Abstract
Tumour pathogenesis is characterized by an immunosuppressive microenvironment that limits the development of effective tumour-specific immune responses. This is in part the result of tumour-dependent recruitment and activation of regulatory cells, such as myeloid-derived suppressor cells and regulatory T cells in the tumour microenvironment and draining lymph nodes. Shedding of gangliosides by tumour cells has immunomodulatory properties, suggesting that gangliosides may be a critical factor in initiating an immunosuppressive microenvironment. To better define the immunomodulatory properties of gangliosides on antigen-specific T-cell activation and development we have developed an in vitro system using ganglioside-treated murine bone-marrow-derived dendritic cells to prime and activate antigen-specific CD4+ T cells from AND T-cell receptor transgenic mice. Using this system, ganglioside treatment promotes the development of a dendritic cell population characterized by decreased CD86 (B7-2) expression, and decreased interleukin-12 and interleukin-6 production. When these cells are used as antigen-presenting cells, CD4 T cells are primed to proliferate normally, but have a defect in T helper (Th) effector cell development. This defect in Th effector cell responses is associated with the development of regulatory T-cell activity that can suppress the activation of previously primed Th effector cells in a contact-dependent manner. In total, these data suggest that ganglioside-exposed dendritic cells promote regulatory T-cell activity that may have long-lasting effects on the development of tumour-specific immune responses.
Keywords: dendritic cells, gangliosides, T helper effector cells, tolerance/suppression
Introduction
Gangliosides are sialic-acid-containing glycosphingolipids located in the outer leaflet of the plasma membrane of eukaryotic cells. Tumour cells, particularly tumours of neuroectodermal cell origin, express high levels of gangliosides and shed them in the microenvironment in vitro and in vivo.1–3 Clinically, increased levels of tumour-derived circulating gangliosides correlate with disease status and are predictive of more rapid disease progression in patients with advanced stage neuroblastoma, suggesting that gangliosides may play a role in tumour development and progression.4,5 In support of this hypothesis, tumour-derived gangliosides have been shown to modulate growth factor signalling of normal cells in the tumour microenvironment such as fibroblasts and vascular endothelial cells that may promote tumour growth and metastasis.6,7 Also, gangliosides may promote tumour development by suppressing immune cell function and promoting immune evasion mechanisms.1,8–10
The mechanisms by which gangliosides suppress tumour immunity, although incompletely understood, probably involve regulation of multiple immune cells. For example, direct T-cell exposure to exogenous gangliosides has been shown to blunt interleukin-2 (IL-2) -dependent proliferation,11,12 to promote an increased susceptibility to apoptotic cell death,13,14 as well as immune deviation toward the development of T helper type 2 (Th2) effector cells.15,16 In addition to acting directly on T lymphocytes, gangliosides may also regulate the development of tumour immunity by the inhibition of antigen-presenting cell function,8,10,17 and affecting immunological activation and priming in vivo.18,19
In our current studies, we have focused on the effect of ganglioside exposure of antigen-presenting cells (APC) found in the tumour microenvironment. Exposed to tumour antigens and shed tumour gangliosides in the tumour itself, APC such as dendritic cells (DC) can migrate to tumour-draining lymph nodes where they have initial contact with T cells. Here, they interact with naive T cells through antigen-specific recognition of peptide–MHC complexes and co-stimulatory molecules on their surface. Once this interaction takes place, naive CD4+ T cells become activated, proliferate and can differentiate into a variety of effector cell subsets, depending on the signals they receive from the APC and the microenvironment.20 Activation and differentiation of CD4+ T cells are critically dependent upon, and regulated by, the initial interaction with DC, which is consistent with the hypothesis that a major effect of gangliosides on T-cell-mediated tumour immunity is the result of the previous exposure of APC to gangliosides shed by tumour cells into the tumour microenvironment.
To address the role of gangliosides in regulating dendritic cell function and subsequent T-cell activation and effector cell development, we used a well-characterized murine model system that can assess primary CD4 T-cell activation and development. In this system purified CD4 T cells obtained from AND T-cell receptor transgenic mice [in which CD4+ T cells express a T-cell receptor specific for the C terminus of Moth Cytochrome c (pMCC) peptide in the context of I-Ek]21 were stimulated with bone-marrow-derived DC (BMDC) pre-treated with GD1a ganglioside. Similar to our previous observations using human monocytes and DC,8,17,22 we show that ganglioside treatment of murine BMDC reduced lipopolysaccharide (LPS) -dependent up-regulation of CD80/86 and reduced IL-12 production. Importantly, priming CD4 T cells with ganglioside-treated DC also resulted in decreased development of Th1 effector cell function upon re-stimulation, when compared with priming with control DC. However, this defect in Th1 differentiation was not fully corrected upon the addition of exogenous IL-12 or agonistic antibody to CD28, suggesting that other factors may be involved in down-regulating T-cell effector function. In cell co-culture experiments we found that pre-treatment of BMDC with gangliosides promoted the development of regulatory T-cell activity that could suppress T-cell effector function of already differentiated effector T cells. These data suggest that ganglioside exposure of DC in the tumour microenvironment may have long-lasting effects on the development and function of tumour antigen-reactive T cells both within and outside the tumour microenvironment.
Materials and methods
Mice
The AND T-cell receptor-transgenic mice in which CD4+ T cells express a T-cell receptor specific for the C terminus of pMCC peptide in the context of I-Ek have been previously described,21 and are maintained in our breeding colony as heterozygotes on a C57BL/6 background. B10.Br mice were used as a source of BMDC for priming naive cell activation, and T-cell-depleted spleen cells were used as APC to re-stimulate previously primed effector cells. All procedures involving these animals were approved by an institutional animal review committee.
