Abstract
Sialic-acid-binding immunoglobulin-like lectins, siglecs, are important immune receptors expressed widely in mammals. A unique feature of siglecs is their ability to bind sialylated glycans and transmit signals to immune cells. The CD33-related siglecs (CD33rSiglecs) form a major subfamily of the siglecs, containing a large, rapidly evolving group of genes that expanded in mammals through an inverse duplication event involving a primordial cluster of siglec genes over 180 million years ago. Humans express a much larger set of CD33rSiglecs than mice and rats, a feature that can be explained by a dramatic loss of CD33rSiglec genes in rodents. Most CD33rSiglecs have immune receptor tyrosine-based inhibitory motifs and signal negatively. Interestingly, novel DAP-12-coupled ‘activating’ CD33rSiglecs have been identified, such as siglec-14 and siglec-16, which are paired with the inhibitory receptors, siglec-5 and siglec-11, respectively. The evolution of these activating receptors may have been driven in part by pathogen exploitation of inhibitory siglecs, thereby providing the host with additional pathways by which to combat these pathogens. Inhibitory siglecs seem to play important and varied roles in the regulation of host immune responses. For example, several CD33rSiglecs have been implicated in the negative regulation of Toll-like receptor signalling during innate responses; siglec-G functions as a negative regulator of B1-cell expansion and appears to suppress inflammatory responses to host-derived ‘danger-associated molecular patterns’. Recent work has also shown that engagement of neutrophil-expressed siglec-9 by certain strains of sialylated Group B streptococci can suppress killing responses, thereby providing experimental support for pathogen exploitation of host CD33rSiglecs.
Keywords: evolution, immunoglobulins, lectins, sialic acids, siglecs
Introduction
Sialic-acid-binding immunoglobulin-like lectins, siglecs, form a family of cell surface receptors expressed on immune cells that mostly mediate inhibitory signalling1–3 (Fig. 1, Table 1). Like other important inhibitory immune receptor families such as killer-cell immunoglobulin-like receptor4,5 and leucocyte immunoglobulin-like receptor,6 siglecs are transmembrane molecules that contain inhibitory signalling motifs named immunoreceptor tyrosine-based inhibitory motifs (ITIMs)7,8 in their cytoplasmic tails and immunoglobulin superfamily domains in their extracellular portions. Compared with other immunoglobulin superfamily proteins, a unique feature of siglecs is that their specific ligands are sialylated carbohydrates, unlike most other immune receptors that bind to protein determinants.
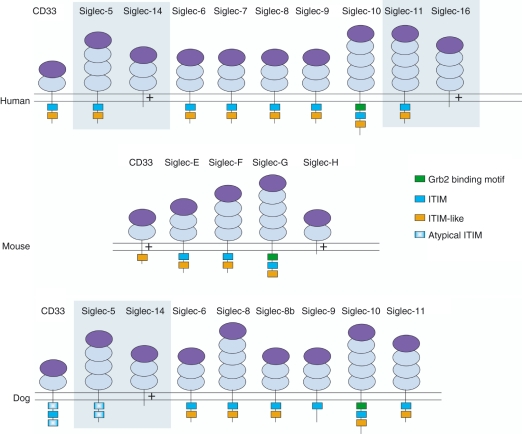
Figure 1.
Domain structure of CD33-related sialic-acid-binding immunoglobulin-like lectins (CD33rSiglecs) in human, mouse and dog. Shading represents paired receptors in which the terminal immunoglobulin domains are > 99% identical but the transmembrane and cytoplasmic tails are distinct. + represents a positively charged residue in the transmembrane domain. Dark purple domains are V-set immunoglobulin superfamily (IgSF) domains that mediate sialic acid binding, light purple domains are C2-set IgSF domains. Siglec-8b is the result of a gene duplication of siglec-8 found in dog but not primates or rodents. Atypical immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in dog CD33 and siglec-5 do not conform to canonical ITIM sequence nor do they share similarity to ITIM-like motifs with other siglecs.
Table 1.
Expression, function and preferred ligands of known human and mouse CD33-related sialic-acid-binding immunoglobulin-like lectins (CD33rSiglecs)
| Siglec | Cellular expression | Known functions | Preferred glycan | ITIM present? | References |
|---|---|---|---|---|---|
| Human Siglec-3 (CD33) | Myeloid progenitors, monocytes | Inhibition of Ca2+ flux, cell growth and apoptosis. Release of cytokines | α-2,6-linked sialic acid | Yes | 54 |
| Mouse CD33 | Neutrophils, macrophage subsets | α-2,6-linked sialic acid | No (transmembrane lysine) | 34 | |
| Human Siglec-5 | Neutrophils, B cells | Increased respiratory burst in neutrophils and uptake of Neisseria meningitidis | α-2,6- and α-2,3-linked sialic acids | Yes | 55 |
| Human Siglec-14 | Neutrophils and monocytes | α-2,6- and α-2,3-linked sialic acids | No (transmembrane arginine) | 20,30 | |
| Human Siglec-6 | Trophoblasts and B cells | Sialyl Tn structures | Yes | 56 | |
| Human Siglec-7 (p75/AIRM-1) | Natural killer cells, minor subset of CD8 T cells, monocytes | Inhibition of cytotoxicity and T-cell receptor signalling | α-2,8-linked sialic acid, internal α-2,6-linked sialic acid; weaker to α-2,3-and α-2,6-linked sialic acids. | Yes | 57 |
| Human Siglec-9 | Neutrophils, monocytes, conventional dendritic cells | Inhibition of T-cell receptor-dependent signalling | α-2,6- and α-2,3-linked sialic acids | Yes | 58 |
| Mouse Siglec-E | Neutrophils, monocytes and minor subsets of natural killer and CD8 T cells induced on macrophages by MyD88-coupled Toll-like receptor ligation | α-2,6- and α-2,3-linked sialic acids | Yes | 38,39 | |
| Human Siglec-8 | Eosinophils, basophils | Induction of apoptosis | 6′-sulphated sialyl Lewis X | Yes | 59 |
| Mouse Siglec-F | Eosinophils and alveolar macrophages | Inhibition of eosinophil expansion in airway inflammation | 6′-sulphated sialyl Lewis X | Yes | 60 |
| Human Siglec-10 | Natural killer-like cells, B cells, monocytes and eosinophils | Binds CD24 and proposed to reduce inflammation in response to tissue damage | α-2,6- and α-2,3-linked sialic acids, vascular adhesion molecule-1, CD24 glycoforms | Yes and one Grb-2-like motif | 61–63 |
| Mouse Siglec-G | B cells | Controls B1 cell expansion and activation; binds CD24 and reduces inflammation in response to tissue damage | CD24 glycoforms | Yes and one Grb-2-like motif | 41,42 |
| Human Siglec-11 | Macrophages especially microglia | Suppression of production of pro-inflammatory mediators and phagocytosis by microglia. | α-2,8-linked sialic acids | Yes | 24 |
| Human Siglec-16 | Macrophages | No (transmembrane lysine) | 22 | ||
| Mouse Siglec-H | Plasmacytoid dendritic cells | Inhibition of type-I interferon production | No known ligand | No (transmembrane lysine) | 31,32 |
Related human and mouse siglecs and known paired siglecs are grouped together.
