Abstract
The immune response to human cytomegalovirus (HCMV) infection is characterized by the accumulation of HCMV-specific CD8+ T cells, particularly in the elderly; such expansions may impair immune responses to other pathogens. We investigated mechanisms underlying HCMV-specific expansions in 12 young and 21 old healthy subjects (although not all analyses were performed on all subjects). Phenotypically, HCMV-pentamer+ CD8+ T cells were characterized by marked Vβ restriction, advanced differentiation (being predominantly CD27− CD28−), and variable CD45RO/RA expression. Although more common and larger in older subjects, expansions had similar phenotypic characteristics in the young. In one old subject, repeated studies demonstrated stability in size and Vβ distribution of pentamer+ populations over 6 years. We tested whether HCMV-specific CD8+ T-cell expansions arose from accelerated proliferation or extended lifespan by in vivo labelling with deuterated glucose and ex vivo Ki-67 expression. Uptake of deuterated glucose was lower in pentamer+ cells than in pentamer– CD8+ CD45RO+ or CD8+ CD45RA+ cells in three old subjects, consistent with reduced proliferation and extended lifespan. Similarly Ki-67 labelling showed no evidence for increased proliferation in HCMV-specific CD8+ expansions in older subjects, although pentamer– CD45RA+ cells from young donors expressed very little Ki-67. We investigated Bcl-2 and CD95 as possible anti-apoptotic mediators, but neither was associated with pentamer-positivity. To investigate whether expansion represents a compensatory response to impaired functionality, we performed two tests of functionality, peptide-stimulated proliferation and CD107 expression; both were intact in pentamer+ cells. Our data suggest that HCMV-specific CD8+ expansions in older subjects accumulate by extended lifespan, rather than accelerated proliferation.
Keywords: CD8/cytotoxic T cells, cytomegalovirus, senescence, T cells, viruses/viral immunity
Introduction
Post-natal human cytomegalovirus (HCMV) infection in humans most often causes a relatively mild and self-limiting illness. After primary infection the virus is not cleared completely but establishes life-long infection, remaining latent in cells of myeloid lineage.1 A vigorous cell-mediated immune response prevents recrudescence2 and infected adults remain asymptomatic unless immunity is suppressed, as in those with HIV infection or in transplant recipients, in whom severe retinitis, colitis, pneumonitis or hepatitis can occur.3 This long asymptomatic period may not be as harmless as once supposed. Evidence is accumulating that chronic HCMV may be an important contributor to age-related impairment of the immune system, or ‘immunosenescence’2,4–6 contributing to the increased susceptibility to infection and reduced vaccine responsiveness known to occur in the elderly.7,8
The thesis that chronic HCMV infection is deleterious is supported by several lines of evidence. First, epidemiological data from large prospective studies of elderly cohorts demonstrate an association between HCMV seropositivity and reduced survival.9,10 Second, the presence of large oligoclonal populations of HCMV-specific T cells, especially within the CD8+ T-cell pool, is associated with reduced survival in the elderly.11 Third, it is recognized that expansion of one subpopulation of lymphocytes may displace or impair the function of others. In mice, for example, the presence of expanded T-cell clones can be shown to reduce overall T-cell diversity12 and, during herpes simplex virus (HSV) infection, HSV-specific CD8+ clones can be shown to out-compete other T-cell clones, particularly those of the same T-cell receptor (TCR) Vβ subtype.13 Similarly, human T-cell responses to Epstein–Barr virus are impaired in those with HCMV-driven clonal expansions.14 A plausible mechanistic model may be constructed whereby HCMV adversely impacts the rest of the T-cell repertoire.15
In the elderly, HCMV-specific expansions are very common, occurring in about one-third of adults > 65 years; in some elderly individuals, they come to dominate the T-cell pool.16 As a result, the immune repertoire, in terms of TCR Vβ family utilization, may become skewed, with one or two families being highly dominant. Such Vβ expansions may also be found in young subjects, suggesting that it is not just chronology that drives their accumulation.17 What is not known, however, is the mechanism by which these HCMV-specific populations become so large. Two alternative models may be considered, one depending on proliferation, the other on accumulation. In the proliferative model, cellular populations continue to divide and expand in response to persistent antigen. Consistent with this model is evidence of ongoing viral reactivation, which is seen more frequently in the elderly than the young.18 However, this model is not supported by evidence that suggests that the replicative capacity of HCMV-specific CD4+ T cells in elderly subjects is severely restricted.19 Alternatively, HCMV-specific T-cell expansions may arise by accumulation, cells having an extended lifespan, possibly as a consequence of failure of ‘normal’ apoptosis. In the latter model, oligoclonal expansions would be expected to be highly stable, changing little over time, whereas in the former model, individual clones may be driven to exhaustion to be replaced by alternative clones over time. Of course, the two models are not mutually exclusive and elements of both may operate concurrently.
Similarly the driving force for clonal expansion remains unclear. Is it driven by persistent and recurrent HCMV reactivation, or does functional activity of HCMV-specific cells fall with time so that an increasing number of HCMV-specific T cells are required to maintain control of chronic infection? Functional activity might either be impaired directly by age or result from reduced avidity of TCRs for peptide–MHC complexes. The latter might be associated with ageing if clonal evolution deleted the highest affinity clones first, possibly by proliferative exhaustion, leaving only low-avidity clones to control persisting HCMV infection later in life.15
To investigate how HCMV-specific expansions occur and persist in elderly individuals, we compared HCMV-specific T cells from healthy old subjects with those from young individuals. First, we characterized the cells making up expansions. We then tested whether expansions occur as a result of accelerated proliferation or extended lifespan (i) using deuterated glucose labelling in vivo,20 (ii) by determining Ki-67 expression ex vivo, and (iii) by examining the expression of genes involved in cell survival and death. Finally, we assessed whether functional responses of HCMV-specific T cells are altered in the old.
Materials and methods
Subjects and sample preparation
Elderly (≥ 65 years) and young (< 40 years) healthy subjects were recruited by local advertisement. All were free from acute or chronic illness and disorders of mobility or cognition; none were taking medication known to affect immune function. To characterize HCMV-specific responses we identified a cohort with measurable HLA-restricted cellular responses by a step-wise screening process. First, we tested for HCMV IgG seropositivity; if seropositive, we identified those with significant cellular responses by measuring interferon-γ production in response to HCMV lysate; this group were HLA-typed to identify HLA-A2-positive and HLA-B7-positive subjects in whom pentamer studies could be performed, as dominant HCMV peptide epitopes have been identified for these alleles. Data are presented from 21 healthy old subjects (mean age 75 years, range 65–91 years) and 12 healthy young subjects (mean age 30 years, range 23–36 years), although not all measurements were performed in all subjects. All procedures were performed in accordance with the principles of the Declaration of Helsinki and after approval from the local Research Ethics Committee.