Ganglioside, cytokines and other reagents
Highly purified GD1a and GM1 gangliosides were purchased from Matreya (Pleasant Gap, PA). Gangliosides were dissolved in EHAA (Click’s) medium (Invitrogen, Carlsbad, CA) without fetal bovine serum and resuspended by mild bath sonication. Recombinant murine granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-2 and IL-12 were obtained from BD Biosciences (San Diego, CA), Roche Diagnostics (Indianapolis, IN) and Peprotech (Rocky Hill, NJ), respectively. Anti-transforming growth factor-β was purchased from R&D (Minneapolis, MN) and anti-IL-10R was provided by Nancy Noben-Trauth (George Washington University). All fluorochrome-conjugated antibodies were purchased from BD Biosciences or eBiosciences. Intracellular cytokine fixation and permeabilization reagents were obtained from Invitrogen/Caltag (Burlingame, CA).
In vitro isolation, activation, and ganglioside treatment of BMDC
Tibias and femurs from B10.Br mice were dissected aseptically, washed first in PBS, twice in ethanol, and finally in EHAA (Click’s) medium supplemented with 10% fetal calf serum (FCS) and 2-mercaptoethanol. Bone marrow cell suspensions were prepared by flushing the inside of the bones with a syringe needle in solution containing Click’s medium containing 10% FCS and dispersed through a 100-μm mesh filter to create a single cell suspension. Viable mononuclear cells were isolated using a Ficoll–Hypaque gradient. Cells (106 cells/ml) were cultured in Clicks medium with 10% FCS and GM-CSF (20 ng/ml) for 3 days, and then non-adherent cells were removed. Fresh medium was added and the remaining, adherent, cells were cultured for an additional 2 days with GM-CSF. After the total culture period of 5 days, BMDC were harvested in PBS/5 mm EDTA, washed and re-suspended in Click’s medium supplemented with 1% FCS with or without the added GD1a ganglioside. After overnight culture the cells were further stimulated with LPS (200 ng/ml) for an additional 24 hr in the continued presence of GD1a.
Purification of CD4+ T cells and APC
CD4+ T cells from lymph nodes and spleens of B6 AND transgenic mice were isolated by immunomagnetic negative selection, using antibodies against CD8, CD32/CD16, B220 and MHC class II, followed by incubation with anti-mouse and anti-rat immunoglobulin-coated magnetic beads as previously described.23 Purity of the recovered Vα11+ CD4+ T cells as determined by staining with anti-CD4 and anti-Vα11 monoclonal antibody is usually 85–95%. T-cell-depleted spleen cells for use as APC were prepared by antibody-mediated complement lysis from total splenocytes as described elsewhere.24 The APC were treated with 50 μg/ml mitomycin C (Sigma, St Louis, MO) and washed extensively before use.
CD4+ T-cell activation and priming
AND CD4+ T cells (5 × 105 cells/ml) were stimulated with GD1a or non-GD1a pre-treated LPS-stimulated BMDC (5 × 104 cells/ml) in the presence of pMCC (5 μg/ml) [agonist peptide of Moth cytochrome c (81–103), pMCC = VFAGLKKANERADLIAYLKQATK] and recombinant IL-2 (25 IU/ml). After 5 days of primary stimulation, primed T cells were harvested, counted and equal numbers were re-stimulated with mitomycin C-treated T-cell-depleted splenocytes (1 × 106 cells/ml) and pMCC (5 μg/ml) and analysed for cytokine production by ELISA and by intracellular staining. For analysis of cell proliferation after T-cell priming, T cells were loaded with 0·5 μm carboxyfluorescein succinimidyl ester (CFSE) and stimulated as above except in the absence of exogenous IL-2.
Intracellular cytokine analysis
Intracellular cytokine analysis was performed following 12–16 hr of stimulation with agonist peptide and the addition of monensin (Golgistop, BD Biosciences) for the final 4–6 hr of culture. Cells were harvested and surface labelled with anti-CD4 before fixation and permeabilization, and then labelled with antibodies to interferon-γ (IFN-γ; BD Biosciences).
ELISA
Cytokine concentrations in cell culture supernatants were determined by ELISA reagents specific for IL-2, IL-4, IFN-γ, IL-12, IL-6, IL-10 (from R&D Systems; Pierce-Endogen, Cambridge, MA; and eBioscience). Supernatants were diluted serially in duplicate, and the average concentration of cytokine (and standard deviation) of two to four replicate dilutions was determined in relation to a reference standard supplied by the manufacturer.
Statistics
Values of P were determined using a t-test comparing average cytokine concentrations from ganglioside-treated cells with those from control cells as indicated.
