Abstract
The lung is one of the commonest sites of exposure to environmental allergen or pathogen, so the expression of a variety of cytokines in the lung is dynamically regulated by inflammatory or structural cells in the lung. In the last decades, characterization of the local lung cytokine milieu in allergic or injury models has identified a collective role of certain cytokines, such as type 1 or type 2 cytokines, driving polarized inflammatory and tissue phenotypes. With the development of transgenic mouse modelling systems, the effector function of individual cytokine and the pathophysiological consequences of cytokine polarization in the lung have been effectively evaluated. Here, we present an overview of the transgenic systems currently used to assess the biological function of cytokine or other mediators in the lung. We discuss the inflammatory and tissue phenotypes detected in the lungs of transgenic mice over-expressing representative T helper type 1 (interferon-γ, interleukin-12), T helper type 2 (interleukins -4, -5, -9, -10 and -13), and T helper type 17 cytokines. The effects of genetic modification of cytokine receptors or transcriptional factors such as GATA-3 and T-bet in pulmonary inflammation and remodelling tissue responses are also discussed because these transcription factors are regarded as essential regulators of cytokine polarization. Finally, we discuss the limitations and future application of transgenic approaches in the studies of human lung diseases characterized by cytokine polarization.
Keywords: cytokine; lung, type 1 (Th1) and type 2 (Th2); polarization; transgenic mice
Introduction
A variety of cytokines are produced by the inflammatory cells and structural cells in response to external stimuli such as infectious agents, particulate matters or noxious gases or as a result of cell-to-cell communication. As molecular messengers of cellular communication, a variety of cytokines play an essential role in innate and adaptive immune responses by recruiting and activating inflammatory cells. They also initiate and regulate local tissue repair processes critical for resolution of inflammation as well as tissue remodelling (for review see ref. 1). Although each cytokine has its own distinct functional role, collectively a group of cytokines, sometimes in redundant fashion, can lead to certain polarized inflammatory and tissue responses.
The lung is one of the primary sites continuously interfacing external environments. The dynamic interactions between host and invading pathogens or allergens in the lung are regulated by the expression of specific cytokines depending on the nature of the stimulation and the type of cells involved in the process. Type 1 or type 2 polarized immune responses mainly driven by T helper type 1 (Th1) [interferon-γ (IFN-γ), interleukin-12 (IL-12)] or Th2 (IL-4, IL-5, IL-9, IL-13) cytokines in the lung have been well-characterized in a variety of animal models of infection or allergic inflammation.2,3 The type 1 or 2 inflammatory responses caused by these polarized cytokines are also closely linked to the characteristic inflammatory and pathological tissue phenotypes in the lung. In general, type 1 inflammation is frequently associated with an acute innate immune response against invading pathogens such as bacterial or viral infection. The macrophages, neutrophils and other innate immune cells such as natural killer (NK) cells are the major cells involved in type 1 inflammation. On the other hand, type 2 inflammation is more frequently associated with the chronic adaptive immune responses related to allergy or tissue fibrosis. The inflammatory cells associated with adaptive immunity, such as eosinophils and B and T lymphocytes, are therefore more frequently implicated in type 2 inflammation. However, the same inflammatory cells can also play a major role in both types of inflammation. It has been demonstrated that macrophages classically and alternatively activated by type 1 and type 2 cytokines, respectively, play a significant role in the development of inflammatory and tissue responses. Accordingly, the tissue phenotypes of type 1 and type 2 inflammatory responses in the lungs are different depending on the nature of the inflammation. Whereas type 1 inflammation is more likely to cause a destructive tissue phenotype such as the emphysematous alveolar destruction observed in cigarette smoke-exposed animal models, type 2 inflammation contributes to tissue repair or the healing process by enhancing cellular proliferation and extracellular matrix production.
The development of transgenic and other genetically modified mice has enabled us to efficiently and accurately define the function of specific genes. In the last decade, our laboratory and others have successfully generated a number of lung-specific transgenic mice that over-express cytokines and other mediators (for review see ref. 2). The transgenic mice with lung-specific over-expression of Th1 or Th2 cytokines developed characteristic type 1 and type 2 inflammatory and tissue responses. This transgenic modelling of cytokine polarization has provided a powerful tool to understand the cellular and molecular mechanisms with characteristic ‘skewed cytokine phenotypes’ that are commonly noted in chronic inflammatory lung diseases such as asthma and chronic obstructive lung disease. Previously, it has been noted that viral or bacterial infection or tumour metastasis in the lung favours certain polarized cytokine environments.4–7 The transgenic or null mutant mice driving cytokine polarization in the lung (Table 1) can be used as important animal models in evaluating the potential contribution of these skewed cytokine environments in the pathogenesis and progression of human lung diseases.
Table 1.
