Abstract
6-Sulpho LacNAc dendritic cells (slanDC) are a major population of human blood DC that are highly pro-inflammatory, as characterized by their outstanding capacity to produce tumour necrosis factor-α and interleukin-12 (IL-12) and to prime antigen-specific T-cell responses. SlanDC were found to be present in inflamed tissue such as atopic dermatitis, where high levels of histamine are also present. As histamine is an important regulator of allergic inflammation we investigated the role of histamine receptors, particularly the most recently identified histamine H4 receptor (H4R), in modulating the pro-inflammatory function of slanDC. The expression of H4R was evaluated by real-time PCR and flow cytometry. Cytokine production in response to H4R stimulation was assessed by intracellular flow cytometric staining and enzyme-linked immunosorbent assay. We show that slanDC express the H1R, H2R and H4R on mRNA and the H4R on protein level. No differences were observed in basal H4R expression in patients with atopic dermatitis and psoriasis, but in atopic dermatitis patients the H4R was up-regulated by interferon-γ. When stimulated with lipopolysaccharide in the presence of histamine, slanDC produced substantially lower levels of the pro-inflammatory cytokines tumour necrosis factor-α and IL-12, mediated solely via the H4R and via the combined action of H2R and H4R, respectively. In contrast, the production of IL-10 was not affected by histamine receptor activation on slanDC. The slanDC express the H4R and its stimulation leads to reduced pro-inflammatory capacity of slanDC. Hence, H4R agonists might have therapeutic potential to down-regulate immune reactions, e.g. in allergic inflammatory skin diseases.
Keywords: H4 receptor, histamine, interleukin-12, 6-sulpho LacNAc dendritic cells, tumour necrosis factor-α
Introduction
6-Sulpho LacNAc expressing dendritic cells (slanDC) were previously identified as a new subset of human DC.1 SlanDC account for 0·5–2% of the peripheral blood mononuclear cells (PBMC) and therefore represent the largest population of DC present in human blood. SlanDC appear as important pro-inflammatory immune cells because they show great capacity to induce primary antigen-specific T-cell responses2 and they up-regulate the expression of the activation marker CD69 and the secretion of IFN-γ (interferon-γ) in natural killer cells.3 Moreover slanDC stand out by their high-level production of tumour necrosis factor-α (TNF-α) and they are the main source of interleukin-12 (IL-12) among blood leucocytes compared with monocytes and CD1c+ DC.4 In contrast to classical CD1c+ DC and plasmacytoid DC, slanDC express anaphylatoxin receptors (C5aR, C3aR) and were shown to migrate in response to C5a stimulation in vivo.5 In T helper type 1 (Th1) -mediated diseases slanDC were shown to infiltrate the inflamed tissue: they have been identified in the dermis of patients suffering from psoriasis vulgaris and in the pannus tissue of rheumatoid arthritis.4 Recently their presence was also shown in skin lesions of the chronic phase of atopic dermatitis (AD) (Knut Schäkel, abstract communicated at the CIA meeting 2008). The chronic phase of AD is characterized by a Th1 phenotype (in contrast to the acute phase, which is more Th2 dominated6), which fosters the hypothesis that slanDC play a role in allergic inflammatory skin diseases, especially in Th1-mediated pathologies.
Histamine represents an important immunomodulatory mediator that plays a role in acute as well as in chronic allergic reactions and is present at high levels in the skin of AD and other inflammatory skin diseases such as psoriasis.7,8 Histamine is released from mast cells and basophils upon IgE-receptor cross-linking and four different G-protein-coupled histamine receptors have been identified.9 Histamine receptors are functionally expressed on many cells involved in inflammatory skin reactions, i.e. on eosinophils10 mast cells,11 keratinocytes,12,13 T cells14 as well as antigen presenting cells.15–17 Especially the H1R and the recently discovered H4R18,19 histamine receptors were shown to have an immunomodulatory function. In this regard we observed that the stimulation of the H4R on in vitro-generated monocyte-derived DC (MoDC) and monocyte-derived inflammatory epidermal DC resulted in chemotaxis and a reduced production of IL-12 and CCL2.15,16 These data support the view of targeting the H4R for therapeutic use. However, native human blood DC such as slanDC, which are direct precursors of inflammatory dermal, mucosal or synovial DC have not been studied in this respect. Moreover, because of their outstanding capacity to induce T-cell responses and pro-inflammatory cytokines and their recruitment to inflamed skin, slanDC are of particular immunological relevance in allergic inflammatory diseases.
Therefore we sought to investigate the expression of histamine receptors on slanDC, especially the H4R, in different groups, including patients with AD and psoriasis and healthy controls. In addition we were interested in the regulation of H4R expression by different cytokines and a possible role of histamine in the modulation of the pro-inflammatory function of slanDC.