ITIM, immunoreceptor tyrosine-based inhibitory motifs.
Interest in siglecs has grown over recent years as it has become increasingly clear that these receptors play a wide range of roles in the immune system. Following the sequencing of the human genome,9 known siglecs have expanded from the well-characterized conserved members: sialoadhesin,10 CD22,11–16 CD3317 and myelin-associated glycoprotein,18 to the rapidly evolving large CD33-related siglec (CD33rSiglec) subfamily (Fig. 1, Table 1)19 and novel potentially activating members of the siglec family.20–22 This review focuses on new ideas about the evolution of the CD33rSiglecs and discusses the functional roles that CD33rSiglecs play in the host as well as their interactions with pathogens.
Evolution of CD33rSiglecs, a history of host and pathogen interaction
Sialic acids are ubiquitously found on the surface of mammalian cells.1,2 CD33rSiglecs form a large cluster on chromosome 19 in humans and this cluster is well conserved in all mammals.2,23 Following a study of different species including primates, rodents, dog, cow, marsupials, amphibians and fish, Cao et al.23 proposed that the CD33rSiglecs cluster in mammals was the product of a major inverse duplication of a smaller sub-cluster that arose early in mammalian evolution 180 million years ago (Fig. 2). This conclusion is based on observations that (i) CD33rSiglecs are encoded in opposing orientations across the cluster, (ii) there are several non-siglec genes found in duplicates across the sub-cluster and (iii) these non-siglec genes are also encoded in opposing orientations and found at locations consistent with a major inverse duplication.23 One of the major implications of this theory is that the small CD33rSiglecs cluster in mice and rats, which was thought to have possibly represented the primordial cluster from which primate CD33rSiglecs evolved,2 is more likely to have arisen from a substantial deletion of a larger inversely duplicated cluster of genes shared among all mammals.2,23 Primates, in contrast, appear to have extended their CD33rSiglecs to include many non-functional pseudogenes, several of which are thought to have once had an activating signalling role in contrast to the rest of the CD33rSiglec family, which are predominantly ITIM-containing inhibitory receptors.22,23 Dog is a more divergent species compared with primates and rodents. Study of dog CD33rSiglecs provides evidence for expansion in primates and deletion in rodents because dog and primates share many CD33rSiglec genes that are missing in rodents (Fig. 1) but primates display a greater number of pseudogenes, which are missing in dog.23
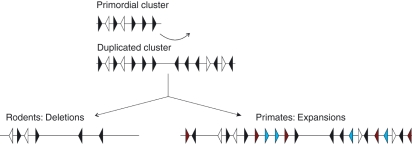
Figure 2.
Simplified inversed duplication model of CD33-related sialic-acid-binding immunoglobulin-like lectin (CD33rSiglec) evolution. A primordial cluster containing five siglec genes arranged in tandem probably existed before mammalian evolution 180 million years ago (siglec genes in black). This cluster is thought to have also contained non-siglec genes (white) located between the siglec genes. This primordial cluster is thought to have undergone an inverse duplication, creating a larger cluster with two sub-clusters, each containing five siglec genes and two non-siglec genes encoded in opposing directions. This is the initial cluster from which CD33rSiglec clusters of different mammalian species evolved. Species differences are most pronounced when comparing rodents and primates. Rodents show a large-scale deletion of genes from the cluster, resulting in four siglecs only. In contrast, primates maintained most siglecs from the initial cluster and also expanded siglec genes giving rise to both activating (red) and inhibitory (blue) forms. However, most of the newly formed siglecs have become pseudogenes, consistent with a strong de-selective drive.
