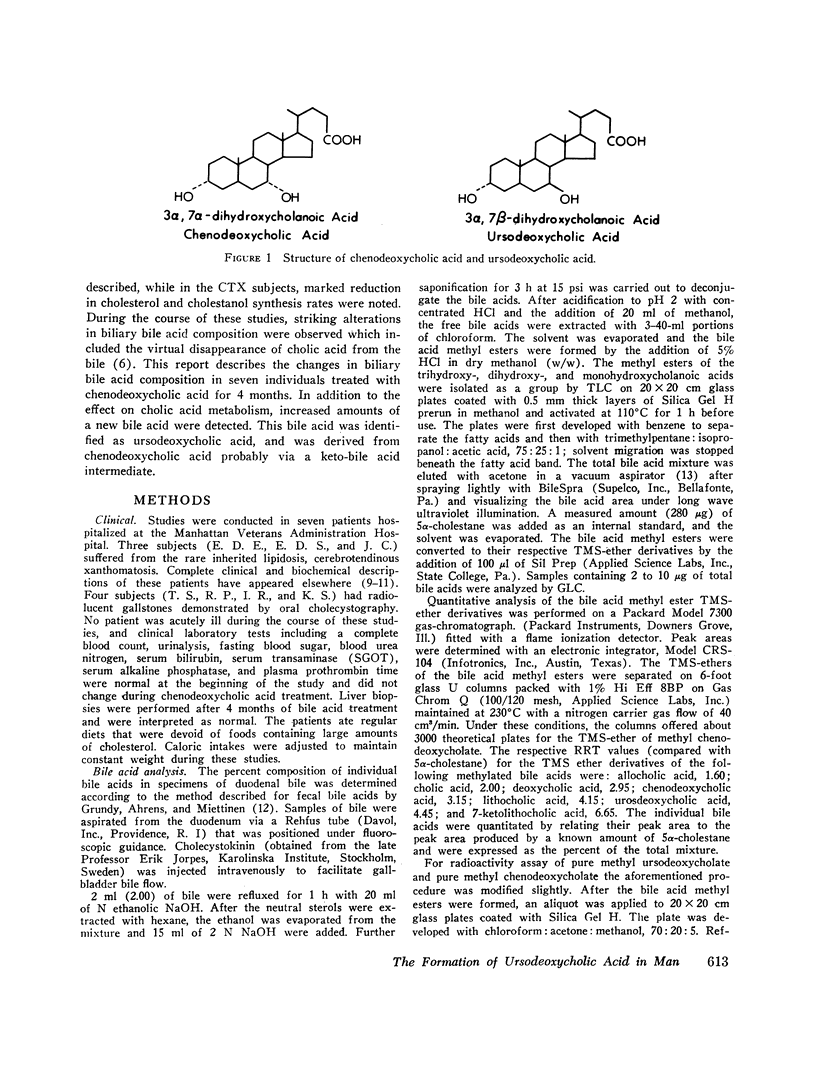
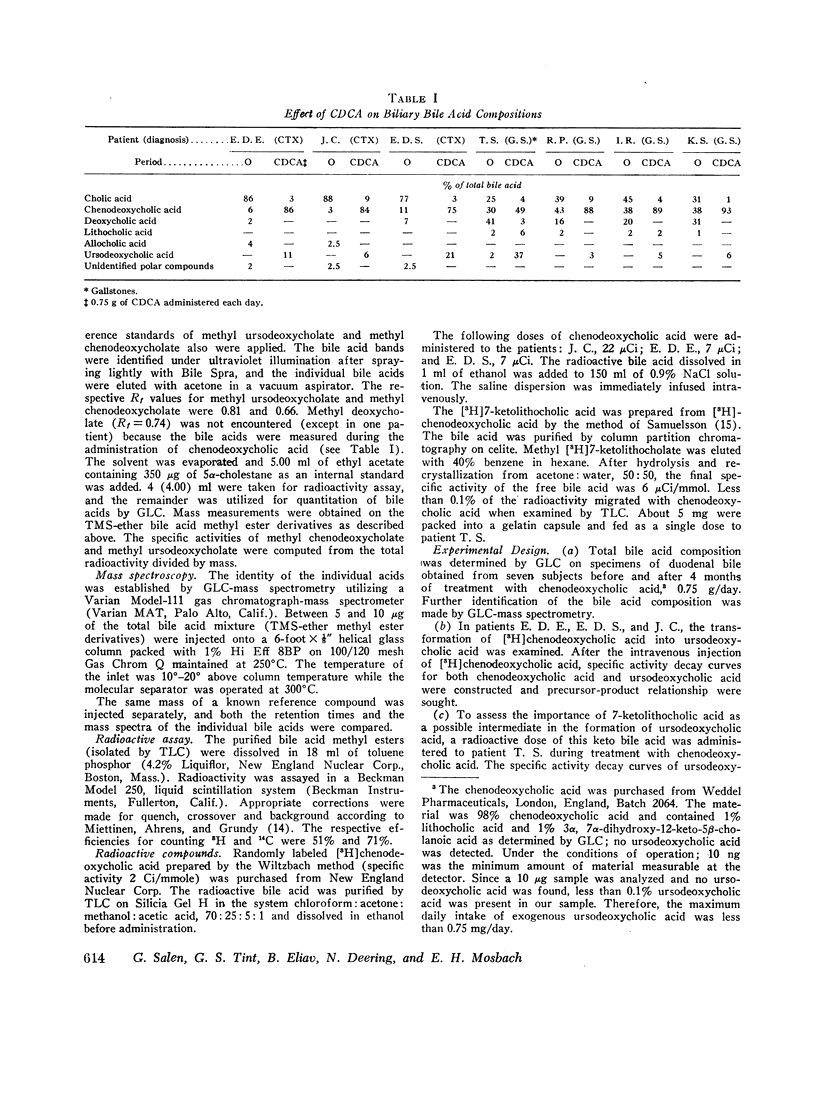
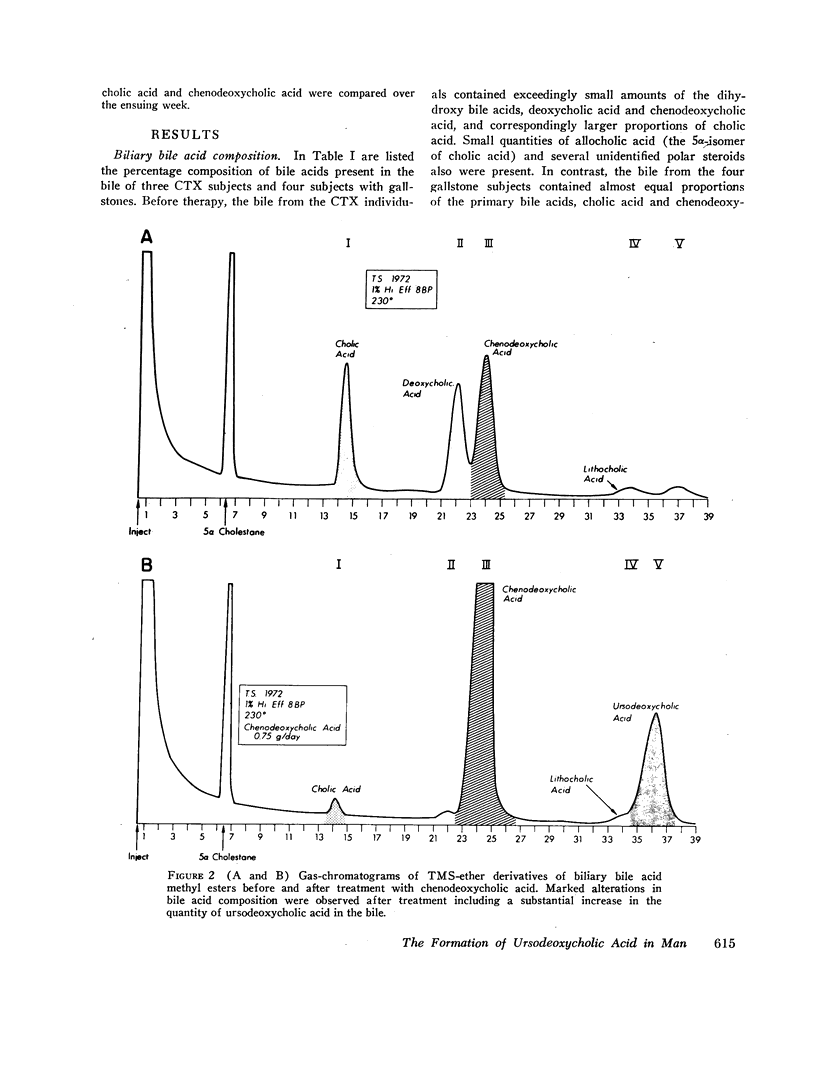
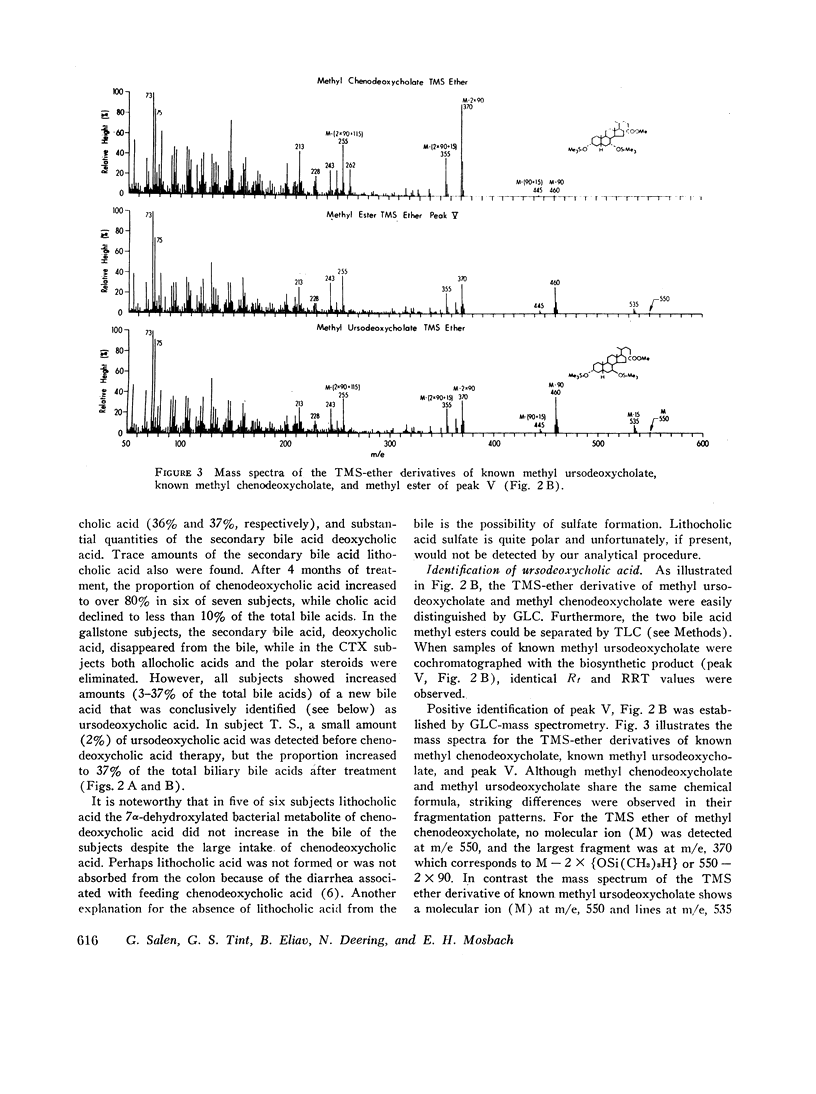
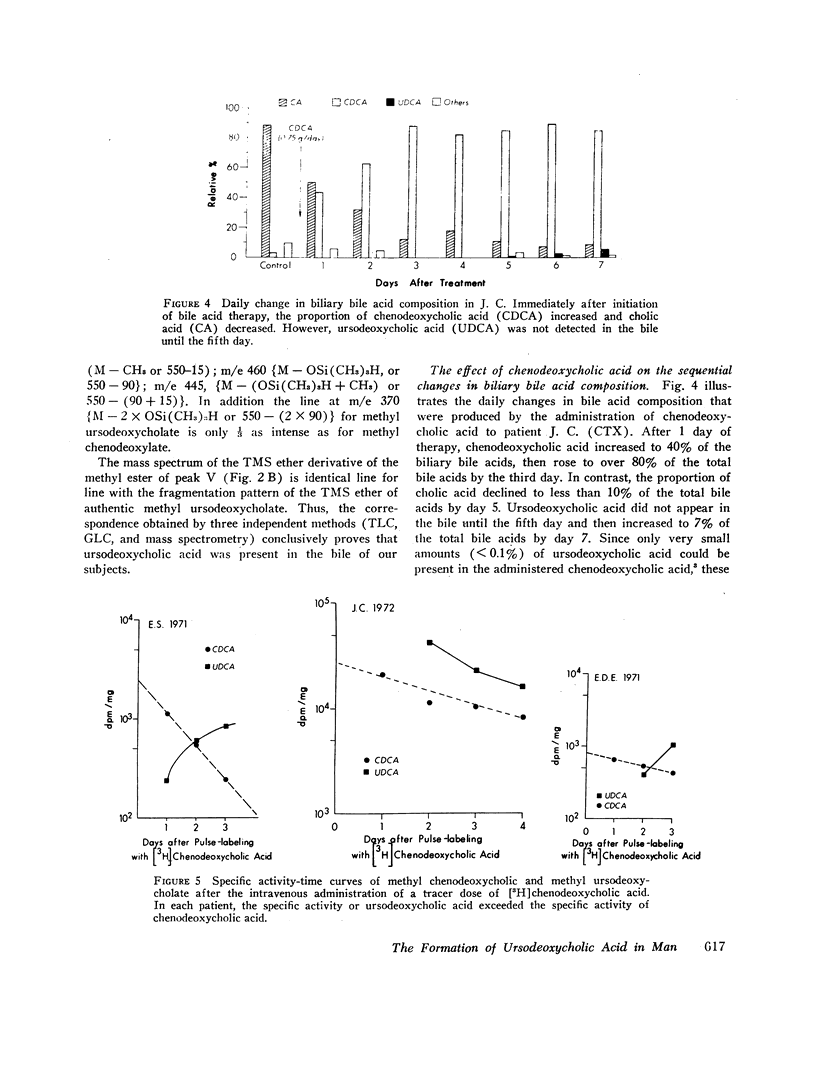
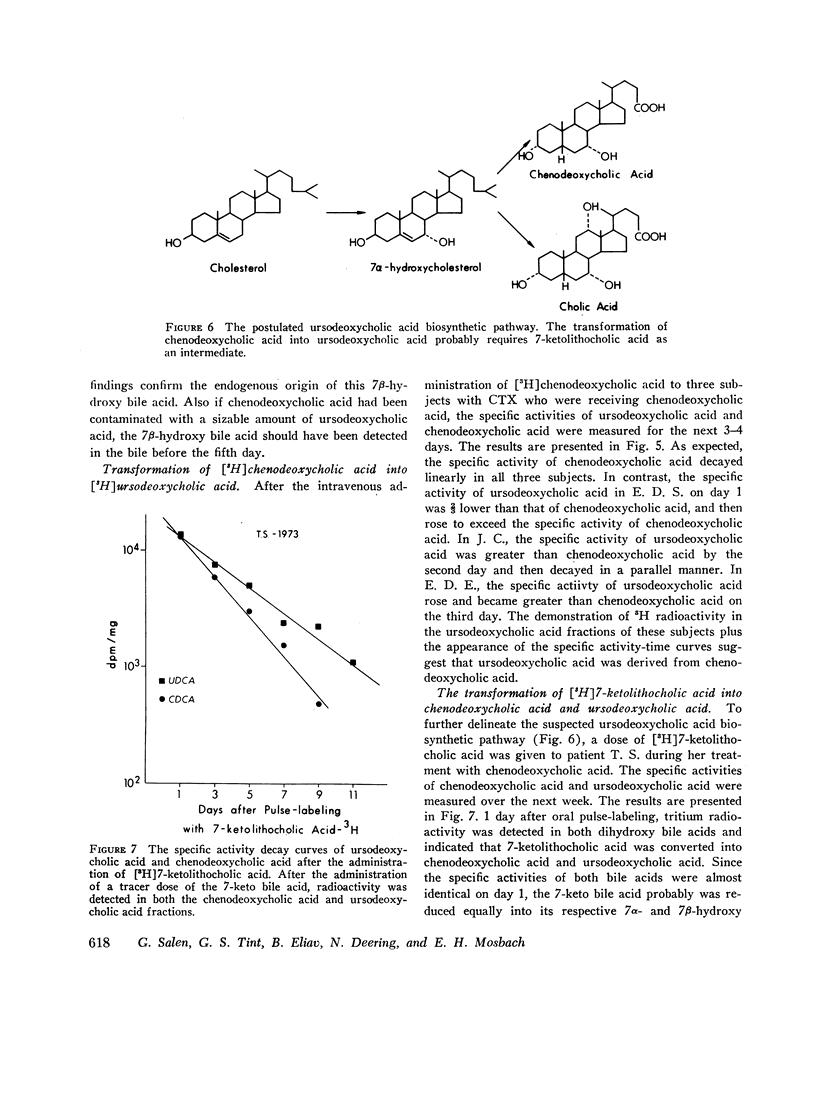
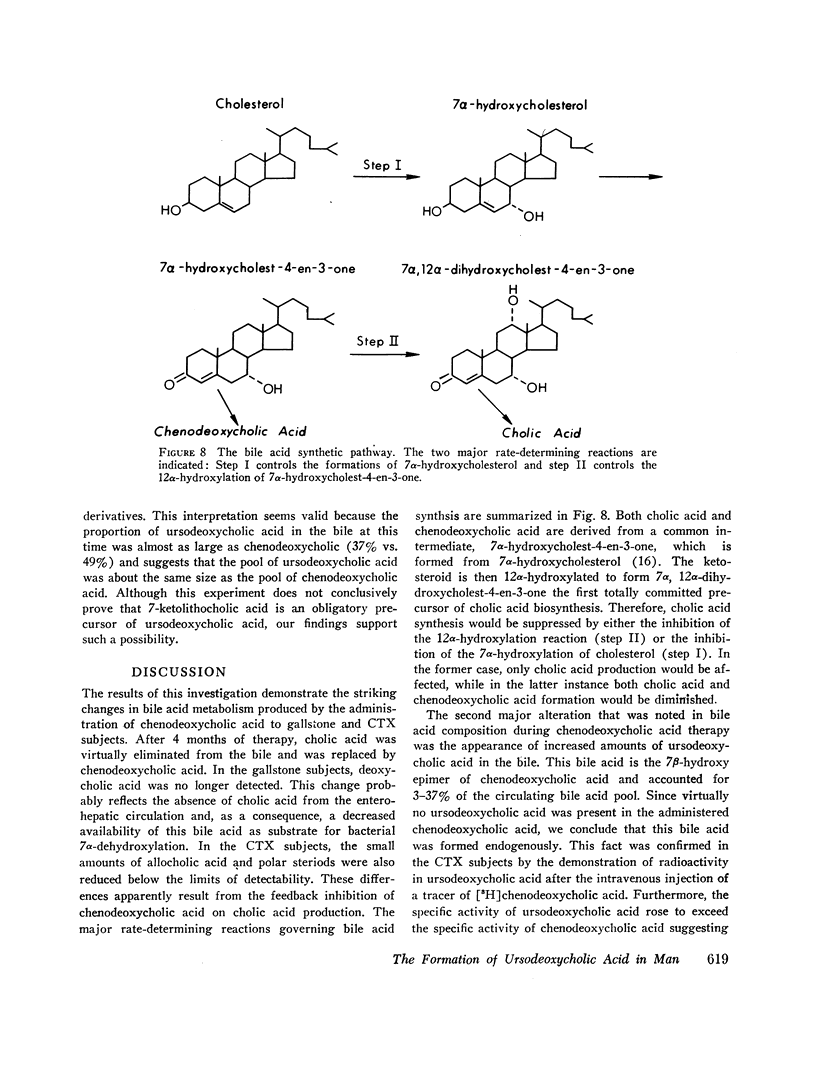
The formation of ursodeoxycholic acid, the 7 beta-hydroxy epimer of chenodeoxycholic acid, was investigated in three subjects with cerebrotendinous xanthomatosis and in four subjects with gallstones. Total biliary bile acid composition was analyzed by gas-liquid chromatography before and after 4 months of treatment with 0.75 g/day of chenodeoxycholic acid. Individual bile acids were identified by mass spectrometry. Before treatment, bile from cerebrotendinous xanthomatosis (CTX) subjects contained cholic acid, 85%; chenodeoxycholic acid, 7%; deoxycholic acid, 3%; allocholic acid, 3%; and unidentified steroids, 2%; while bile from gallstone subjects contained cholic acid, 45%; chenodeoxycholic acid, 43%; deoxycholic acid, 11%, and lithocholic acid, 1%. In all subjects, 4 months of chenodeoxycholic acid therapy increased the proportion of this bile acid to approximately 80% and decreased cholic acid to 3% of the total biliary bile acids, the remaining 17% of bile acids were identified as ursodeoxycholic acid. After the intravenous injection of [3H]chenodeoxycholic acid, the specific activity of biliary ursodeoxycholic acid exceeded the specific activity of chenodeoxycholic acid, and the resulting specific activity decay curves suggested precursor-product relationships. When [3H]7-ketolithocholic acid was administrated to another patient treated with chenodeoxycholic acid, radioactivity was detected in both the ursodeoxycholic acid and chenodeoxycholic acid fractions. These results indicate that substantial amounts of ursodeoxycholic acid are formed in patients treated with chenodeoxycholic acid. The ursodeoxycholic acid was synthesized from chenodeoxycholic acid presumably via 7-ketolithocholic acid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. D., Whitney B., Dowling R. H. Gallstone dissolution in man using chenodeoxycholic acid. Lancet. 1972 Dec 9;2(7789):1213–1216. doi: 10.1016/s0140-6736(72)92266-0. [DOI] [PubMed] [Google Scholar]