Results
Ganglioside exposure of BMDC inhibits LPS-dependent up-regulation of co-stimulatory molecules and cytokine production
Previous studies showed that pre-treatment with gangliosides inhibits LPS-mediated up-regulation of co-stimulatory molecules and cytokine production by human peripheral blood monocytes and blood-derived DC,17,22,25 and tumour gangliosides have also been shown to inhibit DC generation and function.10 In the current study, we extended these findings using an antigen-specific mouse model and murine BMDC. As shown in Fig. 1(a), treatment of BMDC with the ganglioside GD1a inhibits LPS-dependent up-regulation of B7-1 and B7-2 (with B7-2 more affected than B7-1), while the LPS-dependent increase in MHC class II expression is not affected. As a control, CD45 expression was also unchanged upon GD1a treatment. In addition to affecting co-stimulatory molecule expression, GD1a treatment also suppressed LPS-dependent secretion of the pro-inflammatory cytokines IL-12 and IL-6 by BMDC and LPS-induced IL-10 production by BMDC was also suppressed by GD1a exposure (Fig. 1b,c). Ganglioside treatment alone had no effect on cytokine production compared with untreated BMDC. As with GD1a, pre-treatment of BMDC with GM1 ganglioside also inhibited IL-12 production25 (Fig. 1c). The inhibitory effect of ganglioside treatment could be partially overcome by increasing the concentration of LPS, indicating that the ganglioside effects on the BMDC were not non-specifically toxic (Fig. 1c). In these experiments a dose of 50 μm of ganglioside was used, which has been shown to be optimal in previous experiments,25 and does not induce a decrease in the viability of treated DC compared with control DC, as determined by trypan blue exclusion (data not shown).
Figure 1.
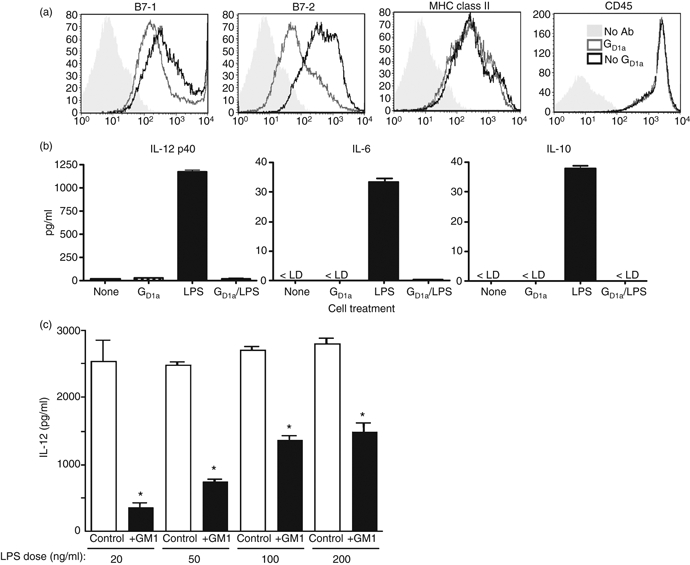
Pre-treatment of bone-marrow-derived dendritic cells (BMDC) inhibits lipopolysaccharide (LPS) -induced co-stimulatory molecule expression and cytokine production. Bone marrow cells grown in the presence of granulocyte–macrophage colony-stimulating factor (GM-CSF) for 5 days were harvested and treated with 50 μm GD1a in EHAA supplemented with 1% fetal calf serum. (a) After 24 hr the cells were stimulated with 200 ng/ml lipopolysaccharide (LPS) and then cells were harvested and protein expression on CD11c+ cells was analysed by flow cytometry. (b) Cells were prepared as above and supernatants from the BMDC treated with or without GD1a and LPS as indicated were analysed by ELISA. (c) BMDC prepared as above were treated with 50 μm GM1 for 24 hr and then exposed to various doses of LPS as indicated for an additional 24 hr. Supernatants were collected and analysed by ELISA. The ELISA data are an average (+ SD) obtained from two to four wells from two independent two-fold serial dilutions for each sample. *P < 0·05 for all groups compared with control. The results are representative of three independent experiments.
T-cell differentiation into Th effector cells is inhibited upon priming with ganglioside-treated DC
So as to determine the effect of ganglioside exposure on the ability of BMDC to prime naive T cells and induce the development of T-cell effector function, purified CD4+ AND T-cell receptor transgenic T cells were stimulated with an agonist peptide from pMCC presented by ganglioside-treated or untreated control BMDC in the presence of exogenous IL-2. After 4–5 days the T cells were harvested and equal numbers of cells were re-stimulated with peptide, presented by normal control T-cell-depleted spleen cells, and then cytokine production was assessed. In these experiments, T cells primed with BMDC pre-treated with ganglioside produced significantly less IFN-γ and IL-2 upon secondary stimulation than did T cells primed with untreated control BMDC, indicating a defect in Th1 effector cell development (Fig. 2a). The extent of the defect in IFN-γ production in these experiments was variable (see also Figs 4–6), but typically ranged from a 50% to 95% decrease in IFN-γ release, compared with parallel control cultures, and was related to the dose of ganglioside used to pre-treat the BMDC (Fig. 2a). Under these neutral conditions, i.e. in the absence of exogenously added cytokines to skew effector cell development, there was typically little or no detectable production of IL-4, IL-10, or IL-17, regardless of whether T cells were primed with ganglioside-treated or control BMDC (data not shown). When T cells were primed with ganglioside-treated BMDC under Th2 cytokine skewing conditions (in the presence of exogenous IL-4 and anti-IFN-γ antibody), there was also a decrease in the development of IL-4-producing cells upon secondary stimulation compared with priming with control BMDCs (Fig. 2b).
Figure 2.