The rise and fall of activating CD33rSiglecs
One example of the newly formed potentially activating siglecs in primates is siglec-16. Siglec-16 was originally reported to contain a 4-bp deletion in the second exon that encodes its first N-terminal immunoglobulin-like domain, rendering it non-functional.24 However, genetic analysis of UK Caucasians showed that siglec-16 is in fact not a pseudogene and encodes a full open reading frame.22 A polymorphism analysis revealed a 50–50% split in the UK population between the two alleles: wild-type and the 4-bp deletion mutant alleles.22
Siglec-16 is paired with siglec-11,24 which is an inhibitory receptor of the CD33rSiglec family.22 Siglecs-11 and -16 share 99% homology in their first three extracellular immunoglobulin superfamily domains22 and both show expression in the brain. However, similarities between the two receptors break down in the transmembrane domain. Siglec-11, like most transmembrane receptors, is neutrally charged in the transmembrane portion, in contrast to siglec-16, which encodes both a positively charged lysine that has been shown to bind the immunoreceptor tyrosine-based activation motif (ITAM) containing adaptor molecule, DAP12, as well as a negatively charged glutamate residue at – 4 position from the lysine.22 The ITAM encoded in the cytoplasmic portion of DAP12 can recruit protein tyrosine kinases such as syk,25 which play a role in cellular activation.8,26
It is generally accepted that sialic acids evolved first in higher animals and were then acquired by several microbial pathogens through various mechanisms,2 but alternative theories also exist.27 It was proposed that activating siglecs arose as a way to combat pathogenic engagement of host inhibitory siglecs and dampening of immune cell signalling22,23,28 (Fig. 3a). However, one can envisage the detrimental effect of uncontrolled over-activation in the immune system that may be experienced by the introduction of activating siglecs that recognize the same ligand as their inhibitory isoforms. This might explain the rapid de-selection of these newly ‘invented’ activating siglecs.23 For example, siglec-11 has been shown to display important neuroprotective properties, such as inhibition of production of pro-inflammatory mediators, interleukin-1β (IL-1β) and nitric oxide synthase-2 and phagocytosis in microglia, the resident macrophage in the brain.29 Engagement of siglec-16 in the brain with the same ligand as siglec-11, could trigger inappropriate immune and inflammatory responses. In fact, for siglec-16, equilibrium is observed between the wild-type and mutant alleles in the population. We could be witnessing a gradual phasing out of the new siglec-16 gene in humans or it might indicate that a balance has already been achieved between the pathogenic pressure to keep siglec-16 in the population and the de-selective pressure against siglec-16 driven by its detrimental effects on immune activation22 (Fig. 3b).
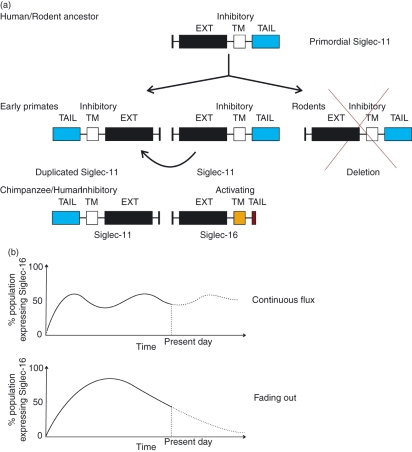
Figure 3.
Evolution of sialic-acid-binding immunoglobulin-like lectin 16 (siglec-16) from siglec-11. An ancestral siglec-11 gene existed before rodents and primates. This gene underwent an inverse duplication in early primates creating two inhibitory siglec-11-like genes. The gene located at the original location of siglec-11 underwent inverse duplication in the chimpanzee to create a new siglec-16 gene containing an activating transmembrane domain and a cytoplasmic tail lacking immunoreceptor tyrosine-based inhibitory motifs (ITIMs). Rodents show a complete deletion of the siglec-11 gene and no inverse duplication (a). Currently siglec-16 wild-type and 4-bp deletion alleles exist at 50%: 50% distribution in the population. We make two hypotheses as to how this ratio came about. In the first hypothesis (upper), after creation of siglec-16, a balance of positive selective pressure from pathogen manipulation of siglec-11 and negative selective pressure from siglec-16 causing an inappropriate immune response has kept the wild-type and 4-bp deletion alleles at continuous flux. In the second hypothesis (lower), after creation of siglec-16, negative selection as the result of inappropriate activation of wild-type siglec-16 has overcome the positive selective effect of the pathogen causing a gradual fading of the wild-type allele and accumulation of the 4-bp deletion mutant in the population. It could also be the case that the pathogen(s) responsible for selection of siglec-16 was simply eliminated from the population after the appearance of siglec-16 (b).
Discovery of other potentially activating siglecs
Besides siglec-16, three other recently characterized siglecs possess charged transmembrane domains and can interact with DAP12: siglec-14 in humans,20,30 siglec-15 in human and mouse21 and siglec-H in rodents only.31–33 Like siglec-11 and siglec-16, human siglec-14 is paired with siglec-5 and both pairs of siglecs share high homology in their extracellular domains. A transmembrane domain in siglec-14, containing a charged arginine residue, allows siglec-14 to interact with DAP12, unlike siglec-5. Siglec-5 also contains inhibitory ITIM-like motifs, which siglec-14 lacks. Recent studies show a fusion at the genomic level in parts of the population between siglec-5 and siglec-14 that results in a functional deletion of siglec-14.30 This phenomenon is consistent with the observation of strong de-selection imposed upon activating siglecs as discussed above.
Siglec-1521 is different among the newly discovered potentially activating siglecs in two ways. First, it is conserved from mammals to fish.21 Second, siglec-15 is the only receptor in the siglec family that encodes both an ITIM and a charged transmembrane residue that has been shown to associate promiscuously with the positive signalling adaptor molecules, DAP10, DAP12 and Fc receptor γ-chain.21 It will be interesting to see how signalling through siglec-15 is regulated and whether siglec-15 survived such a long evolution because of its ability to trigger different types of signalling.
