Abstract
Functional disruption of dendritic cells (DC) is an important strategy for viral pathogens to evade host defences. In this context, porcine circovirus type 2 (PCV2), a single-stranded DNA virus, impairs plasmacytoid DC (pDC) and conventional DC activation by certain viruses or Toll-like receptor (TLR) ligands. This inhibitory capacity is associated with the viral DNA, but the impairment does not affect all signalling cascades; TLR7 ligation by small chemical molecules will still induce interleukin-6 (IL-6) and tumour necrosis factor-α secretion, but not interferon-α or IL-12. In this study, the molecular mechanisms by which silencing occurs were investigated. PP2, a potent inhibitor of the Lyn and Hck kinases, produced a similar profile to the PCV2 DNA interference with cytokine secretion by pDC, efficiently inhibiting cell activation induced through TLR9, but not TLR7, ligation. Confocal microscopy and cytometry analysis strongly suggested that PCV2 DNA impairs actin polymerization and endocytosis in pDC and monocyte-derived DC, respectively. Altogether, this study delineates for the first time particular molecular mechanisms involved in PCV2 interference with DC danger recognition, which may be responsible for the virus-induced immunosuppression observed in infected pigs.
Keywords: dendritic cells, DNA, inhibition, virus
Introduction
Interaction of viruses with the immune system is a key-determinant in the outcome of the infection. Many viruses have developed strategies to escape immune defences, such as altering the immunostimulatory capacity of the dendritic cells (DC).1,2 Porcine circovirus type 2 (PCV2), a small non-enveloped single-stranded (ss) DNA virus, has been associated with post-weaning multisystemic syndrome,3,4 as well as with other diseases in pigs such as porcine dermatitis and nephropathy syndrome,5 for a review see ref. 6. PCV2 associates with cells of the monocytic lineage such as monocyte-derived DC (MoDC), where it can persist for days without any signs of replication.7,8 No sign of apoptosis or cell surface modulation has been observed in these infected cells, suggesting that the virus could employ DC as vectors to spread within the body. Recently, we have demonstrated that PCV2 DNA inhibits cytokine secretion by plasmacytoid dendritic cells (pDC) stimulated with the toll-like receptor (TLR) ligands CpG (TLR9) or R837 (TLR7).9 This is considered important in the context of danger recognition, impairing the initial activation signal for maturation of MoDC and subsequent efficient antigen presentation.10,11 The finding that PCV2 DNA inhibits cytokine secretion by pDC correlates with the description of immunomodulatory CpG motifs in the small circular genome of PCV212,13 proposed to be pertinent to PCV2-associated disease pathogenesis.
To date, the molecular mechanisms involved in the silencing of pDC by PCV2 DNA remain to be defined. Studies in mice and humans have demonstrated that CpG interacts with TLR9 in pDC and B cells,14 which was until recently the only receptor described for CpG.14 However, Sanjuan et al.15 have shown that CpG can also activate a TLR9-independent pathway initiated at the cell surface of monocytes and pDCs. Activation of this pathway induces a tyrosine phosphorylation-mediated signalling cascade mediated by two src family kinases, Hck and Lyn. Upon activation, Hck and Lyn phosphorylate several downstream mechanisms initiating multiple signalling events. The src family kinases are of major importance for endocytic pathways requiring cell-cytoskeleton rearrangement such as non-clathrin-mediated endocytosis or phagocytosis (for a review see ref. 16). The authors demonstrated that triggering of both TLR9-dependant and TLR9-independent src family kinase pathways by CpG was necessary for cell activation by this ligand.15 Accordingly, the present study sought to highlight molecular mechanisms associated with PCV2 inhibition of cytokine secretion by pDC. The influence of PCV2 DNA on actin polymerization in pDC as well as antigen endocytosis in MoDC was analysed. Altogether, the results suggest a link between the immunomodulatory capacity of PCV2 DNA and the cytoskeleton reorganization.
Materials and methods
Virus
Stock pools of PCV2 were generated in PK15A cells from a well-characterized isolate (strain stoon 1010, ref. 3) as described previously.7 Mock antigen was prepared from uninfected PK15A cells in the same manner as for PCV2.
Cell preparation and stimulation for interferon-α and interleukin-6
Plasmacytoid DC, CD172a+ cells and interleukin-4 (IL-4)/granulocyte–macrophage colony-stimulating factor-derived MoDC were prepared as previously described.17 Briefly, for pDC preparation, blood monocytic cells were isolated from total peripheral blood mononuclear cells (PBMC) on a 55% Percoll gradient. The pDC were then enriched with an anti-CD4 antibody using the magnetic antibody cell sorting (MACS) sorting system and stimulated in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum containing 2 × 10−5 m 2-mercaptoethanol (all from Invitrogen, Basel, Switzerland). The final preparation was free of monocytes and contained 27–30% CD172low CD4high pDC.
For CD172a+ cells, blood monocytic cells were sorted from total PBMC with an anti-CD172a antibody (74-22-14A, ATCC, LGC Promochem Sarl, Molsheim, France) using the MACS sorting system. This preparation contained > 90% CD172a+ CD4− monocytic cells and up to 2% CD172low CD4high pDC.