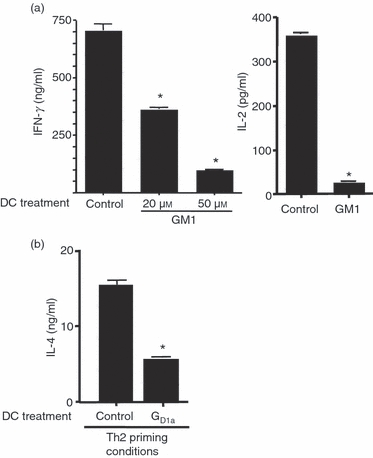
Priming with ganglioside-treated bone-marrow-derived dendritic cells (BMDC) inhibits the development of effector T cells. (a) Naive CD4+ T cells from B6 AND TCR transgenic mice were primed with 5 μg/ml MCC peptide presented by either untreated BMDC or BMDC pre-treated with the indicated concentration of GM1 ganglioside as described in Fig. 1 in the presence of exogenous interleukin-2 (IL-2; neutral conditions). After 4 days the T cells were harvested and equal numbers (5 × 10^5/ml) were re-stimulated with MCC peptide presented by T-cell-depleted spleen cells as antigen-presenting cells. Two days later supernatant was collected and the indicated cytokines were assayed by ELISA as described in Fig. 1. (b) B6 AND T-cell receptor T cells were stimulated as in (a) except exogenous IL-4 and anti-interferon-γ (IFN-γ) antibody was added during the priming phase of the culture to induce T helper type 2 (Th2) development (Th2 skewing conditions) and GD1a (50 μm) was used to treat the BMDC. The level of IL-4 produced under neutral conditions is below the level of detection in the ELISA. *P < 0·005 for all groups compared with control. These results are representative of more than three independent experiments for each cytokine.
Figure 4.
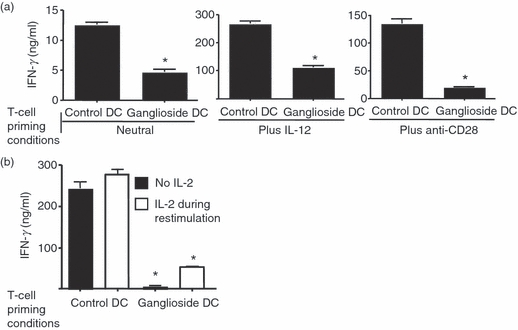
Inhibition of T helper (Th) effector cell development by ganglioside-treated bone-marrow-derived dendritic cells (BMDC) persists despite the addition of exogenous co-stimulation and exogenous IL-12. (a) B6 AND T-cell receptor T cells were stimulated with 50 μm GD1a-treated or untreated control BMDC and pMCC as in Fig. 2, except exogenous 5 ng/ml interleukin-12 (IL-12) or 5 μg/ml exogenous CD28 antibody (clone 37.51) was added as indicated, in addition to control cells which were cultured under neutral conditions as in Fig. 2(a). After 4 days the T cells were harvested and equal numbers were re-stimulated with pMCC and T-cell-depleted spleen cells and interferon-γ (IFN-γ) secretion in the supernatant was assessed by ELISA after 2 days as described for Fig. 2. (b) B6 AND T-cell receptor cells were primed with control or GM1-treated BMDC and pMCC with exogenous IL-2 as in Fig. 2 for 5 days. The cells were then harvested and re-stimulated with pMCC and T-cell-depleted antigen-presenting cell with or without exogenous IL-2 (25 U/m) during re-stimulation. After 48 hr, supernatants were collected and assayed by ELISA as described. *P < 0·005 for all groups compared with control. These data are representative of three independent experiments.
The deficient development of IFN-γ-producing Th effector cells upon priming with ganglioside-treated BMDC does not appear to be related to a defect in initial T-cell activation. This is supported by the equivalent induction of CD69 and CD25 expression on T cells 24 hr after stimulation with either GD1a pre-treated BMDCs or control BMDCs (Fig. 3). In addition, proliferation of T cells after stimulation with pMCC, assessed by CFSE dye dilution, was similar regardless of whether the BMDC were pre-treated with GD1a (Fig. 3). Consistent with the CFSE data, cell recovery at the end of the priming period was not affected when comparing T cells primed with GD1a-pre-treated BMDC with T cells primed with control BMDC (data not shown).
Figure 3.
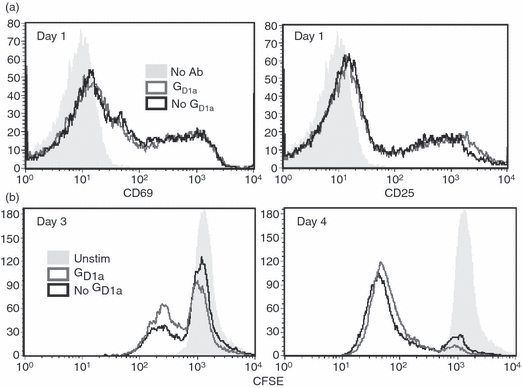
Initial T-cell activation is not affected after priming with ganglioside-treated bone-marrow-derived dendritic cells (BMDC). B6 AND T-cell receptor T cells were stimulated with GD1a-treated or untreated control BMDC and pMCC as in Fig. 2 except in the absence of exogenous interleukin-2 (IL-2). (a) T cells were harvested 24 hr after priming and analysed for CD25 and CD69 expression after gating on CD4+ cells. (b) Before priming, T cells were loaded with 0·5 μm CFSE, stimulated as indicated, and then 3 and 4 days later were harvested and the degree of CFSE dye dilution was assessed on CD4+ T cells. These data are representative of three independent experiments.