Peptide HLA-class I pentamers
Unlabelled Pro5® MHC class I pentamers (Proimmune, Oxford, UK) containing either the HLA-A*0201-restricted peptide NLVPMVATV (HCMV pp65 495–503) or the HLA-B*0702-restricted peptide TPRVTGGGAM (HCMV pp65 417–426) were used for the identification of HCMV peptide-specific CD8+ T cells. Peripheral blood mononuclear cells (PBMC) were stained with the appropriate pentamer (15 min, ambient temperature), washed, then incubated with Pro5® Fluorotag allophycocyanin or R-phycoerythrin (R-PE; Proimmune) for (30 min, 4°) together with peridinin chlorophyll protein (PerCP)-conjugated anti-CD8 (BD Biosciences, Oxford, UK).
Phenotypic analysis
Cryopreserved PBMC were stained with directly conjugated antibodies including CD8-PerCP and a combination of the following monoclonal antibodies [either fluorescein isothiocyanate (FITC) or R-PE-conjugated] CD27, CD28, CD11a, CCR7, CD62L, CD95 (BD Biosciences), CD57 (Beckman Coulter, Brea, CA), CD45RO and CD45RA (Dako, Glostrup, Denmark). The TCR Vβ repertoire was investigated using the IOTest® Beta Mark kit (Beckman Coulter) according to the manufacturer’s instructions. For intracellular Bcl-2 staining, cells were surface stained with MHC class I pentamer, CD8-PerCP and the appropriate TCR Vβ-FITC antibody (BioMed Immunotech, Tampa, FL) and then fixed and permeabilized (Fix & Perm Cell Permeabilization kit; Caltag Laboratories, Buckingham, UK) and stained with Bcl-2-PE (BD Biosciences). All antibodies were pre-titrated to determine optimal staining concentrations. Four-parameter flow cytometric analysis was performed on a FACSCalibur flow cytometer (BD Biosciences) using CellQuest software (BD Biosciences) and data were analysed using Winmdi software (Scripps Research Institute, La Jolla, CA).
Analysis of Ki-67 expression
For ex vivo detection of cells in cycle, cryopreserved PBMC were stained with the appropriate MHC class I pentamer and cell surface markers, treated with eBioscience Fixation and Permeabilization kit according to the manufacturer’s instructions and stained with FITC-conjugated or R-PE-conjugated anti-Ki-67 or isotype control (BD Biosciences; 30 min, 4°), as described elsewhere.21 For measurement of proliferation by Ki-67 expression following stimulation with HCMV peptides, cryopreserved PBMC from HCMV-seropositive donors were plated in 24-well tissue culture plates at 1 × 106/ml in RPMI-1640 containing 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mm l-glutamine (complete medium) (Sigma, St Louis, MO). Cells were stimulated for 3 days at 37° with 5 μg/ml of the relevant HCMV pp65 peptide (Proimmune), collected, washed and stained for Ki-67 as described above.
CFSE staining for assessment of proliferation
Carboxy fluorescein succinimidyl ester (CFSE; Invitrogen, Eugene, OR) was diluted to a concentration of 5 μm in PBS and added to cells suspended in PBS to give a final CFSE concentration of 2·5 μm. Cells were labelled at room temperature for 10 min. Labelling was stopped by adding an equal volume of complete medium for 1 min and the cells were washed extensively in PBS before use.
CD107a staining
Cryopreserved PBMC were stimulated with HCMV pp65 peptides as described above. After 3 days, FITC-conjugated anti-CD107a or isotype control (BD Biosciences) was added to the relevant wells and cells were incubated at 37° for 2 hr. Then, 25 μl of 100 μm monensin per 106 cells was added. After a further 4 hr, cells were washed and co-stained with MHC class I pentamer, anti-CD8 and in some cases the appropriate PE-conjugated anti-TCR Vβ antibody.
In vivo labelling with deuterated glucose, modelling and data analysis
In vivo proliferation/disappearance rates were investigated in three elderly subjects, essentially as previously described.20,22 All received a 60-g dose of 6,6-2H2-glucose as an oral solution in half-hourly aliquots over 10 hr following an initial priming dose. Blood glucose deuterium enrichment was monitored during administration. Follow-up blood samples taken over the ensuing 3 weeks were sorted according to CD3, CD8, CD45RO expression and pentamer binding by flow cytometry (Mo-Flo, Cytomation, Fort Colins, CO). Sorted cells underwent DNA extraction, digestion and derivitization followed by gas chromatography mass spectrometry (GC/MS) analysis for deuterium enrichment as previously described.23 DNA deuterium enrichment data were modelled as a function of time to derive the average proliferation rate (p) for T-cell populations and the disappearance rates (d*) of labelled cells within each sub-population.20,24,25 Statistical significance was evaluated using the Student’s t-test or Mann–Whitney U-tests for comparison of groups according to distribution of data (Prism, GraphPad Software Inc., La Jolla, CA).
Results
HCMV+ expansions develop at all ages, are Vβ restricted and stable over time
Of the old individuals screened, 18 were identified as both HCMV-responsive and HLA A2/B7-expressing. Twelve of these individuals had A2-NLV pentamer-binding populations ranging in size from 0·2 to 14·5% total CD8+ T cells and nine had B7-TPR-binding populations comprising 0·5–32% of CD8+ cells (Fig. 1a,b). Four elderly subjects were both A2- and B7-positive, but all showed restricted responses (two to A2-TPR; two to B7-NLV); none showed measurable pentamer binding to both epitopes. The HCMV-specific pentamer-binding populations were also found in young HCMV-responsive HLA A2/B7 individuals but were significantly smaller in younger subjects, ranging in size from 0·1 to 4% for A2-NLV and 0·4 to 1·7% for B7-TPR (P < 0·05 young versus old).
Figure 1.

CD8+ human cytomegalovirus (HCMV) -specific T-cell receptor (TCR) Vβ expansions in young and old. Peripheral blood mononuclear cells from HCMV-seropositive donors were stained with MHC class I pentamers to determine the percentage of CD8+ HCMV-specific T cells. (a) Representative flow cytometric profiles from young and old donors showing the percentage of total CD8+ cells specific for the HCMV peptides NLV (donors O11 and Y5) or TPR (donors O2 and Y1). (b) Percentage of CD8+ HCMV-specific cells identified for all donors included in the study. The horizontal line represents the median value for each group (young and old for each peptide); comparisons are by Mann–Whitney U-test. (c) TCR Vβ usage of total CD8+ (open bars) and NLV-specific (solid bars) T cells in a representative old donor (O11); cells were co-stained with pentamer and 24 different anti-TCR Vβ antibodies (in order: Vβ 1, 2, 3, 4, 5.1, 5.2, 5.3, 7.1, 7.2, 8, 9, 11, 12, 13.1, 13.2, 13.6, 14, 16, 17, 18, 20, 21.3, 22, 23). (d) As described for (c) but for a representative young donor (Y5).
