Abstract
Human peripheral blood Vγ2Vδ2 T cells are important for host defence and tumour immunity. Their unusual T-cell receptor (TCR) recognizes small molecule phosphoantigens; stimulated cells produce inflammatory cytokines and are potently cytotoxic for a variety of tumours. However, molecular mechanisms linking phosphoantigen stimulation and cytotoxicity are incompletely understood. We know that isopentenyl pyrophosphate (IPP) activates mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/Erk) and phosphoinositide 3-kinase (PI-3K)/Akt pathways; specific inhibition of Erk or Akt significantly impairs the functional response to IPP. We now show that interleukin-2 also activates MEK/Erk and PI-3K/Akt pathways but on its own, fails to induce cytokine expression or cytotoxicity. Hence, MEK/Erk and PI-3K/Akt activation are necessary but not sufficient to induce effector responses in Vγ2Vδ2 T cells and a TCR-dependent signal is still required for tumour cell killing. Cyclosporin A, an inhibitor of calcineurin, blocked calcium-dependent nuclear translocation of nuclear factor of activated T cell (NFAT) and significantly reduced IPP-induced cytokine production, degranulation and cytotoxicity. The IPP-induced calcium mobilization and NFAT translocation were necessary to activate Vγ2Vδ2 effector functions; interleukin-2, acting on the MEK/Erk pathway, regulated the strength of these responses. The TCR has a specific role in Vγ2Vδ2 T-cell killing of tumour cells, which is distinct from its role in triggering cellular proliferation in response to phosphoantigens.
Keywords: interleukin-2, signalling, T-cell receptor, γδ T cell
Introduction
The γδ T cells represent a distinct lymphocyte subset with a T-cell receptor (TCR) composed of γ and δ chains in place of the more familiar TCR-αβ on conventional CD4+ or CD8+ T-cell subsets.1,2 In humans, γδ T cells represent 1–10% of the circulating T cells in blood. The majority (> 80%) of blood γδ T cells express the Vγ2Vδ2 (also termed Vγ9Vδ2) TCR3 that mediates broad reactivity to infectious agents and tumours. The Vγ2Vδ2 TCR recognizes low-molecular-weight, non-peptidic compounds termed ‘phosphoantigens’, including isopentenyl pyrophosphate (IPP),4,5 an intermediate in sterol and isoprenoid biosynthesis. Bisphosphonates, which are used to treat bone resorption disorders, reduce biosynthetic conversion of IPP causing increased levels of this phosphoantigen with corresponding increases in Vγ2Vδ2 T-cell stimulation.6 Following stimulation by phosphoantigens, Vγ2Vδ2 T cells proliferate, produce cytokines [particularly interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α)],7,8 release granzymes or perforin,9,10 and acquire cytotoxic activity against tumour cells9–11 or infected cells.12–14 In contrast to αβ T cells, Vγ2Vδ2 T cells recognize phosphoantigens in an MHC-unrestricted manner and do not require antigen processing by professional antigen-presenting cells.4,5 As they are among the few T-cell subsets that can be stimulated in vivo by drug treatment and are potently cytotoxic for many human tumour cells, the intentional activation of Vγ2Vδ2 T cells represents a promising strategy for immunotherapy of cancer.13,14
Given the unique characteristics of TCR-γδ and the potential clinical importance of Vγ2Vδ2 T cells in cancer, it is crucial to clarify the specific signalling pathways that mediate phosphoantigen activation of the cytotoxic effector phenotype. We and others reported previously that mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/Erk) and phosphoinositide 3-kinase (PI-3K)/Akt pathways were part of the response to IPP.15 Specific inhibition of Erk or Akt significantly impaired IPP-induced cytokine production (IFN-γ and TNF-α) and cytotoxicity against tumour cells. A similar phosphoantigen, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP), also induced MEK/Erk and PI-3K/Akt-mediated signal transduction.16 These studies showed that MEK/Erk and PI-3K/Akt pathways were involved in Vγ2Vδ2 responses to phosphoantigens. It had been shown previously that proliferation responses of murine γδ T cells were also sensitive to cyclosporin A,17 indicating a role for the calcineurin pathway in activation of primary cells. In human Vγ2Vδ2 T cells, cyclosporin treatment altered susceptibility to activation-induced cell death.18 There have been indications that calcium signalling is required for differentiated effector functions of γδ T cells, but this was not studied specifically in human cells that recognize low-molecular-weight, non-peptidic phosphoantigens.
Interleukin-2 (IL-2) also plays a critical role in phosphoantigen-driven Vγ2Vδ2 T-cell responses.14 Activation of signal transducer and activator of transcription 4 (STAT4) and STAT5 along with Erk1/2 and p38 were observed in primary Vγ2Vδ2 T cells that were stimulated to proliferate. Our focus is on mechanisms that control effector functions of Vγ2Vδ2 T-cell lines. The critical difference is that TCR signals in primary cells must first induce the high-affinity IL-2 receptor to enable cytokine signalling, including MEK/Erk and PI3-Akt activation. Vγ2Vδ2 T-cell lines [10–14 days after IPP plus IL-2 stimulation of peripheral blood mononuclear cells (PBMC)] already express the high-affinity IL-2 receptor and can be used to assess the direct effects of phosphoantigen on cytokine expression and cytotoxicity. Our studies showed that IL-2 treatment of Vγ2Vδ2 T-cell lines activated the MEK/Erk and PI-3/Akt pathways but failed to induce effector cell functions including cytokine expression, degranulation and cytotoxicity. A calcium-dependent response, normally triggered by phosphoantigen addition, was required for these effector activities and the function of IL-2 is to increase the magnitude of these responses.