Siglec-H is a rodent CD33rSiglec expressed specifically on plasmacytoid dendritic cells (pDCs) and is a good marker for pDCs.32 Siglec-H contains a transmembrane lysine residue and associates with DAP12.31,32 However, it has not been shown so far to bind sialic acids and when cross-linked at the cell surface it paradoxically triggers inhibition of interferon-α production, as discussed further below. The role of siglec-H as an endocytic receptor has been characterized by Zhang et al.,31 who targeted pDCs using anti-siglec-H IgG coupled to ovalbumin. Siglec-H-dependent uptake led to cross-presentation of ovalbumin antigens to CD8+ T cells via MHC class I molecules on pDCs, resulting in antigen-specific CD8+ T-cell expansion.31
Mouse CD33 differs from human CD33 because it also encodes a charged transmembrane containing a lysine residue. To date, it has not been shown whether this feature enables murine CD33 to associate with adaptor molecules such as DAP12. However, a preliminary analysis of CD33-deficient mice revealed no clear-cut differences in regulation of inflammatory responses.34
CD33rSiglecs and the host immune system
Negative regulatory functions of different CD33rSiglecs have been observed in studies of cell expansion, cytokine production, cellular activation and induction of apoptosis (reviewed in ref. 1). It is likely, although not directly demonstrated in most cases, that the cytoplasmic ITIM and ITIM-like motif are important in these functions via recruitment of downstream targets such as SHP-1 and SHP-2 tyrosine phosphatases as well as other SH2-domain-containing effector molecules.1,35 Below we summarize recent data supporting a role of CD33rSiglecs in the regulation of inflammatory and immune responses.
The role of CD33rSiglecs in regulating Toll-like receptor-driven cytokine production
Using over-expression in mouse RAW and human THP-1 macrophage-like cell lines, siglec-9 expression was shown to suppress the Toll-like receptor (TLR) -dependent production of pro-inflammatory cytokines, tumour necrosis factor-α (TNF-α) and IL-6, in macrophages following lipopolysaccharide (LPS) or peptidoglycan stimulation.35 In contrast, production of the anti-inflammatory cytokine IL-10 was enhanced. These effects were abolished when the critical tyrosine residues in ITIM and ITIM-like motifs of siglec-9 were mutated.35 These observations are consistent with earlier studies of human monocytes in which siRNA-mediated knockdown of CD33 led to spontaneous secretion of pro-inflammatory cytokines36 and collectively they indicate that ITIM-bearing CD33rSiglecs may restrain the pro-inflammatory functions of macrophages.
Cross-talk between CD33rSiglecs and TLR signalling pathways was also demonstrated for siglec-H.32,33 Following cross-linking of siglec-H expressed in pDCs with antibodies, type-I interferon production in response to TLR-9 ligation with CpG was strongly inhibited. This paradoxical inhibition of cytokine production via DAP12-coupled ‘activating’ receptors has been observed with several pDC-expressed receptors and may be the result of a signalling pathway in pDCs shared with B cells that suppresses type 1 interferon production.37
Siglec-E is a typical inhibitory murine siglec expressed on myeloid cells.38,39 Boyd et al.40 have recently demonstrated a TLR- and MyD88-dependent up-regulation of siglec-E on murine bone-marrow-derived macrophages. Cross-linking siglec-E using specific antibodies shows reduction in production of nuclear factor-κB-dependent cytokines, TNF-α and IL-6, in response to LPS stimulation. This suggests that siglec-E up-regulation on macrophages represents a negative feedback pathway that limits the inflammatory response to LPS signalling.
A potential limitation of receptor over-expression and the use of antibodies to cross-link siglecs is that they may trigger non-physiological signalling pathways. Siglecs are normally masked on the cell surface via cis interactions with cell-expressed sialic acids, which limits the ability of exogenous trans ligands to induce clustering at the cell surface. Furthermore, the natural siglec–sialic acid interactions are much weaker than the siglec–antibody interactions and typically in the affinity range of 100–1000 μm. Alternative in vitro approaches include the use of synthetic sialylated carbohydrates to cross-link siglecs, which might better approximate the natural interactions between siglecs and their ligands on other cells in terms of both affinity and avidity. Siglec-deficient mice are proving useful in determining the precise regulatory role of siglecs as discussed further below.
Siglec-G as a negative regulator of mouse B1a cell expansion and antibody production
Siglec-G is predominantly expressed on B cells, including the B1a cell population that is important for making rapid T-independent IgM responses to bacterial carbohydrate antigens as well as natural antibodies.41 Hoffmann et al.41 showed that siglec-G-deficient mice had a large expansion of the B1a population which began early in development and this was independently confirmed by Ding et al.42 The expansion was specific to B1a B cells and not follicular B2 B cells, which also express siglec-G.41,42 Mixed radiation chimeras prepared with 1: 1 ratios of wild-type and siglec-G-deficient bone marrow cells, demonstrated that the effect of siglec-G in controlling cellular expansion is B-cell intrinsic.41 The B1a-cell expansion in siglec-G-deficient mice was not the result of increased cell cycling but rather reduced turnover rate as shown by lower bromodeoxyuridine incorporation.41 These data are suggestive of increased survival of B1a cells in siglec-G−/− mice, possibly through increased B-cell receptor signalling. Over-expression of siglec-G inhibited B-cell-receptor-mediated Ca2+ signalling and the siglec-G-deficient B1a cells exhibited exaggerated calcium signalling and increased IgM production.41 A similar phenotype has been observed in SHP-1-deficient mice, which exhibit expansion of the B1-cell population and higher B-cell receptor-induced calcium signalling in B cells. This suggests that SHP-1 plays a role downstream of siglec-G to give rise to its inhibitory function.43 This newly defined role of siglec-G may explain the naturally muted signalling response of B1a cells when compared with the B2 population in which siglec-G does not seem to play a functional role despite relatively high levels of expression.41
CD22 and siglec-G double knockout mice were created to investigate the potential redundancy between CD22 and siglec-G.44 It was shown that the double knockout mice had an even greater increase in B1-cell expansion, while the B2 population showed a reduction in size.44 Neither CD22 nor siglec-G single knockout mice showed development of autoimmunity whereas aged CD22, siglec-G double knockout mice showed spontaneous development of anti-DNA autoantibodies and displayed a mild form of immune complex glomerulonephritis.44 These data suggest that CD22 and siglec-G may have par-tial overlap in the regulation of B-cell signalling and tolerance.