When NLV+ CD8+ T cells from both young and old were double-stained with a panel of antibodies to TCR Vβ families, within individuals, pentamer-binding CD8+ cells expressed a restricted range of TCR Vβ genes. In three of seven young and four of eight old, the pentamer-binding cells expressed predominantly a single Vβ gene. In the old, highly restricted Vβ usage was associated with large expansions. The HCMV-specific NLV-pentamer-binding cells in this group of individuals expressed predominantly Vβ8, Vβ13.1 and Vβ14 (Fig. 1c,d). Restricted Vβ usage was not representative of the overall CD8 Vβ repertoire in either old or young donors (Fig. 1c,d). Taken together, these results suggest that although HCMV expansions are larger in the old, the processes that result in expansion have already begun in young HCMV-infected individuals and Vβ restriction is established early in this process. Once established, expansions appear to be stable; in one old subject (O3), the size of the Vβ family containing the dominant HCMV-specific TPR+ population (Vβ3), analysed three times over a 6-year period, remained essentially unchanged (2003: 7·5%; 2006: 7·8%; 2009: 7·4%), although some changes were noted in the size of other Vβ families (Fig. 2). Another subject studied twice with a 5-year interval showed a similar pattern of consistency (data not shown).
Figure 2.
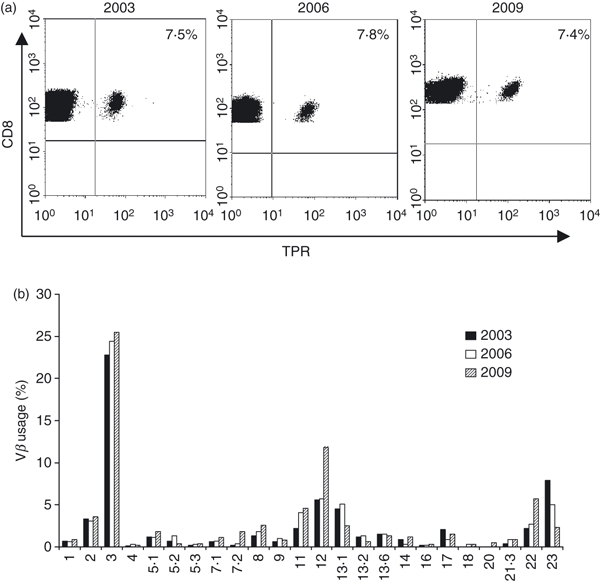
Long-term stability of human cytomegalovirus (HCMV) -specific expansions in an elderly subject. Peripheral blood mononuclear cells taken three times over a 6-year period from a single old HCMV+ B7-restricted donor (O3) were stained with (a) CD8 and MHC class I TPR-specific pentamer and (b) with CD8 and the same Vβ panel as detailed in Fig. 1.
HCMV expansions in young and old show a similarly differentiated phenotype
Changes in the pattern of CD45 isoform expression have been correlated with differentiation of T cells from naive to effector to memory function.26 Similarly progressive loss, first of the co-stimulatory molecule CD28, and then CD27 is associated with differentiation, terminating in the production of CD27/28 double-negative effectors.27,28 When the pattern of CD45 isoform expression of HCMV pentamer+ expansions was investigated, striking heterogeneity was noted both within individual subjects and between different donors. Among all donors, the dominant phenotype of HCMV+ expansions was approximately equally distributed between the three patterns: CD45RO+ alone, CD45RA+ alone and CD45RA+ CD45RO+ double-positives. Although numbers were small, there was no apparent difference in the overall pattern of distribution of phenotypes between young and old donors, nor was the phenotype related to the size of the expansion (Table 1).
Table 1.
Patterns of CD45 isoform expression on pentamer-positive CD8+ T cells from young and old subjects
| Old | Young | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Donor | Pentamer | Size of pentamer+ population (%) | Dominant phenotype | Size of dominant phenotype within pentamer+ (%) | Donor | Pentamer | Size of pentamer+ population (%) | Dominant phenotype | Size of dominant phenotype within pentamer+ |
| O1 | TPR | 32 | DP | 53 | Y1 | TPR | 1·7 | DP | 41 |
| O2 | TPR | 12 | DP | 44 | Y2 | TPR | 1·4 | DP | 41 |
| O3 | TPR | 11 | RA | 80 | Y3 | TPR | 1·2 | RO | 86 |
| O4 | TPR | 7 | RO | 93 | Y4 | TPR | 0·4 | RA | 60 |
| O5 | TPR | 4 | RO | 51 | |||||
| O6 | TPR | 4 | RA | 49 | |||||
| O7 | TPR | 3 | DP | 74 | |||||
| O8 | TPR | 1·5 | RA | 64 | |||||
| O9 | TPR | 0·5 | RO | 49 | |||||
| O10 | NLV | 14 | RO | 90 | Y5 | NLV | 2·4 | RO | 87 |
| O11 | NLV | 9 | DP | 58 | Y7 | NLV | 0·4 | RO | 51 |
| O12 | NLV | 5 | RO | 95 | Y8 | NLV | 0·4 | RA | 72 |
| O13 | NLV | 4 | RA | 75 | Y9 | NLV | 0·2 | DP | 61 |
| O14 | NLV | 3 | RO | 63 | |||||
| O15 | NLV | 2 | RA | 39 | |||||
Numbers shown are the size of the pentamer+ population as a proportion of all CD8+ cells and the proportion of pentamer+ cells expressing the dominant CD45 phenotype.
Abbreviations: RA, CD45RA+ single-positive; RO, CD45RO+ single-positive; DP, CD45RA+ CD45RO+ double-positive. Expansions were larger in old versus young donors for both TPR and NLV tetramers, P= 0·034 and 0·025 repectively (Mann–Whitney U-test, two-tailed).
In terms of CD27/CD28 expression, most HCMV+ expansions showed the highly differentiated (CD27− CD28−) phenotype, by contrast with their variable patterns of CD45 expression; this was true in both old and young donors (Table 2). Again, there was no relationship between the size of the pentamer+ population and the CD27/CD28 phenotype, suggesting that differentiation and expansion are affected independently. Additional staining experiments showed that pentamer+ cells tended to express high levels of CD11a and CD57, with minimal expression of CCR7 and CD62L in both old and young donors (data not shown).
Table 2.