Materials and methods
PBMC and tumour cell lines
Whole blood was obtained from healthy human volunteers who provided written informed consent; all protocols were approved by the Institutional Review Board at the University of Maryland, Baltimore, MD. Total lymphocytes were separated from heparinized peripheral blood by density gradient centrifugation (Ficoll-Paque; Amersham Biosciences, Uppsala, Sweden). The PBMC and TU167 cells (squamous cell carcinoma) were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (GIBCO, Carlsbad, CA), 2 mm l-glutamine, and penicillin–streptomycin (100 U/ml and 100 mg/ml, respectively); for Daudi B cells (CCL-213; American Type Culture Collection, Bethesda, MD), 4·5 g/l glucose, 1·5 g/l NaHCO3, 10 mm HEPES, and 1 mm sodium pyruvate were added.
Generating Vγ2Vδ2 T-cell lines
The PBMC were cultured with complete medium and stimulated with 15 μm IPP (Sigma, St. Louis, MO) plus 100 U/ml human recombinant IL-2 (Tecin, Biological Resources Branch, National Institutes of Health, Bethesda, MD). Fresh complete medium and 100 U/ml IL-2 were added every 3 days. Proliferation of γδ T-cells was measured by staining for CD3 and Vγ2Vδ2, and determining the percentage of γδ T cells within the total lymphocyte population at day 14. Cell lines obtained after in vitro expansion were used for biochemical studies; they were rested for 1 week in 10 U/ml IL-2 before each experiment.
Immunoblot analysis
Cells were lysed in gel loading buffer (Invitrogen, Carlsbad, CA); samples were boiled for 10 min and proteins were separated by SDS–PAGE. Proteins were transferred to nitrocellulose membranes and probed with various primary antibodies. Secondary antibodies including horseradish peroxidase-conjugated, anti-rabbit or anti-mouse (Cell Signaling Technology, Inc., Danvers, MA) were visualized with enhanced chemiluminescence (GE Healthcare, Buckinghamshire, UK) and exposure to Kodak X-ray film. In some experiments, cytoplasmic and nuclear extracts were prepared with NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, Rockford, IL) according to the manufacturer’s directions.
Cytotoxicity assay
A non-radioactive, fluorometric cytotoxicity assay with calcein-acetoxymethyl (Molecular Probes, Eugene, OK) was used to measure cytotoxicity against Daudi B cells. Resting γδ cells (effector cells) were treated with or without IL-2 or cyclosporin A at various concentrations for 1 hr at 37°. Daudi B or TU167 target cells were labelled for 15 min with 2 mm calcein-acetoxymethyl at 37°, then washed once with PBS. Cells were combined at various effector-to-target (E : T) ratios in 96-well, round-bottomed microtitre plates (Corning, NY) and incubated at 37° in 5% CO2 for 4 hr; assays were performed in triplicate. After incubation, supernatants were transferred to a 96-well flat-bottomed microtitre plate and calcein content was measured using a Wallac Victor2 1420 multi-channel counter (l485/535 nm). Per cent specific lysis was calculated as: (test release – spontaneous release) / (maximum release – spontaneous release) × 100.
Flow cytometry and intracellular cytokine assay
Unless noted, cells were stained with fluorophore-conjugated monoclonal antibodies from BD Biosciences, San Jose, CA. Generally, 3 × 105 to 5 × 105 cells were washed, resuspended in 50–100 μl of RPMI-1640, and stained with mouse anti-human Vγ2Vδ2-phycoerythrin (-PE) clone B6, mouse anti-human CD3-fluorescein isothiocyanate (-FITC) clone UCHT1, mouse anti-human CD3-allophycocyanin (-APC) clone UCHT1, mouse anti-human CD107a-FITC clone H4A3, and isotype controls, including rabbit anti-mouse IgG1-FITC clone X40, IgG1-PE clone X40 and IgG1-APC clone X40. For detecting intracellular TNF-α and IFN-γ, cells were stained with mouse anti-human Vγ2Vδ2-FITC clone B6 then fixed, permeabilized and incubated for 45 min at 4° with mouse anti-human TNF-α-PE and mouse anti-human IFN-γ-APC. Intracellular staining solutions were obtained from the Cytofix/Cytoperm Kit (BD Biosciences). Cells were washed with staining buffer and resuspended. Data for at least 1 × 104 lymphocytes (gated on the basis of forward- and side-scatter profiles) were acquired from each sample on a FACSCalibur flow cytometer (BD Biosciences). All samples were analysed using FlowJo software (FlowJo 8.8.2, Tree Star, San Carlos, CA). For stimulation before staining, Vγ2Vδ2 T cells were treated with IPP for 4 hr. In some experiments, Vγ2Vδ2 T cells were treated with IL-2 or cyclosporin A at various concentrations for 1 hr at 37° before stimulation.