Genetic modulation of cytokines and cytokine receptors, transcriptional factors causes cytokine polarization in the lung and other organs
| Cytokine1 | Genetic manipulation | Phenotype | References |
|---|---|---|---|
| IL-4 | TG2 | Lymphoctyic, eosinophilic inflammation; no airway hyper-reactivity (AHR); no airway wall remodelling Airway fibrosis and alveolar destruction in C57BL/6 mice | 16,17 |
| IL-5 | TG | Peribronchial eosinophilic inflammation, goblet cell hyperplasia, subepithelial collagen deposit, AHR | 18 |
| IL-13 | TG | Prominent eosinophils, macrophage and lymphocytic inflammation; airway fibrosis, mucus metaplasia and AHR | 25 |
| IFN-γ | TG | Increased macrophage, neutrophils, emphysematous destruction; matrix metalloproteinases 12 and 9 | 14 |
| IL-12 | TG | Increased natural killer cells, bronchial infiltrate of CD4, B cells | 15 |
| IL-9 | TG | Eosinophilic inflammation, mast cell hyperplasia, bronchial hyper-responsiveness | 19 |
| IL-10 | TG | Reduced lipopolysaccharide response; lymphocytic inflammation, goblet cell metaplasia, subepithelial fibrosis | 20, 21 |
| IL-4Rα | KO2 | Impaired worm control, attenuated IL-4, IL-5, IL-13 Chronic inflammation | 48,47 |
| IL-13R1 | KO | Important for baseline IgE but not T helper type 2 (Th2) and IgE responses to T-cell dependent antigens Increased airway resistance, mucus, transforming growth factor-β and eotaxin Exaggerated Th2 response compared to IL-4Rα KO | 42, 43 |
| IL-13Ra2 | KO | Increased Selective and inhibitor of IL-13 induced inflammation, remodelling in the lung Increased fibrosis despite no change in eosinophilia and decrease in IL-13 levels | 51,52 |
| IL-17A/IL-17F | TG | Increased leucocyte and macrophage recruitment Increased chemokine production Airway changes | 32, 33 |
| IL-17A | KO | Decreased Th2 helper responses | 33 |
| IL-17F | KO | Enhanced type 2 cytokine production and eosinophil function | 33 |
| IL-25 | TG | Increased mucus production Increased airway eosinophils | 36 |
| IL-33 receptor | TG | Abrogated effects of IL-33 | 57 |
| KO | Susceptibility to parasitic infection | 56 | |
| TIR8 | KO | Exacerbation of Th2 response in allergic ovalbumin pulmonary inflammation Hyper-responsiveness to IL-33 Susceptibility to parasitic infection | 59,84,56 |
| T-bet | TG | Lower goblet cell hyperplasia, mucus hypersecretion, eosinophilic airway inflammation Predominant Th1 response, type 1 cytotoxic CD8+ T-cell response, attenuation Th2 response | 75,77 |
| KO | Failure to develop Th1 cells, reduced IFN-γ | 76 | |
| GATA-3 | TG | Th2 cytokine production Increased T1/ST2 expression Attenuated development of hypersensitivity pneumonitis (a Th1 disease) Enhanced fibrosis, airway smooth muscle hyperplasia after allergen challenge | 67, 69, 71, 72, 75 |
| KO | Impaired Th2 cytokine production and proliferation | 64, 65 | |
| c-maf | TG | Increased Th2 cytokine and elevated serum IgG1 and IgE | 73 |
| KO | Impaired IL-4, T cells spontaneously polarize to Th1 phenotype | 74 | |
| Foxoa2 | KO | Spontaneous pulmonary eosinophilic inflammation and goblet cell metaplasia and increased Th2 cytokines | 79 |
| RORγT | KO | Attenuated eosinophilic pulmonary inflammation Lower Th2 cytokine production | 83 |
IL-4, interleukin-4; IFN-γ, interferon-γ; IL-4Rα, interleukin-4 receptor-α; TIR8, Toll-interleukin-1 receptor-8; RORγT, retinoic acid-related orphan receptor γT.
KO, knockout; TG, transgenic.
Transgenic modelling in the lung
The first successful transgenic mice were generated in 1980.8 Since then, the use of genetically modified animals in the study of biology and pathology has become a standard investigative tool. A number of genetic alteration methods have been developed to study the function of a specific gene. The transgenic over-expression and targeted null mutation of specific genes are the most commonly used methods of genetic modification. In general, whereas targeted null mutation is being used to define the role of specific endogenous gene, the over-expressing transgenic approaches focus to evaluate the responses associated with the effector functions of the gene in various experimental conditions. However, transgenic expression of dominant-negative or non-functional mutant forms can be used as an alternative of targeted null mutation. Transgene expression using cell-specific promoters further provides a great advantage for the accurate evaluation of gene function in an organ-specific or cell-specific manner. Furthermore, stable expression of the transgene can be achieved by targeted insertion of the transgene in permissive chromosomal loci, such as Rosa 26 or HPRT, by homologous recombination9–13. In addition, investigators have developed transgenic systems in which transgene expression can be externally regulated by tetracycline-responsive elements such as the one described below.
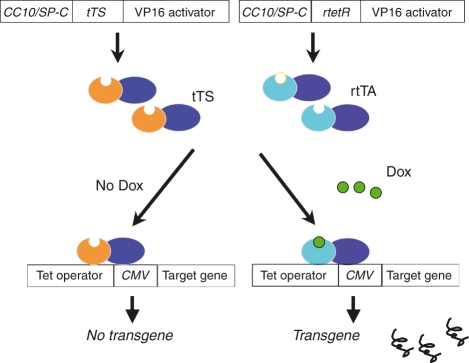
For lung-specific expression, the Clara cell 10 000 molecular weight protein (CC10) and surfactant apoprotein C (SP-C) promoters are frequently used. The former targets transgenes to airway Clara cells and, to a lesser extent, alveolar type II epithelial cells. In contrast, the SP-C promoter targets predominantly to the alveolar epithelium. Lung-targeted transgene can be constitutively expressed and this standard transgenic system has provided remarkable insights into pulmonary biology. However, in utero activation of the transgene frequently causes embryo lethality or growth or developmental abnormalities that can confound phenotypic interpretation. This system is also limited in its ability to model the waxing and waning process in chronic diseases and its ability to assess the natural history of transgene-induced phenotypic alterations. To overcome these problems, double or triple inducible transgenic systems were developed and successfully used for lung-specific transgenic expression of a number of cytokines. In this inducible system, CC10 or SP-C promoter-driven reverse tetracycline-controlled transactivator (rtTA) or tetracycline-controlled silencer (tTS) regulate the tetracycline operator (tet-O), minimal cytomegalovirus (CMV) promoter-driven transgene constructs in a lung-specific manner. By simultaneous microinjection of these three constructs into pronuclei of fertilized eggs, tightly regulated transgenic mice can be generated with minimal leakage of the transgene expression in the developmental stage. In this triple transgenic system, in the presence of doxycycline, rtTA binds, in trans, to the tet-O and VP-16 (a powerful transcriptional activator isolated from herpes simplex virus) activates downstream transgene expression. In the absence of doxycycline, tTS binds with tet-O and actively suppresses transcription of target gene expression (Fig. 1).
Figure 1.
Schematic illustration of the constructs used for the generation of lung-specific triple transgenic system. Lung-specific promoters of Clara Cell 10 000 molecular weight (CC10) or surfactant protein-C (SP-C) drive tetracycline-controlled transcriptional silencer (tTS) or tetracycline-controlled transactivator (rtTA) expression in a lung-specific manner. The tetracycline response element (TRE) consisting of tet operon (tetO) and promoter cytomegalovirus (CMV) drive target transgene expression. By simultaneous micro-injection of these three constructs, the expression of transgene can be tightly regulated by the administration of tetracycline (doxycycline; Dox). In the absence of doxycycline, tTS occupies the TRE region of the target construct and prevents the downstream transgene expression. In the presence of doxycycline, rtTA replaces the tTS with greater affinity on the TRE region and activates the transcription of target transgene.