Methods
Isolation of PBMC and slanDC
Peripheral blood samples were taken from patients with severe extrinsic AD or psoriasis, patients without inflammatory skin disease served as controls. Patients were diagnosed and treated in our department; they did not receive systemic treatment during a 2-week period before blood withdrawal. All participants gave their written informed consent. The PBMC were separated by density centrifugation on lymphoprep (Fresenius Kabi Norge AS, Oslo, Norway) and erythrocytes were removed by incubation with Gey’s lysis buffer. For the isolation of slanDC, buffy coats from anonymous healthy donors were obtained from the local blood bank. SlanDC were isolated from the PBMC using magnetic cell sorting. The positive isolation procedure was performed as described previously.4 Briefly, PBMC were incubated with supernatant of the M-DC8 hybridoma, followed by labelling with anti-mIgM magnetic microbeads (Miltenyi Biotech, Bergisch-Gladbach, Germany) before separation using the MACS Cell Separation technology (Miltenyi Biotech). The PBMC and isolated slanDC (purity of 90–95%) were cultured in Iscove’s medium supplemented with 2 mm l-glutamine, 100 μg/ml penicillin/streptomycin, 1 × non-essential amino acids, 0·05 mg/ml gentamicin and 6% volume/volume fetal calf serum at 37° in a humidified atmosphere containing 5% CO2.
Flow cytometric analysis of H4R expression
The cells were washed twice in PBS and then incubated for 20 min in PBS (Biochrom, Berlin, Germany) containing 500 μg/ml human IgG (Aventis Behring, Marburg, Germany), 0.2% w/V gelatine (Sigma, Deisenhofen, Germany) and 20 mM NaN3 (Sigma) (this buffer is referred to as FcγR-blocking buffer). The cell surface was stained with M-DC8 hybridoma supernatant and allophycocyanin-conjugated rat anti-mIgM (Beckman Coulter, Krefeld, Germany) alone or in combination with phycoerythrin-conjugated CD16 (Beckman Coulter). As isotype control, mIgM (BD Pharmingen, Heidelberg, Germany) and phycoerythrin-conjugated mIgG (Sigma) were used, respectively. Subsequently, cells were fixed and permeabilized (Fixation/Permeabilization kit; eBioscience, San Diego, CA). Intracellular staining was performed with H4R antibody recognizing amino acids 194–303 (SantaCruz Biotechnology, Santa Cruz, CA) or polyclonal rabbit isotype control (R&D Systems, Wiesbaden, Germany), followed by labelling with goat anti-rabbit-FITC (Beckman Coulter). M-DC8, CD16 and H4R positivity of the cells was assessed by flow cytometry (FACSCalibur; Becton Dickinson, Heidelberg, Germany).
For the measurement of H4R expression in response to cytokine stimulation the cells were incubated for 48 hr with 20 ng/ml IFN-γ (R&D Systems), 50 ng/ml IL-13 (Peprotech, Hamburg, Germany) or 10 μg/ml poly I:C (Sigma).
Messenger RNA isolation, reverse transcription, and quantitative real time-PCR
Isolated slanDC were washed in PBS and lysed for RNA isolation using a Mini RNA Isolation II kit (Zymo Research, Orange, CA) and reverse transcription was performed with the First-Strand cDNA Synthesis kit (MBI Fermentas, St Leon-Rot, Germany). As control, cDNA of H3R-transfected HEK cells was prepared analogously. Real-time quantitative PCR was performed on a LightCycler (Roche Molecular Biochemicals, Mannheim, Germany) using SYBR Green with Quantitect primer assays for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; QT01192646), H1R (QT00199857), H2R (QT00210378), H3R (QT00210861) and H4R (QT00032326) according to the manufacturer’s instructions (Qiagen, Hilden, Germany). The following PCR settings were used: an initial activation step of 15 min at 95° with ramp 20° per second was followed by three-step cycling (45 cycles): denaturation 15 seconds, 94°; annealing 20 seconds, 55°; extension 20 seconds, 72° (all three with ramp 2° per second). Melting curve analysis was performed from 60–90° with ramp 20° per second. The amount of target genes relative to the reference GAPDH was quantified using Relative Quantification software (Roche Molecular Biochemicals). To visualize the amplification products after completion of the PCR run, agrose gel electrophoresis was performed with 2% agarose (Roth, Karlsruhe, Germany) in 1 × Tris–borate–EDTA buffer (Roth).