The MoDC were generated form CD172a+ cells for 4–5 days in MoDC medium [DMEM 10% (volume/volume) specific pathogen-free pig serum supplemented with IL-4 (100 U/ml; 17) and granulocyte–macrophage colony-stimulating factor (150 ng/ml, kindly obtained from Shigeki Inumaru, Institute of Animal Health, Ibaraki, Japan)].
From 0·1 × 106 to 0·3 × 106 pDC or 0·2 × 106 MoDC, were incubated with PCV DNA, PCV2 viral preparation, or PP2 (Calbiochem/EMD Biosciences, Inc., La Jolla, CA) as indicated in the text 30 min before stimulation with CpG-oligodeoxynucleotide (ODN) D32 (10 μg/ml) or R837 (10 μg/ml). After 20 hr of incubation, supernatant was harvested and tested by ELISA for interferon-α (IFN-α)18 and IL-6 (R&D Systems, Abingdon, UK).
Preparation of PCV2 DNA replicative form
Either PCV2 or PCV1 DNA circular double-stranded (ds) form (‘replicative form’) was generated in vitro from the whole PCV2 or PCV1 genome cloned in a pGEM or pIC plasmid, respectively.9 The PCV2 genome was excised by a single EcoRI digestion and PCV1 by a single PstI digestion. The ds linear DNA (1·7 kb) was purified from plasmid by gel extraction using Qiaquick gel extraction kit (Qiagen, Hilde, Germany). Circularization of the ds linear DNA was performed using the T4 DNA ligase and linear DNA at a concentration of 1 μg/ml in a total reaction volume of 20 μl. Product corresponding to the ds circular form was excised by gel extraction using a Qiaquick gel extraction kit (Qiagen).
Southern blot
Fifteen microlitres of five different PCV2 stocks was loaded on a 2% agarose gel and subjected to electrophoresis at 20 V overnight. DNA was denatured and transferred on a nitrocellulose membrane by Southern blot using standard methods. Biotinylated probe was generated by nick translation using a gel-purified whole linear ds genomic PCV2 DNA as target. After transfer, the blot lot was washed with 2 × saline sodium citrate washing buffer before cross-linking DNA at 80° for 2 hr. Blocking was performed in 10 ml HyperHyb solution (Invitrogen) + 0·5 mg/ml salmon testis DNA for 30 min at 65°. After blocking, membrane was incubated in 5 ml HyperHyb solution containing 0·2 mg/ml of salmon testis DNA and 300 ng of PCV2 ATP-biotinylated probe at 65° for 1 hr 30 min. After incubation, membrane was subjected to washes of increasing stringency and revealed using streptavidin and Odyssey reader.
Confocal microscopy and flow cytometry
For staining of actin polymerization, pDC were seeded at 250 000 cells/well in 1 ml complete medium (DMEM, 10% fetal bovine serum, 2 × 10−5 m 2-mercaptoethanol) in six-well plates. Cells were untreated or incubated with 10 μg/ml GpC ODN (D32) or 100 ng PCV2 DNA 30 min before the addition of CpG-ODN (D32) at 10 μg/ml for 10 min. Unstimulated cells were used as control. Cells were then transferred in 4 ml polypropylene tubes and fixed with 4% paraformaldehyde, 10 min at room temperature. Cells were then incubated with phalloidin Alexa (Molecular Probes, Leiden, NL) in PBS/0·3% saponin for 20 min on ice, washed twice in PBS/0·1% saponin [weight/volume (w/v)] and once in PBS 1 × before mounting in Moviol.
For PCV2 detection in MoDC by confocal microscopy, cells were seeded in Labtek II (Nunc, Roskilde, Denmark) in 0·25 ml medium and infected with PCV2 high or low inhibitory stocks at a multiplicity of infection (MOI) of 0·1 of the 50% tissue culture infective dose (TCID50)/cell. Every 3 days of culture 0·25 ml fresh MoDC medium was added. After 2 or 8 days, cells were washed once in cold PBS and fixed in 4% paraformaldehyde. PCV2 capsid antigen was stained with anti-PCV2 (ORF2; monoclonal antibody 7G5-G4-A1, IgG2a, kindly provided by Dr Allan Gordon QUB, Belfast19) antibody in PBS containing 0·3% saponin (w/v). After washing twice in PBS containing 0·01% saponin (w/v) cells were incubated for 20 min with isotype-specific Alexa-conjugated anti-mouse IgG (Molecular Probes), diluted in PBS/0·3% saponin, then washed twice in PBS/0·1% saponin (w/v) and once in PBS 1× before mounting in Moviol.
For PCV2 detection in MoDC by flow cytometry, cells were incubated with 100 ng circularized PCV1 or PCV2 DNA for 30 min before addition of 100 ng PCV2 virus-like particles (VLP; kindly provided by Dr Christine Andreoni, Merial, Lyon, France) for 24 hr. After fixation and permeabilization of cells with the Fix and Perm kit (ADG An Der Grub, Bio research GMBH, Kaumberg, Austria), PCV2 capsid antigen was detected with anti-PCV2 antibody and stained using fluorescein, phycoerythrin F(ab’)2 anti-mouse isotype-specific immunoglobulin (Southern Biotechnology Associates, Inc., Birmingham, AL).