Transgenic expression of type 1 cytokines in the lung
Interferon-γ and IL-12 are prototypic type 1 cytokines, mainly produced by Th1 lymphocytes, macrophages, dendritic cells, and granulocytes. Studies from our laboratory demonstrated that the transgenic expression of IFN-γ in the lung induces prominent macrophage-rich and neutrophil-rich inflammation and emphysematous alveolar destruction14 It also induced prominent protease and anti-protease alterations. The IFN-γ in the lung stimulated the expression and activation of proteases such as matrix metalloproteinases -12 or -9, while inhibiting the expression of anti-proteases such as tissue inhibitor of metalloproteinase-1, resulting in emphysematous alveolar destruction.14
Interleukin-12 is a heterodimer (p70) composed of a p40 subunit and a p35 subunit and both subunits are required to be expressed in a cell for it to be bioactive. The IL-12 receptors are composed of β1- and β2-subunits which bind to p40 and p35 of IL-12, respectively. The receptors are mainly expressed on activated T, B and cytotoxic NK cells. Although lung-specific IL-12 transgenic mice have not been generated, transgenic expression of IL-12 in thyroid also increased the IL-12 expression in the lung, resulting in characteristic tissue inflammation and mucous responses.15 The lungs of these transgenic mice were markedly infiltrated by mononuclear cells and prominent bronchially associated lymphoid tissues consist of B cells and CD4+ lymphocytes. The number of lung NK cells in these transgenic mice was biphasic; initially the number of NK cells increased during the first month of life, then, sharply declined and remained significantly lower than controls. With age, these mice demonstrated increased oxidative stress and transforming growth factor-β (TGF-β) signalling and decreased mucociliary clearance, histological and functional characteristics that can be seen in Sjögren’s lung disease.15
Transgenic expression of type 2 cytokines in the lung
Typically, type 2 inflammatory responses are caused by Th2 cytokines that are mainly produced by T helper lymphocytes. However, other inflammatory and structural cells in the lung, such as eosinophils, macrophages or epithelial cells, can also be prominent sources of these cytokines to drive type 2 inflammatory responses. In addition, studies from our laboratory and others demonstrated that transgenic expression of vascular endothelial growth factor (VEGF), a pro-angiogenic cytokine, in the lung induces type 2 inflammatory responses partly by way of an IL-13-dependent pathway. To understand the effects of each type 2 cytokine, lung-specific IL-4, IL-5, IL-9, IL-10 and IL-13 and VEGF transgenic mice have been generated and characterized. In accordance with the belief that Th2 cytokines play an important role in asthma and allergic responses, each of these cytokines generated allergic inflammatory and physiological responses.
Transgenic IL-4 expression in the lung caused lymphocytic and eosinophilic inflammation without airway hyper-reactivity and airway wall remodelling.16 The modest nature of this response is in keeping with other studies that suggest that IL-4 is more active in the initiation phase than in the effector phases of Th2 inflammation. However, there are significant genetic influences controlling IL-4 effector pathways because IL-4 transgenic expression in a C57BL/6 background demonstrated profound airway fibrosis and emphysematous alveolar destruction as well as impressive eosinophilic inflammation.17 Transgenic IL-5 expression caused more prominent pulmonary changes pathognomonic of asthma, which include peribronchial eosinophilic inflammation, goblet cell hyperplasia, subepithelial collagen deposition and airway hyper-responsiveness to methacholine.18 Transgenic IL-9 expression also caused eosinophilic inflammation, mast cell hyperplasia and bronchial hyper-responsiveness.19 Interestingly, many of the effects of IL-9 were the result of the ability of IL-9 to induce other Th2 cytokines, especially IL-13.19 The transgenic expression of IL-10 caused complex pulmonary alterations where it inhibited lipopolysaccharide-induced inflammation and induced lymphocytic inflammation, goblet cell metaplasia and subepithelial fibrosis.20 Interestingly, the IL-10-induced mucus response was also mediated by IL-13. In lipopolysaccharide models, induction of IL-10 in the acute period resulted in an anti-inflammatory phenotype, but when IL-10 is induced in a chronic fashion a similar pro-inflammatory phenotype was observed.21 Among Th2 cytokines, transgenic expression of IL-13 caused the most impressive Th2 inflammatory and tissue responses. In these mice, we observed quite prominent eosinophil-, macrophage- and lymphocyte-rich inflammatory responses, airway fibrosis, mucus metaplasia and airway hyper-responsiveness.22 These responses were not mediated by other Th2 cytokines, suggesting that IL-13 is a final common pathway in Th2 effector responses.23 By breeding these transgenic mice with mice with null mutations of other genes or treating these mice with chemical blockers or neutralizing antibodies, major insights have been obtained into the mechanisms that mediate these IL-13-induced responses. These studies have highlighted the important roles that chemokines and chemokine receptors,24,25 TGF-β1,26 proteases and adenosine play in the IL-13-induced alterations in the lung.27,28 Most recently, it has been shown that the inducible over-expression of VEGF in the lung also induced macrophage and eosinophil-dominant inflammation, mucus metaplasia, smooth muscle hyperplasia, airways hyper-responsiveness and neoangiogenesis.29 These mice also had a defect in tolerance to inhaled antigen and enhanced numbers of activated pulmonary dendritic cells. Interestingly, the induction of mucus in the lungs of VEGF transgenic mice was mediated by VEGF-induced IL-13 production. When viewed in combination, it is clear that the transgenic modelling of Th2 cytokines and growth factors elegantly recapitulates asthma and other allergic response-relevant phenotypes characterized by eosinophilic inflammation, fibrotic tissue responses, and airway hyper-responsiveness.
