Abstract
Relatively little is known about regulatory T (Treg) cells and their functional responses in dogs. We have used the cross-reactive anti-mouse/rat Foxp3 antibody clone FJK-16s to identify a population of canine CD4+ FOXP3high T cells in both the peripheral blood (PB) and popliteal lymph node (LN). FOXP3+ cells in both PB and LN yielded positive staining with the newly developed anti-murine/human Helios antibody clone 22F6, consistent with the notion that they were naturally occurring Treg cells. Stimulation of mononuclear cells of LN origin with concanavalin A (Con A) in vitro yielded increased proportions and median fluorescence intensity of FOXP3 expression by both CD4+ and CD8+ T cells. Removal of the Con A and continued culture disclosed a CD4+ FOXP3high population, distinct from the CD4+ FOXP3intermediate T cells; very few CD8+ FOXP3high T cells were observed, though CD8+ FOXP3intermediate cells were present in equal abundance to CD4+ FOXP3intermediate cells. The CD4+ FOXP3high T cells were thought to represent activated Treg cells, in contrast to the FOXP3intermediate cells, which were thought to be a more heterogeneous population comprising predominantly activated conventional T cells. Co-staining with interferon-γ (IFN-γ) supported this notion, because the FOXP3high T cells were almost exclusively IFN-γ−, whereas the FOXP3intermediate cells expressed a more heterogeneous IFN-γ phenotype. Following activation of mononuclear cells with Con A and interleukin-2, the 5% of CD4+ T cells showing the highest CD25 expression (CD4+ CD25high) were enriched in cells expressing FOXP3. These cells were anergic in vitro, in contrast to the 20% of CD4+ T cells with the lowest CD25 expression (CD4+ CD25−), which proliferated readily. The CD4+ CD25high FOXP3high T cells were able to suppress the proliferation of responder CD4+ T cells in vitro, in contrast to the CD4+ CD25− cells, which showed no regulatory properties.
Keywords: FOXP3, Helios, interferon-γ, interleukin-10, regulatory T cell, suppression
Introduction
Regulatory T (Treg) cells play a crucial role in the maintenance of peripheral tolerance.1,2 Abnormalities of Treg-cell number or function have been implicated in several autoimmune3–5 and allergic6–8 diseases, and Treg cells play a pivotal role in the maintenance of allograft tolerance.9–11 Despite limiting collateral damage in the immune response against certain microbes, Treg cells have also been implicated in the pathogenesis of a number of infectious diseases – either by promoting persistence of the pathogen by inhibiting anti-microbial effector responses or by acting as a cellular reservoir of the pathogen.12–15 Such pathomechanisms have been demonstrated in both rodents and higher mammals, including veterinary species: for example, Treg cells are known to be a reservoir of productive feline immunodeficiency virus infection16–19 and are induced in the periphery by porcine reproductive and respiratory syndrome virus.20,21 The manipulation of Treg-cell number or function therefore holds promise as a novel therapy for infectious disease or as a component of more effective vaccination strategies.22,23
Regulatory T cells may be broadly divided into naturally occurring subsets (nTreg), which develop in the thymus along a regulatory lineage (for example, the canonical CD4+ CD25+ Foxp3+ Treg cells); and induced, or ‘adaptive’, subsets, which arise in the periphery following activation of conventional T (Tcon) cells in a microenvironment rich in regulatory cytokines, or following the interaction of Tcon with nTreg cells in a process called infectious tolerance – for example, the Tr1, Th3 and ‘induced Treg (iTreg)’ cells.24–27 Regulatory T cells have been characterized in mice,24 rats,28,29 humans,5 baboons,30,31 macaques,32 chimpanzees,33 cats16,34,35 and pigs;36–38 furthermore, there is convincing indirect or historical evidence for Treg cells in cows,39–41 sheep42,43 and horses.44 However, relatively little is known about Treg cells in dogs, though indirect evidence for their existence has been available for several years.45–47 We48 and others49–54 have used the anti-mouse/rat Foxp3 antibody clone FJK-16s to identify a population of canine CD4+ T cells that phenotypically resembles Treg cells, but direct evidence for regulatory activity has remained elusive.55 In this study, we have characterized the phenotype and function of canine CD4+ CD25high FOXP3high T cells in vitro, providing direct evidence for the regulatory function of this T-cell subset in dogs – an important veterinary species that also serves as a model for several human diseases, including a number of cancers,56–58 systemic lupus erythematosus59,60 and several genetic diseases of the haemopoietic system.61
Materials and methods
Animals
Blood was collected into potassium EDTA by jugular venepuncture and popliteal lymph nodes (LNs) were aseptically harvested from colony beagles or greyhounds, euthanized for reasons unrelated to this study. All animals were systemically healthy and aged between 12 and 30 months. Routine vaccinations against common pathogens had been performed and prophylactic oral endoparasiticidal treatment had been administered. All protocols had passed scrutiny by the local ethical review committee before work was allowed to commence.
Isolation of neutrophils and mononuclear cells
Mononuclear cells and neutrophils were isolated from blood using a double-density centrifugation protocol, as described by Strasser et al.62 Cells were washed separately in PBS twice, before being re-suspended in complete medium to establish cell count and viability. Mononuclear cells were isolated from LNs via mechanical maceration of the tissue through a 70-μm cell strainer (BD Biosciences, Oxford, UK). The resulting cells were suspended in RPMI-1640 (Sigma Aldrich, Gillingham, UK) supplemented with 100 units/ml penicillin/streptomycin (Gibco, Paisley, UK), 2 mm l-glutamine (Gibco), 10 mm HEPES (Gibco) and 10% volume/volume (v/v) heat-inactivated fetal calf serum (PAA Laboratories, Yeovil, UK) (complete medium) and centrifuged at 600 g for 5 min at room temperature. The cells were washed twice in complete medium before re-suspension to establish cell count and viability.
Cell culture
Mononuclear cells were cultured in 96-well, round-bottom plates in complete medium containing 5 μg/ml concanavalin A (Con A; Sigma Aldrich). Plates were incubated in a humidified atmosphere of 5% v/v CO2 at 37°. At either 72 or 120 hr, the cells were removed and stained for flow cytometric analysis.
Monoclonal antibodies
Canine-specific or cross-reactive fluorochrome-conjugated monoclonal antibodies (mAbs) against cell surface and intracellular markers were used to identify different cell subsets. These included mAbs with specificity for canine CD4 (clone YKIX302.9), CD8 (YCATE55.9) and CD5 (YKIX322.3) (all AbD Serotec, Kiddlington, UK); cross-reactive mAbs with specificity for human CD32 (AT10) and CD79b (AT107-2) (both AbD Serotec); and cross-reactive mAbs with specificity for human CD25 (ACT-1; Dako UK Ltd, Ely, UK), murine Foxp3 (FJK-16s; eBioscience, Hatfield, UK) and murine/human Helios (22F6; BioLegend, San Diego, CA). Appropriate isotype control mAbs in ‘fluorescence minus one’ tubes were used in all staining panels.
Analytical flow cytometry
All incubation steps were performed in the dark on ice, unless otherwise indicated. The manufacturer’s protocol for Foxp3 staining was applied (http://www.ebioscience.com/ebioscience/specs/antibody_77/77-5775.htm). Briefly, cells were pre-incubated with mouse anti-human CD32 mAb for 15 min, washed, and stained with mAbs against surface antigens for 20 min. Cells were washed and incubated overnight in a 1 : 4 v/v fixation/permeabilization solution at 4°. They were then washed again twice, before incubating with a blocking solution containing 10% v/v fetal calf serum (PAA Laboratories) for 20 min and staining with various mAbs against intracellular antigens for 30 min. A final washing step was undertaken, before re-suspension of the cells in PBS. Freshly isolated or activated cells were analysed for the expression of surface and intracellular antigens using FITC-, phycoerythrin- and Alexa Fluor® 647-conjugated mAbs according to the manufacturer’s recommendations. A published protocol was used to analyse interferon-γ (IFN-γ) expression.63 Briefly, cells were cultured with PMA (50 ng/ml; Sigma Aldrich) and ionomycin (500 ng/ml; Sigma Aldrich) for 4 hr, adding brefeldin A (10 μg/ml; Sigma-Aldrich) 2 hr before the end of the assay. Samples were obtained on a FACS Canto II® flow cytometer (BD Biosciences) in a quantitative manner, using standard acquisition gates defined on the basis of forward and side scatter. CALTAG™ Counting Beads (Caltag-Medsystems, Buckingham, UK) were employed to allow comparisons of cell numbers between cultures or between time-points, in all cases normalizing counts to the number of cells per culture well. Results were analysed using Flow-Jo™ software (Tree Star Inc., Ashland, OR).