Patterns of CD27/CD28 expression on pentamer-positive CD8+ T cells from young and old subjects
| Old | Young | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Donor | Pentamer | Size of pentamer+ population (%) | Dominant phenotype | Size of dominant phenotype within pentamer+ (%) | Donor | Pentamer | Size of pentamer+ population (%) | Dominant phenotype | Size of dominant phenotype within pentamer+ (%) |
| O1 | TPR | 32 | DN | 83 | Y1 | TPR | 1·7 | DN | 41 |
| O2 | TPR | 12 | DN | 85 | Y2 | TPR | 1·4 | DN | 47 |
| O3 | TPR | 11 | 27/DP/DN | 27/38/25 | Y3 | TPR | 1·2 | 27/DP/DN | 31/29/36 |
| O4 | TPR | 7 | DN | 61 | Y4 | TPR | 0·4 | DN | 56 |
| O5 | TPR | 4 | DN | 62 | |||||
| O6 | TPR | 4 | DN | 59 | |||||
| O7 | TPR | 3 | DN | 52 | |||||
| O8 | TPR | 1·5 | DN | 84 | |||||
| O9 | TPR | 0·5 | 28/DP | 41/42 | |||||
| O10 | NLV | 14 | DN | 67 | Y5 | NLV | 2·4 | 27/DP | 35/39 |
| O11 | NLV | 9 | DN | 58 | Y6 | NLV | 0·7 | DN | 61 |
| O12 | NLV | 5 | 27 | 53 | Y7 | NLV | 0·4 | 27 | 48 |
| O13 | NLV | 4 | DN | 51 | Y8 | NLV | 0·4 | DN | 40 |
| O14 | NLV | 3 | DN | 83 | Y9 | NLV | 0·2 | DN | 53 |
| O15 | NLV | 2 | 27 | 46 | |||||
| O16 | NLV | 1 | DN | 93 | |||||
| O17 | NLV | 0·2 | DP/DN | 36/28 | |||||
Numbers shown are the size of the pentamer+ population, as a proportion of all CD8+ cells, and the proportion of pentamer+ cells expressing the dominant CD27/28 phenotype. Abbreviations: DN, double-negative, CD27− CD28−; DP, double-positive, CD27+ CD28+; 27, CD27+ CD28− single-positive; 28, CD27− CD28+ single-positive. CD27− CD28−, double-negative cells represent the dominant subset in both young and old, comprising 70·5% in old and 66·7% in young subjects.
HCMV expansions arise by accumulation not increased proliferation
To ascertain whether HCMV expansions arise because of increased proliferation or extended lifespan we used complementary in vivo and ex vivo approaches. In vivo, we used the deuterated glucose approach in three healthy elderly Caucasian women (O3, O4 and O13; aged 77–82 years). After 10 hr of deuterium-labelling with oral glucose, PBMC from blood samples taken over the ensuing 3 weeks were sorted according to pentamer binding and expression of CD3, CD8 and CD45. In all three subjects pentamer+ CD8+ cells had substantially lower rates of incorporation of deuterium than either pentamer– CD8+ CD45RO+ or CD8+ CD45RO− T cells (Table 3). In one subject, (O3), only peak data could be analysed; further follow-up samples were not available. In the other two, labelling curves were plotted on graphs and modelled as a function of time (Fig. 3) to derive proliferation rates (p). These values were lower for pentamer+ cells than for other CD8+ T cells in both subjects (Table 3). Doubling-times (T2) were about 1 year for O13 and O3 and about 7 weeks for O4, who had generally higher turnover rates (Table 3), including CD4+ cells (data not shown). In this study, disappearance rates (d*) tended to be higher than proliferation rates, as generally observed,24 because the parameter d* represents loss only of labelled cells, not all cells. In both old subjects in whom modelling was possible, disappearance rates in pentamer+ cells were lower than in other pentamer– CD8 subsets (Table 3). Taken together, these data give no support to the hypothesis that ongoing or rapid proliferation explains the expansion of pentamer-binding cells in old individuals; conversely, the slow turnover and relatively long disappearance half-lives suggest that accumulation is the major contributor to expansion.
Table 3.
Proliferation and disappearance rates of CD8+ T cells from in vivo labelling in three elderly subjects
| Subject | Peak enrichment (%/day) | Proliferation rate, P (%/day) | Doubling Time (days) | Disappearance rate, d* (%/day) | Half-life (days) |
|---|---|---|---|---|---|
| O13 | |||||
| Pentamer+ | 0·17 | 0·18 | 385 | 1·26 | 55 |
| CD45RO+ | 0·53 | 0·69 | 100 | 7·75 | 9 |
| CD45RO− | 0·69 | 0·82 | 85 | 8·45 | 8 |
| O4 | |||||
| Pentamer+ | 1·3 | 1·44 | 48 | 1·88 | 37 |
| CD45RO+ | 3·92 | 4·71 | 15 | 8·08 | 9 |
| CD45RO− | 2·76 | 3·26 | 21 | 10·89 | 6 |
| O3 | |||||
| Pentamer+ | 0·19 | 3681 | |||
| CD45RO+ | 1·16 | 601 | |||
| CD45RO− | 2·75 | 251 | |||
Data represent measured peak enrichment (a minimum estimate of proliferation rates) and modelled proliferation (p) and disappearance rate constants (d*).
Doubling time is derived from p except for O3, where it is based on the peak enrichment.
Figure 3.

In vivo proliferation and disappearance of CD8+ T cells. Fraction of labelled cells following deuterium-labelled glucose administration for 10 hr at time 0. Peak height indicates proliferation rate and rate of decline indicates disappearance rate. Pentamer+ cells (filled squares) were separated from CD45RO+ (open squares) and CD45RO− (open circles) before analysis. Data are shown from two individual subjects, O13 (a) and O4 (b); expansions were predominantly CD45RO– in O13 but CD45RO+ in O4.
Because the proportion of CD8 T cells is lower in young than old donors and the pentamer+ populations are smaller, it was not possible to separate sufficient cells from young donors to allow deuterated glucose labelling and GC/MS analysis. We therefore investigated expression of the cell cycle-associated marker Ki-67, which is present in G1, S, G2 and M-phase, but not G0, cell nuclei to assess ex vivo proliferation. When Ki-67 labelling was compared between pentamer+ CD8+ cells and all other CD8+ T cells (‘pentamer-negative’) in old subjects, similar proportions of cells were found to be in cycle (Fig. 4a,b). The same comparison in the young revealed a difference, pentamer+ cells having higher rates of Ki-67 expression. We speculated that this difference might relate to specific subsets and performed more detailed analysis of Ki-67 expression according to CD27 and CD45RO expression. We found that Ki-67 primarily correlated with CD45RO expression, being predominantly found on CD45RO+ cells, irrespective of CD27 status (see Fig. 4c for a representative experiment). In five individuals (four old, one young) where pentamer+ CD8 cells had a mixed CD27/CD45RO phenotype, the same pattern was observed (data not shown). Further analysis of CD45RO+/CD45RO− subsets showed that neither CD45RO subset differed in Ki-67 expression in the elderly (Fig. 4d), and that pentamer+ cells in the young had similar rates of Ki-67 expression to pentamer+ cells in the older subjects (Fig. 4d). However, in the young, pentamer– cells in both subsets had lower rates of Ki-67 expression, consistent with a larger population of very slow turnover, true naive cells within this compartment.