Transgenic expression of Th17 in the lung
Recent advances have led to the discovery of a new T-lymphocyte subset, Th17 cells that produce IL-17, playing a role in neutrophilic and macrophage inflammation in the lung.30 These Th17 cells are IL-17-producing cells that form a unique lineage of retinoic acid-related orphan receptor (RORγT) -expressing lymphocytes under polarization driven by cytokines such as TGF-β, IL-6, IL-1β and IL-23. It has been found that the orphan nuclear receptor RORγT directs the differentiation programme of the IL-17-expressing T helper cells.31 Th17 cells can produce both IL-17A and IL-17F. Chronic over-expression ofIL-17A or IL-17F in the mouse lung epithelium resulted in chemokine production, leucocyte and macrophage recruitment and airway changes such as hypertrophic lung epithelium in the bronchial tree and alveolar wall thickening.32,33 The levels of IL-17A and IL-17F correlate positively with severe asthma in humans. However, the role of IL-17 in murine models of asthma has been examined with mixed results. One study suggested different roles for IL-17A and IL-17F, where IL-17A-deficient mice exhibited Th2 helper responses whereas IL-17F-deficient mice displayed enhanced type 2 cytokine production and eosinophil function.33 Interleukin-17 is also critical for host defence against a wide range of pathogens through the recruitment of neutrophils and direct effects on structural lung cells. Mice lacking IL-17, or its receptor IL-17RA, are at increased susceptibility to various pulmonary infections such as those caused by Klebsiella pneumoniae and Mycoplasma pneumoniae.34,35 These mice had decreased neutrophil recruitment, which led to higher bacterial burdens in the lung. Another member of IL-17 is IL-17E, also known as IL-25, which is a product of Th2 cells and epithelial cells. The IL-17 family member IL-25 promotes canonical Th2 signalling pathways and enhances allergic responses. Chronic over-expression of IL-25 in the airway epithelium resulted in asthma features such as mucus production, and airway infiltration of eosinophils and macrophages.36 Work in this area of Th17 cytokines in the lung have actively been pursued for many pulmonary disease models.
Cytokine polarization from genetic modification of cytokine receptors or transcription factors
Cytokine receptors
The various polarizing cytokines interact through their own receptor or receptor complexes to exert their function. Interleukin-13 mediates its functions via its cognate receptor, which is a heterodimer composed of the IL-4Rα and the IL-13 binding protein, IL-13Rα1.37 The IL-13Rα1–IL-4Rα complex can act as an alternative receptor for IL-4, especially in cells that lack the common γ-chain that usually forms a complex with IL-4Rα to bind IL-4.38 The IL-13R complex is formed in a sequential manner whereby IL-13 first binds to IL-13Rα1 before recruiting IL-4Rα to form a high-affinity signalling complex.39–41 Interleukin-4 also binds to IL-13Rα1–IL-4Rα complex, but IL-13Rα1 contributes to a smaller degree to the overall binding affinity of IL-4 to its receptor.39 Over-expression of cytokine receptors is not easy to achieve given that almost all receptors are membrane-bound, which is why some investigators have taken advantage of gene deletion approaches where important cytokine and biological insights have been discovered. Use of these gene-deleted animals also helps to distinguish the differential roles of these cytokine receptors.
In an asthma mouse model using IL-13Rα1 null mice, it was reported that IL-13Rα1 is essential for baseline IgE production, but Th2 and IgE responses to T-cell-dependent antigens are IL-13Rα1-independent.42 The increased airway resistance, mucus, TGF-β and eotaxin production, but not the cellular infiltration, are critically dependent on IL-13Rα1. Through using these gene-deleted animals, the investigators were also able to identify a CCR3- and IL-13Rα1-independent pathway for lung eosinophilia.42 Others, interestingly, have shown that IL-13Rα1 null mice have exacerbated Th2 responses compared with IL-4Rα null mice, which exhibited weak Th2 responses.43
As the common subunit of receptor complexes for both IL-4 and IL-13, IL-4Rα is required for mediating signal transduction of these two cytokines.44–46 As such, IL-4Rα-deficient mice lack responsiveness for both IL-4 and IL-13. In a model of Leishmania major infection, it was found that the initial control of disease in IL-4 null and IL-4Rα null mice was equivalent; however, in contrast to IL-4 null mice, the IL-4Rα null mice developed progressive chronic disease suggesting a protective role for IL-13 signalling.47 In gene-deficient animals, IL-4Rα has been shown to be required for the control of Nippostrongylus brasiliensis, and for Th2 development during infection characterized by cytokine production and GATA-3. These Th2 parameters and the ability to control worm infection were more severely affected in mice deficient for IL-4Rα than in IL-4 gene-deficient mice. These studies help to reveal the importance of IL-13 in regulating Th2 responses to nematode infection because it seems to require IL-4Rα.48
A second IL-13R chain, IL-13Rα2, consists of a soluble form and a membrane-bound form. Structure and function studies have suggested that this IL-13Rα2 acts as a dominant negative inhibitor or a decoy receptor for IL-13.49,50 Highly polarized type 2 cytokine responses can potentially be harmful and even lethal to the host if uncontrolled. Interleukin-13Rα2 has been shown to be an important down-regulatory factor. Using a model of IL-13 transgenic mice, we saw that disruption of IL-13Rα2 in these mice led to significant augmentation of the IL-13-induced pulmonary inflammation, mucus metaplasia, subepithelial fibrosis and airway remodelling in the lung.51 Similar disruption of IL-13Rα2 had no effect on the tissue effects of lung-targeted transgenic IL-4 mice.51 In a parasitic helminth Th2 infection model, the use of IL-13Rα2 null mice showed marked exacerbation of fibrosis despite the fact that the levels of IL-13 were significantly decreased, highlighting the importance of this receptor in the down-regulation of a chronic and pathogenic Th2-mediated immune response.52
Interleukin-33 is a new member of the IL-1 family, and has been shown to be a ligand for the ST2L receptor, which is expressed mostly on mast cells, Th2 cells, basophils and eosinophils.53,54 Interleukin-33 binds to ST2L, and the complex binds to IL-1RAcP, which induces a pathway that leads to the secretion of Th2-type and pro-inflammatory cytokines.55 Gene deletion of this IL-33 receptor (T1/ST2) resulted in increased susceptibility to Toxoplasma gondii infection.56 ST2, the secreted form of the ST2L receptor, has been recently over-expressed in mice, and such over-expression abrogated the effects of IL-33, suggesting that ST2 is induced as a negative regulator.57
Toll-interleukin-1 receptor-8 (TIR8), also known as single immunoglobulin IL-1 receptor-related molecule (SIGIRR), is a member of the IL-1R-like family. Evidence to date suggests that TIR8 inhibits signalling receptor complexes of IL-1 family members associated with Th1 (IL-18), Th2 (IL-33) and Th17 (IL-1) differentiation.58 The TIR8 is expressed in Th2 cells and inhibits IL-133/T1/ST2-mediated signalling and Th2 cytokine production.59 Mice that are TIR8 null had exaggerated Th2 responses in allergic pulmonary inflammation induced by ovalbumin.59 They also exhibited hyper-responsiveness to IL-133 with elevated serum levels of IL-5 and IL-13, splenomegaly and lung inflammation. These findings from the genetically modified mice suggest that TIR8 potentially serves as a negative feedback control in Th2 polarization in the control of allergic inflammatory responses.