Results
Circularized, dsDNA replicative form of PCV2 is required for efficient inhibition of pDC cytokine activation
We previously demonstrated that the capacity of PCV2 to inhibit CpG activation of pDC was associated with the viral nucleic acid.9 Nevertheless, a high amount of PCV2 DNA, which may not be relevant in vivo, was necessary to obtain cytokine secretion inhibition (1 μg for 40% inhibition). These experiments employed PCV2 dsDNA in a linear form,9 which is not the form ‘naturally’ found. Therefore, the relevance of the dsDNA circular structure to the inhibitory activity was analysed. The PCV2 genome was re-circularized after excision from the pGEM plasmid (Fig. 1a). One hundred nanograms of re-circularized PCV2 DNA were sufficient to induce a 40% inhibition of IFN-α secretion in CpG-stimulated CD172+ cells (Fig. 1b), whereas with the linear form, inhibition is already lost with 250 ng linear ds DNA.9 No inhibition was observed when 100 ng PCV1 was employed in its dsDNA circular form (Fig. 1b‘PCV1DNA’). We further tested the inhibitory capacity of re-circularized PCV2 dsDNA on enriched pDC (CD4+ CD172low). This sorting method allows more sensitive detection of IL-6. Thirty-two nanograms of circular dsDNA of PCV2 was still inhibitory for IFN-α and IL-6 pDC responsiveness to CpG-ODN stimulation (Fig. 1c,d). As expected from our previous data,9 the inhibitory potential of the PCV2 dsDNA was less effective at inhibiting IL-6 when signalling through TLR7 than through CpG (Fig. 1d,e). According to these results, 100 ng circular PCV2 dsDNA was used in all subsequent inhibition experiments.
Figure 1.
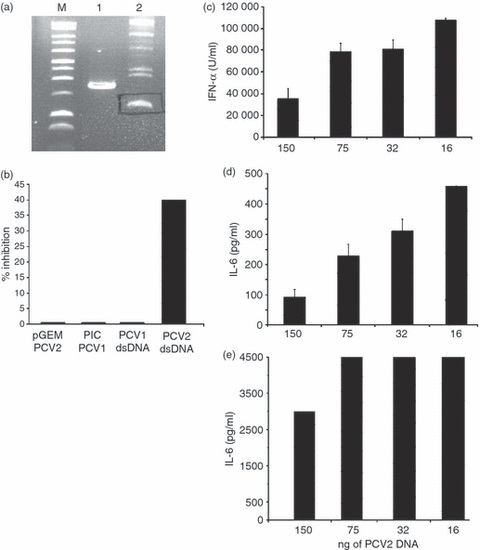
Inhibitory activity of porcine circovirus type 2 (PCV2) or type 1 (PCV1) genomes. (a) Isolation of re-circularized PCV genome. After excision of PCV2 or PCV1 genome from the pGEM or pIC plasmids, respectively, genomes are re-circularized using the DNA ligase. The product of ligation (see lane 2) corresponding to the double-stranded (ds) circular form is isolated on a 1% agarose gel. Lane 1, migration of ds linear form; lane M, marker. The data are representative of three independent experiments. (b) CD172a+ cells were incubated with 100 ng of the PCV2 or PCV1 genome before (pGEM PCV2 or PIC PCV1) and after (PCV2 or PCV1 dsDNA) excision and circularization and stimulated with 10 μg/ml of CpG-oligodeoxynucleotide (ODN; D32). Unstimulated cells or cells stimulated with 10 μg/ml CpG-ODN were used as negative and positive controls, respectively (data not shown). Supernatants were harvested after 16 hr and interferon-α (IFN-α) was measured by ELISA. The percentage of inhibition is calculated as: 100 – [(IFN-α produced by DNA pretreated cells + CpG)/(IFN-α produced by cells + CpG) × 100]. Dose-dependent inhibition of cytokine secretion; plasmacytoid dendritic cells isolated after cell fractionation on a 55% Percoll gradient and CD4+ selection, were incubated with two-fold dilutions of PCV2 dsDNA. After 30 min, cells were stimulated with CpG (10 μg/ml; c, d) or R837 (5 μg/ml; D32; e). Supernatants were harvested after 16 hr and IFN-α (c) and interleukin-6 (IL-6; d and e) were measured by ELISA. The data are representative of four independent experiments. The data are representative of three independent experiments.
Comparison of PCV2 DNA inhibition with inhibitors of actin polymerization
Whereas TLR9 is well documented as a DNA receptor, we observed that PCV2 dsDNA also inhibits cytokine induction in porcine cells such as fibrocytes or conventional DC, which do not express TLR9.20,21 Accordingly, the involvement of TLR9 as the receptor for PCV2 DNA-induced inhibition of cytokine signalling pathways is unlikely.