Statistical analysis
Differences among groups were analysed using Student’s t-test. P < 0·05 was considered to be significant.
Results
IL-2 induced rapid Erk and Akt phosphorylation in Vγ2Vδ2 T cells
Interleukin-2 and phosphoantigen are required for Vγ2Vδ2 T-cell proliferation in vitro and in vivo.14 Here, we compared responses to either IL-2 or phosphoantigen in Vγ2Vδ2 T-cell lines obtained from human PBMC, where Vγ2Vδ2 T cells were > 90% of cells in culture (Fig. 1a). Vγ2Vδ2 T-cell lines were washed twice and cultured in fresh medium for 24 hr before they were treated with IPP or IL-2. After stimulation, cells were collected for Western blotting assays to detect signal transduction intermediates. The IPP induced rapid activation of Erk and Akt, as expected (Fig. 1b). Interleukin-2 alone activated these same signalling molecules in our cell lines (Fig. 1b). As a control, IL-2 but not IPP induced rapid phosphorylation of STAT5 (Fig. 1b), as expected.
Figure 1.
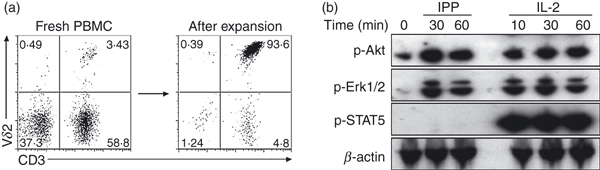
Interleukin-2 (IL-2) promoted Erk and Akt phosphorylation in Vδ2 T cells. (a) Freshly isolated peripheral blood mononuclear cells (PBMC) contained 1–10% of Vγ2Vδ2 T cells (left panel), we generated a Vδ2 T-cell line by in vitro expansion with isopentenyl pyrophosphate (IPP) plus IL-2 and the percentage of Vγ2Vδ2 T cells was > 90% (right panel). (b) Vγ2Vδ2 T-cell lines were rested after washing twice and culturing in fresh medium for 24 hr before being treated with IPP (15 μm) or IL-2 (100 U/ml) for 10–60 min. After stimulation, cells were collected for western blotting assays with antibodies specific for signal transduction molecules. Data are representative of at least three independent experiments with PBMC from unrelated donors.
IL-2 activated Erk and Akt but did not induce effector responses
As IL-2 activates Erk and Akt, we next wanted to know whether IL-2 induced effector responses. Vγ2Vδ2 T-cell lines or fresh PBMC were treated with IL-2, IPP or both at various concentrations for 4 hr. Cytokine (IFN-γ and TNF-α) production and CD107a expression were measured in the Vδ2 subset. CD107a (lysosomal-associated membrane protein 1) is present in membranes of cytotoxic granules and is expressed transiently on cell surfaces during degranulation.19,20 Interleukin-2 alone did not induce cytokine production or degranulation (CD107a expression). In contrast, IPP alone triggered both cytokine production, in both fresh and expanded Vδ2 T cells, and CD107a expression in Vδ2 T-cell lines (Fig. 2a,b). When IPP and IL-2 were used together, cytokine production (Fig. 2a) and degranulation (Fig. 2b) were increased in a dose-dependent manner depending on the IL-2 concentration.
Figure 2.
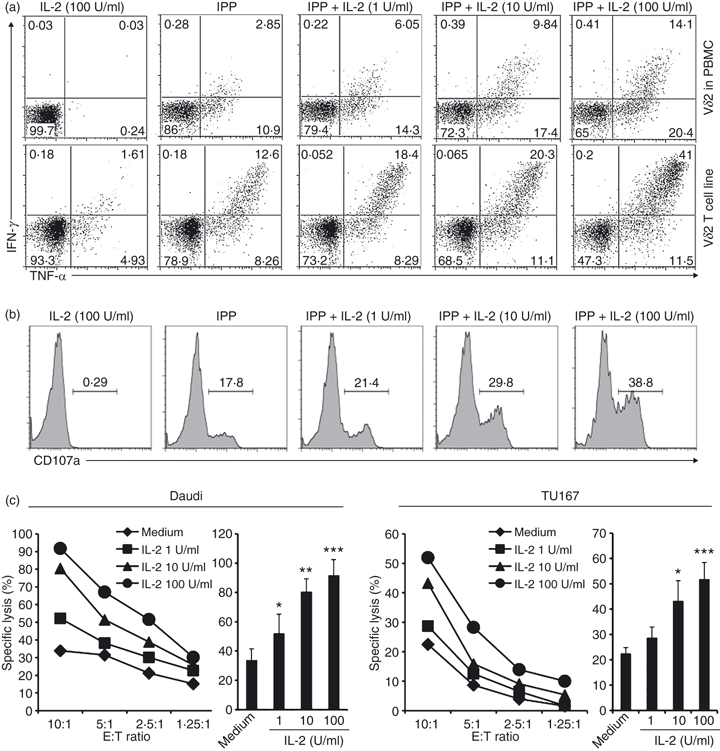
Phosphoantigen elicits cytokine, degranulation and cytotoxicity responses that are amplified by interleukin-2 (IL-2). (a,b) Peripheral blood mononuclear cells (PBMC) or Vγ2Vδ2 T-cell lines were treated with IL-2, isopentenyl pyrophosphate (IPP) or both for 4 hr. Interferon-γ (IFN-γ) or tumour necrosis factor-α (TNF-α) production (a) and CD107a expression (b) were detected by flow cytometry. Cells were gated on Vδ2+ cells. (c) Resting Vγ2Vδ2 T-cell lines were pretreated with IL-2 at various concentrations for 1 hr. The cytotoxicity of Vγ2Vδ2 T cells against Daudi (left panel) or TU167 (right panel) was evaluated at several effector to target (E : T) ratios with triplicate wells for each condition. At E : T = 10 : 1 there was a significant effect of increasing IL-2 on target cell lysis; *P < 0·05; **P < 0·01; ***P < 0·001. Data are representative of three independent experiments with PBMC from unrelated donors.