Transcription factors
The cytokine environment can also be polarized by modulating important transcription factors. The differentiation of naive helper T cells towards Th1 or Th2 is regulated by the transcription factors T-box expressed in T cells (or T-bet) and GATA-binding protein-3 (GATA-3), respectively.60 T-bet is a Th1-specific transcription factor known to initiate Th1 development as a result of its transactivation of the Th1 cytokine IFN-γ while inhibiting Th2 cell differentiation.61 T-bet expression has also been shown to regulate cytolytic effector mechanisms of CD8+ T cells.62 GATA-3 is a member of the GATA family of zinc finger proteins because they bind to consensus DNA sequences that comprise A/T-GATA-A/G and it plays an important role in the development of the Th2 phenotype while reciprocally inhibiting Th1 cells.63
Germ-line deletion of GATA-3 is lethal to embryos, which is why investigators have made conditionally deficient mice using the Cre-lox system. These models demonstrated that GATA-3-deficient Th2 cells have impaired Th2 cytokine production and proliferation compared with wild-type Th2 cells.64,65 GATA-3 null mice when infected with Nippostrongylus brasilienesis exhibited reduced levels of serum IgE and their T helper cells displayed a Th1 cytokine profile, instead of the expected Th2 profile, after infection.65 This nicely parallels observations in humans that carry only one functional GATA-3 allele and suffer from hypoparathyroidsm, deafness and renal dysplasia syndrome. Patients with this syndrome exhibit attenuated levels of IgE and Th2-dependent IgGs but elevated Th1-dependent IgGs.66 Forced expression of GATA-3 has been shown to be sufficient to induce the production of type 2 cytokines in cells that lack signal transducer and activator of transcription 6 (known to be important for Th2 induction), and in the presence of IFN-γ and absence of IL-4 (a cytokine environment that favours driving Th1 differentiation).67 GATA-3 on its own can completely restore Th2 development, c-af induction, Th2-specific DNase I hypersensitive sites in the IL-4 locus, and Th2 expression, allowing for Th2 commitment. Over-expression of GATA-3 in mice resulted in strong expression of T1/ST2 in T helper cells, especially preferentially expressed on Th2 cells. These T helper cells from GATA-3 transgenic mice also produce type 2 cytokines even after cultivation under Th1 polarizing conditions.68 These mice exhibited exaggerated Th2 immune responses and reciprocal attenuated Th1 responses in models of delayed-type hypersensitivity, parasitic worm infection and airway allergic inflammation.69–71 In a mouse model of hypersensitivity pneumonitis or farmer’s lung, a Th1-mediated lung inflammation that involves repeated exposure to the bacterium Saccharopolysporarectivirgula, over-expression of GATA-3 attenuated the development of hypersensitivity pneumonitis by correcting the Th1-polarizing condition.72
Transgenic mice have also been made to over-express c-maf (a proto-oncogene important in optimizing GATA-3) under a T-cell-specific promoter. These c-maf transgenic mice exhibited increase Th2 cytokine production, specifically IL-4, and elevated serum IgG1 and IgE.73 On the other hand, c-maf null mice have greatly impaired IL-4 production and the T cells spontaneously polarize to a Th1 phenotype.74 Over-expressing c-maf, in the absence of GATA-3, did not cause airway inflammation and airway hyper-reactivity. This indicated a different role for c-Maf in regulating cytokine gene expression in lung T cells versus peripheral T cells. Without GATA-3, c-Maf selectively induced IL-5 for Th2 differentiation but suppressed IL-13 production by lung CD4+ T cells.74
Work using transgenic mice over-expressing either GATA-3 or T-bet showed that these specific transcription factors by themselves can shift the lung Th1/Th2 bias after ovalbumin allergen challenges. The degrees of subepithelial fibrosis and airway smooth muscle hyperplasia after repeated allergen exposure were significantly enhanced in mice over-expressing GATA-3 compared with wild-type mice. On the other hand, allergen-induced goblet cell hyperplasia, mucus hypersecretion and eosinophilic airway inflammation were significantly lower in mice over-expressing T-bet.75
Mice lacking T-bet, on the other hand, fail to develop Th1 cells and display a dramatic reduction of IFN-γ production by CD4+ T cells.76 T-bet transgenic mice have been shown to generate a predominant Th1 response by promoting IFN-γ induction, IgG2a class switching over IgG1, and attenuation of the Th2 response. These T-bet over-expressing mice also generated a predominant type 1 cytotoxic CD8+ T-cell response with spontaneous skin inflammation and exaggerated contact dermatitis, a skin disease known to be driven by Th1 immunity.77 In an autoimmune glomerulonephritis model, where a shift in Th1 condition is thought to be a contributing factor in the kidney inflammation, over-expressing T-bet in T cells in this mouse exacerbated the disease, whereas over-expressing GATA-3 improved the glomerulonephritis in the Yaa mice.78
Foxa2, a winged helix/forkhead box transcription factor, is selectively expressed in respiratory epithelial cells and is important in the programming of Th2-cell-mediated innate immunity in the lung.79 Deletion of Foxa2 caused spontaneous pulmonary eosinophilic inflammation and goblet cell metaplasia, and increased production of Th2 cytokines and chemokines, which seems to be dependent on IL-4R signalling. The Th2-associated inflammatory mediators such as acidic chitinase (Chi1) and chitinase 3-like 1 (Chi3li) are increased in these Foxa2 null mice.
In recent years, the lineage-specific transcription factor studies have been extended by the identification of FoxP3, RORγt and Bcl6, as key regulators of T regulatory, Th17 and follicular T helper cells.80–82 Knockout of RORγT (required for Th17 cell production), for example, led to attenuated eosinophilic pulmonary inflammation and lower Th2 cytokine production in an allergic asthma mouse model, revealing the importance of this Th17 transcription factor in Th1 and Th2 balance.83 It will be interesting to use transgenic models that express these newly discovered lineage-specific transcription factors to study the cytokine polarization that leads to these new T-cell lineage populations. These animal models provide a system to modulate the level of T-bet and/or GATA-3 and other important transcription factors, and so the balance of Th1, Th2 and other T helper activities. They are useful for revealing potential important links between immunological diseases such as asthma, dermatitis, colitis and the Th1/Th2 dysbalance.