The negative regulatory role of CD22 on B cells is well characterized but whether siglecs play a role in inducing tolerance in immune cells had not been explored until recently. Duong et al.45 showed that decoration of TI-2 antigens with sialic acids induces poor immune responses and leads to tolerance. Both siglec-G and CD22 have been shown to play a role in inducing tolerance, preventing plasma cell differentiation and survival.45 This is the first report of tolerance being induced through siglecs in addition to their established role in dysregulation of cell signalling.
Siglec-G/CD24 complex and danger signal recognition
Host response to injury is a relatively neglected component of innate immunity that is often viewed simply as a system that discriminates between self and non-self. Matzinger first proposed the ‘danger theory’ in 1994, in which she argued that rather than differentiating between self and non-self, the immune system discriminates between dangerous and non-dangerous signals, whether it is from an external or internal source.46 Like pathogen-associated molecular patterns (PAMPs), which interact with TLRs to stimulate immune response against pathogens, danger-associated molecular patterns (DAMPs) are released during injury and are thought also to bind TLRs and induce an inflammatory response.47 The DAMPs include heat-shock protein 70, heat-shock protein 90, high mobility group box 1 (HMGB1) and cellular RNA.47,48 Using a paracetamol-induced liver necrosis model, CD24, a glycosylphosphatidylinositol-anchored protein, has been identified as a receptor that interacts with the danger signal, HMGB1 and acts to protect against paracetamol-induced hepatotoxicity.48 CD24-deficient mice showed strong pro-inflammatory responses to paracetamol treatment: increase in IL-6, monocyte chemotactic protein-1 and TNF-α.48 Liver damage was indicated by an increase in serum alanine transaminase, indicative of liver haemorrhage and necrosis.48 Siglec-10 was shown to bind to CD24 and proposed to transduce inhibitory signalling that protects the mice against a lethal response to liver cell death.48 This was supported in studies of siglec-G (mouse orthologue of siglec-10) deficient mice which also showed greater inflammatory responses to high-dose paracetamol injections.48 The response of dendritic cells cultured from wild-type, CD24−/− and siglec-G−/− mice to the DAMP signal HMGB1 was compared with the PAMP signal LPS.48 There appeared to be an increased cytokine production in response to HMGB1 in dendritic cells from CD24−/− and siglec-G−/− but the difference was not observed in response to LPS, which was only used at a single concentration.48 This selective regulation of immune response to DAMPs over PAMPs identified here provides a potential mechanism to explain how the host can discriminate between endogenous danger signals and exogenous pathogen-derived signals (Fig. 4).
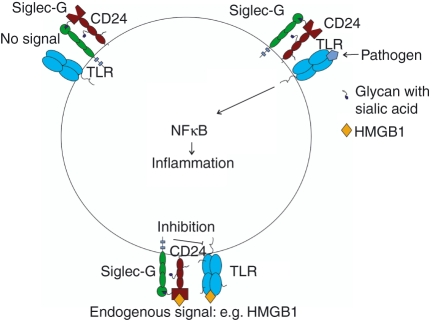
Figure 4.
Model for the involvement of sialic-acid-binding immunoglobulin-like lectin G (siglec-G) in danger signalling. It is proposed48 that CD24 is complexed with siglec-G but does not exhibit inhibitory signalling in the absence of a danger signal such as HMGB1. When there is only pathogen-derived signal, the CD24–siglec-G complex is not engaged and the Toll-like receptor (TLR) is able to deliver activating signals to the immune cell without inhibitory signals from siglec-G. When there is a danger signal, such as HMGB1, both the CD24–siglec-G complex and TLR are engaged. Siglec-G becomes active and is able to suppress signalling from TLR and dampen pro-inflammatory responses.
CD33rSiglecs and pathogens
It seems likely that the primary function of ITIM-bearing CD33rSiglecs is to regulate host immune functions via siglec–sialic acid interactions and downstream signalling. A potential secondary consequence of this function is exploitation by pathogens that capture or synthesize their own sialic acid and subvert immune responses by engaging inhibitory siglecs. In turn, this could provide an explanation as to why expansion of activating siglecs that resemble inhibitory siglecs in their extracellular domains took place, to allow the host immune system to engage sialylated pathogens and trigger protective immune responses.22,23,28 We discuss a recent example of how pathogenic incorporation of sialic acids is thought to engage and manipulate host CD33rSiglecs.
CD33rSiglec interaction with group B Streptococcus
Two CD33rSiglecs, siglec-5 and siglec-9, have been shown to be targeted by group B Streptococcus (GBS) to promote immune evasion. Different strains of GBS have been shown to bind these two siglecs in distinct fashions. Whereas several sialylated GBS strains bind siglec-9 and other CD33rSiglecs49 through their sialyated capsular polysaccharides (Siaα2-3Galβ1-4GlcNAc), a particular strain, serotype Ia, of GBS can bind siglec-5 via its cell wall-anchored β protein and this does not involve glycan recognition.50 The GBS binding to siglec-5 was shown to induce SHP-2 recruitment and negatively regulate receptor-mediated phagocytosis. The GBS β protein is therefore a new immune target in addition to the Fc portion of serum IgA and factor H.51 In a recent study, neutrophils were shown to interact with serotype III GBS sialylated capsular polysaccharides in a siglec-9-dependent fashion.52 In the presence of sialic acid-binding site blocking antibodies, neutrophils produced a stronger oxidative burst, showed increased granule protease release and generated more neutrophil extracellular traps.52 Hence, the GBS capsular polysaccharide appears to dampen neutrophil responses in a sialic-acid- and siglec-9-dependent manner.