Fluorescence-activated cell sorting (FACS™)
Before sorting, mononuclear cells were activated as previously described for 96 hr. The activated cells were washed with complete medium, stained with mAbs against CD4 and CD25, and sorted using a MoFlo™ XDP Cell Sorter (Beckman Coulter, High Wycombe, UK). Cells were sorted on the basis of the lymphocyte gate – as determined by forward and side scatter characteristics – and differential expression of the antigen identified by the anti-human CD25 mAb, assumed to be canine CD25,64 identifying CD25high and CD25− populations. The CD25high gate incorporated the 5% of CD4+ T cells showing the brightest fluorescence signal for CD25, while the CD25− gate incorporated the 20% of CD4+ T cells showing the dimmest fluorescence signal for CD25.
Reverse transcription–quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from CD25high and CD25− CD4+ T cells by means of a phenol-bromochloropropane-isopropanol protocol using TRI Reagent™ (Applied Biosystems, Warrington, UK) according to the manufacturer’s recommendations. Taqman™ gene expression assays (Applied Biosystems) were performed in triplicate for each transcript, using a one-step Cells-to-CT™ kit (Applied Biosystems) and a cycling protocol of 48° for 15 min (reverse transcription), 95° for 10 min (activation of DNA polymerase) and then 50 cycles of 95° for 15 seconds (denaturation) and 60° for 1 min (annealing/extension) in a real-time thermal cycler (CHROMO4™ Continuous Fluorescence Detector; GRI Ltd, Essex, UK). The qPCR mixture contained 100 ng/μl RNA template, 900 nm forward and reverse primers, 250 nm probe, 2 × TaqMan™ RT-PCR Mix (10 μl) and 40 × TaqMan™ RT enzyme mix (0·5 μl) in a total reaction volume of 20 μl.
Opticon 3.0 software™ (Bio-Rad Ltd, Hemel Hempstead, UK) was employed to determine Ct values. Two additional, control reactions – respectively lacking the RNA template or the enzyme mix – were performed in each experiment. Data were analysed using the ‘Gene Expression Ct Difference’ (GED) formula,65 normalizing transcript abundance to that of β2-microglobulin. Reactions failing to yield a signal were assigned a Ct value of 40.
In vitro suppression assay
Following FACS™ the CD25high and CD25− fractions were rested in complete medium containing 50 U/ml interleukin-2 (IL-2; R&D Systems, Abingdon, UK) for 48 hr. Positive immunomagnetic selection of third-party CD4+ cells yielded a conventional (target) cell population. Magnetic separation was performed according to the manufacturer’s instructions, using anti-CD4-phycoerythrin and phycoerythrin-streptavidin Microbeads (Miltenyi Biotec, Bisley, UK). The CD4+ cells were activated with Con A (2·5 μg/ml) in complete medium for 48 hr, in parallel with the CD25high and CD25− cells previously isolated by FACS™ which were activated in complete medium containing both Con A (2·5 μg/ml) and IL-2 (20 U/ml). All cells were cultured at a density of 1 × 106/ml in 96-well, round-bottom plates. Following activation, the CD25high and CD25− cells were washed and cultured for a further 72 hr in fresh complete medium, either alone or following admixture with the washed CD4+ T cells. Additional control cultures were established, including monocultures of different cell populations with and without supplemental IL-2 (10 U/ml). Proliferation was measured by the incorporation of [3H]TdR (37MB q/ml; GE Healthcare Life Sciences, Little Chalfont, UK), pulsing the plates (1 μCi/well) 18 hr before the end of the assays and subsequent cell harvesting.
Statistical analysis
Bartlett’s test was used to assess whether groups of data showed homogeneity of variance. Group homogeneity was not observed, prompting use of the Friedman test for paired data or the Kruskal–Wallis test for unpaired data, followed in both cases by Dunn’s Multiple Comparison testing if P< 0·05; P-values are shown for pairwise comparisons that were significantly different.
Results
A population of CD4+ CD25+ FOXP3+ Helios+ T cells is present in the blood and peripheral lymph nodes of dogs
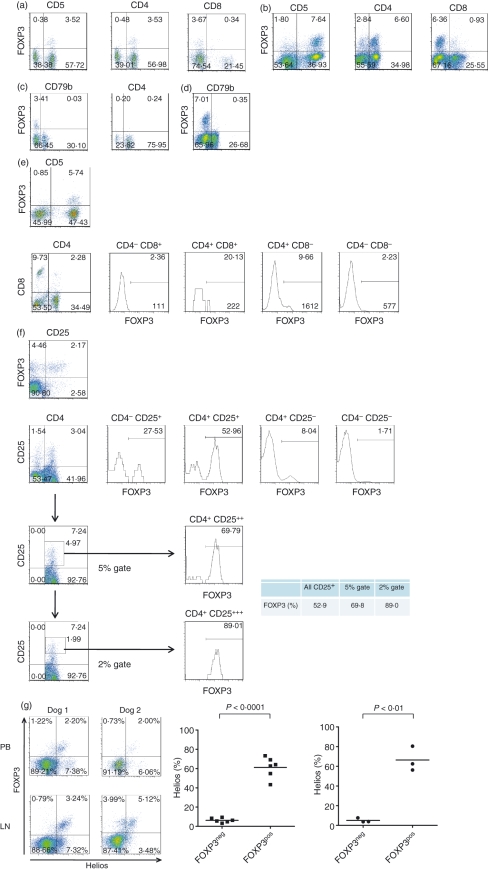
Three-colour flow cytometry revealed populations of FOXP3+ T cells in both the peripheral blood (PB; Fig. 1a) and popliteal LNs (Fig. 1b) of systemically healthy greyhounds and beagles. A mean of 4·3% of all lymphocytes in PB were FOXP3+, of which the majority were T cells [3·4 ± 0·2% (mean ± SEM) CD5+ versus 0·9 ± 0·2% CD5−; n= 10]. Similarly, 6·2 ± 0·6% of LN-derived cells were CD5+ FOXP3+ versus 1·1 ± 0·2% CD5− FOXP3+ (n= 10). The FOXP3+ cells were both CD4+ and CD4−, though the former predominated: in PB, 3·4 ± 0·2% of lymphocytes were CD4+ FOXP3+ versus 1·1 ± 0·1% CD4− FOXP3+ (n= 12) and in LNs, 4·8 ± 0·6% of cells were CD4+ FOXP3+ versus 3·2 ± 0·6% CD4− FOXP3+ (n= 9). Relatively few CD8+ FOXP3+ T cells were observed in either PB (0·4 ± 0·1%; n= 10) or LNs (1·0 ± 0·1%; n= 9), suggesting the existence of a CD4− CD8− FOXP3+ T-cell population; indeed, the CD8− FOXP3+ populations in both PB (4·4 ± 0·4%; n= 10) and LNs (7·1 ± 0·8%; n= 9) were, respectively, larger than the CD4+ FOXP3+ populations. Negligible FOXP3 expression was observed in B cells (CD79b+) (Fig. 1c,d) and neutrophils (CD5− CD4+) (Fig. 1c). When FOXP3 expression by lymphocytes defined on the basis of CD4 and CD8 co-staining was examined, FOXP3+ cells could be identified in the CD4− CD8− gate, again supporting the existence of double-negative FOXP3+ cells (Fig. 1e); these cells were likely to be T cells because the majority of FOXP3+ cells were CD5+.
Figure 1.