Limitations and future application of transgenic approaches in the studies of human diseases
Overall, the transgenic expression of type 1 or type 2 cytokines provides impressive insights into the effector functions of these polarizing cytokines. However, data from these animal models need to be carefully interpreted because of the inherent features of transgenic modelling systems. First, the random transgene integration into the chromosome may disrupt or modulate other untargeted gene expression. These alterations can lead to phenotypes that are not mediated by the transgene in question. Also, the variable nature of the transgene expression because of the variation in the insertion site and copy numbers of transgene may complicate the accurate evaluation of the transgene’s effects. However, by employing targeted expression of transgene, the issue of expression variation as the result of random transgene integration can be resolved as noted above. In addition, the generation and evaluation of multiple lines of transgenic mice with different levels of expression could help to address the phenotypic variance originating from variable transgene expression. Lastly, structural and physiological differences between humans and mice need to be considered in the interpretation of inflammatory and tissue phenotypes of mice.
Even with these limitations, transgenic approaches will be useful to understand the pathogenesis of human lung diseases characterized by the polarized chronic inflammation as typified by diseases such as asthma, chronic obstructive lung disease and pulmonary fibrosis. The transgenic mouse model is a powerful biological tool because certain studies cannot be performed because of the lack of potent antagonists of transcription factors or blocking antibodies for receptors and cytokines. This makes the transgenic technology an attractive option. As a disease model, transgenic mice can also be used for screening and evaluation of potential therapeutic interventions. Interestingly, recent studies revealed that chronic inflammation with skewed cytokine expression is predisposed to certain viral or bacterial infections or tumour metastases, suggesting that there is an intimate relationship between innate immunity and local cytokine milieu. These emerging concepts may explain the mechanism of super-infection or enhanced tumour metastasis that lead to exacerbated progression of human diseases such as asthma or chronic obstructive lung disease. In this regard, these transgenic mouse models provide an excellent system with which to investigate the cellular and molecular mechanisms of these complex disease progressions and interactions between innate and adaptive immune responses.
Acknowledgments
This work was supported by National Institutes of Health grant RO1-HL084225 (to C.G. Lee), and grants from the Parker B. Francis Foundation and the Flight Attendants Medical Research Institute (FAMRI) (to C.S. Dela Cruz).
Disclosures
The authors have no financial disclosure.
References
- 1.Moore BB, Moore TA, Toews GB. Role of T- and B-lymphocytes in pulmonary host defences. Eur Respir J. 2001;18:846–56. doi: 10.1183/09031936.01.00229001. [DOI] [PubMed] [Google Scholar]
- 2.Lee CG, Elias JA. Transgenic models. In: Laurent GL, Shapiro S, editors. Encyclopedia of Respiratory Medicine. Maryland Heights, MO, USA: Elsevier; 2006. pp. 283–7. [Google Scholar]
- 3.Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003;111:291–7. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raymond T, Schaller M, Hogaboam CM, Lukacs NW, Rochford R, Kunkel SL. Toll-like receptors, Notch ligands, and cytokines drive the chronicity of lung inflammation. Proc Am Thorac Soc. 2007;4:635–41. doi: 10.1513/pats.200706-067TH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruth JH, Warmington KS, Shang X, Lincoln P, Evanoff H, Kunkel SL, Chensue SW. Interleukin 4 and 13 participation in mycobacterial (type-1) and schistosomal (type-2) antigen-elicited pulmonary granuloma formation: multiparameter analysis of cellular recruitment, chemokine expression and cytokine networks. Cytokine. 2000;12:432–44. doi: 10.1006/cyto.1999.0595. [DOI] [PubMed] [Google Scholar]
- 6.Nevala WK, Vachon CM, Leontovich AA, Scott CG, Thompson MA, Markovic SN. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res. 2009;15:1931–9. doi: 10.1158/1078-0432.CCR-08-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strieter RM, Keane MP. Innate immunity dictates cytokine polarization relevant to the development of pulmonary fibrosis. J Clin Invest. 2004;114:165–8. doi: 10.1172/JCI22398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon JW, Scangos GA, Plotkin DJ, Barbosa JA, Ruddle FH. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980;77:7380–4. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palais G, Nguyen Dinh Cat A, Friedman H, et al. Targeted transgenesis at the HPRT locus: an efficient strategy to achieve tightly controlled in vivo conditional expression with the tet system. Physiol Genomics. 2009;37:140–6. doi: 10.1152/physiolgenomics.90328.2008. [DOI] [PubMed] [Google Scholar]
- 10.Cvetkovic B, Yang B, Williamson RA, Sigmund CD. Appropriate tissue- and cell-specific expression of a single copy human angiotensinogen transgene specifically targeted upstream of the HPRT locus by homologous recombination. J Biol Chem. 2000;275:1073–8. doi: 10.1074/jbc.275.2.1073. [DOI] [PubMed] [Google Scholar]
- 11.Nyabi O, Naessens M, Haigh K, et al. Efficient mouse transgenesis using Gateway-compatible ROSA26 locus targeting vectors and F1 hybrid ES cells. Nucleic Acids Res. 2009;37:e55. doi: 10.1093/nar/gkp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hohenstein P, Slight J, Ozdemir DD, Burn SF, Berry R, Hastie ND. High-efficiency Rosa26 knock-in vector construction for Cre-regulated overexpression and RNAi. Pathogenetics. 2008;1:3. doi: 10.1186/1755-8417-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Zheng T, Zhu Z, Homer RJ, Riese RJ, Chapman HA, Jr, Shapiro SD, Elias JA. Interferon γ induction of pulmonary emphysema in the adult murine lung. J Exp Med. 2000;192:1587–600. doi: 10.1084/jem.192.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGrath-Morrow S, Laube B, Tzou SC, Cho C, Cleary J, Kimura H, Rose NR, Caturegli P. IL-12 overexpression in mice as a model for Sjögren lung disease. Am J Physiol Lung Cell Mol Physiol. 2006;291:L837–46. doi: 10.1152/ajplung.00134.2006. [DOI] [PubMed] [Google Scholar]