Intracellular staining of TNF-α and IL-12
For the analysis of intracellular cytokine production PBMC were stimulated with 10 μm histamine (Alk-Scherax, Wedel, Germany) or 4-methylhistamine (Tocris Bioscience, Bristol, UK) for 6 hr, then the cells were activated by addition of 100 ng/ml lipopolysaccharide (LPS; Sigma-Aldrich, Deisenhofen, Germany) and 1 μg/ml Brefeldin (BD Biosciences, Heidelberg, Germany) for another 18 hr. For blocking experiments cells were treated with JNJ7777120 (Sigma-Aldrich) 30 min before the stimulation with histamine receptor agonists. Before staining, the cells were washed in PBS and after incubation with FcγR-blocking buffer the surface was stained with anti-M-DC8 and allophycocyanin-conjugated rat anti-mIgM (Beckman Coulter). After fixation and permeabilization (Fixation/Permeabilization kit; eBioscience), intracellular staining was performed with anti-TNF-α (eBioscience) and anti-IL-12 (BD Pharmingen) or mIgG isotype controls (Sigma).
Enzyme-linked immunosorbent assay
Isolated slanDC were stimulated with 10 μm histamine (Alk-Scherax), the H1R agonist 2-pyridylethylamine, the H2R agonist amthamine or the H4R agonist 4-methylhistamine (all from Tocris Bioscience) for 6 hr, then the cells were activated by addition of 100 ng/ml LPS (Sigma-Aldrich) and the supernatants were taken at the indicated time-points. For blocking experiments, cells were treated with the H4R antagonist JNJ7777120 (Sigma-Aldrich) 30 min before the stimulation with histamine receptor agonists. Cell-free supernatants were used to detect the cytokines TNF-α, IL-12 and IL-10 in ELISA performed according to the manufacturer’s instructions (eBioscience).
Statistical analysis
For statistical analysis the paired t-test was used; P < 0·05 was regarded as significant. The program GraphPad Prism® version 3.02 (GraphPad Software, Inc, San Diego, CA) was used for statistical analysis.
Ethics
The investigation of the role of histamine receptors in allergic skin inflammation was approved by the local ethics committee of the Hannover Medical School (Vote Nr. 4253) and was conducted according to the Declaration of Helsinki Principles.
Results
SlanDC express histamine receptors H1R, H2R and H4R
The mRNA for the histamine receptors H1R, H2R and H4R, but not that for H3R, was detected in isolated human slanDC by real-time LightCycler PCR (Fig. 1). Flow cytometric analysis of slanDC showed H4R-positive staining, which did not change during a 1-day culture of the cells, whereas the expression of CD16 was down-regulated (as described previously1) (Fig. 2).
Figure 1.
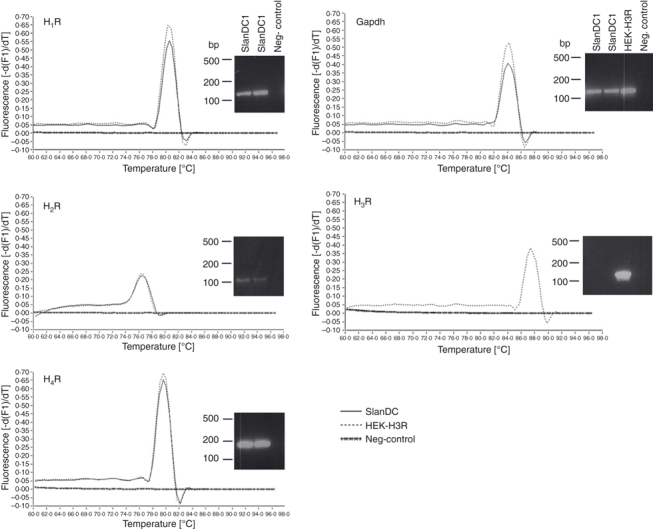
6-Sulpho LacNAc dendritic cells (SlanDC) express the histamine receptors H1R, H2R and H4R at the mRNA level. Representative real-time PCR melting peaks and agarose gel bands of the PCR products out of two independent experiments are depicted (negative control is without reverse transcription, H3R-transfected HEK cells were used as positive control for H3R amplification).
Figure 2.