We also measured the effect of IL-2 on Vγ2Vδ2 T-cell cytotoxicity. We used Daudi B (a Burkitt’s lymphoma) and TU167 (a squamous carcinoma) cell lines as targets; IL-2 significantly increased cell lysis by Vγ2Vδ2 T cells and the effect was dose-dependent (Fig. 2c). The IPP alone, but not IL-2 alone, induced cytokine expression and degranulation. When antigen (IPP or tumour cells) was present, IL-2 had a dose-dependent effect on the response. Recognition of antigen by TCR is important for Vγ2Vδ2 cytotoxicity and is distinct from natural killer cell NK killing, even though both require activation through NKG2D.21,22
IPP but not IL-2 induced calcium-dependent nuclear translocation of nuclear factor of activated T cells
We sought to define pathway(s) that are activated by IPP but not IL-2, and may be required for triggering effector functions. Calcium signals have been described in various cells of the immune system, including αβ T cells, B cells, NK cells, mast cells, dendritic cells, monocytes and macrophages, where they regulate cell differentiation, gene transcription and effector functions.23 We tested whether calcium signalling might help to distinguish a unique contribution of the TCR-γδ. The nuclear factor of activated T cells (NFAT) proteins are transcription factors expressed mainly in cells of the immune system.24 In resting cells, NFAT proteins are heavily phosphorylated and sequestered in the cytoplasm. When the calcium pathway is activated, increased intracellular calcium ions (Ca2+) activate calcineurin, a Ca2+/calmodulin-dependent serine phosphatase, which dephosphorylates NFAT proteins and promotes their translocation to the nucleus.25 We measured NFAT nuclear translocation as a marker for Ca2+ signalling in IL-2- or IPP-treated Vγ2Vδ2 T cells. Resting Vγ2Vδ2 T-cell lines were treated with IL-2 or IPP for 15 min. Cytoplasmic and nuclear extracts were prepared, then NFAT1 and NFAT4 were measured by Western blotting. We detected nuclear NFAT1 and NFAT4 only after IPP treatment; IL-2 did not trigger nuclear localization of these proteins (Fig. 3). As a control, we detected phosphorylated STAT5 in nuclei but not cytoplasm from IL-2-treated cells (Fig. 3). Signalling through the TCR, upon IPP addition, uniquely affected the activation and nuclear localization of NFAT proteins.
Figure 3.
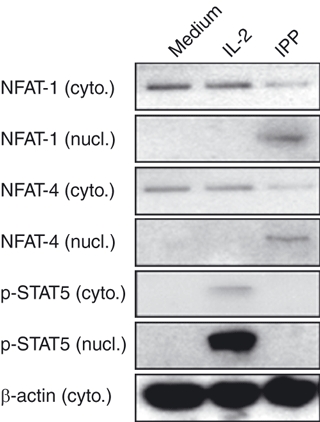
Only phosphoantigen induced calcium-dependent nuclear translocation of nuclear factor of activated T cells (NFAT). Resting Vδ2 T-cell lines were treated with interleukin-2 (IL-2; 100 U/ml) or isopentenyl pyrophosphate (IPP; 15 μm) for 15 min. NFAT1, NFAT4, phosphorylated signal transducer and activator of transcription 5 (STAT5) and β-actin were measured by Western blotting in cytoplasmic or nuclear extracts. Data are representative of two independent experiments with peripheral blood mononuclear cells from unrelated donors.