A population of CD4+ CD25+ FOXP3+ T cells is present in the blood and peripheral lymph nodes of dogs. Three-colour flow cytometry revealed populations of FOXP3+ T cells in both the peripheral blood (a) and popliteal lymph nodes (b) of systemically healthy greyhounds and beagles. [In all dot plots, proportions of cells lying within each of the quadrants are indicated in the corners of the quadrants, sampling the peripheral blood (a, c, g) and popliteal lymph nodes (b, d–g)] These cells were both CD4+ and CD4−, though the former predominated; relatively lower proportions of CD8+ than CD4− FOXP3+ T cells were observed, suggesting the existence of a CD4− CD8− FOXP3+ T-cell population. Negligible FOXP3 expression was observed in B cells (CD79b+) (c, d) and neutrophils (CD5− CD4+) (c). When FOXP3 expression by lymphocytes, defined on the basis of CD4 and CD8 co-staining, was examined in a second dog (e), FOXP3+cells could be identified in the CD4− CD8− gate, again supporting the existence of double-negative FOXP3+cells (numbers of cells within the FOXP3+ gates are shown in the bottom right corner of each histogram, normalized to counts per well); these cells were likely to be T cells as the majority of FOXP3+ cells were CD5+. Staining for CD25 using the monoclonal antibody ACT-1 revealed that FOXP3+ cells were enriched in the CD4+ CD25+ population, but a surprising number of FOXP3+ T cells were also observed in the ACT-1-negative fraction, as shown in the upper dot plot in part (f). The lower three dot plots analyse the expression of FOXP3, shown by the adjacent histograms, within each of the four un-gated quadrants (upper row) or within the gates representing the upper 5% (second row) or upper 2% (third row) of CD4+ T cells, assessed on the basis of CD25 expression. Hence, FOXP3+ cells comprised 52·9% of all CD4+ CD25+ cells, but 69·8% of the upper 5% of CD4+ T cells and 89·0% of the upper 2% of CD4+ T cells, representing cells showing the highest CD25 expression: FOXP3+ T cells were therefore enriched in the CD4+ CD25high population. In most cases, the majority of FOXP3+ cells yielded a positive stain with the anti-murine/human Helios monoclonal antibody 22F6, suggesting that they were naturally occurring regulatory T cells (g). The dot plots to the left in (g) show the results of 22F6 (Helios) and FOXP3 co-staining of peripheral blood (PB) and (LN) cells from two dogs. The graphs to the right summarize the proportion of 22F6 (Helios)+ cells in the FOXP3− (FOXP3neg) and FOXP3+ (FOXP3pos) populations derived from peripheral blood (square symbols) or lymph nodes (circular symbols); the data were analysed with a paired Student's t-test. Initial data shown are from two greyhounds (dog 1: a–d, f; dog 2: e), but are also representative of results from the beagles; a total of 12 dogs were sampled, though not all staining reactions were performed in every case. Helios staining (g) was performed only on samples derived from beagles.
Staining for CD25 using the mAb ACT-1 revealed that FOXP3+ cells were enriched in the CD25+ population, especially the CD4+ CD25high (Fig. 1f). However, surprisingly, the majority of FOXP3+ cells were ACT-1-negative (Fig. 1f): in PB, 0·7 ± 0·2% of lymphocytes were CD25+ FOXP3+ versus 4·2 ± 0·3% CD25− FOXP3+ (n= 5) and in LNs, 1·5 ± 0·4% of cells were CD25+ FOXP3+ versus 5·9 ± 1·6% CD25− FOXP3+ (n= 3).
The newly developed anti-murine/human Helios mAb66 was used to stain PB and LN preparations (Fig. 1g). Although variable, at least 50% of FOXP3+ cells were Helios+ in most cases: in PB, 2·5 ± 0·5% of cells were FOXP3+ Helios+ versus 2·3 ± 0·9% FOXP3+ Helios− (n = 6), while in LN, 3·92 ± 0·6% of cells were FOXP3+ Helios+ versus 2·3 ± 0·9% FOXP3+ Helios− (n = 3) (Fig. 1g).
Polyclonal stimulation of canine T cells up-regulates FOXP3 in both CD4+ and CD8+ T cells and discloses a CD4+ FOXP3high IFN-γ− subpopulation
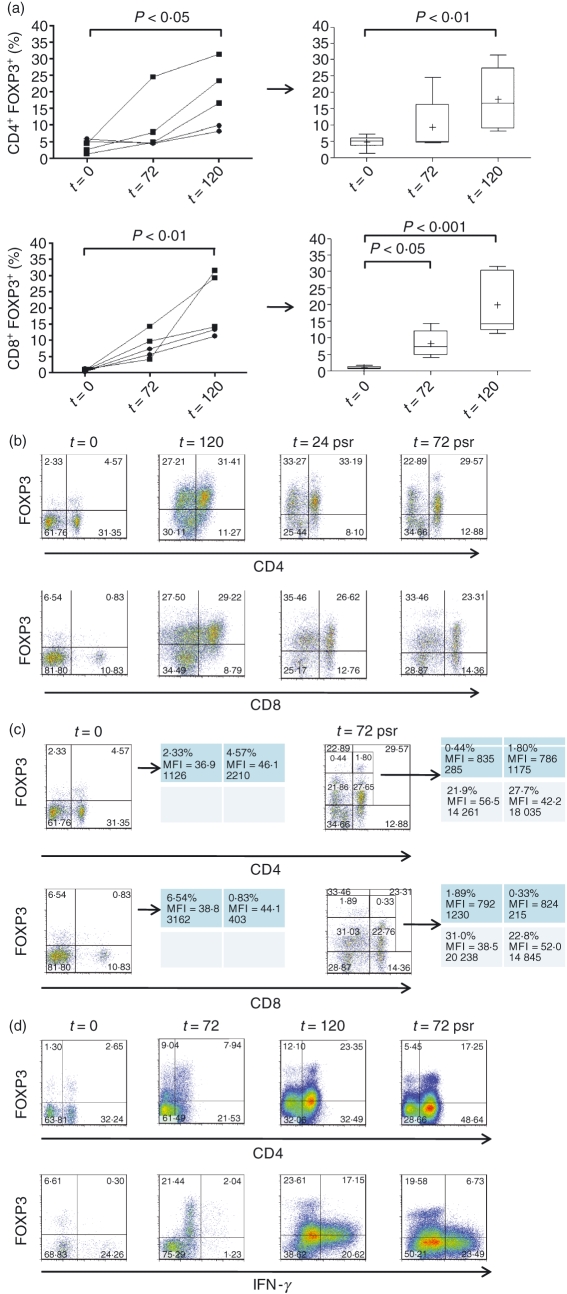
Mononuclear cells derived from the popliteal LNs of systemically healthy greyhounds and beagles showed increased proportional expression of FOXP3 when cultured with Con A for periods of up to 120 hr (Fig. 2a). The fold-increase in proportional FOXP3 expression was particularly striking in the CD8+ population: while 4·8 ± 0·6% of cells were CD4+ FOXP3+ at time 0 (n= 9), increasing to 9·3 ± 3·9% at 72 hr (n= 5) and 17·9 ± 4·3% at 120 hr (n= 5), only 1·0 ± 0·1% of cells were CD8+ FOXP3+ at time 0 (n= 9), increasing to 8·2 ±1·8% at 72 hr (n= 5) and 19·9 ± 4·3% at 120 hr (n= 5).
Figure 2.
Polyclonal stimulation of canine T cells up-regulates FOXP3 in both CD4+ and CD8+ T cells and discloses a CD4+ FOXP3highIFN-γ− subpopulation. Mononuclear cells derived from the popliteal lymph nodes of systemically healthy greyhounds and beagles showed increased proportional expression of FOXP3 when cultured with concanavalin A (Con A) for periods of up to 120 hr (a; t = 120). Data shown represent proportions of CD4+ or CD8+ FOXP3+ cells, expressed as a percentage of total lymphocytes gated on the basis of forward and side scatter characteristics. Square symbols relate to greyhounds and circular symbols to beagles; the lines connect data from individual dogs. The box-and-whisker plots on the right summarize the time–courses but incorporate additional time 0 data from animals whose cells were not cultured (CD4+, n= 9; CD8+, n= 9); P-values indicate statistical differences between respective time-points (other time-points yielded P-values > 0·05). (The whiskers mark the 5th and 95th percentiles, boxes mark the 25th and 75th percentiles, horizontal lines show the median values, and crosses show the mean values.) When the cells were washed and re-cultured in the absence of Con A (post-stimulus removal: psr) for up to 72 hr (b), a population of FOXP3high cells, predominantly CD4+ and distinct from the FOXP3intermediate cells, became apparent. (c) Analysis of the proportions of cells in the FOXP3high and FOXP3intermediate populations, along with their median fluorescence intensity (MFI) and absolute number. We speculated that the FOXP3high population represented activated regulatory T cells, in contrast to the FOXP3intermediate, which were thought to be predominantly activated conventional T cells. Co-staining with interferon-γ (IFN-γ) supported this notion because the CD4+ FOXP3high T cells were almost exclusively IFN-γ−, while the CD4+ FOXP3intermediate cells expressed a more heterogeneous IFN-γ phenotype (d; bottom panel gated on CD4+ cells). [FOXP3 MFI values were generally lower in the experiments incorporating intracellular staining for IFN-γ (d), than in those in which FOXP3 was the only intracellular antigen (c), but distinct FOXP3high and FOXP3intermediate cells were nevertheless consistently appreciated.] Data shown are from a greyhound, but are also representative of results from the beagles (total animals: a, n= 5; b, c, n= 3; d, n= 4).