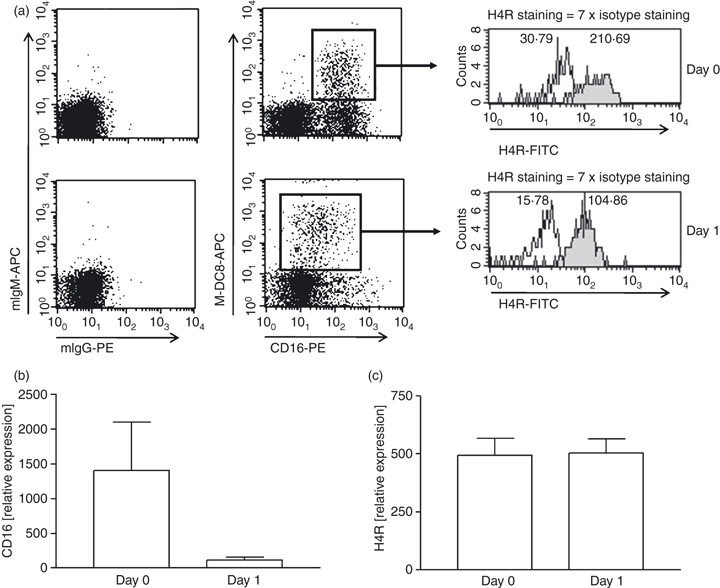
6-Sulpho LacNAc dendritic cells (SlanDC) express the histamine receptor H4R on protein level. CD16 and H4R expression on M-DC8-positive slanDC were measured by flow cytometry at day 0 and after 1 day in culture. In (a) one representative experiment of four which are summarized in (b and c) is shown. (white: isotype; grey: specific staining; the numbers given in the histograms are mean fluorescence intensity; relative expression levels of CD16 and H4R were calculated as follows: specific staining times hundred divided by isotype staining).
Regulation of H4R expression
SlanDC from individuals without inflammatory skin diseases, patients with AD and patients with psoriasis expressed similar levels of H4R as determined by flow cytometry (Fig. 3a). Stimulation with the Th1 cytokine IFN-γ resulted in up-regulation of the H4R on slanDC isolated from patients with AD (Fig. 3b), whereas in healthy controls and psoriasis patients no effect of IFN-γ was observed (Fig. 3c,d). The Th2 cytokine IL-13 and the toll-like receptor ligand poly I:C had no significant effect on H4R expression in any of the studied groups (data not shown).
Figure 3.
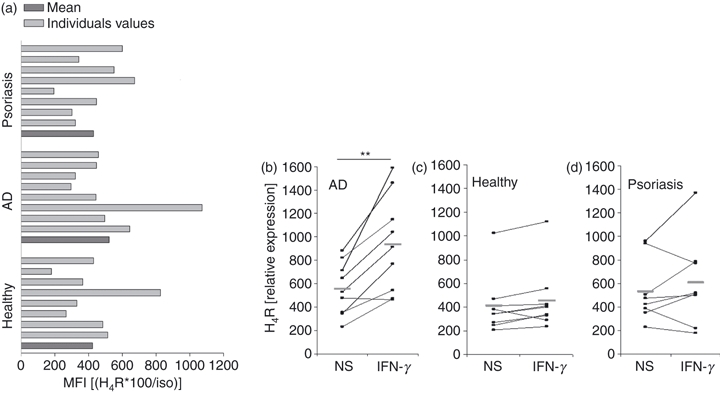
Regulation of expression of the histamine receptor H4R in disease and by inflammatory cytokines. (a) Basal H4R expression does not vary in different disease groups [psoriasis, atopic dermatitis (AD) and healthy controls, eight individual measurements and their mean are depicted]. (b–d) PBMC were stimulated for 48 hr with 20 ng/ml interferon-γ (IFN-γ) and H4R expression on slanDC was determined flow cytometrically. The relative mean fluorescence intensity of non-stimulated cells versus IFN-γ-stimulated cells is shown for 6-sulpho LacNAc dendritic cells (slanDC) isolated from AD patients (b), healthy controls (c) and psoriasis patients (d). (The relative expression levels of H4R were calculated as follows: specific staining times hundred divided by isotype staining.) **P < 0·005.
H4R stimulation leads to down-regulation of LPS-induced TNF-α and IL-12 production
It has been described previously, that slanDC are the principal producers of IL-12 and also produce high levels of TNF-α upon activation with the toll-like receptor ligand LPS.2 Stimulation of PBMC with histamine or the H4R-specific agonist 4-methylhistamine significantly down-regulated the production of TNF-α and IL-12 in slanDC, as measured by intracellular cytokine staining (Fig. 4a,b). The down-regulation of TNF-α could be fully blocked by pre-incubation of the cells with the H4R selective antagonist JNJ7777120, showing that the effect is specific for H4R (Fig. 4a); for IL-12 only partial blockage was achieved (Fig. 4b). In addition to studies with PBMC the effect of histamine on the release of cytokines into the cell culture supernatant was investigated in isolated slanDC. We could observe histamine-induced down-regulation of TNF-α and IL-12 secretion into the supernatant at three consecutive time points: 24, 48 and 72 hr (Fig. 5). The H4R agonist 4-methylhistamine also led to decreased cytokine secretion and the H4R receptor antagonist JNJ7777120 could selectively block the down-regulation (Fig. 6a). As slanDC also express the H1R and H2R (Fig. 1) we tested in addition agonists at these receptors. The TNF-α secretion was not down-regulated after stimulation with the H1R agonist 2-pyridylethylamine and the H2R agonist amthamine, indicating that the down-regulation of TNF-α is solely mediated via the H4R. For IL-12 we observed down-regulation after stimulation with the H2R agonist indicating that the down-regulation of IL-12 is mediated by two histamine receptors H2R and H4R (Fig. 6b). We did not observe significant differences in the secretion of the anti-inflammatory cytokine IL-10 (Figs 5 and 6).
