Abstract
We and others have previously shown that reactive microglia express the major histocompatibility complex (MHC) class I and class II antigens in the hippocampus of patients suffering from epilepsy. Although the MHC glycoproteins serve as restriction elements for T lymphocytes, there is little information available regarding T lymphocytes in hippocampal sclerosis. In the present study, we investigated T lymphocyte infiltration in human hippocampi in four cases of epilepsy with hippocampal sclerosis, as well as in four control cases without neurological disease. No CD8- or CD4-positive T lymphocytes were seen in hippocampi from the control cases. In contrast, CD8- and CD4-positive T lymphocytes had infiltrated into the hippocampi of patients with hippocampal sclerosis. In addition, expression of intercellular adhesion molecule-1 was diffusely upregulated in the hippocampi with hippocampal sclerosis. These results indicate that T lymphocyte infiltration is involved in the pathology of hippocampal sclerosis.
Keywords: epilepsy, hippocampus, lymphocyte, microglia
I. Introduction
Hippocampal sclerosis (HS), the most common pathological finding in temporal lobe epilepsy, is characterized by prominent neuronal loss, mossy fiber sprouting, and astroglial proliferation [22]. In addition, reactive microglia in the hippocampi of epilepsy patients express major histocompatibility complex (MHC) class I and class II antigens [6]. The MHC I and II antigens are also expressed in rat models of epilepsy, such as those with kainic acid (KA)-induced seizures [1, 3, 8, 14]. The expression of MHC antigens on microglia has been reported in several neurodegenerative disorders, including Alzheimer’s disease (AD) and amyotrophic lateral sclerosis [11, 15, 20, 23]. These conditions are characterized by microglial activation in the affected brain regions. Reactive microglia produce several inflammatory factors associated with immune responses [4, 16].
The MHC glycoproteins serve as restriction elements for T lymphocytes. The MHC I and II antigens play a role in antigen presentation for T cytotoxic/suppressor lymphocytes (CD8) and T helper (Th)/inducer (CD4) lymphocytes, respectively [12]. Although these lymphocytes are not observed in the parenchyma of normal brains, they do infiltrate the parenchyma in AD [10] and chronic encephalitis (e.g. Rasmussen encephalitis) [13]. However, it remains uncertain whether these T lymphocytes are found in the hippocampi of patients with temporal lobe epilepsy. In animal models of epilepsy, it has been reported that leukocytes, including T lymphocytes, infiltrate the hippocampus [3, 25]. In the hippocampi of epilepsy model rats, intercellular adhesion molecule-1 (ICAM-1) is expressed in the blood vessels, followed by leukocyte infiltration [3]. Because leukocytes express lymphocyte function-associated antigen-1 (LFA-1), a ligand for ICAM-1, we proposed that the expression of ICAM-1 may be a trigger for the infiltration of T lymphocytes into the hippocampus when epilepsy occurs [3]. In the present study, we investigated whether the same phenomenon occurs in the hippocampi of patients with HS. We also evaluated the presence of T lymphocytes and ICAM-1 in the hippocampi of epileptic patients with and without HS, as well as in neurologically normal controls.
II. Materials and Methods
Tissue preparation
The present study was approved by the Ethics Committee at Shiga University of Medical Science. Four cases of HS were compared with one surgically excised hippocampus and three non-HS hippocampi obtained at autopsy. All HS patients satisfied commonly accepted criteria for surgery, with intractable seizures emanating from temporal lobe foci. All autopsy cases died suddenly without any history of neurological disease. The clinicopathological features of all cases are given in Table 1. Brain tissues were sliced, fixed by immersion in formalin (4% formaldehyde, phosphate buffered, pH 7.4) for 2 days, and then placed in 10 mM phosphate-buffered saline (PBS) containing 15% sucrose and 0.1% sodium azide at 4°C for cryoprotection. Tissues were cut into 40-µm sections on a freezing microtome. Sections were collected in 10 mM PBS containing 0.1% sodium azide and stored at 4°C until use.
Table 1.