- 16.Rankin JA, Picarella DE, Geba GP, et al. Phenotypic and physiologic characterization of transgenic mice expressing interleukin 4 in the lung: lymphocytic and eosinophilic inflammation without airway hyperreactivity. Proc Natl Acad Sci U S A. 1996;93:7821–5. doi: 10.1073/pnas.93.15.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma B, Blackburn MR, Lee CG, et al. Adenosine metabolism and murine strain-specific IL-4-induced inflammation, emphysema, and fibrosis. J Clin Invest. 2006;116:1274–83. doi: 10.1172/JCI26372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JJ, McGarry MP, Farmer SC, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–56. doi: 10.1084/jem.185.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Temann UA, Ray P, Flavell RA. Pulmonary overexpression of IL-9 induces Th2 cytokine expression, leading to immune pathology. J Clin Invest. 2002;109:29–39. doi: 10.1172/JCI13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CG, Homer RJ, Cohn L, et al. Transgenic overexpression of interleukin (IL)-10 in the lung causes mucus metaplasia, tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J Biol Chem. 2002;277:35466–74. doi: 10.1074/jbc.M206395200. [DOI] [PubMed] [Google Scholar]
- 21.Spight D, Zhao B, Haas M, Wert S, Denenberg A, Shanley TP. Immunoregulatory effects of regulated, lung-targeted expression of IL-10 in vivo. Am J Physiol Lung Cell Mol Physiol. 2005;288:L251–65. doi: 10.1152/ajplung.00122.2004. [DOI] [PubMed] [Google Scholar]
- 22.Zhu Z, Homer RJ, Wang Z, Chen Q, Geba GP, Wang J, Zhang Y, Elias JA. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–88. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elias JA, Zheng T, Lee CG, Homer RJ, Chen Q, Ma B, Blackburn M, Zhu Z. Transgenic modeling of interleukin-13 in the lung. Chest. 2003;3(Suppl):339S–45S. [PubMed] [Google Scholar]
- 24.Ma B, Liu W, Homer RJ, Lee PJ, Coyle AJ, Lora JM, Lee CG, Elias JA. Role of CCR5 in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol. 2006;176:4968–78. doi: 10.4049/jimmunol.176.8.4968. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Z, Ma B, Zheng T, Homer RJ, Lee CG, Charo IF, Noble P, Elias JA. IL-13-induced chemokine responses in the lung: role of CCR2 in the pathogenesis of IL-13-induced inflammation and remodeling. J Immunol. 2002;168:2953–62. doi: 10.4049/jimmunol.168.6.2953. [DOI] [PubMed] [Google Scholar]
- 26.Lee CG, Homer RJ, Zhu Z, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor β1. J Exp Med. 2001;194:809–21. doi: 10.1084/jem.194.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackburn MR, Lee CG, Young HW, Zhu Z, Chunn JL, Kang MJ, Banerjee SK, Elias JA. Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. J Clin Invest. 2003;112:332–44. doi: 10.1172/JCI16815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanone S, Zheng T, Zhu Z, et al. Overlapping and enzyme-specific contributions of matrix metalloproteinases-9 and -12 in IL-13-induced inflammation and remodeling. J Clin Invest. 2002;110:463–74. doi: 10.1172/JCI14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CG, Link H, Baluk P, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol. 2010;72:495–516. doi: 10.1146/annurev-physiol-021909-135926. [DOI] [PubMed] [Google Scholar]
- 31.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 32.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang XO, Chang SH, Park H, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–75. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–27. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angkasekwinai P, Park H, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–17. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hilton DJ, Zhang JG, Metcalf D, Alexander WS, Nicola NA, Willson TA. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci U S A. 1996;93:497–501. doi: 10.1073/pnas.93.1.497. JID – 7505876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obiri NI, Debinski W, Leonard WJ, Puri RK. Receptor for interleukin 13. Interaction with interleukin 4 by a mechanism that does not involve the common γ chain shared by receptors for interleukins 2, 4, 7, 9, and 15. J Biol Chem. 1995;270:15. doi: 10.1074/jbc.270.15.8797. [DOI] [PubMed] [Google Scholar]
- 39.Andrews AL, Holloway JW, Holgate ST, Davies DE. IL-4 receptor α is an important modulator of IL-4 and IL-13 receptor binding: implications for the development of therapeutic targets. J Immunol. 2006;176:7456–61. doi: 10.4049/jimmunol.176.12.7456. [DOI] [PubMed] [Google Scholar]
- 40.Andrews AL, Holloway JW, Puddicombe SM, Holgate ST, Davies DE. Kinetic analysis of the interleukin-13 receptor complex. J Biol Chem. 2002;277:46073–8. doi: 10.1074/jbc.M209560200. [DOI] [PubMed] [Google Scholar]
- 41.Andrews AL, Nordgren IK, Kirby I, Holloway JW, Holgate ST, Davies DE, Tavassoli A. Cytoplasmic tail of IL-13Rα2 regulates IL-4 signal transduction. Biochem Soc Trans. 2009;37(Pt 4):873–6. doi: 10.1042/BST0370873. [DOI] [PubMed] [Google Scholar]
- 42.Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor α1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci U S A. 2008;105:7240–5. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramalingam TR, Pesce JT, Sheikh F, et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor α1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–38. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 45.Wills-Karp M, Finkelman FD. Untangling the complex web of IL-4- and IL-13-mediated signaling pathways. Sci Signal. 2008;1:pe55. doi: 10.1126/scisignal.1.51.pe55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–90. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 47.Mohrs M, Ledermann B, Kohler G, Dorfmuller A, Gessner A, Brombacher F. Differences between IL-4- and IL-4 receptor α-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J Immunol. 1999;162:7302–8. [PubMed] [Google Scholar]
- 48.Barner M, Mohrs M, Brombacher F, Kopf M. Differences between IL-4R α-deficient and IL-4-deficient mice reveal a role for IL-13 in the regulation of Th2 responses. Curr Biol. 1998;8:669–72. doi: 10.1016/s0960-9822(98)70256-8. [DOI] [PubMed] [Google Scholar]
- 49.Donaldson DD, Whitters MJ, Fitz LJ, et al. The murine IL-13 receptor α2: molecular cloning, characterization, and comparison with murine IL-13 receptor α1. J Immunol. 1998;161:2317–24. [PubMed] [Google Scholar]
- 50.Kawakami K, Taguchi J, Murata T, Puri RK. The interleukin-13 receptor α2 chain: an essential component for binding and internalization but not for interleukin-13-induced signal transduction through the STAT6 pathway. Blood. 2001;97:2673–9. doi: 10.1182/blood.v97.9.2673. [DOI] [PubMed] [Google Scholar]
- 51.Zheng T, Liu W, Oh SY, et al. IL-13 receptor α2 selectively inhibits IL-13-induced responses in the murine lung. J Immunol. 2008;180:522–9. doi: 10.4049/jimmunol.180.1.522. [DOI] [PubMed] [Google Scholar]