Figure 4.
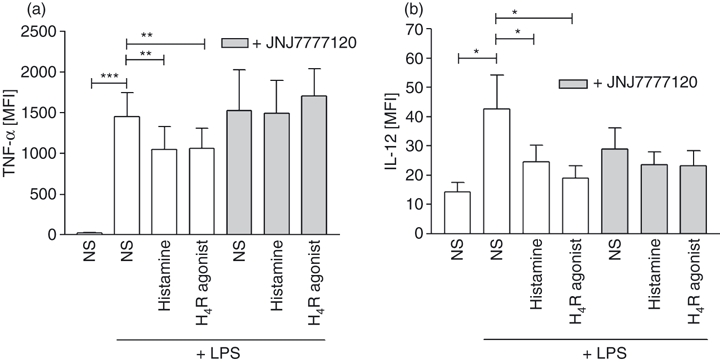
Stimulation of the histamine receptor H4R reduces the production of tumour necrosis factor-α (TNF-α) and interleukin-12 (IL-12) as determined by intracellular cytokine staining. PBMC were stimulated for 6 hr with 10 μm histamine or the H4R agonist 4-methylhistamine, then 100 ng/ml lipopolysaccharide (LPS) and 1 μg/ml Brefeldin were added and intracellular (a) TNF-α production and (b) IL-12 production were measured by flow cytometry after overnight incubation. Pre-incubation with JNJ7777120 fully blocked the H4R-induced down-regulation of TNF-α and partially IL-12 (mean and SEM of 11 independent experiments are shown). *P < 0·05; **P < 0·005; ***P < 0·001.
Figure 5.
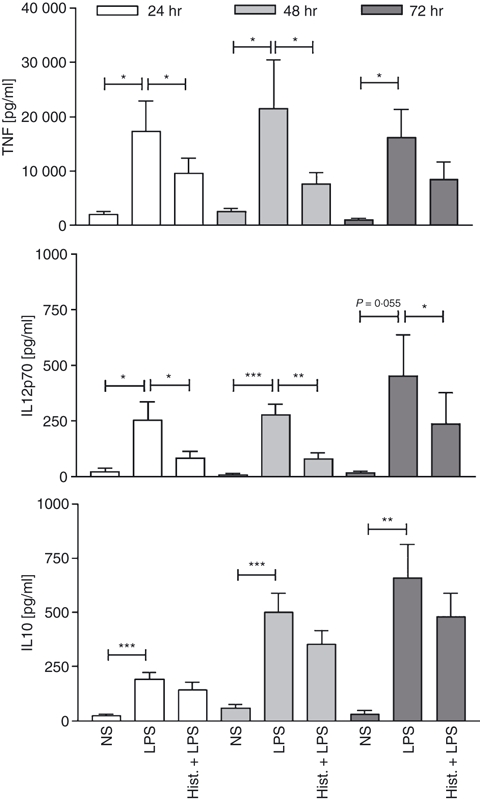
Histamine decreases tumour necrosis factor-α (TNF-α) and interleukin-12 (IL-12) secretion into the cell supernatant. Isolated 6-sulpho LacNAc dendritic cells (slanDC) were stimulated for 6 hr with 10 μm histamine, then 100 ng/ml LPS was added and after 24, 48 and 72 hr the cytokine content in the cell culture supernatant was determined by ELISA (mean and SEM of 11 independent experiments are shown). *P < 0·05; **P < 0·005; ***P < 0·001.
Figure 6.