When the cells were washed and re-cultured in the absence of Con A for up to 72 hr, a population of FOXP3high cells – predominantly CD4+ and distinct from the FOXP3intermediate cells – became apparent (Fig. 2b). In the example shown, the median fluorescence intensity (MFI) of the CD4+ FOXP3high T-cell population was ∼ 19-fold higher than that of the CD4+ FOXP3intermediate cells, though the latter were ∼ 15-fold more numerous (Fig. 2c); very few CD8+ FOXP3high T cells were observed but CD8+ FOXP3intermediate cells were present in equal abundance to CD4+ FOXP3intermediate cells. We speculated that the FOXP3high population represented activated Treg cells, in contrast to the FOXP3intermediate, which were thought to be a more heterogeneous population containing predominantly activated Tcon cells. Co-staining with IFN-γ supported this notion, because the FOXP3high T cells were almost exclusively IFN-γ− whereas the FOXP3intermediate cells expressed a more heterogeneous IFN-γ phenotype (Fig. 2d).
Peripheral CD4+ CD25high FOXP3high T cells in the dog show a regulatory phenotype and suppressive function in vitro
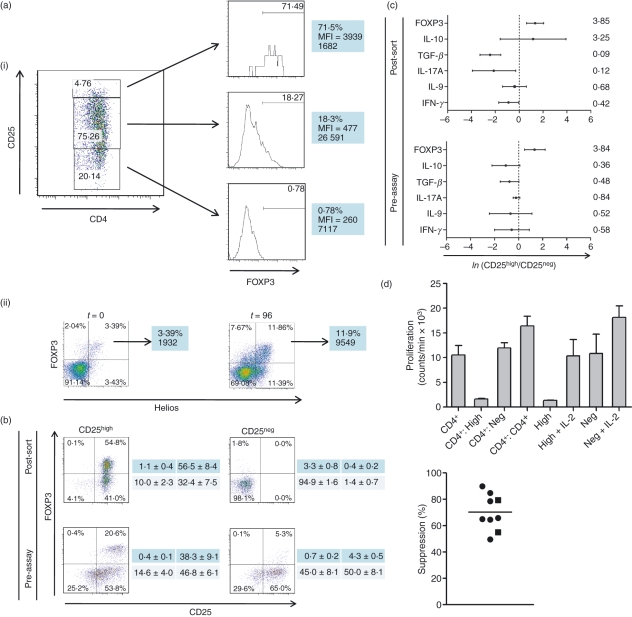
Activation of mononuclear cells with both Con A and IL-2 (10 U/ml) augmented up-regulation of CD25 expression beyond that seen with Con A alone [data not shown and Fig. 3a(i)]. Furthermore, the activation protocol appeared to expand the population of FOXP3+ Helios+ cells [Fig. 3a(ii)]: whereas 3·9 ± 0·6% of LN cells were FOXP3+ Helios+ at time 0, with an absolute number of 3176 ± 777 FOXP3+ Helios+ cells per culture well, 9·6 ± 1·5% of the cells were FOXP3+ Helios+ after 96 hr, with an absolute number of 12 223 ± 1360 FOXP3+ Helios+ cells per well. This strategy was therefore employed to generate a population of activated T cells, from which FACS™ was used to sort the 5% of CD4+ T cells with the highest, and the 20% of CD4+ T cells with the lowest, CD25 expression. The CD25high cells were consistently enriched in cells expressing FOXP3 relative to the CD25intermediate or CD25− (CD25neg) cells [Fig. 3a(i)]. Thus, 66·8 ± 5·7% of the CD4+ CD25high T cells were FOXP3+, in contrast to only 15·2 ± 2·9% of the CD25intermediate and 2·9 ± 0·9% of the CD25low T cells (n = 7). Comparison of the phenotype of CD4+ T cells immediately following FACS™ (‘post-sort’) and before inception of the Treg-cell assay (‘pre-assay’) revealed that the CD25high fraction retained a population of FOXP3high cells at the point of cellular admixture, whereas the CD25− fraction contained only a small population of FOXP3intermediate cells – despite expressing CD25 with exposure to Con A – that were likely to represent activated Tcon cells (Fig. 3b). Although the CD4+ CD25high T cells were mixed with activated CD4+ responder T cells at a ratio of 1 : 1, the ratio of (CD4+ FOXP3high): (FOXP3− + FOXP3intermediate) CD4+ T cells at the inception of the co-culture assays was likely to be ∼ 1 : 6, because the CD4+ CD25high T cells were often only ∼ 30% FOXP3+ at this time (Fig. 3b). The CD4+ T-cell populations were further evaluated by means of RT-qPCR assays, which revealed that the ‘post-sort’ CD25high T cells showed greater expression of transcripts encoding FOXP3 (geometric mean GED ratio 3·85; n= 4) and IL-10 (3·25; n= 4) than the CD25− cells at the same time-point; over-expression of FOXP3 (3·84; n= 4) was also evident at the point of admixture of the cells (‘pre-assay’), but transcripts encoding transforming growth factor-β (TGF-β) and pro-inflammatory cytokines generally appeared to be less abundant in the CD25high T cells at both time-points (Fig. 3c). The CD4+ CD25high T cells were able to suppress the proliferation of activated CD4+ responder T cells in vitro, whereas the CD4+ CD25− cells showed no suppressive properties: proliferation was suppressed by 70·2 ± 4·6% (mean ± SEM) in a total of nine independent experiments performed with T cells derived from both PB and LNs (Fig. 3d). When cultured alone, the CD4+ CD25high T cells showed anergy that could be broken by the addition of IL-2 (20 U/ml), whereas the CD4+ CD25− cells proliferated robustly with or without exogenous IL-2 (Fig. 3d).
Figure 3.
Peripheral CD4+ CD25high FOXP3high T cells in the dog show a regulatory phenotype and suppressive function in vitro. Following activation of mononuclear cells with concanavalin A (Con A) and interleukin-2 (IL-2; 10 U/ml), FACS™ was used to sort the 5% of CD4+ T cells with the highest and the 20% of CD4+ T cells with the lowest CD25 expression. (A 5% rather than a 2% gate was chosen to optimize both FOXP3 enrichment and numbers of cells sorted for functional assays.) The CD25high cells were consistently enriched in cells expressing FOXP3 relative to the CD25intermediate or CD25− (CD25neg) cells [a(i)]. The histograms show respective expression of FOXP3 of each population, with median fluorescence intensity (MFI) and absolute cell counts; these results are representative of seven independent experiments. The 96-hr (t = 96) activation protocol increased the proportion and absolute number of FOXP3+ Helios+ cells, as revealed by co-staining with the anti-murine/human Helios monoclonal antibody 22F6 [a(ii)]. (b) The phenotype of CD4+ T cells from a dog both immediately following FACS™ (‘post-sort’) and on the day of CD25high and CD25neg cell admixture following pre-activation with Con A, for the purposes of the regulatory T-cell assay (‘pre-assay’). The CD4+ CD25high fraction, in this case approximately 55% FOXP3+, retains a population of FOXP3high cells at the point of admixture, whereas the CD25neg fraction contains only a small population of FOXP3intermediate cells, likely to represent activated conventional T cells. The tables to the right of the dot plots show respective summary data (mean ± SEM [%]) for each of the quadrants from five independent experiments. Reverse-transcription quantitative polymerase chain reaction (RT-qPCR) assays (c) reveal that the ‘post-sort’ CD25high T cells show greater expression of transcripts encoding FOXP3 and IL-10; greater expression of FOXP3 is also evident at the point of admixture of the cells (‘pre-assay’). All RT-qPCR assays were performed on lymph-node-derived cells and qPCR data are expressed as the natural logarithm (ln) of the ratio of gene expression Ct difference of CD25high : CD25neg cells, normalized to β2-microglobulin; mean and standard deviation ln expression ratios are depicted, with geometric mean ratios shown on the right of the figure (n= 4). (An ln expression ratio of 0 represents equally abundant transcript in both populations). When the cells depicted in (b) were co-cultured with activated third party responder CD4+ T cells at a 1 : 1 ratio, the CD4+ CD25high T cells were able to suppress the proliferation of the responder cells, whereas the CD4+ CD25neg cells showed no suppressive properties (d). When cultured alone, the CD4+ CD25high T cells showed anergy that could be broken by the addition of IL-2 (20 U/ml), while the CD4+ CD25neg cells proliferated robustly with or without exogenous IL-2. Control co-cultures of CD25high or CD25neg CD4+ T cells maintained in complete medium without Con A admixed with similarly unstimulated third-party CD4+ T cells showed only background counts, ruling out any influence of a mixed leukocyte reaction. Error bars indicate standard errors of the mean of triplicate cultures (CD4+ T cells and co-cultures) or (difference in values/2) for duplicate monocultures of CD25high or CD25neg T cells. The figure to the right of the panel summarizes proportional suppression of proliferation for nine independent experiments; square symbols represent assays performed on peripheral blood mononuclear cells, circles represent assays performed on lymph node-derived cells, and the horizontal line indicates the mean value. (All co-culture assays were performed on cells derived from beagles.)