Figure 4.
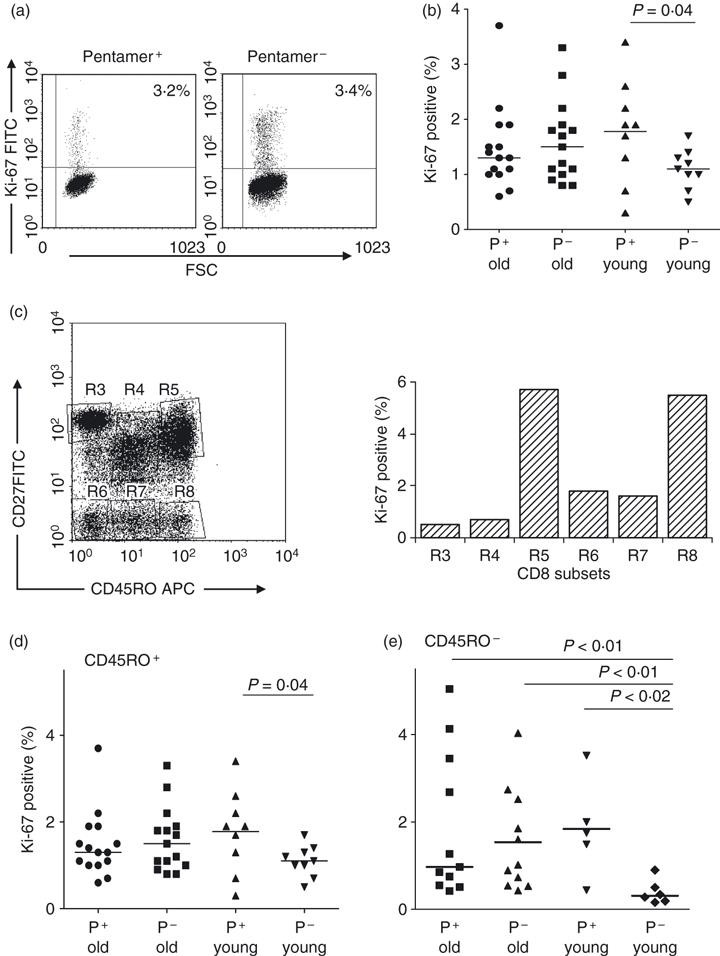
Ex vivo proliferation measured by Ki-67 expression. Peripheral blood mononuclear cells from young and old human cytomegalovirus (HCMV) -positive donors were stained for CD8, specific pentamer and Ki-67 and gated on pentamer+ CD8+ T cells, control cells from an HCMV-negative old donor gated on CD8+ T cells were stained for Ki-67. (a) Representative dot plots comparing the pentamer+ CD8+ T-cell population with the CD8+ pentamer− population from the same old donor (O2). (b) Comparison of Ki-67 expression on all CD8+ cells derived from old (n = 15) and young (n = 9) donors. P-value by Wilcoxon signed rank test. (c) Comparison of Ki-67 activity on subsets defined by CD27 × CD45RO staining. (d) Expression of Ki-67 on subsets of CD45RO+ cells stained with CD8, MHC class I HCMV-specific pentamer and CD45RO from panels of old (n = 15) and young (n = 9) donors. Each data point represents the value from one donor; horizontal bars are medians. P-value quoted is by pairwise comparison (Wilcoxon signed rank test); no other significant differences between groups by analysis of variance (anova; Kruskal–Wallace). (e) As for (d) but for CD45RO− cells (n = 11 and six respectively). P-value by anova (Kruskal–Wallace) = 0.019. Comparisons between groups by Mann–Whitney U-test.
Altered Bcl-2 or CD95 expression do not account for the extended lifespan of HCMV-specific expansions
Some of the data above suggested that HCMV-specific expansions have an extended lifespan in old subjects, so we addressed whether this might be related to altered expression of Bcl-2 or CD95, components of two major signalling pathways for lymphocyte survival/death. In four young and four old subjects, all pentamer+ cells were CD95+ and showed similar levels of Bcl-2 expression to that of the total CD8+ T-cell population (Fig. 5). No differences were apparent between young and old donors.
Figure 5.

Altered expression of Bcl-2 and CD95 does not explain the extended lifespan of CD8+ human cytomegalovirus (HCMV) -specific T cells within T-cell receptor (TCR) Vβ expansions. Peripheral blood mononuclear cells from four young and four old subjects were stained with MHC class I pentamers to identify HCMV-specific CD8+ T cells and the apoptotic potential of this population was assessed by flow cytometry after staining for CD95 and Bcl-2. Representative flow cytometric profiles from one old (left panel) and one young (right panel) donor showing CD95 (upper row, donors O15 and Y5) and Bcl-2 (bottom row donors O14 and Y8) expression on HCMV pentamer+ cells. Plots are gated on total CD8+ T cells.
Altered proliferative and functional responses do not account for the accumulation of HCMV-specific expansions
To investigate the role of impaired functionality in HCMV responses, we employed two complementary approaches. First, we determined whether cells could respond by proliferation to stimulation with specific peptides. CFSE-labelled cells were stimulated with NLV peptide for 6 days then stained with NLV pentamer. We found that only pentamer-specific cells responded and proliferated (Fig. 6a). When fresh PBMC were stimulated with either peptide mix or specific peptide (NLV or TPR) for 3 days, only pentamer+ cells up-regulated Ki-67 (Fig. 6b). The possibility that responses might differ in pentamer+ populations in young and old was examined but no significant differences were detected (Fig. 6c). Furthermore there was no evidence that donors whose pentamer+ population was of a single Vβ type respond less well than those with a mixed Vβ, where there might be more actively expanding clones (Fig. 6c). Second, CD107 expression was measured as an index of cytotoxic potential; when combined with pentamer staining and specific peptide stimulation, we found no evidence for impaired cytotoxic functionality in such cells in the elderly (Fig. 6d). These studies were performed in two young and four elderly donors, who all showed similar patterns of response.
Figure 6.