Recent studies have highlighted the presence of a cell surface receptor for small DNA molecules such as CpG-ODNs on cells of the monocytic lineage and on pDC.15 Blocking the activation of this receptor strongly impedes CpG signalling, and blocks cytoskeletal reorganization.
Therefore, the effect of PCV2 dsDNA on IFN-α and IL-6 secretion by pDC was compared with PP2, a specific src family kinase inhibitor. Cytokine (IL-6 or IFN-α) secretion induced by R837 was more weakly inhibited by 0·5 or 1 μm PP2 (Fig. 2a,b) than by cytokine secretion induced by CpG-ODN (Fig. 2c). Indeed, IFN-α secretion induced by CpG-ODN was efficiently inhibited by both concentrations of PP2 with a high percentage of cytokine inhibition already clear with 0·5 μm PP2 (Fig. 2c). These data suggest a similarity between cytokine inhibition by PCV2 dsDNA and PP2, affecting mainly cytokine activation through the TLR9 pathway. This indicates that cytokine inhibition by PCV2 dsDNA may be caused by a defect in cytoskeletal reorganization.
Figure 2.
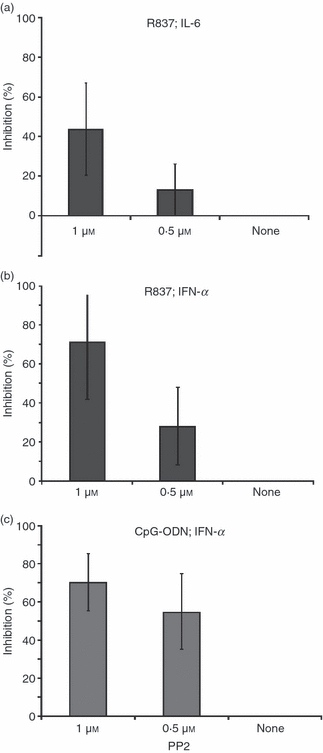
Inhibition of cytokine secretion by PP2. Plasmacytoid dendritic cells isolated after cell fractionation on a 55% Percoll gradient and CD4+ selection were incubated with PP2 at 1 μm, 0.5 μm or none before stimulation with R837 (5 μg/ml; a, b) or CpG ODN (10 μg/ml; c). Supernatants were harvested after 16 hr and interleukin-6 (IL-6; a) or interferon-α (IFN-α; b, c) were measured by ELISA. The data represent a mean of three independent experiments.
PCV2 dsDNA acts on CpG-induced actin poylmerization and cell endocytosis
To confirm the effect of PCV2 dsDNA on pDC actin polymerization, pDC were enriched from PBMC, and their microfilaments were stained with phalloidin after stimulation with CpG-ODN in the presence or absence of PCV2 dsDNA. Analysis focuses on large CD4+ cells (10 μm). Actin polymerization and microfilament reorganization were observed in cells stimulated with CpG-ODN (Fig. 3a,b). Synapse-like formations were observed in adjacent cells (Fig. 3a) and cytosolic meshes were found in isolated cells (Fig. 3b). Such cytoskeletal reorganization, in particular synapse-like formations, were not observed in cells treated with PCV2 dsDNA or GpC-ODN before CpG-ODN stimulation (Fig. 3c–d) or in unstimulated cells (Fig. 3e).
Figure 3.
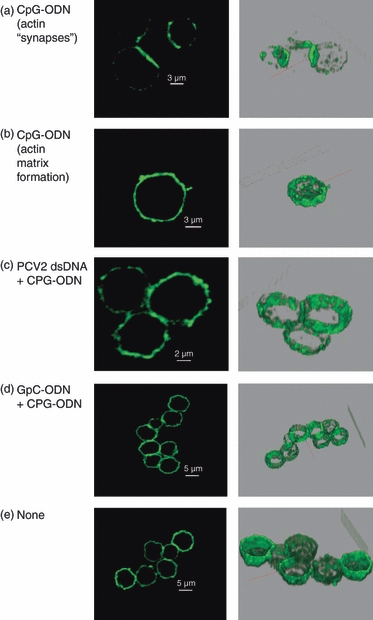
Confocal microscopy. Sectional scanning of plasmacytoid dendritic cells showing actin staining. Cells were isolated by cell fractionation on a 55% Percoll gradient and CD4+ selection. Cells were incubated fro 30 min with 100 ng porcine circovirus type 2 (PCV2) DNA, 10 μg/ml GpC, or untreated (none) before stimulation with 10 μg/ml CpG. Cells were then fixed and permeabilized and stained for actin with phalloidin. (a) CpG-oligodeoxynucleotide (ODN) stimulation of microfilament re-organization seen in terms of synaptic-like actin formations between cells. (b) CpG-ODN stimulation cytoplasmic microfilament matrix formation. (c, d) PCV2 double-stranded DNA and GpC treatment prevents CpG-ODN-induced microfilament re-organization (no ‘synaptic’ or cytoplasmic matrix formation). (e) Untreated. The right column shows three-dimensional images of cells sections. The data are representative of two independent experiments.