- 52.Chiaramonte MG, Mentink-Kane M, Jacobson BA, et al. Regulation and function of the interleukin 13 receptor α2 during a T helper cell type 2-dominant immune response. J Exp Med. 2003;197:687–701. doi: 10.1084/jem.20020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kondo Y, Yoshimoto T, Yasuda K, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 54.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–90. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurowska-Stolarska M, Kewin P, Murphy G, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–90. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- 56.Jones LA, Roberts F, Nickdel MB, Brombacher F, McKenzie AN, Henriquez FL, Alexander J, Roberts CW. IL-33 receptor (T1/ST2) signalling is necessary to prevent the development of encephalitis in mice infected with Toxoplasma gondii. Eur J Immunol. 2010;40:426–36. doi: 10.1002/eji.200939705. [DOI] [PubMed] [Google Scholar]
- 57.Ohto-Ozaki H, Kuroiwa K, Mato N, Matsuyama Y, Hayakawa M, Tamemoto H, Tominaga SI. Characterization of ST2 transgenic mice with resistance to IL-33. Eur J Immunol. 2010;40:2632–42. doi: 10.1002/eji.200940291. [DOI] [PubMed] [Google Scholar]
- 58.Garlanda C, Anders HJ, Mantovani A. TIR8/SIGIRR: an IL-1R/TLR family member with regulatory functions in inflammation and T cell polarization. Trends Immunol. 2009;30:439–46. doi: 10.1016/j.it.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Bulek K, Swaidani S, Qin J, et al. The essential role of single Ig IL-1 receptor-related molecule/Toll IL-1R8 in regulation of Th2 immune response. J Immunol. 2009;182:2601–9. doi: 10.4049/jimmunol.0802729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fields PE, Kim ST, Flavell RA. Cutting edge: changes in histone acetylation at the IL-4 and IFN-γ loci accompany Th1/Th2 differentiation. J Immunol. 2002;169:647–50. doi: 10.4049/jimmunol.169.2.647. [DOI] [PubMed] [Google Scholar]
- 61.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 62.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A. 2003;100:15818–23. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem. 1997;272:21597–603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 64.Pai SY, Truitt ML, Ho IC. GATA-3 deficiency abrogates the development and maintenance of T helper type 2 cells. Proc Natl Acad Sci U S A. 2004;101:1993–8. doi: 10.1073/pnas.0308697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu J, Min B, Hu-Li J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–65. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 66.Skapenko A, Leipe J, Niesner U, et al. GATA-3 in human T cell helper type 2 development. J Exp Med. 2004;199:423–8. doi: 10.1084/jem.20031323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ouyang W, Lohning M, Gao Z, Assenmacher M, Ranganath S, Radbruch A, Murphy KM. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12:27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 68.Nawijn MC, Dingjan GM, Ferreira R, Lambrecht BN, Karis A, Grosveld F, Savelkoul H, Hendriks RW. Enforced expression of GATA-3 in transgenic mice inhibits Th1 differentiation and induces the formation of a T1/ST2-expressing Th2-committed T cell compartment in vivo. J Immunol. 2001;167:724–32. doi: 10.4049/jimmunol.167.2.724. [DOI] [PubMed] [Google Scholar]
- 69.Ozawa H, Tamauchi H, Ito M, Terashima M, Inoue M, Hozumi K, Habu S, Watanabe N. Immune responses to Nippostrongylus brasiliensis and tuberculin protein in GATA-3-transgenic mice. Immunol Lett. 2005;99:228–35. doi: 10.1016/j.imlet.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Watanabe N, Tamauchi H, Ozawa H, Ito MF, Ovary Z, Habu S. Th2 immune responses in GATA-3-transgenic mice infected with Heligmosomoides polygyrus. Int Arch Allergy Immunol. 2003;131(Suppl 1):11–4. doi: 10.1159/000070475. JID – 9211652. [DOI] [PubMed] [Google Scholar]
- 71.Yamashita N, Tashimo H, Ishida H, et al. Involvement of GATA-3-dependent Th2 lymphocyte activation in airway hyperresponsiveness. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1045–51. doi: 10.1152/ajplung.00195.2005. [DOI] [PubMed] [Google Scholar]
- 72.Matsuno Y, Ishii Y, Yoh K, et al. Overexpression of GATA-3 protects against the development of hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2007;176:1015–25. doi: 10.1164/rccm.200612-1887OC. [DOI] [PubMed] [Google Scholar]
- 73.Ho IC, Lo D, Glimcher LH. c-maf promotes T helper cell type 2 (Th2) and attenuates Th1 differentiation by both interleukin 4-dependent and -independent mechanisms. J Exp Med. 1998;188:1859–66. doi: 10.1084/jem.188.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hausding M, Sauer K, Maxeiner JH, Finotto S. Transgenic models in allergic responses. Curr Drug Targets. 2008;9:503–10. doi: 10.2174/138945008784533570. [DOI] [PubMed] [Google Scholar]
- 75.Kiwamoto T, Ishii Y, Morishima Y, et al. Transcription factors T-bet and GATA-3 regulate development of airway remodeling. Am J Respir Crit Care Med. 2006;174:141–51. doi: 10.1164/rccm.200601-079OC. JID – 9421642. [DOI] [PubMed] [Google Scholar]
- 76.Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci U S A. 2002;99:5545–50. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishizaki K, Yamada A, Yoh K, et al. Th1 and type 1 cytotoxic T cells dominate responses in T-bet overexpression transgenic mice that develop contact dermatitis. J Immunol. 2007;178:605–12. doi: 10.4049/jimmunol.178.1.605. [DOI] [PubMed] [Google Scholar]
- 78.Shimohata H, Yamada A, Yoh K, Ishizaki K, Morito N, Yamagata K, Takahashi S. Overexpression of T-bet in T cells accelerates autoimmune glomerulonephritis in mice with a dominant Th1 background. J Nephrol. 2009;22:123–9. [PubMed] [Google Scholar]
- 79.Chen G, Wan H, Luo F, Zhang L, Xu Y, Lewkowich I, Wills-Karp M, Whitsett JA. Foxa2 programs Th2 cell-mediated innate immunity in the developing lung. J Immunol. 2010;184:6133–41. doi: 10.4049/jimmunol.1000223. [DOI] [PubMed] [Google Scholar]
- 80.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 81.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 82.Yu D, Rao S, Tsai LM, et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–68. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 83.Tilley SL, Jaradat M, Stapleton C, et al. Retinoid-related orphan receptor γ controls immunoglobulin production and Th1/Th2 cytokine balance in the adaptive immune response to allergen. J Immunol. 2007;178:3208–18. doi: 10.4049/jimmunol.178.5.3208. [DOI] [PubMed] [Google Scholar]
- 84.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]