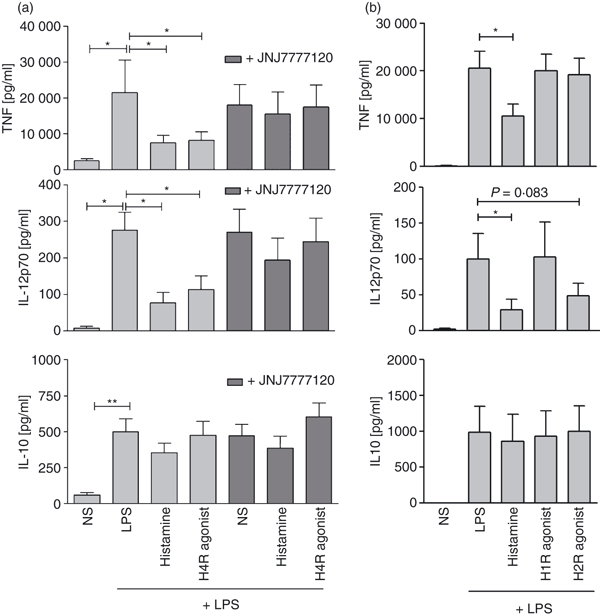
The down-regulation of tumour necrosis factor-α (TNF-α) and interleukin-12 (IL-12) is mediated by the histamine receptor H4R. (a)After 48 hr of stimulation histamine and the H4R agonist 4-methylhistamine reduced the level of lipopolysaccharide (LPS) -induced TNF-α and IL-12, but not IL-10. Pre-incubation with the H4R antagonist JNJ7777120 restored LPS-induced cytokine production (mean and SEM of 11 independent experiments is shown). (b) The H1R agonist 2-pyrilethylamine did not reduce LPS-induced TNF-α and IL-12 production. The H2R agonist amthamine had no effect on TNF-α secretion, but down-regulated IL-12 production. The cytokine IL-10 was not affected by histamine receptor stimulation (mean and SEM of seven independent experiments are shown). *P < 0·05; **P < 0·005.
Discussion
Several studies show that slanDC are pro-inflammatory cells producing large amounts of inflammatory cytokines and inducing antigen-specific T-cell responses.2,4 As a result of their presence in chronic lesions of AD and psoriasis they are thought to be involved in the pathogenesis of inflammatory skin diseases. However, relatively little is known about the regulation of their function. We chose to investigate the effect of histamine on slanDC, because histamine is an important inflammatory mediator present in the lesions of AD and psoriasis and histamine has been shown to modulate the function of other types of antigen-presenting cells such as monocytes17 and MoDC.15 Here we show for the first time, that slanDC express histamine receptors and that their pro-inflammatory capacity is down-regulated in response to stimulation with histamine.
SlanDC express mRNA for three histamine receptors H1R, H2R and H4R, but not for the H3R. The absence of H3R expression is not surprising as the H3R mainly plays a role in the regulation of neurotransmission in the central nervous system.20 Moreover the histamine receptor expression pattern is similar to what is known for other DC subtypes, such as MoDC.15 The newly described H4R is of particular interest in inflammatory skin diseases21 and immunomodulatory effects on DC were already identified so we decided to study this receptor in more detail. By flow cytometry we could show that slanDC express the H4R on the protein level and that the expression level does not change during culture of the cells. We did not observe differences in the basal H4R expression level in diseases like AD and psoriasis, but the Th1-associated cytokine IFN-γ led to an up-regulation of H4R expression of slanDC isolated from patients with AD, whereas in healthy and psoriatic cells no difference was observed. The Th2-associated cytokine IL-13 and the toll-like receptor ligand poly I:C could not significantly modulate the expression of H4R in any of the studied groups. The increase of H4R expression upon IFN-γ stimulation was also described for inflammatory dendritic epidermal cells,16 a subset of DC only present in the inflamed skin of AD patients.22 In chronic lesions of AD, predominantly IFN-γ and other Th1 cytokines are present, therefore it is likely that slanDC up-regulate the expression of the H4R during and after the infiltration to these tissues. Interestingly we did not find up-regulation of the H4R on slanDC derived from psoriasis patients, although this disease is also Th1-mediated. Possible explanations for this observation could be disease-dependent differences in IFN-γ-mediated signalling or variations in the expression density of IFN-γ receptors. It has been shown for example that atopic diseases are associated with genetic polymorphisms in the IFN-γ receptor 1 gene leading to higher transcription of this receptor.23
To study the functional effects of histamine on slanDC, we stimulated PBMC as well as isolated slanDC with histamine and H4R agonists. After histamine stimulation we observed impaired intracellular production and release into the supernatant of the pro-inflammatory cytokines TNF-α and IL-12 in response to slanDC activation by the toll-like receptor agonist LPS. Although the down-regulation of TNF-α was solely mediated via the H4R, we observed a dual H2R and H4R mediated effect for IL-12, which is in accordance with previous findings on MoDC.15 These observations strongly suggest that histamine impairs the pro-inflammatory capacity of slanDC, because the key cytokines of early immune responses are no longer produced in high amounts. Interleukin-12 is an important activator of