Clinicopathological features of patients
| Case no. | Age (years) | Sex | Postmortem delay | Diagnosis |
|---|---|---|---|---|
| Hippocampal sclerosis | ||||
| 1 | 27 | M | Surgical specimen | Hippocampal sclerosis |
| 2 | 29 | M | Surgical specimen | Hippocampal sclerosis |
| 3 | 45 | M | Surgical specimen | Hippocampal sclerosis |
| 4 | 39 | F | Surgical specimen | Hippocampal sclerosis |
| Control cases | ||||
| 5 | 31 | M | Surgical specimen | Head trauma |
| 6 | 21 | M | 10 hr | Acute morphine overdose |
| 7 | 44 | M | 4 hr | Suicide |
| 8 | 24 | F | 8 hr | Heart trauma |
Immunohistochemistry
For immunohistochemistry, sections were incubated in 0.5% hydrogen peroxide in 0.1 M PBS (pH 7.4) containing 0.3% Triton-X100 (PBST) for 30 min at room temperature to eliminate endogenous peroxidase. After washing with PBST, sections were incubated for 30 min with PBST containing 2% bovine serum albumin to block non-specific protein binding. Sections were then incubated for 1–3 days at 4°C with a mouse monoclonal antibody directed against CD4 (1:100 dilution; Nichirei, Tokyo, Japan) or CD8 (1:100 dilution; Nichirei) or a rabbit polyclonal antibody against ICAM-1 (1:500 dilution; Beckman Coulter, Marseille, France). After washing with PBST, sections were incubated at room temperature for another 1 hr with biotinylated anti-mouse or anti-rabbit IgG (1:1000 dilution; Vector Laboratories, Burlingame, CA, USA), followed by 1 hr incubation at room temperature with avidin-biotinylated peroxidase complex (1:4000 dilution; Vector Laboratories). Sections were then incubated with 0.02% 3,3'-diaminobenzidine (DAB) and 0.3% nickel ammonium sulfate in 50 mM Tris-HCl buffer (pH 7.6) to precipitate a purple chromogen.
For double immunostaining, some sections were incubated with 0.5% hydrogen peroxidase in 0.1 M PBST for 30 min at room temperature after the first cycle of staining for CD4 or CD8. After washing with PBST, the sections were incubated with mouse monoclonal antibody against CD31 (1:500 dilution; Dako, Glostrup, Denmark) or rabbit polyclonal antibody against Iba1 (1:1000 dilution; Wako Pure Chemicals, Osaka, Japan). After washing with PBST, sections were incubated with peroxidase-labeled anti-mouse or anti-rabbit IgG (1:10 dilution; Histofine; Nichirei) for 1 hr at room temperature. Peroxidase labeling was detected by incubating the sections with 0.02% DAB and 0.0045% hydrogen peroxide in 50 mM Tris HCl buffer (pH 7.6) to precipitate a brown chromogen.
The specificity of ICAM-1 staining was examined by an immunoabsorption test. The sections were stained using the ICAM-1 antibody pre-incubated for 2 hr at room temperature with 5 µg/ml of ICAM-1 protein (Abcam Japan, Tokyo, Japan).
Cell counting
The number of CD4- or CD8-immunopositive cells was determined in three 20-µm sections. The entire area of each section was divided into 4400×3300 µm grids and each grid was then serially entered into a computer using a microscopic image analysis system (Biozero; Keyence, Osaka, Japan). The entire area of each section was then reconstructed by the computer and the total area of the hippocampal formation was determined using ImageJ 1.43 medical imaging software (National Institutes of Health, Maryland, USA). The number of cells was determined visually and cell counts were corrected for cell size [9]. The actual calculated number of cells (N) was obtained using the following formula:
N = n × (T/(T + h))
where n is the number of cell bodies, T is the thickness of the section, and h is the average diameter of the counted cell.