To determine if the defect in the development of Th effector cells could be attributed to the decrease in co-stimulation (B7-2 expression) or to the decreased IL-12 production observed upon ganglioside treatment (Fig. 1), T cells were primed in the presence of an agonistic antibody to CD28 or in an excess of exogenously added IL-12. As shown in Fig. 4a, the addition of either IL-12 or anti-CD28, despite causing an increase in overall Th1 effector development, did not reverse the effect of incubation with ganglioside-exposed BMDC: T cells primed in the presence of ganglioside pre-treated BMDCs continued to exhibit a > 50% decrease in IFN-γ production compared with control cultures. These data suggested that the immunosuppressive effect of ganglioside-treated BMDC cannot be fully corrected by enhancing the stimulatory microenvironment, and may involve other factors in addition to decreased IL-12 production and decreased co-stimulation.
As the Th effector cells produced less IL-2 upon re-stimulation, we asked if the addition of exogenous IL-2 during re-stimulation could rescue the ganglioside-dependent defect in IFN-γ production. As shown in Fig. 4b, exogenous IL-2 did not restore the IFN-γ production by Th effector cells primed with ganglioside-treated BMDC.
Priming with ganglioside-treated DC induces regulatory T-cell activity
The development of regulatory T cells is a major mechanism involved in down-regulating tumour-specific immune responses.26 To determine if ganglioside-treated BMDC were inducing regulatory T-cell activity in our system, we performed a series of ‘mixing’ studies. In these experiments, T cells primed for 5 days with ganglioside-treated BMDC were harvested and then mixed with effector T cells primed in parallel control cultures (i.e. primed with untreated BMDC). As shown in Fig. 5a, the T cells primed with ganglioside-treated BMDC exhibited dose-dependent suppressive activity on the ability of previously activated effector T cells to secrete IFN-γ upon secondary stimulation.
Figure 5.
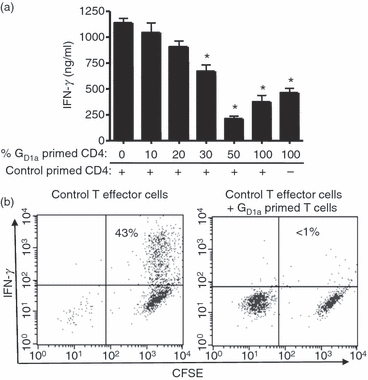
T cells primed with GD1a-treated bone-marrow-derived dendritic cells (BMDC) inhibit interferon-γ (IFN-γ) production by previously primed Th effector cells. B6 AND T-cell receptor T cells were stimulated with GD1a treated or untreated control BMDC and pMCC as in Fig. 2 for 4 days. The T cells from each group were harvested and re-stimulated with T-cell-depleted splenocytes and pMCC. (a) Increasing numbers of T cells originally primed with GD1a-treated BMDC were mixed with a fixed number of control T cells (5 × 105) primed with untreated BMDC (No GD1a) before re-stimulation. These cells were re-stimulated for 48 hr with T-cell-depleted splenocytes in the presence of pMCC and IFN-γ production was measured by ELISA. *P < 0·01 for the indicated groups compared with control. (b) T cells primed with control, untreated BMDC or as above were harvested after 5 days and labelled with CFSE, whereas T cells primed with ganglioside-treated DC were harvested in parallel and left unlabelled to distinguish the two populations of T cells. The cells were then re-stimulated for approximately 24 hr in the absence or presence of equal numbers of T cells primed with GD1a-treated BMDC (unlabelled by CFSE). Intracellular IFN-γ production was assessed by flow cytometry. Key: Left panel (control): CFSE-labelled T cells primed with control BMDC combined with unlabelled T cells primed with control BMDC; right panel: CFSE-labelled T cells primed with control BMDC combined with equal numbers of unlabelled T cells primed with GD1a-treated BMDC. These data are representative of three independent experiments.
The decrease in IFN-γ production was directly attributed to decreased production by the control T effector cells as demonstrated by intracellular flow cytometric analysis of IFN-γ production by individual T effector cells 24 hr after re-stimulation (Fig. 5b). In this experiment, cells primed with ganglioside-treated BMDC (not labelled with CFSE) were mixed with CFSE-labelled control T cells primed with untreated BMDC and then re-stimulated with pMCC peptide and T-cell-depleted spleen cells as APC. After 24 hr the cultures were harvested and intracellular IFN-γ production was assessed in both T-cell populations. As shown in Fig. 5b, approximately 40% of the CFSE-labelled T effector cells produced IFN-γ in the absence of T cells primed with ganglioside-treated BMDC. In contrast, IFN-γ production by the same CFSE-labelled T effector cells was markedly inhibited upon mixing with equal numbers of T cells primed with ganglioside-treated BMDC.
The necessity of cell contact for the observed immunosuppressive activity of T cells primed by ganglioside-treated BMDC was assessed in transwell experiments. When control T effector cells were mixed with T effector cells primed by ganglioside-treated BMDC and re-stimulated with peptide, there was significant suppression of IFN-γ production (Fig. 6, lane C) similar to that seen in Fig. 5. However, when the same number of T cells primed with ganglioside-treated BMDC are mixed with control T effector cells, but separated by a permeable membrane, no suppressive activity is observed (Fig. 6, lane D).
Figure 6.