Discussion
This study has characterized the phenotype and function of canine CD4+ CD25high FOXP3high T cells, providing direct evidence of their suppressive function in vitro. The existence of canine Treg cells has been surmised for several years, initially in studies of radiation chimaeras,47 progressive myelopathy of German shepherd dogs46 and the action of a novel anti-arthritic drug in beagles.45 A population of canine CD4+ T cells with the phenotypic characteristics of Treg cells has been identified using an anti-mouse/rat Foxp3 mAb.48–52 However, direct evidence of regulatory function has remained elusive until now. The current study has documented FOXP3 expression by subpopulations of both CD4+ and CD8+ T cells, though the former predominated; furthermore, we provide indirect evidence for the existence of a peripheral CD4− CD8− FOXP3+ T cell population (Fig. 1a,b,e). The antibody clone used in this and other studies, FJK-16s, has been assumed to cross-react with canine FOXP3,49–52 supported by a pattern of staining resembling that in other species, including negligible reactivity with B cells and neutrophils. Studies have also demonstrated specific staining of cell lines transfected with a construct encoding the canine protein.64 The CD4− CD8− FOXP3+ cells were thought to be T cells, although four-colour staining – currently challenging owing to the limited availability of commercial mAbs in suitable formats – would need to be performed to confirm this notion. Double-negative (DN) Treg cells have been described in both mice67 and humans,68 but in both species they are FOXP3−, prompting the intriguing possibility that canine DN FOXP3+ cells represent a unique regulatory population – although an alternative possibility is that these cells are DN Tcon cells that have up-regulated FOXP3 with activation in vivo. Functional interrogation of these cells would be required to distinguish these possibilities, necessitating five-colour staining with mAbs against CD5, CD4, CD8, CD25 and FOXP3 to confirm that the DN FOXP3+ cells are CD25high, followed by four-colour staining on the basis of the extracellular antigens for the purpose of FACS™ and subsequent in vitro assays. Owing to the limited availability of commercial mAbs in suitable formats and the number of cells required to undertake functional assays, such studies would currently present a number of significant challenges.
An antibody against Helios, a member of the Ikaros transcription factor family that has been associated with Treg-cell ontogeny and function,69–71 has recently been developed, showing reactivity with both the murine and human proteins.66 Helios was able to differentiate naturally occurring from peripherally induced Foxp3+/FOXP3+ Treg cells in both of these species.66 The majority of the FOXP3+ cells identified in PB and LNs in the current study yielded a positive staining reaction with the anti-Helios mAb, suggesting that they were nTreg cells. Although we did not specifically confirm that the anti-Helios mAb cross-reacts with the canine protein, its ability to distinguish Helios in species as phylogenetically distinct as mice and humans suggests that the epitope to which it binds is highly conserved and is therefore likely to be present in the canine molecule.
Interestingly, populations of CD5− FOXP3+ cells were observed in both PB and LNs in the current study. In the dog, CD5 – a type I transmembrane glycoprotein of the scavenger receptor cysteine-rich superfamily72 – is expressed by both T cells73 and, at low levels, natural killer cells;74 in contrast to those of other species, canine B cells of the B1a lineage do not appear to express CD5,75 justifying its use as a pan-T-cell marker in the dog. Indeed, in our hands anti-CD5 mAbs yielded a brighter, more consistent signal than anti-CD3 (data not shown). The expression of FOXP3 by CD5− cells therefore suggested that either there was a sub-population of FOXP3+ T cells lacking CD5 expression or FOXP3 expression occurred in cells other than lymphocytes. Ectopic expression of FOXP3 in non-lymphoid cells has been documented in neoplastic tissue76,77 and under experimental conditions,78,79 but not to our knowledge in the healthy, unmanipulated organism. Further investigations will be required to define the phenotype and function of these cells.
We and others have used the anti-human CD25 mAb clone ACT-1 to detect canine CD25.64,80,81 Recent studies using GL-1 cells transduced with a construct encoding canine CD25 have confirmed that this antibody reacts with the canine protein.64 We found that FOXP3 expression was enriched in the CD25+ population and could be enriched further by gating CD25high cells, in a manner similar to human CD25+ T cells, in which the subpopulation showing the highest CD25 expression is regulatory.82 In contrast to previous studies,64 we were able to detect CD4+ CD25+ T cells in both LNs and PB mononuclear cells examined ex vivo without previous stimulation, but were surprised to find that the majority of FOXP3+ cells were ACT-1-negative. We postulate that the affinity of this mAb for the canine epitope is low, a view supported by a recent study in which a specific anti-canine CD25 mAb was developed in mice.55 A proportion of the ACT-1-negative cells may therefore be CD25+, which would reconcile this apparent anomaly with the observation that the majority of Foxp3/FOXP3+ T cells in both rodents and humans are CD25+.
Stimulation of mononuclear cells derived from peripheral LNs with Con A for up to 120 hr elicited a significant increase in percentage and MFI of FOXP3 expression by both CD4+ and CD8+ T cells (Fig. 2). This phenomenon occurred in the absence of exogenous IL-2 or TGF-β, though the addition of low concentrations of IL-2 augmented CD25 and FOXP3 expression (Fig. 3a). Robust increases in CD25 expression were also observed in a recent study of CD4+ T cells derived from PB stimulated with Con A, yielding parallel increases in FOXP3 expression.64 However, similar experiments performed in an earlier study failed to elicit significant increases in the proportions of FOXP3+ CD4+ T cells without the addition of IL-2 and TGF-β,49 presumably reflecting differences in experimental conditions. Interestingly, in our study removal of the stimulus and continued culture disclosed a FOXP3high population of lymphocytes that was IFN-γ− and predominantly CD4+ (Fig. 2d). Both the high level of FOXP383,84 and the lack of IFN-γ expression – Foxp3 directly represses the Ifng gene85,86 – suggested that this population was regulatory in nature, supported by our subsequent functional studies in vitro (Fig. 3d).
Two alternative, non-mutually exclusive explanations for the increased proportion and absolute numbers of FOXP3+ T cells with polyclonal stimulation were considered – namely, up-regulation of FOXP3 in cells that were originally either FOXP3intermediate or FOXP3−, or proliferation of pre-existing FOXP3+ T cells. The impressive increase in MFI of FOXP3 suggested that up-regulation of this molecule had occurred in individual cells, but parallel proliferation of pre-existing Treg cells could not be excluded. Reasoning that in both mice and humans Helios expression is restricted to nTreg cells and is not induced by stimulation, even in the presence of TGF-β, we explored the expression of Helios in cells that had been stimulated in an identical manner to those for the functional studies. We observed an impressive increase in the number of FOXP3+ Helios+ cells with Con A stimulation, arguing for the proliferation of pre-existing nTreg cells. However, Helios expression was not limited to the FOXP3high population, which we speculated were Treg cells on the basis of their IFN-γ− phenotype in earlier studies (Fig. 2d). Two possible explanations for this observation were considered: either the FOXP3intermediate Helios+ cells were also Treg cells, but in a less activated state – which could reconcile with the observation of a FOXP3intermediate IFN-γ− population in the previous studies (Fig. 2d) – or Helios may not allow such definitive distinction of nTreg cells in the dog as in mice and humans, perhaps being induced alongside FOXP3 in non-regulatory T cells. Further studies are required to confirm the cross-reactivity of the anti-murine/human Helios mAb with the canine protein, which will then allow the distribution and kinetics of Helios expression in this species to be explored in detail, to provide answers to these questions.