Non-acetylated sialic acids on GBS are vulnerable to sialidase attack and the bacteria are susceptible to complement binding and lysis.53 It was shown that partial O-acetylation (80%) of sialic acids prevents enzymatic removal and does not significantly affect complement C3b accumulation on the surface of GBS.53 The O-acetylated sialic acid is not able to engage siglec-9 as shown by binding assays involving siglec-9–Fc fusion proteins.53 Interestingly, these findings led to the hypothesis that GBS might display an intermediate level of O-acetylation to optimize between complement evasion and immune dampening through engaging host siglec-9.
Concluding remarks
CD33rSiglecs evolved from an ancient small cluster of a few genes arranged in tandem and underwent a large-scale inverse duplication to create a much larger cluster. Whereas rodents appear to have lost many CD33rSiglecs, primates show expansion. New potentially activating CD33rSiglecs such as siglec-14 and siglec-16 appeared in dog and primates. These are paired with inhibitory molecules siglec-5 and siglec-11, respectively. These widely differing CD33rSiglec repertoires between mammals may reflect the ongoing evolutionary arms race between host and pathogen. CD33rSiglecs are expressed broadly in the innate immune system and growing evidence suggests that their primary function is to dampen host immune responses and set appropriate activation thresholds for regulating cellular growth, survival and the production of soluble mediators. This inhibitory function could be targeted by sialylated pathogens to evade immune responses and growing evidence supports this tenet. Potentially activating CD33rSiglecs might have arisen in response to the manipulation by pathogens of inhibitory CD33rSiglecs. These newly evolved receptors resemble the inhibitory CD33rSiglecs in the extracellular portions that are involved in ligand binding but encode charged transmembrane domains and associate with ITAM-containing adaptor molecules such DAP12. A de-selective force, perhaps as the result of inappropriate immune activation caused by these new activating receptors, may explain why most novel potentially activating CD33rSiglecs are currently pseudogenes. Siglec-16, in fact, has one functional and another non-functional mutant allele in humans, both distributed evenly in the population, suggestive of a balance of evolutionary forces that select and de-select for the new activating gene.
Acknowledgments
Work in the authors’ laboratory is supported by a Wellcome Trust Senior Fellowship (WT081882MA) awarded to P.R.C.
Disclosures
The authors have no conflicts of interests to declare.
References
- 1.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–66. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 2.Varki A, Angata T. Siglecs – the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 3.Avril T, Attrill H, Zhang J, Raper A, Crocker PR. Negative regulation of leucocyte functions by CD33-related siglecs. Biochem Soc Trans. 2006;34(Pt 6):1024–7. doi: 10.1042/BST0341024. [DOI] [PubMed] [Google Scholar]
- 4.Parham P. The genetic and evolutionary balances in human NK cell receptor diversity. Semin Immunol. 2008;20:311–6. doi: 10.1016/j.smim.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bashirova AA, Martin MP, McVicar DW, Carrington M. The killer immunoglobulin-like receptor gene cluster: tuning the genome for defense. Annu Rev Genomics Hum Genet. 2006;7:277–300. doi: 10.1146/annurev.genom.7.080505.115726. [DOI] [PubMed] [Google Scholar]
- 6.Brown D, Trowsdale J, Allen R. The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens. 2004;64:215–25. doi: 10.1111/j.0001-2815.2004.00290.x. [DOI] [PubMed] [Google Scholar]
- 7.Daeron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev. 2008;224:11–43. doi: 10.1111/j.1600-065X.2008.00666.x. [DOI] [PubMed] [Google Scholar]
- 8.Barrow AD, Trowsdale J. You say ITAM and I say ITIM, let’s call the whole thing off: the ambiguity of immunoreceptor signalling. Eur J Immunol. 2006;36:1646–53. doi: 10.1002/eji.200636195. [DOI] [PubMed] [Google Scholar]
- 9.Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 10.Crocker PR, Kelm S, Hartnell A, Freeman S, Nath D, Vinson M, Mucklow S. Sialoadhesin and related cellular recognition molecules of the immunoglobulin superfamily. Biochem Soc Trans. 1996;24:150–6. doi: 10.1042/bst0240150. [DOI] [PubMed] [Google Scholar]
- 11.Nitschke L. The role of CD22 and other inhibitory co-receptors in B-cell activation. Curr Opin Immunol. 2005;17:290–7. doi: 10.1016/j.coi.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Lajaunias F, Nitschke L, Moll T, Martinez-Soria E, Semac I, Chicheportiche Y, Parkhouse RM, Izui S. Differentially regulated expression and function of CD22 in activated B-1 and B-2 lymphocytes. J Immunol. 2002;168:6078–83. doi: 10.4049/jimmunol.168.12.6078. [DOI] [PubMed] [Google Scholar]
- 13.Kelm S, Gerlach J, Brossmer R, Danzer CP, Nitschke L. The ligand-binding domain of CD22 is needed for inhibition of the B cell receptor signal, as demonstrated by a novel human CD22-specific inhibitor compound. J Exp Med. 2002;195:1207–13. doi: 10.1084/jem.20011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nitschke L, Floyd H, Crocker PR. New functions for the sialic acid-binding adhesion molecule CD22, a member of the growing family of Siglecs. Scand J Immunol. 2001;53:227–34. doi: 10.1046/j.1365-3083.2001.00868.x. [DOI] [PubMed] [Google Scholar]