Considering the above observations with pDC, we investigated the effect of PCV2 dsDNA on MoDC endocytosis, a mechanism strongly dependent on actin polymerization. Therefore, MoDC were incubated with purified circularized PCV2 dsDNA before incubation with PCV2 VLP. After 24 hr, cells were stained for PCV2 capsid protein and analysed by flow cytometry. A 50% decrease of the mean fluorescence intensity was obtained when cells were pre-incubated with PCV2 dsDNA before addition of VLP (Fig. 4) in comparison with untreated cells. When cells were pre-incubated with PCV1 dsDNA, a 19% decrease of the mean fluorescence intensity was observed (Fig. 4).
Figure 4.
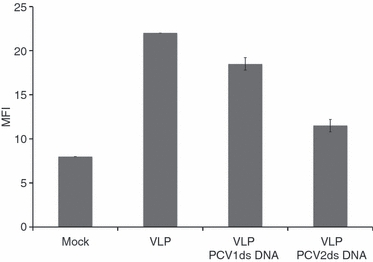
Monocyte-derived dendritic cell endocytosis: flow cytometry. Monocyte-derived dendritic cells were incubated with 100 ng porcine circovirus type 1 (PCV1) or type 2 (PCV2) DNA 30 min before addition of 100 ng PCV2 virus-like particles (VLP). After 24 hr cells were fixed and permeabilized and stained for PCV2 capsid protein. The data are representative of four independent experiments.
Similar results were obtained by confocal microscopy. The MoDC were incubated with two different PCV2 stocks chosen among five different stocks (all obtained by classical virus preparation on PK15A; see Materials and methods section) that displayed a different potential to inhibit cytokine secretion by pDC (Fig. 5a; shown as preparations ‘A’, ‘B’, ‘C’, ‘D’, ‘E’). Analysis of the PCV2 DNA content of these five preparations by Southern blot showed, as expected, that the inhibitory capacity of the PCV2 preparation strongly correlated with the amount of PCV2 dsDNA present in the preparation (Fig. 5b; these preparations were not treated to release the ss circular genomic DNA from the virions, and may contain only the non-encapsulated dsDNA replicative forms). These data confirmed, moreover, that relatively low levels of DNA, in circular conformation, should be sufficient for an efficient inhibition.
Figure 5.
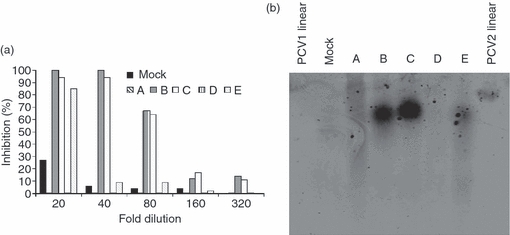
(a) Inhibitory potential of various porcine circavirus type 2 (PCV2) preparations. CD172a+ enriched peripheral blood mononuclear cells were incubated with two-fold dilutions of different PCV2 preparations (A, B, C, D, E) or a mock preparation (‘mock’, black histogram) and further stimulated with 10 μg/ml of CpG-oligodeoxynucleotide. Supernatant were harvested after 16 hr and tested for interferon-α (IFN-α) by ELISA. Results are expressed as percentage of inhibition compared with cells stimulated with CpG alone. (b) Southern blot: 15 μl of the PCV2 preparations tested for inhibition of CpG stimulation (a) were loaded on a 2% agarose gel run overnight at 20 V. DNA was then transferred to a nitrocellulose membrane and detected with a PCV2-specific biotinylated probe. As controls, 70 ng double-stranded linear PCV1 (‘PCV1 linear’) or PCV2 (‘PCV2 linear’) were used. The data are representative of two independent experiments.
According to the above results, one ‘low inhibitory’ (prep E) and one ‘high inhibitory’ (prep B) preparation containing, respectively, low or high amounts of PCV2 dsDNA were employed at identical MOI (0·01 TCID50/ml) on MoDC. The uptake of PCV2 virions was monitored at 2 or 8 days post-infection. Cells incubated with the PCV2 stock containing low amounts of viral DNA were positive for PCV2 capsid antigen 2 and 8 days post-infection (Fig. 6a,b). In contrast, PCV2 capsid antigen was hardly detectable in cells incubated with the PCV2 stock containing high levels of viral DNA (Fig. 6c,d). As a control, a lysate from uninfected cells was employed (Fig. 6f). These results indicate that antigen uptake by MoDC is impaired in the presence of PCV2 dsDNA, suggesting a deficiency in endocytic processes.
Figure 6.