Taken together, our results were compatible with a model in which the mechanism of increased FOXP3 expression with stimulation was likely to be a combination of (i) up-regulation and recruitment of Tcon cells into a FOXP3+, but not necessarily regulatory, T-cell pool, in a similar manner to the behaviour of human Tcon cells, and (ii) proliferation of pre-existing Treg cells. Whether the CD4+ FOXP3high T cells represented activated nTreg cells or a more heterogeneous population, perhaps including contributions from Tcon cells that had undergone conversion to iTreg cells in vitro, remained unclear. However, notwithstanding the uncertainties of Helios expression by activated T cells in the dog, iTreg cells were unlikely to be a significant component of this FOXP3high population because the majority of comparable studies of activated human Tcon cells have failed to generate bona fide iTreg cells in vitro.87–93
Further phenotypic analysis by means of RT-qPCR (Fig. 3c), coupled with co-culture assays in vitro (Fig. 3d), suggested that expression of FOXP3 was pivotal to the suppressive phenomenon we observed. Transcripts encoding a number of pro-inflammatory cytokines were all less abundant in the CD25high versus CD25− cells, whereas the expression of IL-10 mRNA was variable, with a mean GED ratio of > 1 at the point of FACS™ but < 1 at the point of admixture of the cells for co-culture assays; similarly, the GED ratio for TGF-β was also < 1 at the point of cellular admixture, providing no support for a significant role of either of these cytokines in the regulatory function of these cells in vitro. Proportional suppression of up to ∼ 85% was observed when the CD25high cells were co-cultured with responder CD4+ T cells at a ratio of 1 : 1, but the actual ratio of CD4+ CD25high FOXP3high T cells (putative Treg cells) to Tcon cells was likely to be ∼ 1 : 6, arguing for the potency of suppressor–effector function of these cells in vitro – at least as high as that of similar assays of human Treg cells.94,95 Cells originating from both the PB and LNs were regulatory in nature, suggesting the presence of Treg cells in both of these compartments of the canine peripheral immune system. Further studies will be required to explore the mechanistic basis of suppression mediated by canine CD4+ CD25high FOXP3high Treg cells, but this work and a recently published paper using a canine-specific anti-CD25 mAb55 provide the first direct evidence of Treg cells in dogs and represent an important advance in this field. Given the exciting immunotherapeutic potential of manipulating Treg-cell function in the context of infectious disease, autoimmune disorders, cancer and allotransplantation,96,97 studies of these cells in the dog have never been more timely.
Acknowledgments
O.A.G. gratefully acknowledges funding in his laboratory for work on canine regulatory T cells from the Biotechnology and Biological Sciences Research Council and Novartis Animal Health. We thank Dr John E. Peel for insightful discussions during the course of this work, Dr Iain Peters and Mr Daniel Lowther for practical tips on RT-qPCR, Drs Ayad Eddaoudi and Philip Hexley for help with FACS™, and Professors Julian Dyson and Dirk Werling for help with tritiated thymidine assays.
Glossary
Abbreviations:
- Con A
concanavalin A
- DN
double-negative (CD4− CD8−)
- GED
Gene Expression Ct (threshold cycle) Difference
- IFN
interferon
- IL
interleukin
- iTreg
induced regulatory T
- LN
lymph node
- mAb
monoclonal antibody
- MFI
median fluorescence intensity
- nTreg
naturally occurring regulatory T
- mAb
monoclonal antibody
- PB
peripheral blood
- PMA
phorbol myristate acetate
- Tcon
conventional T
- TGF
transforming growth factor
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Wing K, Yamaguchi T. Dynamics of peripheral tolerance and immune regulation mediated by Treg. Eur J Immunol. 2009;39:2331–6. doi: 10.1002/eji.200939688. [DOI] [PubMed] [Google Scholar]
- 3.Vila J, Isaacs JD, Anderson AE. Regulatory T cells and autoimmunity. Curr Opin Hematol. 2009;16:274–9. doi: 10.1097/MOH.0b013e32832a9a01. [DOI] [PubMed] [Google Scholar]
- 4.Piccirillo CA. Regulatory T cells in health and disease. Cytokine. 2008;43:395–401. doi: 10.1016/j.cyto.2008.07.469. [DOI] [PubMed] [Google Scholar]
- 5.Costantino CM, Baecher-Allan CM, Hafler DA. Human regulatory T cells and autoimmunity. Eur J Immunol. 2008;38:921–4. doi: 10.1002/eji.200738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–46. doi: 10.1016/j.jaci.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 7.Ryanna K, Stratigou V, Safinia N, Hawrylowicz C. Regulatory T cells in bronchial asthma. Allergy. 2009;64:335–47. doi: 10.1111/j.1398-9995.2009.01972.x. [DOI] [PubMed] [Google Scholar]
- 8.Nouri-Aria KT, Durham SR. Regulatory T cells and allergic disease. Inflamm Allergy Drug Targets. 2008;7:237–52. doi: 10.2174/187152808786848405. [DOI] [PubMed] [Google Scholar]
- 9.Feng G, Chan T, Wood KJ, Bushell A. Donor reactive regulatory T cells. Curr Opin Organ Transplant. 2009;14:432–8. doi: 10.1097/MOT.0b013e32832c58f1. [DOI] [PubMed] [Google Scholar]
- 10.Cobbold SP. Regulatory T cells and transplantation tolerance. J Nephrol. 2008;21:485–96. [PubMed] [Google Scholar]
- 11.Gokmen MR, Lombardi G, Lechler RI. The importance of the indirect pathway of allorecognition in clinical transplantation. Curr Opin Immunol. 2008;20:568–74. doi: 10.1016/j.coi.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Majlessi L, Lo-Man R, Leclerc C. Regulatory B and T cells in infections. Microbes Infect. 2008;10:1030–5. doi: 10.1016/j.micinf.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Robertson SJ, Hasenkrug KJ. The role of virus-induced regulatory T cells in immunopathology. Springer Semin Immunopathol. 2006;28:51–62. doi: 10.1007/s00281-006-0019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–60. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 15.Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat Rev Immunol. 2004;4:841–55. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- 16.Vahlenkamp TW, Tompkins MB, Tompkins WA. Feline immunodeficiency virus infection phenotypically and functionally activates immunosuppressive CD4+ CD25+ T regulatory cells. J Immunol. 2004;172:4752–61. doi: 10.4049/jimmunol.172.8.4752. [DOI] [PubMed] [Google Scholar]
- 17.Joshi A, Vahlenkamp TW, Garg H, Tompkins WA, Tompkins MB. Preferential replication of FIV in activated CD4+ CD25+T cells independent of cellular proliferation. Virology. 2004;321:307–22. doi: 10.1016/j.virol.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 18.Joshi A, Garg H, Tompkins MB, Tompkins WA. Different thresholds of T cell activation regulate FIV infection of CD4+ CD25+ and CD4+ CD25− cells. Virology. 2005;335:212–21. doi: 10.1016/j.virol.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Mexas AM, Fogle JE, Tompkins WA, Tompkins MB. CD4+ CD25+ regulatory T cells are infected and activated during acute FIV infection. Vet Immunol Immunopathol. 2008;126:263–72. doi: 10.1016/j.vetimm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wongyanin P, Buranapraditkun S, Chokeshai-Usaha K, Thanawonguwech R, Suradhat S. Induction of inducible CD4+ CD25+ Foxp3+ regulatory T lymphocytes by porcine reproductive and respiratory syndrome virus (PRRSV) Vet Immunol Immunopathol. 2009;133:170–82. doi: 10.1016/j.vetimm.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Silva-Campa E, Flores-Mendoza L, Resendiz M, Pinelli-Saavedra A, Mata-Haro V, Mwangi W, Hernandez J. Induction of T helper 3 regulatory cells by dendritic cells infected with porcine reproductive and respiratory syndrome virus. Virology. 2009;387:373–9. doi: 10.1016/j.virol.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Mills KH. Designer adjuvants for enhancing the efficacy of infectious disease and cancer vaccines based on suppression of regulatory T cell induction. Immunol Lett. 2009;122:108–11. doi: 10.1016/j.imlet.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Joosten SA, Ottenhoff TH. Human CD4 and CD8 regulatory T cells in infectious diseases and vaccination. Hum Immunol. 2008;69:760–70. doi: 10.1016/j.humimm.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10:689–95. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 25.Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells. Cell Mol Life Sci. 2009;66:2603–22. doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003;171:6323–7. doi: 10.4049/jimmunol.171.12.6323. [DOI] [PubMed] [Google Scholar]