- 15.Nitschke L, Carsetti R, Ocker B, Kohler G, Lamers MC. CD22 is a negative regulator of B-cell receptor signalling. Curr Biol. 1997;7:133–43. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 16.Nitschke L. CD22 and Siglec-G: B-cell inhibitory receptors with distinct functions. Immunol Rev. 2009;230:128–43. doi: 10.1111/j.1600-065X.2009.00801.x. [DOI] [PubMed] [Google Scholar]
- 17.Freeman SD, Kelm S, Barber EK, Crocker PR. Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood. 1995;85:2005–12. [PubMed] [Google Scholar]
- 18.Schnaar RL, Lopez PH. Myelin-associated glycoprotein and its axonal receptors. J Neurosci Res. 2009;87:3267–76. doi: 10.1002/jnr.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angata T, Margulies EH, Green ED, Varki A. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc Natl Acad Sci U S A. 2004;101:13251–6. doi: 10.1073/pnas.0404833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angata T, Hayakawa T, Yamanaka M, Varki A, Nakamura M. Discovery of Siglec-14, a novel sialic acid receptor undergoing concerted evolution with Siglec-5 in primates. FASEB J. 2006;20:1964–73. doi: 10.1096/fj.06-5800com. [DOI] [PubMed] [Google Scholar]
- 21.Angata T, Tabuchi Y, Nakamura K, Nakamura M. Siglec-15: an immune system Siglec conserved throughout vertebrate evolution. Glycobiology. 2007;17:838–46. doi: 10.1093/glycob/cwm049. [DOI] [PubMed] [Google Scholar]
- 22.Cao H, Lakner U, de Bono B, Traherne JA, Trowsdale J, Barrow AD. SIGLEC16 encodes a DAP12-associated receptor expressed in macrophages that evolved from its inhibitory counterpart SIGLEC11 and has functional and non-functional alleles in humans. Eur J Immunol. 2008;38:2303–15. doi: 10.1002/eji.200738078. [DOI] [PubMed] [Google Scholar]
- 23.Cao H, de Bono B, Belov K, Wong ES, Trowsdale J, Barrow AD. Comparative genomics indicates the mammalian CD33rSiglec locus evolved by an ancient large-scale inverse duplication and suggests all Siglecs share a common ancestral region. Immunogenetics. 2009;61:401–17. doi: 10.1007/s00251-009-0372-0. [DOI] [PubMed] [Google Scholar]
- 24.Angata T, Kerr SC, Greaves DR, Varki NM, Crocker PR, Varki A. Cloning and characterization of human Siglec-11. A recently evolved signaling molecule that can interact with SHP-1 and SHP-2 and is expressed by tissue macrophages, including brain microglia. J Biol Chem. 2002;277:24466–74. doi: 10.1074/jbc.M202833200. [DOI] [PubMed] [Google Scholar]
- 25.Underhill DM, Goodridge HS. The many faces of ITAMs. Trends Immunol. 2007;28:66–73. doi: 10.1016/j.it.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Tomasello E, Vivier E. KARAP/DAP12/TYROBP: three names and a multiplicity of biological functions. Eur J Immunol. 2005;35:1670–7. doi: 10.1002/eji.200425932. [DOI] [PubMed] [Google Scholar]
- 27.Angata T, Varki A. Chemical diversity in the sialic acids and related alpha-keto acids: an evolutionary perspective. Chem Rev. 2002;102:439–69. doi: 10.1021/cr000407m. [DOI] [PubMed] [Google Scholar]
- 28.Angata T. Molecular diversity and evolution of the Siglec family of cell-surface lectins. Mol Divers. 2006;10:555–66. doi: 10.1007/s11030-006-9029-1. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Neumann H. Alleviation of neurotoxicity by microglial human Siglec-11. J Neurosci. 2010;30:3482–8. doi: 10.1523/JNEUROSCI.3940-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamanaka M, Kato Y, Angata T, Narimatsu H. Deletion polymorphism of SIGLEC14 and its functional implications. Glycobiology. 2009;19:841–6. doi: 10.1093/glycob/cwp052. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Raper A, Sugita N, Hingorani R, Salio M, Palmowski MJ, Cerundolo V, Crocker PR. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107:3600–8. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
- 32.Blasius AL, Cella M, Maldonado J, Takai T, Colonna M. Siglec-H is an IPC-specific receptor that modulates type I IFN secretion through DAP12. Blood. 2006;107:2474–6. doi: 10.1182/blood-2005-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blasius AL, Colonna M. Sampling and signaling in plasmacytoid dendritic cells: the potential roles of Siglec-H. Trends Immunol. 2006;27:255–60. doi: 10.1016/j.it.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Brinkman-Van der Linden EC, Angata T, Reynolds SA, Powell LD, Hedrick SM, Varki A. CD33/Siglec-3 binding specificity, expression pattern, and consequences of gene deletion in mice. Mol Cell Biol. 2003;23:4199–206. doi: 10.1128/MCB.23.12.4199-4206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ando M, Tu W, Nishijima K, Iijima S. Siglec-9 enhances IL-10 production in macrophages via tyrosine-based motifs. Biochem Biophys Res Commun. 2008;369:878–83. doi: 10.1016/j.bbrc.2008.02.111. [DOI] [PubMed] [Google Scholar]
- 36.Lajaunias F, Dayer JM, Chizzolini C. Constitutive repressor activity of CD33 on human monocytes requires sialic acid recognition and phosphoinositide 3-kinase-mediated intracellular signaling. Eur J Immunol. 2005;35:243–51. doi: 10.1002/eji.200425273. [DOI] [PubMed] [Google Scholar]
- 37.Sjolin H, Robbins SH, Bessou G, et al. DAP12 signaling regulates plasmacytoid dendritic cell homeostasis and down-modulates their function during viral infection. J Immunol. 2006;177:2908–16. doi: 10.4049/jimmunol.177.5.2908. [DOI] [PubMed] [Google Scholar]