Cyclosporin A, an inhibitor of calcineurin, significantly inhibited TCR-dependent functions of Vγ2Vδ2 T cells
To test whether the calcium signalling pathway is necessary for TCR-dependent functions of Vγ2Vδ2 T cells, we used cyclosporin A to inhibit calcineurin.26 Vγ2Vδ2 T-cell lines were treated with cyclosporin A for 30 min, then stimulated with IPP or IPP + IL-2 or used for cytotoxicity assays as described above. Cyclosporin A significantly reduced IPP-stimulated and IPP + IL2-stimulated cytokine production (Fig. 4a), degranulation (Fig. 4b) and cytotoxicity against Daudi or TU167 tumour cell lines (Fig. 4c).
Figure 4.
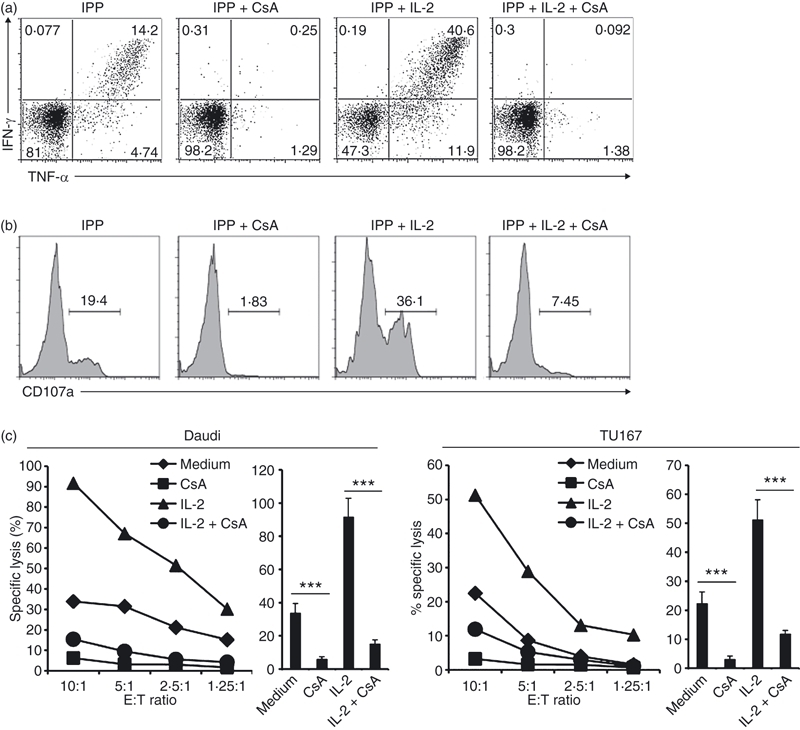
Cyclosporin A (CsA) blocks T-cell receptor (TCR) -dependent functions in Vδ2 T cells. (a,b) Resting Vγ2Vδ2 T-cell lines were pretreated with CsA for 1 hr, then stimulated with isopentenyl pyrophosphate (IPP; 15 μm) or IPP plus interleukin-2 (IL-2; 100 U/ml) for 4 hr. Interferon-γ (IFN-γ) or tumour necrosis factor-α (TNF-α) production (a) and CD107a expression (b) were detected by flow cytometry. (c) Resting Vγ2Vδ2 T-cell lines were pretreated with CsA (100 nm) or CsA + IL-2 (100 U/ml) for 1 hr. The cytotoxicity of Vγ2Vδ2 T cells against Daudi (left panel) or TU167 (right panel) targets were evaluated at several effector to target (E : T) ratios with triplicate wells for each condition. The statistical significance of specific lysis compared with control at E : T = 10 : 1 was analysed. ***P < 0·001. Data are representative of three independent experiments with peripheral blood mononuclear cells from unrelated donors.
Effects of ionomycin on functional responses of Vγ2Vδ2 T cells
To investigate whether activation of the calcium pathway was sufficient to induce functional responses, we tested the effects of ionomycin on Vγ2Vδ2 T-cell lines. Ionomycin is a potent and selective calcium ionophore.27,28 Ionomycin alone did not stimulate significant cytokine production (Fig. 5a) or degranulation (Fig. 5b) in Vγ2Vδ2 T-cell lines. Mostly probably, calcium-dependent, MEK/Erk and PI-3K/Akt pathways work together to induce functional responses. To test this idea, we treated Vγ2Vδ2 T cells with ionomycin (to activate the calcium pathway) and IL-2 (to activate MEK/Erk and PI-3K/Akt pathways). When used together, ionomycin and IL-2 induced strong cytokine production (Fig. 5a) but still did not induce degranulation (Fig. 5b). Only TCR engagement induced degranulation.
Figure 5.