- 27.Stassen M, Schmitt E, Jonuleit H. Human CD4+ CD25+ regulatory T cells and infectious tolerance. Transplantation. 2004;77(1 Suppl.):S23–5. doi: 10.1097/00007890-200401151-00009. [DOI] [PubMed] [Google Scholar]
- 28.Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25− subpopulations. J Immunol. 2000;165:3105–10. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 29.Holm TL, Lundsgaard D, Markholst H. Characteristics of rat CD4+ CD25+ T cells and their ability to prevent not only diabetes but also insulitis in an adoptive transfer model in BB rats. Scand J Immunol. 2006;64:17–29. doi: 10.1111/j.1365-3083.2006.01760.x. [DOI] [PubMed] [Google Scholar]
- 30.Porter CM, Horvath-Arcidiacono JA, Singh AK, Horvath KA, Bloom ET, Mohiuddin MM. Characterization and expansion of baboon CD4+ CD25+ Treg cells for potential use in a non-human primate xenotransplantation model. Xenotransplantation. 2007;14:298–308. doi: 10.1111/j.1399-3089.2007.00416.x. [DOI] [PubMed] [Google Scholar]
- 31.Singh AK, Horvath KA, Mohiuddin MM. Rapamycin promotes the enrichment of CD4+ CD25hi FoxP3+ T regulatory cells from naive CD4+ T cells of baboon that suppress antiporcine xenogenic response in vitro. Transplant Proc. 2009;41:418–21. doi: 10.1016/j.transproceed.2008.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartigan-O’connor DJ, Abel K, Mccune JM. Suppression of SIV-specific CD4+ T cells by infant but not adult macaque regulatory T cells: implications for SIV disease progression. J Exp Med. 2007;204:2679–92. doi: 10.1084/jem.20071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manigold T, Shin EC, Mizukoshi E, Mihalik K, Murthy KK, Rice CM, Piccirillo CA, Rehermann B. Foxp3+ CD4+ CD25+ T cells control virus-specific memory T cells in chimpanzees that recovered from hepatitis C. Blood. 2006;107:4424–32. doi: 10.1182/blood-2005-09-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petty CS, Tompkins MB, Tompkins WA. Transforming growth factor-beta/transforming growth factor-betaRII signaling may regulate CD4+ CD25+ T-regulatory cell homeostasis and suppressor function in feline AIDS lentivirus infection. J Acquir Immune Defic Syndr. 2008;47:148–60. doi: 10.1097/QAI.0b013e318160df70. [DOI] [PubMed] [Google Scholar]
- 35.Lankford S, Petty C, Lavoy A, Reckling S, Tompkins W, Dean GA. Cloning of feline FOXP3 and detection of expression in CD4+ CD25+ regulatory T cells. Vet Immunol Immunopathol. 2008;122:159–66. doi: 10.1016/j.vetimm.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ierino FL, Yamada K, Hatch T, Rembert J, Sachs DH. Peripheral tolerance to class I mismatched renal allografts in miniature swine: donor antigen-activated peripheral blood lymphocytes from tolerant swine inhibit antidonor CTL reactivity. J Immunol. 1999;162:550–9. [PubMed] [Google Scholar]
- 37.Kaser T, Gerner W, Hammer SE, Patzl M, Saalmuller A. Phenotypic and functional characterisation of porcine CD4+ CD25high regulatory T cells. Vet Immunol Immunopathol. 2008;122:153–8. doi: 10.1016/j.vetimm.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Pilon C, Meurens F, Dauba A, Salmon H, Velge-Roussel F, Lebranchu Y, Baron C. Induction of porcine regulatory cells by mycophenolic acid-treated dendritic cells. Transplant Proc. 2009;41:700–2. doi: 10.1016/j.transproceed.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Shafer-Weaver KA, Sordillo LM. Bovine CD8+ suppressor lymphocytes alter immune responsiveness during the postpartum period. Vet Immunol Immunopathol. 1997;56:53–64. doi: 10.1016/s0165-2427(96)05725-x. [DOI] [PubMed] [Google Scholar]
- 40.Smith WG, Usinger WR, Splitter GA. Bovine con A-induced suppressor cells: generation, macrophage requirements and possible mechanisms of regulatory action. Immunology. 1981;43:91–100. [PMC free article] [PubMed] [Google Scholar]
- 41.de Almeida DE, Colvin CJ, Coussens PM. Antigen-specific regulatory T cells in bovine paratuberculosis. Vet Immunol Immunopathol. 2008;125:234–45. doi: 10.1016/j.vetimm.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Ellis JA, Demartini JC. Ovine concanavalin A-induced suppressor cells: generation, assay, age-related effects and re-evaluation of mechanism of suppression. Immunology. 1985;54:353–62. [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X, Shelton J, Mccullagh P. Suppression of anti-thyrocyte autoreactivity by the lymphocytes of normal fetal lambs. J Autoimmun. 1995;8:539–59. doi: 10.1016/0896-8411(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 44.Wagner B, Hillegas JM, Brinker DR, Horohov DW, Antczak DF. Characterization of monoclonal antibodies to equine interleukin-10 and detection of T regulatory 1 cells in horses. Vet Immunol Immunopathol. 2008;122:57–64. doi: 10.1016/j.vetimm.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan JM, Badger AM, Ruggieri EV, Swift BA, Bugelski PJ. Effects of SK&F 105685, a novel anti-arthritic agent, on immune function in the dog. Int J Immunopharmacol. 1993;15:113–23. doi: 10.1016/0192-0561(93)90087-f. [DOI] [PubMed] [Google Scholar]
- 46.Waxman FJ, Clemmons RM, Hinrichs DJ. Progressive myelopathy in older German shepherd dogs II. Presence of circulating suppressor cells. J Immunol. 1980;124:1216–22. [PubMed] [Google Scholar]
- 47.Weiden PL, Storb R, Tsoi MS, Graham TC, Lerner KG, Thomas ED. Infusion of donor lymphocytes into stable canine radiation chimeras: implications for mechanism of transplantation tolerance. J Immunol. 1976;116:1212–9. [PubMed] [Google Scholar]
- 48.Sacchini F, Pinheiro DY, Singh Y, Tisato V, Armitage-Chan E, Cunningham F, Garden OA. Characterising regulatory T cells in healthy dogs: preliminary studies. Immunology. 2007;120(Suppl. 1):11. [Google Scholar]
- 49.Biller BJ, Elmslie RE, Burnett RC, Avery AC, Dow SW. Use of FoxP3 expression to identify regulatory T cells in healthy dogs and dogs with cancer. Vet Immunol Immunopathol. 2007;116:69–78. doi: 10.1016/j.vetimm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Keppel KE, Campbell KL, Zuckermann FA, Greeley EA, Schaeffer DJ, Husmann RJ. Quantitation of canine regulatory T cell populations, serum interleukin-10 and allergen-specific IgE concentrations in healthy control dogs and canine atopic dermatitis patients receiving allergen-specific immunotherapy. Vet Immunol Immunopathol. 2008;123:337–44. doi: 10.1016/j.vetimm.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 51.O’Neill K, Guth A, Biller B, Elmslie R, Dow S. Changes in regulatory T cells in dogs with cancer and associations with tumor type. J Vet Intern Med. 2009;23:875–81. doi: 10.1111/j.1939-1676.2009.0333.x. [DOI] [PubMed] [Google Scholar]
- 52.Horiuchi Y, Tominaga M, Ichikawa M, et al. Increase of regulatory T cells in the peripheral blood of dogs with metastatic tumors. Microbiol Immunol. 2009;53:468–74. doi: 10.1111/j.1348-0421.2009.00144.x. [DOI] [PubMed] [Google Scholar]
- 53.Tominaga M, Horiuchi Y, Ichikawa M, Yamashita M, Okano K, Jikumaru Y, Nariai Y, Kadosawa T. Flow cytometric analysis of peripheral blood and tumor-infiltrating regulatory T cells in dogs with oral malignant melanoma. J Vet Diagn Invest. 2010;22:438–41. doi: 10.1177/104063871002200317. [DOI] [PubMed] [Google Scholar]
- 54.Horiuchi Y, Tominaga M, Ichikawa M, et al. Relationship between regulatory and type 1 T cells in dogs with oral malignant melanoma. Microbiol Immunol. 2010;54:152–9. doi: 10.1111/j.1348-0421.2009.00194.x. [DOI] [PubMed] [Google Scholar]