natural killer cells and induces the differentiation of CD4+ T cells into Th1 cells. TNF-α belongs to the family of acute-phase proteins and is known to induce inflammation and apoptosis, to lead to vasodilatation and increased vascular permeability and to be a potent activator of endothelial cells. The activity of slanDC in the bloodstream is tightly controlled by erythrocytes, which might prevent harmful systemic over-production of inflammatory mediators. It has been shown that the addition of erythrocytes to cultured slanDC blocks their capacity to produce IL-12 and TNF-α via the interaction of CD47 on erythrocytes and the corresponding ligand signal regulatory protein α on slanDC.4 After slanDC leave the bloodstream and infiltrate the tissue, as it was shown in inflamed skin of AD lesions, the control by erythrocytes is lost. Our study suggests that histamine might take over this control function in the tissue because we could show that histamine reduces the highly pro-inflammatory capacity of slanDC, particularly via activation of the H4R. To be sure that histamine stimulation does not reduce cytokine production in general, we investigated IL-10 as a cytokine that is associated with anti-inflammatory actions. Interleukin-10 reduces the production of IL-2, TNF-α, IFN-γ and co-stimulatory molecules and was shown to counteract the inflammatory response in allergic contact dermatitis.24 In our study we could not observe a significant effect of histamine receptor activation on the release of IL-10. As a result, it can be assumed that H4R and H2R stimulation on slanDC specifically down-regulate the production of the pro-inflammatory mediators TNF-α and IL-12, whereas the level of the anti-inflammatory mediator IL-10 is not affected. The differential regulation of cytokine release by histamine might be explained by varying signalling processes involved. For example, it was shown that the activation of mitogen-activated protein kinase signalling mediates histamine-induced down-regulation of IL-12p70 in monocytes,15 but on the other hand induces IL-10 production in monocytes.25
Our observations fit the current understanding of the role of the H4R on antigen-presenting cells. Several studies have shown that the H4R on DC has an anti-inflammatory role: on MoDC, monocytes and inflammatory dendritic epidermal cells the production of IL-12 and CCL2 was down-regulated after H4R activation.15–17 In response to the reduced presence of these mediators, Th1 polarization is impaired and a lower number of macrophages and T cells is attracted to the site of the immune response, respectively.
We can draw the conclusion that the stimulation of the H4R on DC, and as shown here in particular on slanDC, could greatly reduce the inflammatory responses taking place in the course of inflammatory skin diseases like AD and H4R agonists therefore might represent potential therapeutic tools in these kinds of diseases.
Acknowledgments
This study was supported by grants from the Deutsche Forschungsgemeinschaft DFG: Gu434/5-1 and GRK1441/1 and the European Community (COST action BM0806). Maria Gschwandtner was supported by a grant from the Hannover Biomedical Research School.
Glossary
Abbreviations
- H4R
H4 receptor
- MoDC
monocyte-derived dendritic cell
- slanDC
6-sulpho LacNAc dendritic cells
Disclosures
The authors declare no conflict of interest.
References
- 1.Schakel K, Mayer E, Federle C, Schmitz M, Riethmuller G, Rieber EP. A novel dendritic cell population in human blood: one-step immunomagnetic isolation by a specific mAb (M-DC8) and in vitro priming of cytotoxic T lymphocytes. Eur J Immunol. 1998;28:4084–93. doi: 10.1002/(SICI)1521-4141(199812)28:12<4084::AID-IMMU4084>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Schmitz M, Zhao S, Schakel K, Bornhauser M, Ockert D, Rieber EP. Native human blood dendritic cells as potent effectors in antibody-dependent cellular cytotoxicity. Blood. 2002;100:1502–4. [PubMed] [Google Scholar]
- 3.Wehner R, Lobel B, Bornhauser M, et al. Reciprocal activating interaction between 6-sulfo LacNAc+ dendritic cells and NK cells. Int J Cancer. 2009;124:358–66. doi: 10.1002/ijc.23962. [DOI] [PubMed] [Google Scholar]
- 4.Schakel K, von Kietzell M, Hansel A, et al. Human 6-sulfo LacNAc-expressing dendritic cells are principal producers of early interleukin-12 and are controlled by erythrocytes. Immunity. 2006;24:767–77. doi: 10.1016/j.immuni.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Schakel K, Kannagi R, Kniep B, et al. 6-Sulfo LacNAc, a novel carbohydrate modification of PSGL-1, defines an inflammatory type of human dendritic cells. Immunity. 2002;17:289–301. doi: 10.1016/s1074-7613(02)00393-x. [DOI] [PubMed] [Google Scholar]