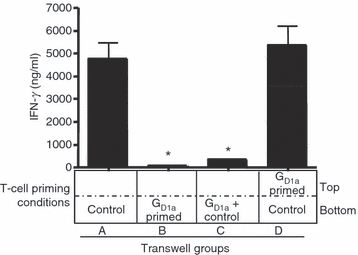
Inhibitory effect of T cells primed with GD1a-treated bone-marrow-derived dendritic cells (BMDC) requires cell–cell contact. B6 AND T-cell receptor T cells were primed with GD1a-treated or untreated control BMDC and pMCC as in Fig. 2 for 5 days. Viable cells were harvested and re-stimulated in transwell culture dishes with 5 μg/ml pMCC. Wells contain T cells primed with control BMDC (control) and/or T cells primed with GD1a-treated BMDC (GD1a), as shown. Both the upper and lower wells in each condition (A–D) also contain 6 × 105 T-cell-depleted spleen cells as antigen-presenting cells. After 48 hr of culture, the supernatant was collected and analysed for interferon-γ (IFN-γ) by ELISA. Key: A, 3 × 105 T cells primed with control BMDC; B, 3 × 105 T cells primed with GD1a-treated BMDC; C, 3 × 105 T cells primed with control BMDC, mixed with 3 × 105 T cells primed with GD1a-treated BMDC; D, both as in C, but separated by the membrane. *P < 0·005 for all groups compared with control. These data are representative of two independent experiments.
These results were confirmed by a second approach, i.e. by culturing effector T cells primed with ganglioside-treated BMDC either at the usual cell density or at a much lower (10-fold lower) density to determine whether minimizing T-cell–T-cell contact during re-stimulation would affect the diminished Th1 effector function observed upon priming with ganglioside-treated BMDC. In this case, re-stimulation of T cells that were primed with ganglioside-treated BMDC at high cell density resulted in marked suppression of the production of IFN-γ (only 2% of control levels), whereas re-stimulation of the same cells at low-density resulted in IFN-γ production at 74% of control levels.
Consistent with the requirement for cell-contact indicated in the transwell experiments, the addition of blocking antibodies to the IL-10 receptor and transforming growth factor-β to T cells during the priming phase of the cell cultures or during secondary stimulation did not reverse the inhibitory effect observed following priming with ganglioside-treated BMDC (not shown). Taken together, our findings suggest that priming with ganglioside-treated BMDC induces the development of a subset of T cells that have suppressive activity, and that these cells can inhibit the function of previously primed effector cells in a cell-contact-dependent manner.
Perhaps the best-characterized population of T regulatory cells are cells expressing the FoxP3 transcription factor. However, comparison of CD4+ FoxP3+ cells following priming with ganglioside-treated or control T cells consistently failed to show an increase in the number of FoxP3+ cells in the ganglioside-treated cultures. In fact the percentage of FoxP3+ cells was less in the ganglioside-treated group (Fig. 7a). It is possible that the FoxP3+ cells that were present were more active when primed with ganglioside-treated rather than control APC, because FoxP3 expression itself may not necessarily reflect suppressor activity.27,28 To test this possibility we removed the CD25+ FoxP3+ cells from both control and ganglioside-primed T-cell populations by cell sorting before re-stimulation. As shown in Fig. 7b, depletion of CD25+ cells did not rescue the defect in IFN-γ production seen in T effector cells primed with ganglioside-treated DC, or reverse the suppressive activity when these cells were mixed with the control population of effector T cells.
Figure 7.
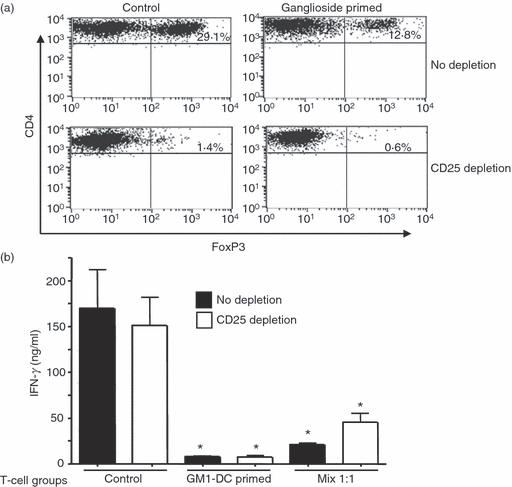
Depletion of FoxP3+ cells does not correct the defect in interferon-γ (IFN-γ) production following priming with ganglioside-treated dendritic cells (DC). B6 AND T-cell receptor T cells were primed with GM1-treated or untreated control bone-marrow-derived DC (BMDC) and pMCC as in Fig. 2 for 5 days. (a) T cells primed with GM-1-treated or control DC were depleted of CD25+ cells by cell sorting on a FACAria following labelling with CD25 antibody. Control and CD25-depleted T cells were then labelled with antibody to CD4, CD25 and FoxP3. Expression of FoxP3 in the control and CD25-depleted cell populations is shown. (b) CD4 T cells prepared as above were then re-stimulated and IFN-γ production was assessed by ELISA. *P < 0·005 for all groups compared with control. These data are representative of two independent cell sorting experiments.
Discussion
There is a large body of evidence indicating that the microenvironment at the tumour site and within the tumour-draining lymph node is immunosuppressive. This is in part the result of tumour production of cytokines and other factors, such as gangliosides that affect the APC expression of co-stimulatory or inhibitory molecules, cytokine production, and production of other immunomodulatory factors. These can in turn inhibit T-cell activation and the generation of tumour immunity.26 The precise role and relative importance of these tumour-derived factors is not well understood. Our current study suggests that exposure of DC to tumour-derived gangliosides may be a critical initiating and long-lasting factor in suppressing an effective tumour immune response. The data suggest that priming of T cells with ganglioside-treated BMDC induced the development of a population of regulatory T cells that could suppress cytokine production by previously primed effector T cells in a contact-dependent manner. Although we focused primarily on IFN-γ production in the current study, the effect of using ganglioside-exposed DC during T-cell priming appears to be relatively broadly immunosuppressive as inhibition of Th2 effector responses was also observed when T cells were stimulated in the presence of exogenous IL-4. Hence the effect of ganglioside-treated APC was not specific for Th1 responses.