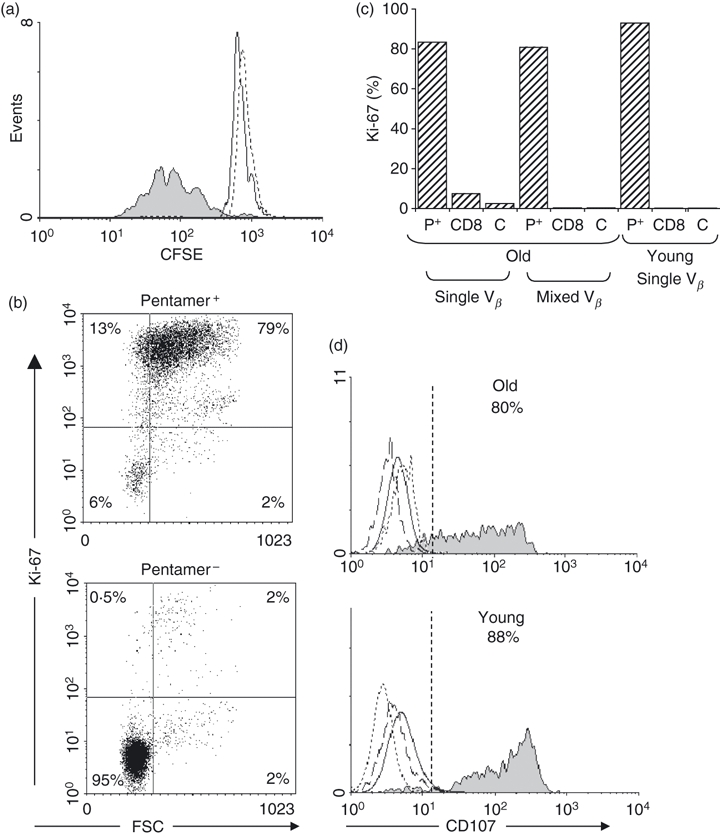
Proliferative responses to human cytomegalovirus (HCMV) peptides and functional capacity of HCMV-specific CD8+ T cells from old and young. (a) Peripheral blood mononuclear cells (PBMC) from an HCMV+ A2-restricted donor (donor O11) with an NLV+ CD8+ T cell expansion, consisting predominantly of Vβ16, were labelled with CFSE and incubated with NLV peptide for 6 days before analysis on a FACScalibur. Solid fill represents CD8+ NLV+ peptide-stimulated cells on day 6, solid line represents CD8+ NLV+ cells on day 0 and dashed line represents CD8+ NLV− peptide-stimulated cells on day 6. (b) PBMC from an old donor with a pentamer+ population containing a small Vβ expansion (donor O14) were stimulated with specific HCMV peptide for 3 days and then stained for Ki-67 (dot plot). (c) Histogram of Ki-67 expression in stimulated cells from one old donor (O6) one with a single dominant Vβ expansion and one young donor (Y9) with a dominant Vβ expansion; P+, pentamer+ cells; CD8, pentamer− CD8+ cells, C, isotype control. (d) The functional profile of HCMV-specific CD8+ T cells was assessed in response to peptide stimulation by measuring surface mobilization of CD107a by flow cytometry. PBMC from one old (donor O6) and one young HLA-B7+ donor (Y1) were stimulated for 3 days with HCMV-B7 restricted peptides then stained with CD107 monoclonal antibodies, incubated with monensin for 6 hr, then stained with TPR and CD8 mAb. Solid fill represents CD8+ TPR+ peptide-stimulated cells; solid line represents control CD8+ TPR− peptide-stimulated cells; dotted line represents CD8+ TPR+ isotype; dashed line represents TPR+ CD8+ cells incubated in medium (no peptide) for 3 days. Data are representative of studies in two young and four elderly donors.
Discussion
For most viral pathogens, memory lymphocyte populations remaining after resolution of the primary infection are relatively small, rarely comprising more than 1% of circulating CD8+ T cells or 0·1% of CD4+ cells; most are lymphoid-homing CD45RO+ CD27+‘central memory’ T cells.28 By contrast, HCMV infection evokes a quite different pattern of response.2,29,30 First, populations of persisting HCMV-specific memory cells are unusually large, sometimes very large, up to 32% of all CD8+ in this study (subject O1, Table 1). Second, they tend to increase with age/time (Fig. 1). Third, they show diversity of CD45 isoform expression, as previously described.16,31,32 The significance of this is unclear because the pattern of isoform expression does not appear to correlate with the age of the donor or other phenotypic features. Fourth, they express a highly differentiated phenotype, lacking CCR7, CD27, CD28 and CD62L, and expressing CD11a and CD57.19,27 These observations, the large size of HCMV-specific memory populations, their stability and their highly differentiated phenotype, together with the observation that CMV infection seems to drive telomere shortening in CD8+ cells,30 as well as in CD4+ cells,19 raise several questions. Specifically, how are such large HCMV-specific expansions generated? How are they maintained over long periods, despite having short telomeres and a highly differentiated phenotype, and are such cells functionally competent?
In terms of how these expansions arise, our data suggest that an accumulation model, characterized by prolonged survival, is more likely to describe events in vivo, than a proliferation model, in which accelerated cell division is ongoing. Three strands of evidence lead us to this conclusion. First, in vivo proliferation rates directly measured using deuterated glucose were significantly lower for HCMV-specific T cells than for CD8 cells of other specificities, regardless of whether they expressed predominantly CD45RO or RA. Although our conclusions are based on only three subjects, the results were consistent in all three.
Second, analysis of Ki-67 expression on antigen-specific CD45RO+ CD8+ T cells (defined by pentamer binding) showed no evidence for accelerated proliferation in the elderly (Fig. 4). For the young, interpretation is more complex; it appears that young pentamer+ cells may have a higher fraction in cycle than young pentamer− cells, particularly among CD45RO− cells (Fig. 4). This difference may derive from differences in antigen-priming rather than from specific effects of HCMV as most CD8+ CD45RO− cells in the young are naive T cells, which turn over slowly,25 whereas pentamer+ CD45RO− cells are antigen-primed effector cells.28,29,33 In the elderly, where pentamer+ CD45RO− cells, in contrast, do not differ in expression of Ki-67 from pentamer− CD45RO− cells, the comparison is appropriate, because in the old, the bulk of CD8+ CD45RO− cells are likely to be effector cells.34
Third, in two elderly subjects studied over an interval of at least 5 years, the stability of the size of the Vβ families containing the dominant HCMV pentamer+ population would be consistent with long-lived oligoclonal populations. (Of course, the same observation would follow if short-lived clones disappeared to be replaced by similar numbers of different clones of exactly the same Vβ specificity, but this appears less likely.) Such data are consistent with previously published data showing relative stability in the clonal response to CMV over a 2-year period.11
Our data demonstrate highly restricted Vβ distribution of HCMV-specific cells; this does not prove that few CD8 clones are present but is consistent with previous studies showing that HCMV responses are often limited to small numbers of clones.2,35 Indeed single clones may dominate; in one individual in this study we used heteroduplex analysis to examine a pentamer-binding population comprising a single Vβ family and found a single dominant clone (data not shown). Vβ staining of pentamer-binding populations also suggests that greater size of the expansion is associated with more restricted Vβ usage.