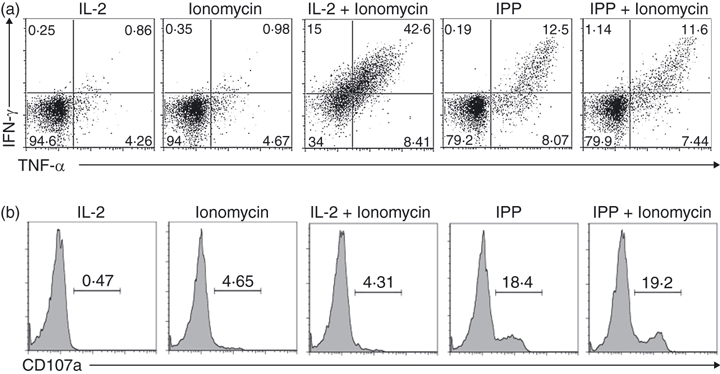
Effects of ionomycin on functional responses of Vδ2 T cells. (a,b) Resting Vγ2Vδ2 T-cell lines were stimulated with ionomycin (1 μm), interleukin-2 (IL-2; 100 U/ml), isopentenyl pyrophosphate (IPP) or combinations as indicated, for 4 hr. Interferon-γ (IFN-γ) or tumour necrosis factor-α (TNF-α) production (a) and CD107a expression (b) were detected by flow cytometry. Data are representative of three independent experiments with peripheral blood mononuclear cells from unrelated donors.
NFAT was involved in both cytokine production and degranulation of Vγ2Vδ2 T cells
Some studies on αβ T cells indicated that NFAT might be involved in Ca2+-dependent cytokine production but not degranulation.29,30 To clarify the question in Vγ2Vδ2 T cells, we used a highly selective inhibitor that prevents NFAT activation without affecting calcineurin phosphatase activity.29,31,32 Vγ2Vδ2 T-cell lines were treated with NFAT inhibitor for 1 hr, then stimulated with IPP. The NFAT inhibitor suppressed both IPP-stimulated cytokine production (Fig. 6a) and degranulation (Fig. 6b) in a dose-dependent manner. In Vγ2Vδ2 T cells, NFAT activation seems to regulate both cytokine expression and degranulation, despite the mobility of ionomycin to trigger degranulation.
Figure 6.
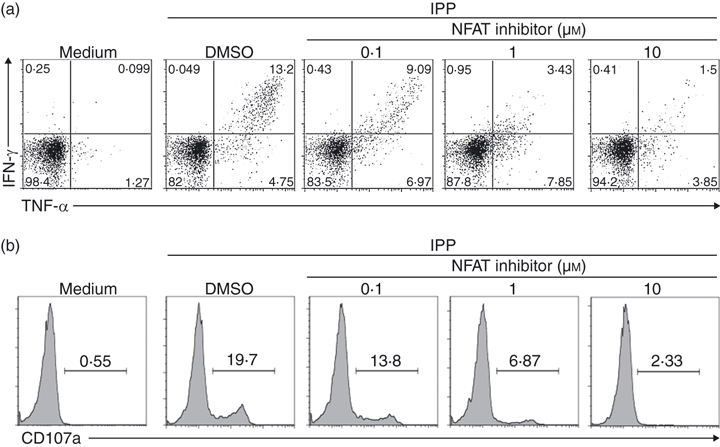
Nuclear factor of activated T cells (NFAT) was involved in Vδ2 T-cell cytokine production and degranulation. (a,b) Resting Vγ2Vδ2 T-cell lines were pretreated with NFAT inhibitor or vehicle control (DMSO) for 1 hr, then stimulated with isopentenyl pyrophosphate (IPP; 15 μm) for 4 hr. Interferon-γ (IFN-γ) or tumour necrosis factor-α (TNF-α) production (a) and CD107a expression (b) were detected by flow cytometry. Data are representative of three independent experiments with peripheral blood mononuclear cells from unrelated donors.
Discussion
In fresh PBMC, phosphoantigen plus IL-2 stimulates rapid proliferation of Vγ2Vδ2 T cells,8,15 which often exceed 90% of cells in culture within 10–14 days. The resulting cell lines respond to restimulation with phosphoantigen plus IL-2, by increasing cytokine expression, degranulation and cytotoxicity. Using Vγ2Vδ2 T-cell lines, which already express the high-affinity IL-2 receptor, we studied the interdependence of antigen and cytokine signalling for inducing effector responses including cytokine expression, degranulation and cytotoxicity. Interleukin-2 alone failed to induce any of these effector functions in Vγ2Vδ2 T-cell lines, despite potent activation of MEK/Erk and PI-3/Akt pathways, and strong activation of STAT5. Even in Vγ2Vδ2 T-cell lines with strong expression of IL-2 receptor, cytokine alone did not induce effector functions. Phosphoantigen alone induced cytokine expression, degranulation and cytotoxicity; all responses were increased by IL-2 in a dose-dependent manner. The induction of cytokines or cytotoxicity was sensitive to cyclosporin A or NFAT inhibitors. Substituting ionomycin for phosphoantigen still induced cytokine expression but failed to induce degranulation; this may be related to the potency of ionomycin in Vγ2Vδ2 T cells.
In αβ T cells, calcium pathways play critical roles in TCR-dependent functions. Ca2+ influx is an absolute requirement for perforin-dependent killing by cytotoxic T lymphocytes and cytokine expression by helper T cells.23,33 Vγ2Vδ2 T cells also demonstrated granule release and cytokine production following stimulation,7–10 but the signalling pathways and interplay of antigen and cytokine binding had not been reported previously.