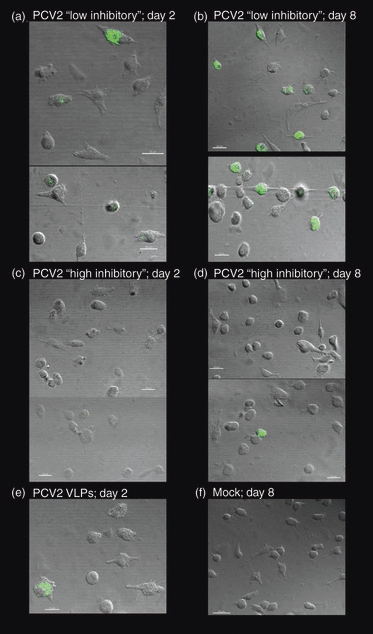
Monocyte-derived dendritic cell endocytosis: confocal microscopy. Monocyte-derived dendritic cell were incubated with porcine circovirus type 2 (PCV2) low (a, b) or high (c, d) inhibitory stocks at a multiplicity of infection of 0.1 of the 50% tissue culture infective dose/cell and incubated for 2 days (a, c) or 8 days (b, d) at 39°. Cells were washed and stained for the PCV2 capsid antigen. A positive control (100 ng of PCV2 virus-like particle; VLP) stained after 2 days is shown in (e), and a negative control (mock cell lysate) stained after 8 days of incubation is shown in (f). The data are representative of two independent experiments.
Discussion
Porcine circovirus type 2 is an important pathogen, shown to be associated with particular diseases of economic significance in pigs.3,6 Certain characteristics of an immunosuppressive nature have been related to the presence of the PCV2 infection.22 The virus was shown to have a tropism for monocytic cells and DC7,23 but it did not replicate in these cells, but rather accumulated in them over prolonged periods of time. Such characteristics pointed to a capacity to regulate the functioning of DC. However, PCV2 do not affect cell surface molecule expression of MoDC nor antigen presentation.7,9 We have shown that PCV2 DNA was able to regulate the responsiveness of pDC to certain danger signals.9 This has subsequently been related to hairpin structure containing CpG motifs in the PCV2 genome.12,24 Nevertheless, the molecular mechanisms involved in the PCV2 DNA-dependent inhibition of danger recognition by pDC have not been defined.
We demonstrated that the circularization of the linear dsDNA further potentiates the inhibitory effect of PCV2 DNA. In this circular conformation, the inhibition was still observable with 32 ng PCV2 circular dsDNA, whereas 250 ng of the linear ds form was ineffective.9 Such characteristics suggest that the inhibitory effect of PCV2 DNA is dependent on its tertiary structure, which would relate to the report by Hasslung Wikström et al.24 These authors showed that chosen oligodeoxyribonucleotides representing PCV2 genomic sequences could be inhibitory, but this depended on the hairpin structure rather than the CpG-ODN motif. They further demonstrated that a change in the PCV2 genome inhibitory sequence did not alter the inhibitory activity, unless it interfered with a loop structure in ODN. It is possible that such loop structures occurs only when the circular genome is produced.
The involvement of the TLR9 receptor in the PCV2 DNA-mediated cytokine inhibition appeared unlikely. First, PCV2 DNA inhibits pDC cytokine induction by a number of pathogen-associated molecular patterns (PAMPs) in addition to TLR9 ligands.9 Second, PCV2 DNA mediated inhibition of cytokine secretion was also observed in porcine cells, which do not express TLR9 such as fibrocytes20 or conventional DC.21 Therefore, it is unlikely that the inhibition occurs through simple competition with this receptor. PCV2 DNA displays a similar competitive antagonism with the stimulatory CpG ODN as inhibitory ODN motifs, acting specifically on immune activation via the TLR9 pathway.25,26
It has recently been demonstrated that TLR9 cascade activation requires activation of kinases involved in cytoskeletal reorganization.15 This phenomenon was described in pDC, monocytic and macrophage cell lines15 as well as in mouse macrophages.27 Upon triggering of an as yet unknown cell surface receptor, the Lyn and Hck kinases associated with cytoskeletal reorganization and phosphorylation of immunoreceptor tyrosine-based activation motifs, become phosphorylated.28 Inhibition of Lyn and Hck phosphorylation leads to a loss of IFN-α induction, but not of IL-6.15 The potent inhibitor of cytoskeletal reorganization, PP2, inhibits IFN-α secretion induced in pDC by CpG-ODN, whereas IL-6 secretion induced by TLR7 ligation remained functionally active. This suggested a relationship between the PCV2 dsDNA mechanism for impairing PAMP recognition and the default in src family kinase phosphorylation induced by PP2. Our data suggest that, unlike IFN-α, the IL-6 cascade induced by TLR7 ligation does not require immunoreceptor tyrosine-based activation motif phosphorylation. However, it is also important to consider the uptake of the small imiquimod molecule R837 used in this study to stimulate TLR7. This may well be different to the reported endocytic uptake and processing of CpG-ODN. Indeed, the R837 is likely to be micropinocytosed, and therefore its uptake is less influenced by modulation of cytoskeletal reorganization. Consequently, PCV2 dsDNA interference with PAMP recognition may be consequential to an influence on microfilament activity. Indeed, PCV2 dsDNA did impede the microfilament reorganization, induced in pDC following recognition of CpG-ODN. The influence on cytoskeleton reorganization was also observed in MoDC. Cells treated with PCV2 dsDNA but not PCV1 dsDNA, displayed a lower efficiency to internalize PCV2 VLP, compared with untreated cells.