Recent findings suggest that a mechanism by which ganglioside exposure alters APC function to induce regulatory T-cell development or activation may be related to ganglioside-dependent alterations in TLR signalling. Using a variety of TLR ligands that stimulate a broad range of TLRs, including TLR2, TLR3, TLR4, TLR5, TLR6 and TLR7/8, we have found that ganglioside pre-treatment inhibits TLR-dependent pro-inflammatory cytokine production.25 In these studies, we found that ganglioside pre-treatment of monocytes before stimulation with TLR agonists promoted the induction of IRAK-M expression. IRAK-M is a negative regulator of TLR signalling and is associated with endotoxin tolerance.29 Importantly, a recent study by Xie et al.30 found that in IRAK-M-deficient mice, the immune response to tumours was enhanced, with a concordant decrease in regulatory T cells. Taken together, these data suggest that IRAK-M induction by gangliosides may promote the development of a regulatory T-cell phenotype.
Myeloid-derived suppressor cells (MDSC) have also been implicated in down-regulating T-cell tumour immune responses and may also be regulated by ganglioside exposure, although we think it unlikely that these cells are playing an important role in our current studies. We cannot definitively exclude the presence of low levels of MDSC in our cultures, but > 80% of the cells in the BMDC cultures used for T-cell stimulation have a DC phenotype and are typically characterized as expressing CD11c and MHC class II and lacking Fc-γ receptor, unlike most MDSC populations. Furthermore, as we are using MHC-II-restricted T cells to analyse T-cell effector function, APC function is restricted to class II MHC-expressing cells.
The precise nature of the regulatory T-cell activity induced following priming with ganglioside-treated DCs remains to be elucidated. Although we have shown that cell contact is required, we have not been able to phenotypically distinguish the specific cell population that is associated with immunosuppressive activity in the cells primed with ganglioside-treated BMDC. Analysis of FoxP3 expression in T cells primed with ganglioside-treated BMDC did not reveal any increase in the generation of FoxP3+ cells when comparing T cells primed with control and ganglioside-pre-treated BMDC (Fig. 7). Furthermore, depletion of CD25+ FoxP3+ cells from the T effector cell population primed with ganglioside-treated DC did not rescue the immunosuppressive effect of ganglioside exposure. These data suggest that there is an atypical FoxP3-independent regulatory cell being generated by ganglioside-treated APC, although future experiments will be needed to characterize further the regulatory activity present following ganglioside treatment of DC. The ganglioside-dependent immunosuppression we observe could be partly the result of direct cytotoxic effects of the regulatory T-cell population, as has been recently described in regulatory T cells isolated from the tumour microenvironment,31 or may be related to the induction of T-cell populations that express negative co-stimulator molecules such as programmed death-1 and cytotoxic T lymphocyte antigen-4.
Although the mechanism of immunoregulation requires further investigation, our data indicate that priming with ganglioside-treated BMDC induces the development of a regulatory cell population that inhibits previously activated effector T cells in a contact-dependent manner. These data provide further support for the concept that gangliosides, shed by tumours into the microenvironment, are a potent immunomodulatory agent that may initiate tumour immune-evasion mechanisms.
Acknowledgments
This work was supported by National Institutes of Health Grant RO1 CA42361, RO1 CA61010 (S.L.) and by The Children’s Cancer Foundation.
Disclosures
The Authors declare that there is no conflict of interest.