In an earlier study we showed that CD8+ CD45RA+ T cells from young and elderly donors divided at a similar slow rate, but that survival of labelled CD8+ CD45RA+ cells in the elderly appeared to be extended.33 We hypothesized that such prolonged survival might contribute to the expansion of this subset in the elderly. As HCMV-specific T cells had been reported to contain a high proportion of these effector cells,27 we speculated that prolonged survival might contribute to the expansion of HCMV-specific populations in the elderly. The present data accord with this view, because we show that pentamer+ cells divide in vivo more slowly than other populations assayed and exhibit slow disappearance kinetics (Fig. 3, Table 3). Such a model would be consistent with other data showing that effector memory CD45RA+ T cells are relatively resistant to CD95-mediated apoptosis.36
At present we are more limited in our analysis of cell survival in the young because we were not able to separate enough pentamer-binding cells from young donors to enable us to measure deuterated glucose incorporation into DNA. However, young and old pentamer-binding cells are strikingly similar in the other phenotypic characteristics studied so it seems likely that similar mechanisms apply in both settings although HCMV-specific cells may divide more rapidly in the young.
In terms of drivers for HCMV-specific clonal expansion, persisting viral stimulation has been postulated but this does not explain why other persistent viruses, e.g. HSV, varicella zoster virus, Epstein–Barr virus, do not seem to generate similar CD8 responses.27 Repeated class I presentation of HCMV antigens without co-stimulation may induce a state of persistence37,38 and this might explain the accumulation of HCMV-specific cells. If this were so, alterations in apoptotic markers might be anticipated. We found no change in expression of Bcl-2 or CD95 in this study (Fig. 5) but these are only two among many pathways regulating survival/death so an impaired apoptosis model is still consistent with our findings. A consistent finding was a phenotype characteristic of advanced differentiation (CD27− CD28−).28 Such populations may be resistant to in vivo proliferative responses because of their requirement for CD137/4-1BBL co-stimulation, rather than CD28.2,39 Furthermore, slow division rates may be necessary for survival of cells with the replicative constraints of short telomere length.19,30 We found no evidence to support the hypothesis that HCMV-specific populations expand as a compensatory mechanism for reduced functionality. However, we only tested two modalities (in vitro peptide-stimulated proliferation and up-regulation of CD107); other indices of function may be abnormal. In addition, the use of an index of functionality in screening subjects (interferon-γ production in response to HCMV lysate) may have excluded subjects who mount anti-CMV responses that are non-functional. Despite such caveats, pentamer+ cells are clearly not effete, although they may have a restricted functionality in other domains.
Several limitations of this study are notable. It was only possible to study a limited number of elderly subjects by in vivo labelling in this study and further confirmatory studies are planned together with comparative studies in young subjects once cell number constraints are overcome. The assumptions and limitations of the deuterated-glucose-labelling approach are discussed elsewhere.20 Some disparity between in vivo turnover and Ki-67 expression was noted; specifically, we did not see a reduction in Ki-67 expression in pentamer+ cells in the old, commensurate with the in vivo labelling studies. This difference may relate to greater sensitivity with in vivo measurements or may be a sampling issue; in vivo labelling captures dividing cells from all compartments, provided they re-circulate freely, whereas Ki-67 FACS only measures recently divided cells at the time they are in the blood. Furthermore, we note a degree of selectivity inherent in our comparisons; pentamer positivity only captures part of the HCMV-responsive CD8 population; not all CMV-responsive cells may behave identically to those recognizing these specific peptides. We selected subjects with large HCMV responses; those with very small responses may have different kinetics.
In summary, our data are more consistent with a model in which HCMV-specific expansions are comprised of cells that have the characteristics of T-cell senescence, being long-lived with infrequent cell divisions, whilst also retaining at least some functional capacity. They are likely to have short telomeres and be resistant to apoptosis. Further studies might explore the mechanisms that confer this apparent resistance to apoptosis including more extensive analyses of apoptotic pathways and studies of alternative markers of functionality. In terms of therapeutic considerations, one might speculate that an environment promoting appropriate cell differentiation and apoptosis may benefit individuals with very large expansions.
Acknowledgments
We are grateful to Toni Sobande for assistance with analyses and to David Price for helpful comments on the manuscript. We are grateful to the donors who were willing to give blood samples and to U3A and Research in Ageing for help with recruitment. We acknowledge the support of Research in Ageing, the Wellcome Trust, BBSRC and the Medical Research Council (UK) in carrying out these studies.
Disclosures
The authors have no financial conflict of interest to disclose.