- 6.Werfel T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J Invest Dermatol. 2009;129:1878–91. doi: 10.1038/jid.2009.71. [DOI] [PubMed] [Google Scholar]
- 7.Simons FE, Simons KJ. The pharmacology and use of H1-receptor-antagonist drugs. N Engl J Med. 1994;330:1663–70. doi: 10.1056/NEJM199406093302307. [DOI] [PubMed] [Google Scholar]
- 8.Krogstad AL, Lonnroth P, Larson G, Wallin BG. Increased interstitial histamine concentration in the psoriatic plaque. J Invest Dermatol. 1997;109:632–5. doi: 10.1111/1523-1747.ep12337620. [DOI] [PubMed] [Google Scholar]
- 9.Bakker RA, Timmerman H, Leurs R. Histamine receptors: specific ligands, receptor biochemistry, and signal transduction. Clin Allergy Immunol. 2002;17:27–64. [PubMed] [Google Scholar]
- 10.Ling P, Ngo K, Nguyen S, et al. Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. Br J Pharmacol. 2004;142:161–71. doi: 10.1038/sj.bjp.0705729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther. 2003;305:1212–21. doi: 10.1124/jpet.102.046581. [DOI] [PubMed] [Google Scholar]
- 12.Giustizieri ML, Albanesi C, Fluhr J, Gisondi P, Norgauer J, Girolomoni G. H1 histamine receptor mediates inflammatory responses in human keratinocytes. J Allergy Clin Immunol. 2004;114:1176–82. doi: 10.1016/j.jaci.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 13.Gschwandtner M, Purwar R, Wittmann M, et al. Histamine upregulates keratinocyte MMP-9 production via the histamine H1 receptor. J Invest Dermatol. 2008;128:2783–91. doi: 10.1038/jid.2008.153. [DOI] [PubMed] [Google Scholar]
- 14.Gutzmer R, Mommert S, Gschwandtner M, Zwingmann K, Stark H, Werfel T. The histamine H4 receptor is functionally expressed on T(H)2 cells. J Allergy Clin Immunol. 2009;123:619–25. doi: 10.1016/j.jaci.2008.12.1110. [DOI] [PubMed] [Google Scholar]
- 15.Gutzmer R, Diestel C, Mommert S, et al. Histamine H4 receptor stimulation suppresses IL-12p70 production and mediates chemotaxis in human monocyte-derived dendritic cells. J Immunol. 2005;174:5224–32. doi: 10.4049/jimmunol.174.9.5224. [DOI] [PubMed] [Google Scholar]
- 16.Dijkstra D, Stark H, Chazot PL, et al. Human inflammatory dendritic epidermal cells express a functional histamine H4 receptor. J Invest Dermatol. 2008;128:1696–703. doi: 10.1038/sj.jid.5701250. [DOI] [PubMed] [Google Scholar]
- 17.Dijkstra D, Leurs R, Chazot P, et al. Histamine downregulates monocyte CCL2 production through the histamine H4 receptor. J Allergy Clin Immunol. 2007;120:300–7. doi: 10.1016/j.jaci.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Itadani H, Hidaka Y, Ohta M, Tanaka K. Molecular cloning and characterization of a new human histamine receptor, HH4R. Biochem Biophys Res Commun. 2000;279:615–20. doi: 10.1006/bbrc.2000.4008. [DOI] [PubMed] [Google Scholar]
- 19.Oda T, Morikawa N, Saito Y, Masuho Y, Matsumoto S. Molecular cloning and characterization of a novel type of histamine receptor preferentially expressed in leukocytes. J Biol Chem. 2000;275:36781–6. doi: 10.1074/jbc.M006480200. [DOI] [PubMed] [Google Scholar]
- 20.Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302:832–7. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- 21.Zampeli E, Tiligada E. The role of histamine H4 receptor in immune and inflammatory disorders. Br J Pharmacol. 2009;157:24–33. doi: 10.1111/j.1476-5381.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wollenberg A, Kraft S, Hanau D, Bieber T. Immunomorphological and ultrastructural characterization of Langerhans cells and a novel, inflammatory dendritic epidermal cell (IDEC) population in lesional skin of atopic eczema. J Invest Dermatol. 1996;106:446–53. doi: 10.1111/1523-1747.ep12343596. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda A, Ebihara N, Kumagai N, et al. Genetic polymorphisms in the promoter of the interferon gamma receptor 1 gene are associated with atopic cataracts. Invest Ophthalmol Vis Sci. 2007;48:583–9. doi: 10.1167/iovs.06-0991. [DOI] [PubMed] [Google Scholar]
- 24.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 25.Dobreva ZG, Miteva LD, Stanilova SA. The inhibition of JNK and p38 MAPKs downregulates IL-10 and differentially affects c-Jun gene expression in human monocytes. Immunopharmacol Immunotoxicol. 2009;31:195–201. doi: 10.1080/08923970802626276. [DOI] [PubMed] [Google Scholar]