References
- 1.Ladisch S, Gillard B, Wong C, Ulsh L. Shedding and immunoregulatory activity of YAC-1 lymphoma cell gangliosides. Cancer Res. 1983;43:3808–13. [PubMed] [Google Scholar]
- 2.Bernhard H, Meyer Zum Buschenfelde KH, Dippold WG. Ganglioside GD3 shedding by human malignant melanoma cells. Int J Cancer. 1989;44:155–60. doi: 10.1002/ijc.2910440127. [DOI] [PubMed] [Google Scholar]
- 3.Portoukalian J, David MJ, Gain P, Richard M. Shedding of GD2 ganglioside in patients with retinoblastoma. Int J Cancer. 1993;53:948–51. doi: 10.1002/ijc.2910530614. [DOI] [PubMed] [Google Scholar]
- 4.Ladisch S, Wu ZL. Detection of a tumour-associated ganglioside in plasma of patients with neuroblastoma. Lancet. 1985;1:136–8. doi: 10.1016/s0140-6736(85)91906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valentino L, Moss T, Olson E, Wang HJ, Elashoff R, Ladisch S. Shed tumour gangliosides and progression of human neuroblastoma. Blood. 1990;75:1564–7. [PubMed] [Google Scholar]
- 6.Liu Y, Mccarthy J, Ladisch S. Membrane ganglioside enrichment lowers the threshold for vascular endothelial cell angiogenic signaling. Cancer Res. 2006;66:10408–14. doi: 10.1158/0008-5472.CAN-06-1572. [DOI] [PubMed] [Google Scholar]
- 7.Li R, Liu Y, Ladisch S. Enhancement of epidermal growth factor signaling and activation of SRC kinase by gangliosides. J Biol Chem. 2001;276:42782–92. doi: 10.1074/jbc.M101481200. [DOI] [PubMed] [Google Scholar]
- 8.Ladisch S, Ulsh L, Gillard B, Wong C. Modulation of the immune response by gangliosides: inhibition of adherent monocyte accessory function in vivo. J Clin Invest. 1984;74:2074–81. doi: 10.1172/JCI111631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uzzo RG, Rayman P, Kolenko V, et al. Renal cell carcinoma-derived gangliosides suppress nuclear factor-kappaB activation in T cells. J Clin Invest. 1999;104:769–76. doi: 10.1172/JCI6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shurin GV, Shurin MR, Bykovskaia S, Shogan J, Lotze MT, Barksdale EM., Jr Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001;61:363–9. [PubMed] [Google Scholar]
- 11.Robb RJ. The suppressive effect of gangliosides upon IL-2-dependent proliferation as a function of inhibition of IL-2 receptor association. J Immunol. 1986;136:971–6. [PubMed] [Google Scholar]
- 12.Chu JW, Sharom FJ. Gangliosides inhibit T-lymphocyte proliferation by preventing the interaction of interleukin-2 with its cell surface receptors. Immunology. 1993;79:10–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Biswas K, Richmond A, Rayman P, et al. GM2 expression in renal cell carcinoma: potential role in tumor-induced T cell dysfunction. Cancer Res. 2006;66:6816–25. doi: 10.1158/0008-5472.CAN-06-0250. [DOI] [PubMed] [Google Scholar]
- 14.Das T, Sa G, Hilston C, et al. GM1 and tumor necrosis factor-alpha, overexpressed in renal cell carcinoma, synergize to induce T-cell apoptosis. Cancer Res. 2008;68:2014–23. doi: 10.1158/0008-5472.CAN-07-6037. [DOI] [PubMed] [Google Scholar]
- 15.Crespo FA, Sun X, Cripps JG, Fernandez-Botran R. The immunoregulatory effects of gangliosides involve immune deviation favoring type-2 T cell responses. J Leukoc Biol. 2006;79:586–95. doi: 10.1189/jlb.0705395. [DOI] [PubMed] [Google Scholar]
- 16.De Leon J, Fernandez A, Clavell M, Labrada M, Yanin B, Mesa C, Fernandez LE. Differential influence of the tumour-specific non-human sialic acid containing GM3 ganglioside on CD4+ CD25– effector and naturally occurring CD4+ CD25+ regulatory T cells function. Int Immunol. 2008;20:591–600. doi: 10.1093/intimm/dxn018. [DOI] [PubMed] [Google Scholar]
- 17.Shen W, Ladisch S. Ganglioside GD1a impedes lipopolysaccharide-induced maturation of human dendritic cells. Cell Immunol. 2002;220:125–33. doi: 10.1016/s0008-8749(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 18.Mond JJ, Witherspoon K, Yu RK, Perera PY, Vogel SN. Inhibition of LPS-mediated cell activation in vitro and in vivo by gangliosides. Circ Shock. 1994;44:57–62. [PubMed] [Google Scholar]
- 19.Mckallip R, Li R, Ladisch S. Tumor gangliosides inhibit the tumor-specific immune response. J Immunol. 1999;163:3718–26. [PubMed] [Google Scholar]
- 20.Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129:33–6. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 21.Kaye J, Hsa ML, Sauron ME, Jameson SC, Gasciogne N, Hendrik SM. Selective development of CD4+ T cells in transgenic mice express a class II MHC restricted antigen receptor. Nature. 1989;341:746–9. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- 22.Shen W, Falahati R, Stark R, Leitenberg D, Ladisch S. Modulation of CD4 Th cell differentiation by ganglioside GD1a in vitro. J Immunol. 2005;175:4927–34. doi: 10.4049/jimmunol.175.8.4927. [DOI] [PubMed] [Google Scholar]
- 23.Leitenberg D, Bottomly K. Regulation of naive T cell differentiation by varying the potency of TCR signal transduction. Semin Immunol. 1999;11:283–92. doi: 10.1006/smim.1999.0184. [DOI] [PubMed] [Google Scholar]
- 24.Leitenberg D, Boutin Y, Constant S, Bottomly K. CD4 regulation of T cell receptor signaling and T cell differentiation following stimulation with peptides of different affinities for the T cell receptor. J Immunol. 1998;161:1194–203. [PubMed] [Google Scholar]
- 25.Shen W, Stone K, Jales A, Leitenberg D, Ladisch S. Inhibition of TLR activation and up-regulation of IL-R-associated kinase-M expression by exogenous gangliosides. J Immunol. 2008;180:4425–32. doi: 10.4049/jimmunol.180.7.4425. [DOI] [PubMed] [Google Scholar]
- 26.Rabinovitch GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–71. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seidel MG, Ernst U, Printz D, et al. Expression of the putatively regulatory T-cell marker FoxP3 by CD4+ CD25+ T cells after pediatric hematopoietic stem cell transplantation. Haematologica. 2006;91:566–9. [PubMed] [Google Scholar]
- 29.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 30.Xie Q, Gan L, Wang J, Wilson I, Li L. Loss of the innate immunity negative regulator IRAK-M leads to enhanced host immune defense against tumor growth. Mol Immunol. 2007;44:3453–61. doi: 10.1016/j.molimm.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–46. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]