Similarly, when a PCV2 infectious preparation containing a high level of PCV2 DNA was employed, the cytokine secretion and the uptake of the virions were impaired, whereas both were still observable with a virus preparation containing a low level of DNA. We have no explanation for why different stocks may contain different amounts of viral DNA. It probably depends on the confluence of the cells at the time of lysis, with the highest DNA content found in cells with a lower confluence (then still replicating) at this moment. The different amounts of DNA in the PCV2 viral stocks were easily visualized by Southern blot. Only one form, the ds form, was detected, suggesting that only very low levels of free ssDNA, the viral encapsidated genomic form, were present. This ssDNA genomic form can usually be detected in infectious PCV2 preparations prepared in PK15A cells.7 However, the method in this article for preparing PCV2 stocks was different to that in the present analysis, in that it employs DNA extraction from infected cells, which allow release of encapsidated DNA. Nevertheless, the results suggest that the dsDNA replicative form is responsible for the observed inhibitory effects, but we cannot exclude the possibility that PCV2 ssDNA may have similar inhibitory capacities.
The present work elaborates on our understanding of how PCV2 may interfere with the activities of innate immune defences. Therein, the dsDNA replicative form of the virus was found to have a high immunomodulatory capacity, interfering with cytoskeletal re-arrangements and the endocytic processes. With respect to innate immune cells, it allows activation of cytokine secretion, efficient internalization of pathogens leading to cellular activation, presentation of the antigens to promote adaptive immune responses, as well as migration to effector sites.29,30 Inhibiting these critical functions of innate immune defence cells may lead to a strong immunosuppression, permitting a facilitated entry and progression of other opportunistic pathogens. The present study suggests for the first time that a deficiency in cytoskeletal rearrangement may be linked to the processes leading to PCV2-associated diseases. This also provides a model for other pathogenic problems associated with ssDNA viruses, including the transfusion transmitted virus (TTV) in humans.
Acknowledgments
This work was funded by the EU sixth framework project PCVD (no. 513928). Special thanks go to Dr Christine Andreoni (Merial, Lyon, France) for the ORF2 VLPs. We are also indebted to Daniel Brechbühl, Veronika Ayala and Andreas Michel for caring for and bleeding the animals.
Glossary
Abbreviations:
- DMEM
Dulbecco’s modified Eagle’s medium
- ds
double-stranded
- IFN
interferon
- IL
interleukin
- MACS
magnetic antibody sorting
- MoDC
monocyte-derived DC
- MOI
multiplicity of infection
- ODN
oligodeoxynucleotide
- PBMC
peripheral blood mononuclear cells
- PCV2
porcine circovirus type 2
- pDC
plasmacytoid DC
- ss
single-stranded
- TCID50
50% tissue culture infective dose
- TLR
Toll-like receptor
Disclosures
None.
References
- 1.Kaiserlian D, Dubois B. Dendritic cells and viral immunity: friends or foes? Semin Immunol. 2001;13:303–10. doi: 10.1006/smim.2001.0326. [DOI] [PubMed] [Google Scholar]
- 2.Klagge IM, Schneider-Schaulies S. Virus interactions with dendritic cells. J Gen Virol. 1999;80:823–33. doi: 10.1099/0022-1317-80-4-823. [DOI] [PubMed] [Google Scholar]
- 3.Allan GM, McNeilly F, Kennedy S, et al. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J Vet Diagn Invest. 1998;10:3–10. doi: 10.1177/104063879801000102. [DOI] [PubMed] [Google Scholar]
- 4.Ellis J, Hassard LE, Clark EG, et al. Isolation of circovirus from lesions of pigs with postweaning multisystemic syndrome. Can Vet J. 1998;39:44–51. [PMC free article] [PubMed] [Google Scholar]
- 5.Rosell C, Segales J, Ramos-Vara JA, et al. Identification of porcine circovirus in tissues of pigs with porcine dermatitis and nephropathy syndrome. Vet Rec. 1997;146:40–3. doi: 10.1136/vr.146.2.40. [DOI] [PubMed] [Google Scholar]
- 6.Segales J, Allan GM, Domingo M. Porcine circovirus diseases. Anim Health Res Rev. 2005;6:119–42. doi: 10.1079/ahr2005106. [DOI] [PubMed] [Google Scholar]
- 7.Vincent IE, Carrasco CP, Hermann B, Meehan BM, Allan GM, Summerfield A, McCullough KC. Dendritic cells harbor infectious PCV2 in the absence of apparent cell modulation or replication of the virus. J Virol. 2003;77:13288–300. doi: 10.1128/JVI.77.24.13288-13300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent IE, Carrasco CP, Guzylack-Piriou L, McCullough KC, Summerfield A. Subset-dependent modulation of dendritic cell activity by circivirus type 2. Immunology. 2005;115:388–98. doi: 10.1111/j.1365-2567.2005.02165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent IE, Balmelli C, Meehan BM, Allan G, Summerfield A, McCullough KC. Silencing of natural interferon producing cell activation by porcine circovirus type 2 DNA. Immunology. 2007;212:693–9. doi: 10.1111/j.1365-2567.2006.02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieg AM. A role of Toll in autoimmunity. Nat Immunol. 2002;3:423–4. doi: 10.1038/ni0502-423. [DOI] [PubMed] [Google Scholar]