III. Results
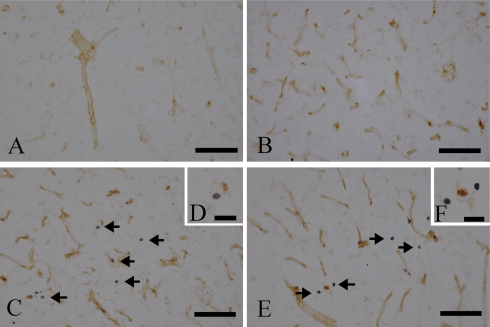
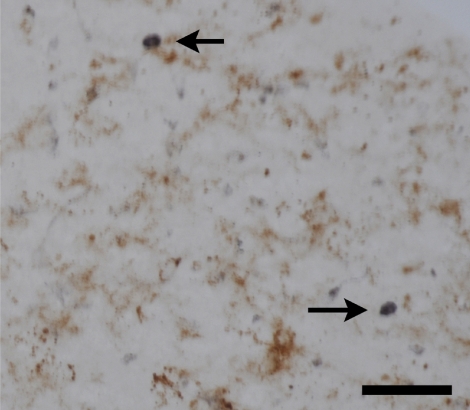
In control cases, no CD4- or CD8-positive lymphocytes (Fig. 1A, B) were found in the hippocampal parenchyma. However, in all epileptic patients with HS, CD4-positive (Fig. 1C, D) and CD8-positive (Fig. 1E, F) lymphocytes were observed to have infiltrated into the hippocampus (Table 2). Double-staining for T lymphocytes and CD31 showed CD4- and CD8-positive T lymphocytes within the parenchyma adjacent to blood vessels (Fig. 1C–F). Although the number of positive lymphocytes varied from case to case, in all cases the number of CD8-positive cells was significantly greater than the number of CD4-positive cells (p<0.01; Table 2). Figure 2 shows double immunostaining for CD4 and Iba1, a marker for microglia/macrophages. It can be seen that the CD4-positive cells constitute a separate subpopulation of cells to the Iba1-positive microglia/macrophages (Fig. 2).
Fig. 1.
Immunohistochemical staining for CD4 (A, C) and CD8 (B, D) in hippocampal sections from control patients (A, B) and patients with hippocampal sclerosis (C, D). Vessels were counter-stained with CD31 (brown). (A, B) No CD-4- or CD8-positive lymphocytes are seen in control sections. (C–F) Many CD-4-positive (C, D; arrows) and CD8-positive (E, F; arrows) lymphocytes are seen in the parenchyma in hippocampal sclerosis. Bars=100 µm (A, B, C, E) and 50 µm (D, F).
Table 2.
Number of T lymphocytes in hippocampal sections
| Case no. | No. CD4 lymphocytes (/mm2) | No. CD8 lymphocytes (/mm2) |
|---|---|---|
| Hippocampal sclerosis | ||
| 1 | 119 | 174 |
| 2 | 6 | 278 |
| 3 | 8 | 16 |
| 4 | 21 | 68 |
| Control cases | ||
| 5 | 0 | 2 |
| 6 | 0 | 0 |
| 7 | 1 | 7 |
| 8 | 4 | 12 |
Fig. 2.
Double immunostaining for CD4 and Iba1 showing that the CD4-positive cells (purple; arrows) are separate to the Iba1-positive cells (brown). Bar=50 µm.
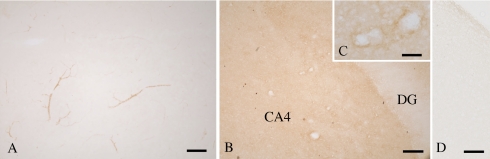
Weak ICAM-1 expression was observed in the blood vessels of hippocampi from control cases (Fig. 3A). In hippocampi from patients with HS (Fig. 3B), diffuse ICAM-1 staining appeared in the parenchyma with the exception of the dentate gyrus (Fig. 3B). At higher magnification, it was evident that ICAM-1 staining was increased in vascular walls and diffuse staining was observed from the vascular walls to the parenchyma (Fig. 3C). The preincubation of the ICAM-1 antibody with 5 µg/ml of ICAM-1 protein abolished the staining (Fig. 3D).
Fig. 3.