References
- 1.Sinclair J, Sissons P. Latency and reactivation of human cytomegalovirus. J Gen Virol. 2006;87:1763–79. doi: 10.1099/vir.0.81891-0. [DOI] [PubMed] [Google Scholar]
- 2.Waller EC, Day E, Sissons JG, Wills MR. Dynamics of T cell memory in human cytomegalovirus infection. Med Microbiol Immunol. 2008;197:83–96. doi: 10.1007/s00430-008-0082-5. [DOI] [PubMed] [Google Scholar]
- 3.Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873–9. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- 4.Pawelec G, Larbi A, Derhovanessian E. Senescence of the human immune system. J Comp Pathol. 2010;142S:S39–44. doi: 10.1016/j.jcpa.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence: is it infectious? Immunol Rev. 2005;205:257–68. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 6.Akbar AN, Beverley PC, Salmon M. Will telomere erosion lead to a loss of T-cell memory? Nat Rev Immunol. 2004;4:737–43. doi: 10.1038/nri1440. [DOI] [PubMed] [Google Scholar]
- 7.Goronzy JJ, Fulbright JW, Crowson CS, Poland GA, O’Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol. 2001;75:12182–7. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishimoto S, Tomino S, Mitsuya H, Fujiwara H, Tsuda H. Age-related decline in the in vitro and in vivo syntheses of anti-tetanus toxoid antibody in humans. J Immunol. 1980;125:2347–52. [PubMed] [Google Scholar]
- 9.Olsson J, Wikby A, Johansson B, Lofgren S, Nilsson BO, Ferguson FG. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech Ageing Dev. 2000;121:187–201. doi: 10.1016/s0047-6374(00)00210-4. [DOI] [PubMed] [Google Scholar]
- 10.Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp Gerontol. 2002;37:445–53. doi: 10.1016/s0531-5565(01)00212-1. [DOI] [PubMed] [Google Scholar]
- 11.Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, Thor SP, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–53. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 12.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–58. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clambey ET, van Dyk LF, Kappler JW, Marrack P. Non-malignant clonal expansions of CD8+ memory T cells in aged individuals. Immunol Rev. 2005;205:170–89. doi: 10.1111/j.0105-2896.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 14.Khan N, Hislop A, Gudgeon N, Cobbold M, Khanna R, Nayak L, Rickinson AB, Moss PA. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol. 2004;173:7481–9. doi: 10.4049/jimmunol.173.12.7481. [DOI] [PubMed] [Google Scholar]
- 15.Akbar AN, Fletcher JM. Memory T cell homeostasis and senescence during aging. Curr Opin Immunol. 2005;17:480–5. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–92. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 17.Clambey ET, Kappler JW, Marrack P. CD8 T cell clonal expansions & aging: a heterogeneous phenomenon with a common outcome. Exp Gerontol. 2007;42:407–11. doi: 10.1016/j.exger.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol. 2007;42:563–70. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher JM, Vukmanovic-Stejic M, Dunne PJ, et al. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J Immunol. 2005;175:8218–25. doi: 10.4049/jimmunol.175.12.8218. [DOI] [PubMed] [Google Scholar]
- 20.Macallan DC, Asquith B, Zhang Y, de Lara CM, Ghattas H, Defoiche J, Beverley PC. Measurement of proliferation and disappearance of rapid turnover cell populations in human studies using deuterium-labelled glucose. Nat Protoc. 2009;4:1313–27. doi: 10.1038/nprot.2009.117. [DOI] [PubMed] [Google Scholar]
- 21.Vukmanovic-Stejic M, Agius E, Booth N, et al. The kinetics of CD4+Foxp3+ T cell accumulation during a human cutaneous antigen-specific memory response in vivo. J Clin Invest. 2008;118:3639–50. doi: 10.1172/JCI35834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghattas H, Darboe BM, Wallace DL, Griffin GE, Prentice AM, Macallan DC. Measuring lymphocyte kinetics in tropical field settings. Trans R Soc Trop Med Hyg. 2005;99:675–85. doi: 10.1016/j.trstmh.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Busch R, Neese RA, Awada M, Hayes GM, Hellerstein MK. Measurement of cell proliferation by heavy water labeling. Nat Protoc. 2007;2:3045–57. doi: 10.1038/nprot.2007.420. [DOI] [PubMed] [Google Scholar]
- 24.Asquith B, Debacq C, Macallan DC, Willems L, Bangham C. Lymphocyte kinetics: the interpretation of labelling data. Trends Immunol. 2002;23:596–601. doi: 10.1016/s1471-4906(02)02337-2. [DOI] [PubMed] [Google Scholar]
- 25.Macallan DC, Asquith B, Irvine A, et al. Measurement and modeling of human T cell kinetics. Eur J Immunol. 2003;33:2316–26. doi: 10.1002/eji.200323763. [DOI] [PubMed] [Google Scholar]
- 26.Akbar AN, Terry L, Timms A, Beverley PC, Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988;140:2171–8. [PubMed] [Google Scholar]
- 27.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–85. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 28.Appay V, Van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–83. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 29.Kuijpers TW, Vossen MT, Gent MR, et al. Frequencies of circulating cytolytic, CD45RA+CD27–, CD8+ T lymphocytes depend on infection with CMV. J Immunol. 2003;170:4342–8. doi: 10.4049/jimmunol.170.8.4342. [DOI] [PubMed] [Google Scholar]
- 30.van de Berg PJ, Griffiths SJ, Yong SL, et al. Cytomegalovirus infection reduces telomere length of the circulating T cell pool. J Immunol. 2010;184:3417–23. doi: 10.4049/jimmunol.0903442. [DOI] [PubMed] [Google Scholar]
- 31.Gillespie GM, Wills MR, Appay V, et al. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8+ T lymphocytes in healthy seropositive donors. J Virol. 2000;74:8140–50. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wills MR, Carmichael AJ, Weekes MP, Mynard K, Okecha G, Hicks R, Sissons JG. Human virus-specific CD8+ CTL clones revert from CD45ROhigh to CD45RAhighin vivo: CD45RAhighCD8+ T cells comprise both naive and memory cells. J Immunol. 1999;162:7080–7. [PubMed] [Google Scholar]
- 33.Wallace DL, Zhang Y, Ghattas H, et al. Direct measurement of T cell subset kinetics in vivo in elderly men and women. J Immunol. 2004;173:1787–94. doi: 10.4049/jimmunol.173.3.1787. [DOI] [PubMed] [Google Scholar]
- 34.Hamann D, Kostense S, Wolthers KC, Otto SA, Baars PA, Miedema F, Van Lier RA. Evidence that human CD8+CD45RA+CD27– cells are induced by antigen and evolve through extensive rounds of division. Int Immunol. 1999;11:1027–33. doi: 10.1093/intimm/11.7.1027. [DOI] [PubMed] [Google Scholar]
- 35.Weekes MP, Wills MR, Mynard K, Hicks R, Sissons JG, Carmichael AJ. Large clonal expansions of human virus-specific memory cytotoxic T lymphocytes within the CD57+ CD28– CD8+ T-cell population. Immunol. 1999;98:443–9. doi: 10.1046/j.1365-2567.1999.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta S, Gollapudi S. CD95-mediated apoptosis in naive, central and effector memory subsets of CD4+ and CD8+ T cells in aged humans. Exp Gerontol. 2008;43:266–74. doi: 10.1016/j.exger.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Redmond WL, Marincek BC, Sherman LA. Distinct requirements for deletion versus anergy during CD8 T cell peripheral tolerance in vivo. J Immunol. 2005;174:2046–53. doi: 10.4049/jimmunol.174.4.2046. [DOI] [PubMed] [Google Scholar]
- 38.Rocha B, Grandien A, Freitas AA. Anergy and exhaustion are independent mechanisms of peripheral T cell tolerance. J Exp Med. 1995;181:993–1003. doi: 10.1084/jem.181.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waller EC, McKinney N, Hicks R, Carmichael AJ, Sissons JG, Wills MR. Differential costimulation through CD137 (4-1BB) restores proliferation of human virus-specific “effector memory” (CD28– CD45RAHI) CD8+ T cells. Blood. 2007;110:4360–6. doi: 10.1182/blood-2007-07-104604. [DOI] [PubMed] [Google Scholar]