- DANIELSSON H. PRESENT STATUS OF RESEARCH ON CATABOLISM AND EXCRETION OF CHOLESTEROL. Adv Lipid Res. 1963;1:335–385. doi: 10.1016/b978-1-4831-9937-5.50015-6. [DOI] [PubMed] [Google Scholar]
- Danzinger R. G., Hofmann A. F., Schoenfield L. J., Thistle J. L. Dissolution of cholesterol gallstones by chenodeoxycholic acid. N Engl J Med. 1972 Jan 6;286(1):1–8. doi: 10.1056/NEJM197201062860101. [DOI] [PubMed] [Google Scholar]
- GOLDRICK B., HIRSCH J. A TECHNIQUE FOR QUANTITATIVE RECOVERY OF LIPIDS FROM CHROMATOPLATES. J Lipid Res. 1963 Oct;4:482–483. [PubMed] [Google Scholar]
- GRUNDY S. M., AHRENS E. H., Jr, MIETTINEN T. A. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL FECAL BILE ACIDS. J Lipid Res. 1965 Jul;6:397–410. [PubMed] [Google Scholar]
- HELLSTROM K., SJOVALL J. On the origin of lithocholic and ursodeoxycholic acids in man. Bile acids and steroids 106. Acta Physiol Scand. 1961 Feb-Mar;51:218–223. doi: 10.1111/j.1748-1716.1961.tb02130.x. [DOI] [PubMed] [Google Scholar]
- MAHOWALD T. A., YIN M. W., MATSCHINER J. T., HSIA S. L., DOISY E. A., Jr, ELLIOTT W. H., DOISY E. A. Bile acids. VIII. Metabolism of 7-ketolithocholic acid-24-C14 in the rat. J Biol Chem. 1958 Feb;230(2):581–588. [PubMed] [Google Scholar]
- MIETTINEN T. A., AHRENS E. H., Jr, GRUNDY S. M. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL DIETARY AND FECAL NEUTRAL STEROIDS. J Lipid Res. 1965 Jul;6:411–424. [PubMed] [Google Scholar]
- Nakayama F. Quantitative microanalysis of bile. J Lab Clin Med. 1967 Apr;69(4):594–609. [PubMed] [Google Scholar]
- Palmer R. H. Bile acids, liver injury, and liver disease. Arch Intern Med. 1972 Oct;130(4):606–617. [PubMed] [Google Scholar]
- Salen G. Cholestanol deposition in cerebrotendinous xanthomatosis. A possible mechanism. Ann Intern Med. 1971 Dec;75(6):843–851. doi: 10.7326/0003-4819-75-6-843. [DOI] [PubMed] [Google Scholar]
- Salen G., Grundy S. M. The metabolism of cholestanol, cholesterol, and bile acids in cerebrotendinous xanthomatosis. J Clin Invest. 1973 Nov;52(11):2822–2835. doi: 10.1172/JCI107478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salen G., Polito A. Biosynthesis of 5 -cholestan-3 -ol in cerebrotendinous xanthomatosis. J Clin Invest. 1972 Jan;51(1):134–140. doi: 10.1172/JCI106783. [DOI] [PMC free article] [PubMed] [Google Scholar]