- 38.Yu Z, Maoui M, Wu L, Banville D, Shen S. mSiglec-E, a novel mouse CD33-related siglec (sialic acid-binding immunoglobulin-like lectin) that recruits Src homology 2 (SH2)-domain-containing protein tyrosine phosphatases SHP-1 and SHP-2. Biochem J. 2001;353(Pt 3):483–92. doi: 10.1042/0264-6021:3530483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang JQ, Biedermann B, Nitschke L, Crocker PR. The murine inhibitory receptor mSiglec-E is expressed broadly on cells of the innate immune system whereas mSiglec-F is restricted to eosinophils. Eur J Immunol. 2004;34:1175–84. doi: 10.1002/eji.200324723. [DOI] [PubMed] [Google Scholar]
- 40.Boyd CR, Orr SJ, Spence S, et al. Siglec-E is up-regulated and phosphorylated following lipopolysaccharide stimulation in order to limit TLR-driven cytokine production. J Immunol. 2009;183:7703–9. doi: 10.4049/jimmunol.0902780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann A, Kerr S, Jellusova J, et al. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8:695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- 42.Ding C, Liu Y, Wang Y, Park BK, Wang CY, Zheng P. Siglecg limits the size of B1a B cell lineage by down-regulating NF-κB activation. PLoS ONE. 2007;2:e997. doi: 10.1371/journal.pone.0000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pao LI, Lam KP, Henderson JM, et al. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 44.Jellusova J, Wellmann U, Amann K, Winkler TH, Nitschke L. CD22 × Siglec-G double-deficient mice have massively increased B1 cell numbers and develop systemic autoimmunity. J Immunol. 2010;184:3618–27. doi: 10.4049/jimmunol.0902711. [DOI] [PubMed] [Google Scholar]
- 45.Duong BH, Tian H, Ota T, et al. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J Exp Med. 2010;207:173–87. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 47.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 48.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–5. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carlin AF, Lewis AL, Varki A, Nizet V. Group B streptococcal capsular sialic acids interact with siglecs (immunoglobulin-like lectins) on human leukocytes. J Bacteriol. 2007;189:1231–7. doi: 10.1128/JB.01155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlin AF, Chang YC, Areschoug T, Lindahl G, Hurtado-Ziola N, King CC, Varki A, Nizet V. Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J Exp Med. 2009;206:1691–9. doi: 10.1084/jem.20090691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Areschoug T, Stalhammar-Carlemalm M, Karlsson I, Lindahl G. Streptococcal beta protein has separate binding sites for human factor H and IgA-Fc. J Biol Chem. 2002;277:12642–8. doi: 10.1074/jbc.M112072200. [DOI] [PubMed] [Google Scholar]
- 52.Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–6. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiman S, Dahesh S, Carlin AF, Varki A, Nizet V, Lewis AL. Genetic and biochemical modulation of sialic acid O-acetylation on group B Streptococcus: phenotypic and functional impact. Glycobiology. 2009;19:1204–13. doi: 10.1093/glycob/cwp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peiper SC, Ashmun RA, Look AT. Molecular cloning, expression, and chromosomal localization of a human gene encoding the CD33 myeloid differentiation antigen. Blood. 1988;72:314–21. [PubMed] [Google Scholar]
- 55.Cornish AL, Freeman S, Forbes G, et al. Characterization of siglec-5, a novel glycoprotein expressed on myeloid cells related to CD33. Blood. 1998;92:2123–32. [PubMed] [Google Scholar]
- 56.Patel N, Brinkman-Van der Linden EC, Altmann SW, et al. OB-BP1/Siglec-6. A leptin- and sialic acid-binding protein of the immunoglobulin superfamily. J Biol Chem. 1999;274:22729–38. doi: 10.1074/jbc.274.32.22729. [DOI] [PubMed] [Google Scholar]
- 57.Nicoll G, Ni J, Liu D, Klenerman P, Munday J, Dubock S, Mattei MG, Crocker PR. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J Biol Chem. 1999;274:34089–95. doi: 10.1074/jbc.274.48.34089. [DOI] [PubMed] [Google Scholar]
- 58.Zhang JQ, Nicoll G, Jones C, Crocker PR. Siglec-9, a novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. J Biol Chem. 2000;275:22121–6. doi: 10.1074/jbc.M002788200. [DOI] [PubMed] [Google Scholar]
- 59.Floyd H, Ni J, Cornish AL, Zeng Z, Liu D, Carter KC, Steel J, Crocker PR. Siglec-8. A novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–6. doi: 10.1074/jbc.275.2.861. [DOI] [PubMed] [Google Scholar]
- 60.Zhang M, Angata T, Cho JY, Miller M, Broide DH, Varki A. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–7. doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whitney G, Wang S, Chang H, et al. A new siglec family member, siglec-10, is expressed in cells of the immune system and has signaling properties similar to CD33. Eur J Biochem. 2001;268:6083–96. doi: 10.1046/j.0014-2956.2001.02543.x. [DOI] [PubMed] [Google Scholar]
- 62.Munday J, Kerr S, Ni J, et al. Identification, characterization and leucocyte expression of Siglec-10, a novel human sialic acid-binding receptor. Biochem J. 2001;355(Pt 2):489–97. doi: 10.1042/0264-6021:3550489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kivi E, Elima K, Aalto K, et al. Human Siglec-10 can bind to vascular adhesion protein-1 and serves as its substrate. Blood. 2009;114:5385–92. doi: 10.1182/blood-2009-04-219253. [DOI] [PMC free article] [PubMed] [Google Scholar]