Immunohistochemical staining for intercellular adhesion molecule-1 (ICAM-1) in the hippocampus. (A) Control; (B–D) hippocampal sclerosis (HS). In the control sections (A), vessels are weakly stained for ICAM-1. In contrast, in sections from patients with HS (B, C), ICAM-1 staining is diffusely increased in the fourth sector of the cornus ammonium (CA4), but not in the dentate gyrus (DG). (D) The preincubation of the ICAM-1 antibody with 5 µg/ml of ICAM-1 protein abolish the staining. Bars=200 µm (A, B, D) and 50 µm (C).
IV. Discussion
The present study has demonstrated that CD4- and CD8-positive T lymphocytes infiltrate into the parenchyma of sclerotic hippocampi. Previous studies have reported that MHC class I and class II antigens are expressed in the hippocampi of epileptic patients [6], as well as in rat models of epilepsy, including those with KA-induced seizures [1, 3, 8, 14]. These observations, together with the results of the present study, suggest that immunoinflammation occurs in the hippocampus in HS. Interestingly, in all cases examined in the present study, the number of CD8-positive cells was significantly greater than the number of CD4-positive cells. These results are consistent with recent observations in an animal model of epilepsy [21].
A previous study has reported that, in addition to microglial activation, perivascular cells are observed in the hippocampus in HS [6]. The results of the present study indicate that some of these perivascular cells are T lymphocytes. Quantitatively, the number of T lymphocytes varied among cases of HS. This suggests that there is some unknown factor/s influencing T cell infiltration.
A previous study has reported no T cells in the parenchyma of HS cases [19]. The reason for the apparent discrepancy between that study and the present study remains unknown. However, there are several possibilities. First, the previous study investigated 6-µm formalin-fixed and paraffin-embedded sections [19], whereas in the present study we fixed hippocampal tissues for 2 days in 4% paraformaldehyde with 0.1 M phosphate buffer (pH 7.4) before cutting 20-µm sections from the tissue blocks. The weak fixation process we used may have preserved antigenicity compared with formalin fixation and paraffin embedding. In addition, the T cell makers used in the two studies differ. The previous report used CD3 [19], whereas in the present study we used CD4 and CD8. Thus, technical differences between the two studies may explain the discrepancy.
In patients with Rasmussen’s encephalitis, a refractory seizure disorder, T (CD4, CD8) and B lymphocytes are present in the brain [17]. In these patients, cytotoxic T cells kill neurons [7]. In the present study, we found that the number of cytotoxic T cells (CD8) was greater than the number of Th cells (CD4) in samples from patients with HS. This observation is consistent with a recent study using KA [21]. Together, the results suggest that T cell infiltration may be involved in neuronal loss in HS. However, in order to clarify this issue, further studies are needed.
As reported previously [2], we found weak ICAM-1 expression in blood vessels in samples from control cases. Interestingly, ICAM-1 expression was increased in the parenchyma of hippocampal sections from patients with HS. It has been reported that ICAM-1 expression is increased by inflammatory responses [25]. Therefore, the results of the present study may suggest that immunoinflammation occurs in the sclerotic hippocampus.
In AD cases, increased ICAM-1 expression has been reported in the parenchyma [2], as well as T cell infiltration [10]. Similar findings have been made in the cortex of AD cases [2]. Because lymphocytes express a ligand for ICAM-1 (i.e. LFA-1), ICAM-1 in the parenchyma may induce T cell infiltration in both HS and AD. In the mouse brain, ICAM-1 expression in vessels increases following transient cerebral ischemia [18] and, in addition to being expressed in the blood vessels, ICAM-1 is found in the astrocytes 24 hr after treatment [18]. However, we did not find any ICAM-1-positive astrocytes in samples from patients with HS, nor were ICAM-1-positive astrocytes found in rat brains following KA treatment [3]. The differences between these studies suggest that ICAM-1 has different pathological roles in cerebral ischemia compared with HS.