Vγ2Vδ2 T-cell lines model the behaviour of Vγ2Vδ2 T cells during active immunotherapy for cancer. Clinical strategies have used bisphosphonate drugs, which increase phosphoantigen levels, plus repeated IL-2 injections to activate and expand the Vγ2Vδ2 T-cell subset. Disease stabilization or clinical regression was reported in metastatic prostate cancer34 and multiple myeloma35 trials where Vγ2Vδ2 T cells were activated by bisphosphonate plus IL-2. An obstacle to greater progress in this area is the observed anergy that develops after multiple bisphosphonate treatments.36 It will be important to examine cell signalling during these in vivo studies to understand what causes anergy, whether this is sensitive to cyclosporin A18 or rapamycin (Li and Pauza, submitted for publication) and what new approaches might be needed to overcome this problem.
In some models of αβ T cells, Ca2+-dependent cytokine production by helper T cells or degranulation in cytotoxic T lymphocytes may be regulated differently.33 In helper T cells, calcineurin dephosphorylates the transcription factor NFAT which then translocates to the nucleus and activates gene expression, a process that has been studied in considerable detail.24,25,37,38 Our results show that calcium mobilization (by ionomycin) plus activation of MEK/Erk and PI-3K/Akt pathways (by IL-2) are sufficient to induce TNF-α and IFN-γ expression. It is likely that NFAT works together with downstream transcription factors including Akt/Erk to initiate gene transcription of TNF-α and IFN-γ. In αβ T cells, NFAT regulates gene expression in concert with activating protein 1 (Jun/Fos) to orchestrate effective responses. In our studies, neither IPP nor IL-2 activated JNK and there was weak expression of Jun (data not shown). Correia et al.16 reported Jun N-terminal kinase (JNK) activation after stimulation with the potent bacterial phosphoantigen (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate or anti-CD3 antibody, and there was no additional impact of adding IL-2. Our inability to detect JNK activation may be the result of using the less potent phosphoantigen IPP. Cytotoxicity was inhibited by cyclosporin A and was increased by IL-2 in a dose-dependent effect manner. In Vγ2Vδ2 cells, NFAT is involved both in cytokine expression and granule release; a result that differs slightly from the regulatory mechanisms reported for αβ T cells.30
Our studies also show an important distinction between NK and γδ cell cytotoxicity. Both cell types depend on NKG2D signalling but only γδ cell killing could be inhibited by TCR-blocking antibodies39 or treating target cells with statin drugs to decrease phosphoantigen levels.40 We now understand that TCR engagement is needed to trigger degranulation even without binding to MHC.
Acknowledgments
We would like to thank Cheryl Armstrong for excellent technical assistance. This work was supported by PHS grant CA142458 (C.D.P.).
Disclosures
The authors have no financial conflicts of interest related to this study.
References
- 1.Carding SR, Egan PJ. γδ T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–45. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 2.Bonneville M, Janeway CA, Jr, Ito K, Haser W, Ishida I, Nakanishi N, Tonegawa S. Intestinal intraepithelial lymphocytes are a distinct set of γδ T cells. Nature. 1988;336:479–81. doi: 10.1038/336479a0. [DOI] [PubMed] [Google Scholar]
- 3.Evans PS, Enders PJ, Yin C, Ruckwardt TJ, Malkovsky M, Pauza CD. In vitro stimulation with a non-peptidic alkylphosphate expands cells expressing Vγ2-Jγ1.2/Vδ2 T-cell receptors. Immunology. 2001;104:19–27. doi: 10.1046/j.0019-2805.2001.01282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–70. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature. 1995;375:155–8. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 6.Roelofs AJ, Jauhiainen M, Monkkonen H, Rogers MJ, Monkkonen J, Thompson K. Peripheral blood monocytes are responsible for γδ T cell activation induced by zoledronic acid through accumulation of IPP/DMAPP. Br J Haematol. 2009;144:245–50. doi: 10.1111/j.1365-2141.2008.07435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Das H, Kamath A, Bukowski JF. Human Vγ2Vδ2 T cells produce IFN-γ and TNF-α with an on/off/on cycling pattern in response to live bacterial products. J Immunol. 2001;167:6195–201. doi: 10.4049/jimmunol.167.11.6195. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Luo K, Pauza CD. TNF-α is a positive regulatory factor for human Vγ2Vδ2 T cells. J Immunol. 2008;181:7131–7. doi: 10.4049/jimmunol.181.10.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Guo BL, Gehrs BC, Nan L, Lopez RD. Ex vivo expanded human Vγ9Vδ2 + γδ-T cells mediate innate antitumor activity against human prostate cancer cells in vitro. J Urol. 2005;173:1552–6. doi: 10.1097/01.ju.0000154355.45816.0b. [DOI] [PubMed] [Google Scholar]
- 10.Viey E, Fromont G, Escudier B, Morel Y, Da Rocha S, Chouaib S, Caignard A. Phosphostim-activated γδ T cells kill autologous metastatic renal cell carcinoma. J Immunol. 2005;174:1338–47. doi: 10.4049/jimmunol.174.3.1338. [DOI] [PubMed] [Google Scholar]