- 11.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 12.Hasslung FC, Berg M, Allan GM, Meehan BM, McNeilly F, Fossum C. Identification of a sequence from the genome of porcine circovirus type 2 with an inhibitory effect on IFN-α production by porcine PBMCs. J Gen Virol. 2003;84:2937–45. doi: 10.1099/vir.0.19362-0. [DOI] [PubMed] [Google Scholar]
- 13.Kekarainen T, Montoya M, Dominguez J, Mateu E, Segalés J. Porcine circovirus type 2 (PCV2) viral components immunomodulate recall antigen responses. Vet Immunol Immunopathol. 2008;124:41–9. doi: 10.1016/j.vetimm.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 14.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 15.Sanjuan MA, Rao N, Lai K-TA, et al. CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J Cell Biol. 2006;172:1057–68. doi: 10.1083/jcb.200508058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conner SD, Schmid SL. Regulated portal of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 17.Carrasco CP, Ridgen RC, Schaffner R, et al. Porcine dendritic cells generated in vitro: morphological, phenotypic and functional properties. Immunology. 2001;104:175–84. doi: 10.1046/j.0019-2805.2001.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz DA, Artursson K, L’Haridon R, Perers R, La Bonardiere C, Alm GV. A sensitive immunoassay for porcine interferon-α. Vet Immunol Immunopathol. 1992;30:319–27. doi: 10.1016/0165-2427(92)90102-v. [DOI] [PubMed] [Google Scholar]
- 19.McNeilly F, McNair L, Mackie DP, et al. Production, characterisation and applications of monoclonal antibodies to porcine circovirus type 2. Arch Virol. 2001;146:909–22. doi: 10.1007/s007050170124. [DOI] [PubMed] [Google Scholar]
- 20.Balmelli C, Alves MP, Steiner E, et al. Responsiveness of fibrocytes to Toll like receptor danger signals. Immunobiology. 2007;212:693–9. doi: 10.1016/j.imbio.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Guzylack-Piriou L, Balmelli C, McCullough KC, Summerfield A. Type-A CpG oligonucleotides activate exclusively porcine natural interferon-producing cells to secrete inteferon-α, tumor necrosis factor-α and interleukin-12. Immunology. 2004;112:28–37. doi: 10.1111/j.1365-2567.2004.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen J, Vincent IE, Botner A, Ladekjaer-Mikkelsen A-S, Allan G, Summerfield A, McCullough KC. Association of lymphopenia with the porcine circovirus type 2 induced postweaning multisystemic wasting syndrome. Vet Immunol Immunopathol. 2003;92:97–111. doi: 10.1016/s0165-2427(03)00031-x. [DOI] [PubMed] [Google Scholar]
- 23.Gilpin DF, McCullough KC, Meehan BM, et al. In vitro studies on the infection and replication of porcine circovirus type 2 in cells of the porcine immune system. Vet Immunol Immunopathol. 2003;94:149–61. doi: 10.1016/s0165-2427(03)00087-4. [DOI] [PubMed] [Google Scholar]
- 24.Hasslung Wikström F, Meehan BM, Berg M, et al. Structure-dependent modulation of alpha interferon production by porcine circovirus type 2 oligodeoxyribonucleotide and CpG DNAs in porcine peripheral blood mononuclear cells. J Virol. 2007;81:4919–27. doi: 10.1128/JVI.02797-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenert P. Inhibitory oligonucleotides – therapeutic promise for systemic autoimmune diseases? Clin Exp Immunol. 2005;140:1–10. doi: 10.1111/j.1365-2249.2004.02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stunz LL, Lenert P, Peckham D, Yi AK, Haxhinasto S, Chang M, Krieg AM, Ashman RF. Inhibitory oligonucleotides specifically block effects of stimulatory CpG oligonucleotides in B cells. Eur J Immunol. 2002;32:1212–22. doi: 10.1002/1521-4141(200205)32:5<1212::AID-IMMU1212>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 27.Achuthan A, Elsegood C, Masendycz P, Hamilton JA, Scholz GM. CpG DNA enhances macrophage cell spreading by promoting the src-family kinase-mediated phosphorylation of paxillin. Cell Signal. 2006;18:2252–61. doi: 10.1016/j.cellsig.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Bolen JB, Brugge JS. Leucocyte protein tyrosine kinases: potential target for drug delivery. Annu Rev Immunol. 1997;15:371–404. doi: 10.1146/annurev.immunol.15.1.371. [DOI] [PubMed] [Google Scholar]
- 29.Resnati M, Guttinger M, Valcamonica S, Sidenius N, Blasi F, Fazioli F. Proteolytic cleavage of the urokinase receptor substitutes for the agonist-induced chemotactic effect. EMBO J. 1996;15:1572–82. [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki T, Kono H, Hirose N, Okada M, Yamamoto T, Yamamoto K, Honda Z. Differential involvement of Src family kinases in Fcg receptor-mediated phagocytosis. J Immunol. 2000;165:473–82. doi: 10.4049/jimmunol.165.1.473. [DOI] [PubMed] [Google Scholar]