Quantitative values for CD4- and CD8-positive cells varied among cases. This suggests that there may be some unknown factor/s influencing T cell infiltration. Bauer et al. have reported that seizure activity itself may trigger the release of chemical factors, such as cytokines, into the surrounding tissue in addition to epinephrine secretion [5]. Uchikado et al. reported that systemic inflammation affects the expression of several cytokines, including ICAM-1, in the human brain [25]. T cell infiltration in HS may be influenced by these factors.
We have reported previously that activated microglia expressing MHC class II antigens were found in HS with temporal lobe epilepsy [6]. Animal studies have shown that such activation can occur in response to a number of different forms of injury, all of which may act by inducing neuronal cell death or degeneration of neuronal processes. In epileptic rat models, microglial activation is closely related to neuronal death processes [24]. The activated microglia found in HS suggest ongoing degenerative processes. The T cell infiltration demonstrated in the present study supports this possibility.
As noted above, Bauer et al. have reported that seizure activity itself may trigger the release of chemical factors in addition to epinephrine, thereby inducing inflammation [5]. Thus, it may be that several cytokines and epinephrine are secreted in HS, although this possibility requires further investigation.
In conclusion, the present study has shown increased ICAM-1 expression and infiltration of CD8- and CD4-positive T lymphocytes in the hippocampus of patients with HS.
V. References
- 1.Akiyama H., Itagaki S., McGeer P. L. Major histocompatibility complex antigen expression on rat microglia following epidural kainic acid lesions. J. Neurosci. Res. 1988;20:147–157. doi: 10.1002/jnr.490200202. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama H., Kawamata T., Yamada T., Tooyama I., Ishii T., McGeer P. L. Expression of intercellular adhesion molecule (ICAM)-1 by a subset of astrocytes in Alzheimer disease and some other degenerative neurological disorders. Acta Neuropathol. 1993;85:628–634. doi: 10.1007/BF00334673. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama H., Tooyama I., Kondo H., Ikeda K., Kimura H., McGeer E. G., McGeer P. L. Early response of brain resident microglia to kainic acid-induced hippocampal lesions. Brain Res. 1994;635:257–268. doi: 10.1016/0006-8993(94)91447-8. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G. M., Cooper N. R., Eikelenboom P., Emmerling M., Fiebich B. L., Finch C. E., Frautschy S., Griffin W. S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I. R., McGeer P. L., O’Banion M. K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F. L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer S., Cepok S., Todorova-Rudolph A., Nowak M., Köller M., Lorenz R., Oertel W. H., Rosenow F., Hemmer B., Hamer H. M. Etiology and site of temporal lobe epilepsy influence postictal cytokine release. Epilepsy Res. 2009;86:82–88. doi: 10.1016/j.eplepsyres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Beach T. G., Woodhurst W. B., MacDonald D. B., Jones M. W. Reactive microglia in hippocampal sclerosis associated with human temporal lobe epilepsy. Neurosci. Lett. 1995;191:27–30. doi: 10.1016/0304-3940(94)11548-1. [DOI] [PubMed] [Google Scholar]
- 7.Bien C. G., Bauer J., Deckwerth T. L., Wiendl H., Deckert M., Wiestler O. D., Schramm J., Elger C. E., Lassmann H. Destruction of neurons by cytotoxic T cells: a new pathogenic mechansm in Rasmussen’s encephalitis. Ann. Neurol. 2002;51:311–318. doi: 10.1002/ana.10100. [DOI] [PubMed] [Google Scholar]