- 11.Alexander AA, Maniar A, Cummings JS, et al. Isopentenyl pyrophosphate-activated CD56+γδ T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin Cancer Res. 2008;14:4232–40. doi: 10.1158/1078-0432.CCR-07-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin G, Mao H, Zheng J, et al. Phosphoantigen-expanded human γδ T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J Infect Dis. 2009;200:858–65. doi: 10.1086/605413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonneville M, Scotet E. Human Vγ9Vδ2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol. 2006;18:539–46. doi: 10.1016/j.coi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Caccamo N, Meraviglia S, Scarpa F, et al. Aminobisphosphonate-activated γδ T cells in immunotherapy of cancer: doubts no more. Expert Opin Biol Ther. 2008;8:875–83. doi: 10.1517/14712598.8.7.875. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Pauza CD. Effects of 15-deoxy-δ12,14-prostaglandin J2 (15d-PGJ2) and rosiglitazone on human Vδ2 T cells. PLoS ONE. 2009;4:e7726. doi: 10.1371/journal.pone.0007726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correia DV, d’Orey F, Cardoso BA, et al. Highly active microbial phosphoantigen induces rapid yet sustained MEK/Erk- and PI-3K/Akt-mediated signal transduction in anti-tumor human γδ T-cells. PLoS ONE. 2009;4:e5657. doi: 10.1371/journal.pone.0005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen BG, Wada H, Matsutake T, Uenaka A, Obata Y, Nakayama E. Inhibition by CsA and FK506 of the in vitro proliferative response of γδ T cells on stimulation with anti-TCR δ monoclonal antibody. Transpl Immunol. 1996;4:158–62. doi: 10.1016/s0966-3274(96)80010-4. [DOI] [PubMed] [Google Scholar]
- 18.Gan YH, Lui SS, Malkovsky M. Differential susceptibility of naive and activated human γδ T cells to activation-induced cell death by T-cell receptor cross-linking. Mol Med. 2001;7:636–43. [PMC free article] [PubMed] [Google Scholar]
- 19.Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–82. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 20.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 21.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 22.Poccia F, Cipriani B, Vendetti S, et al. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive Vγ9Vδ2 T lymphocytes. J Immunol. 1997;159:6009–17. [PubMed] [Google Scholar]
- 23.Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 24.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat Rev Immunol. 2005;5:472–84. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 25.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–32. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 26.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nat Immunol. 2001;2:316–24. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Hermann TE. Characterization of ionomycin as a calcium ionophore. J Biol Chem. 1978;253:5892–4. [PubMed] [Google Scholar]
- 28.Kauffman RF, Taylor RW, Pfeiffer DR. Cation transport and specificity of ionomycin. Comparison with ionophore A23187 in rat liver mitochondria. J Biol Chem. 1980;255:2735–9. [PubMed] [Google Scholar]
- 29.Aramburu J, Yaffe MB, Lopez-Rodriguez C, Cantley LC, Hogan PG, Rao A. Affinity-driven peptide selection of an NFAT inhibitor more selective than cyclosporin A. Science. 1999;285:2129–33. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 30.Grybko MJ, Bartnik JP, Wurth GA, Pores-Fernando AT, Zweifach A. Calcineurin activation is only one calcium-dependent step in cytotoxic T lymphocyte granule exocytosis. J Biol Chem. 2007;282:18009–17. doi: 10.1074/jbc.M702222200. [DOI] [PubMed] [Google Scholar]
- 31.Noguchi H, Matsushita M, Okitsu T, et al. A new cell-permeable peptide allows successful allogeneic islet transplantation in mice. Nat Med. 2004;10:305–9. doi: 10.1038/nm994. [DOI] [PubMed] [Google Scholar]
- 32.Kiani A, Garcia-Cozar FJ, Habermann I, Laforsch S, Aebischer T, Ehninger G, Rao A. Regulation of interferon-γ gene expression by nuclear factor of activated T cells. Blood. 2001;98:1480–8. doi: 10.1182/blood.v98.5.1480. [DOI] [PubMed] [Google Scholar]
- 33.Pores-Fernando AT, Zweifach A. Calcium influx and signaling in cytotoxic T-lymphocyte lytic granule exocytosis. Immunol Rev. 2009;231:160–73. doi: 10.1111/j.1600-065X.2009.00809.x. [DOI] [PubMed] [Google Scholar]
- 34.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–6. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 36.Sicard H, Ingoure S, Luciani B, Serraz C, Fournie JJ, Bonneville M, Tiollier J, Romagne F. In vivo immunomanipulation of Vγ9Vδ2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175:5471–80. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- 37.Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–56. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Oh-hora M, Rao A. Calcium signaling in lymphocytes. Curr Opin Immunol. 2008;20:250–8. doi: 10.1016/j.coi.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisch P, Malkovsky M, Braakman E, et al. γδ T cell clones and natural killer cell clones mediate distinct patterns of non-major histocompatibility complex-restricted cytolysis. J Exp Med. 1990;171:1567–79. doi: 10.1084/jem.171.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De LiberoG. Human T cell receptor γδ cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–8. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]