- 55.Abrams VK, Hwang B, Lesnikova M, Gass MJ, Wayner E, Castilla-Llorente C, Georges GE, Nash RA. A novel monoclonal antibody specific for canine CD25 (P4A10): selection and evaluation of canine Tregs. Vet Immunol Immunopathol. 2009;135:257–65. doi: 10.1016/j.vetimm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gordon I, Paoloni M, Mazcko C, Khanna C. The Comparative Oncology Trials Consortium: using spontaneously occurring cancers in dogs to inform the cancer drug development pathway. PLoS Med. 2009;6:e1000161. doi: 10.1371/journal.pmed.1000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paoloni M, Davis S, Lana S, et al. Canine tumor cross-species genomics uncovers targets linked to osteosarcoma progression. BMC Genomics. 2009;10:625. doi: 10.1186/1471-2164-10-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–56. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 59.Wilbe M, Jokinen P, Hermanrud C, Kennedy LJ, Strandberg E, Hansson-Hamlin H, Lohi H, Andersson G. MHC class II polymorphism is associated with a canine SLE-related disease complex. Immunogenetics. 2009;61:557–64. doi: 10.1007/s00251-009-0387-6. [DOI] [PubMed] [Google Scholar]
- 60.Wilbe M, Jokinen P, Truve K, et al. Genome-wide association mapping identifies multiple loci for a canine SLE-related disease complex. Nat Genet. 2010;42:250–4. doi: 10.1038/ng.525. [DOI] [PubMed] [Google Scholar]
- 61.Bauer TR, Jr, Adler RL, Hickstein DD. Potential large animal models for gene therapy of human genetic diseases of immune and blood cell systems. ILAR J. 2009;50:168–86. doi: 10.1093/ilar.50.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strasser A, Kalmar E, Niedermuller H. A simple method for the simultaneous separation of peripheral blood mononuclear and polymorphonuclear cells in the dog. Vet Immunol Immunopathol. 1998;62:29–35. doi: 10.1016/s0165-2427(97)00150-5. [DOI] [PubMed] [Google Scholar]
- 63.Openshaw P, Murphy EE, Hosken NA, Maino V, Davis K, Murphy K, O’garra A. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J Exp Med. 1995;182:1357–67. doi: 10.1084/jem.182.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mizuno T, Suzuki R, Umeki S, Okuda M. Crossreactivity of antibodies to canine CD25 and Foxp3 and identification of canine CD4+ CD25+ Foxp3+ cells in canine peripheral blood. J Vet Med Sci. 2009;71:1561–8. doi: 10.1292/jvms.001561. [DOI] [PubMed] [Google Scholar]
- 65.Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel ‘gene expression’s CT difference’ formula. J Mol Med. 2006;84:901–10. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- 66.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–41. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang D, Yang W, Degauque N, Tian Y, Mikita A, Zheng XX. New differentiation pathway for double-negative regulatory T cells that regulates the magnitude of immune responses. Blood. 2007;109:4071–9. doi: 10.1182/blood-2006-10-050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mciver Z, Serio B, Dunbar A, et al. Double-negative regulatory T cells induce allotolerance when expanded after allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2008;141:170–8. doi: 10.1111/j.1365-2141.2008.07021.x. [DOI] [PubMed] [Google Scholar]
- 69.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 70.Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T, Sakaguchi S. Foxp3-dependent and -independent molecules specific for CD25+ CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18:1197–209. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- 71.Getnet D, Grosso JF, Goldberg MV, et al. A role for the transcription factor Helios in human CD4+ CD25+ regulatory T cells. Mol Immunol. 2010;47:1595–600. doi: 10.1016/j.molimm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dalloul A. CD5: a safeguard against autoimmunity and a shield for cancer cells. Autoimmun Rev. 2009;8:349–53. doi: 10.1016/j.autrev.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 73.Byrne KM, Kim HW, Chew BP, Reinhart GA, Hayek MG. A standardized gating technique for the generation of flow cytometry data for normal canine and normal feline blood lymphocytes. Vet Immunol Immunopathol. 2000;73:167–82. doi: 10.1016/s0165-2427(99)00163-4. [DOI] [PubMed] [Google Scholar]
- 74.Huang YC, Hung SW, Jan TR, Liao KW, Cheng CH, Wang YS, Chu RM. CD5-low expression lymphocytes in canine peripheral blood show characteristics of natural killer cells. J Leukoc Biol. 2008;84:1501–10. doi: 10.1189/jlb.0408255. [DOI] [PubMed] [Google Scholar]
- 75.Cobbold S, Metcalfe S. Monoclonal antibodies that define canine homologues of human CD antigens: summary of the First International Canine Leukocyte Antigen Workshop (CLAW) Tissue Antigens. 1994;43:137–54. doi: 10.1111/j.1399-0039.1994.tb02315.x. [DOI] [PubMed] [Google Scholar]
- 76.Hinz S, Pagerols-Raluy L, Oberg HH, et al. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res. 2007;67:8344–50. doi: 10.1158/0008-5472.CAN-06-3304. [DOI] [PubMed] [Google Scholar]
- 77.Ebert LM, Tan BS, Browning J, et al. The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res. 2008;68:3001–9. doi: 10.1158/0008-5472.CAN-07-5664. [DOI] [PubMed] [Google Scholar]
- 78.Lipscomb MW, Taylor JL, Goldbach CJ, Watkins SC, Wesa AK, Storkus WJ. DC expressing transgene Foxp3 are regulatory APC. Eur J Immunol. 2010;40:480–93. doi: 10.1002/eji.200939667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Munn DH. Lineage-specific transcription factors in unexpected places. Eur J Immunol. 2010;40:315–7. doi: 10.1002/eji.200940238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masuda K, Yasuda N. The antibody against human CD25, ACT-1, recognizes canine T-lymphocytes in the G2/M and G0/G1 phases of the cell cycle during proliferation. J Vet Med Sci. 2008;70:1285–7. doi: 10.1292/jvms.70.1285. [DOI] [PubMed] [Google Scholar]
- 81.Rissetto KC, Rindt H, Selting KA, Villamil JA, Henry CJ, Reinero CR. Cloning and expression of canine CD25 for validation of an anti-human CD25 antibody to compare T regulatory lymphocytes in healthy dogs and dogs with osteosarcoma. Vet Immunol Immunopathol. 2010;135:137–45. doi: 10.1016/j.vetimm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+ CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 83.Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182:148–53. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guo L, Tian J, Marinova E, Zheng B, Han S. Inhibition of clonal expansion by Foxp3 expression as a mechanism of controlled T-cell responses and autoimmune disease. Eur J Immunol. 2010;40:71–80. doi: 10.1002/eji.200939404. [DOI] [PubMed] [Google Scholar]
- 85.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–40. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 86.Ono M, Yaguchi H, Ohkura N, et al. Foxp3 controls regulatory T-cell function by interacting with AML1/Runx1. Nature. 2007;446:685–9. doi: 10.1038/nature05673. [DOI] [PubMed] [Google Scholar]
- 87.Morgan ME, van Bilsen JH, Bakker AM, et al. Expression of FOXP3 mRNA is not confined to CD4+ CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 88.Roncador G, Brown PJ, Maestre L, et al. Analysis of FOXP3 protein expression in human CD4+ CD25+ regulatory T cells at the single-cell level. Eur J Immunol. 2005;35:1681–91. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 89.Gavin MA, Torgerson TR, Houston E, et al. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci USA. 2006;103:6659–64. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–38. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 91.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 92.Kmieciak M, Gowda M, Graham L, Godder K, Bear HD, Marincola FM, Manjili MH. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory/suppressor function. J Transl Med. 2009;7:89. doi: 10.1186/1479-5876-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+ FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–90. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yates J, Rovis F, Mitchell P, et al. The maintenance of human CD4+ CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro. Int Immunol. 2007;19:785–99. doi: 10.1093/intimm/dxm047. [DOI] [PubMed] [Google Scholar]
- 95.Di Ianni M, Del Papa B, Cecchini D, et al. Immunomagnetic isolation of CD4+ CD25+ FoxP3+ natural T regulatory lymphocytes for clinical applications. Clin Exp Immunol. 2009;156:246–53. doi: 10.1111/j.1365-2249.2009.03901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–65. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miyara M, Wing K, Sakaguchi S. Therapeutic approaches to allergy and autoimmunity based on FoxP3+ regulatory T-cell activation and expansion. J Allergy Clin Immunol. 2009;123:749–55. doi: 10.1016/j.jaci.2009.03.001. quiz 56–7. [DOI] [PubMed] [Google Scholar]