- 8.Finsen B. R., Jørgensen M. B., Diemer N. H., Zimmer J. Microglial MHC antigen expression after ischemic and kainic acid lesions of the adult rat hippocampus. Glia. 1993;7:41–49. doi: 10.1002/glia.440070109. [DOI] [PubMed] [Google Scholar]
- 9.Guillery R. W. On counting and counting errors. J. Comp. Neurol. 2002;447:1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- 10.Itagaki S., McGeer P. L., Akiyama H. Presence of T-cytotoxic suppressor and leucocyte common antigen positive cells in Alzheimer’s disease brain tissue. Neurosci. Lett. 1988;9:259–264. doi: 10.1016/0304-3940(88)90690-8. [DOI] [PubMed] [Google Scholar]
- 11.Kawamata T., Akiyama H., Yamada T., McGeer P. L. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am. J. Pathol. 1992;140:691–707. [PMC free article] [PubMed] [Google Scholar]
- 12.Lampson L. A. Molecular bases of the immune response to neural antigens. Trends Neurosci. 1987;10:211–216. [Google Scholar]
- 13.Li Y., Uccelli A., Laxer K. D., Jeong M. C., Vinters H. V., Tourtellotte W. W., Hauser S. L., Oksenberg J. R. Local-clonal expansion of infiltrating T lymphocytes in chronic encephalitis of Rasmussen. J. Immunol. 1997;158:1428–1437. [PubMed] [Google Scholar]
- 14.Matsuoka Y., Kitamura Y., Okazaki M., Kakimura J., Tooyama I., Kimura H., Taniguchi T. Kainic acid induction of heme oxygenase in vivo and in vitro. Neuroscience. 1998;85:1223–1233. doi: 10.1016/s0306-4522(97)00678-7. [DOI] [PubMed] [Google Scholar]
- 15.McGeer P. L., Itagaki S., McGeer E. G. Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol. 1988;76:550–557. doi: 10.1007/BF00689592. [DOI] [PubMed] [Google Scholar]
- 16.McGeer P. L., Kawamata T., Walker D. G., Akiyama H., Tooyama I., McGeer E. G. Microglia in degenerative neurological disease. Glia. 1993;7:84–92. doi: 10.1002/glia.440070114. [DOI] [PubMed] [Google Scholar]
- 17.McNamara J. O. B cells and epilepsy: the odd couple. Neurology. 2002;58:677–678. doi: 10.1212/wnl.58.5.677. [DOI] [PubMed] [Google Scholar]
- 18.Ohtaki H., Endo S., Nakamachi T., Yin L., Dohi K., Kudo Y., Iwai Y., Matsunaga M., Goto H., Shioda S. Increased expression of intercellular adhesion molecule-1 (ICAM-1) in mouse brain following transient cerebral ischemia. Acta Histochem. Cytochem. 2003;4:385–391. [Google Scholar]
- 19.Ravizza T., Gagliardi B., Noé F., Boer K., Aronica E., Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Rogers J., Luber-Narod J., Styren S. D., Civin W. H. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol. Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- 21.Silverberg J., Ginsburg D., Orman R., Amassian V., Durkin H. G., Stewart M. Lymphocyte infiltration of neocortex and hippocampus after a single brief seizure in mice. Brain Behav. Immun. 2010;24:263–272. doi: 10.1016/j.bbi.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Thom M., Sisodiya S., Najm I. In “Greenfield’s Neuropathology, 8th Edition”, Vol. 1, ed. by S. Love, D. N. Louis and D. W. Ellison. Oxford University Press; USA: 2008. Neuropathology of epilepsy; pp. 833–887. [Google Scholar]
- 23.Tooyama I., Kimura H., Akiyama H., McGeer P. L. Reactive microglia express class I and class II major histocompatibility complex antigens in Alzheimer’s disease. Brain Res. 1990;523:273–280. doi: 10.1016/0006-8993(90)91496-4. [DOI] [PubMed] [Google Scholar]
- 24.Tooyama I., Nishimura T., Nakagawa E., Morita H., Uemura S., Aimi Y., Yasuhara O., Kimura H. Microglial reaction and neuronal death in the hippocampus of rat models of epilepsy. Neuropathology. 1999;19:203–208. [Google Scholar]
- 25.Uchikado H., Akiyama H., Kondo H., Ikeda K., Tsuchiya K., Kato M., Oda T., Togo T., Iseki E., Kosaka K. Activation of vascular endothelial cells and perivascular cells by systemic inflammation: an immunohistochemical study of postmortem human brain tissues. Acta Neuropathol. 2004;107:341–351. doi: 10.1007/s00401-003-0815-x. [DOI] [PubMed] [Google Scholar]