Abstract
Emerging evidence suggests that haematopoietic CD34+ progenitor cells migrate from bone marrow (BM) to sites of allergen exposure where they can undergo further proliferation and final maturation, potentially augmenting the degree of tissue inflammation. In the current study we used a well-characterized mouse model of allergen-induced airway inflammation to determine the role of CCR3 receptor–ligand interactions in the migration and function of CD34+ cells. Allergen exposure significantly increased BM, blood and airway CD34+ CCR3+ cells as well as airway CD34+ CCR3+ stem cell antigen-1-positive (Sca-1+) and CD34+ CD45+ interleukin-5 receptor-α-positive (IL-5Rα+) cells. A portion of the newly produced CD34+ CCR3+, Sca-1+ CCR3+ and IL-5Ralpha+ lung cells showed a significant proliferative capacity in response to allergen when compared with saline-treated animals. In addition, in vitro colony formation of lung CD34+ cells was increased by IL-5 or eotaxin-2 whereas eotaxin-2 had no effect on BM CD34+ cells. Furthermore, both eotaxin-1 and eotaxin-2 induced migration of BM and blood CD34+ CCR3+ cells in vitro. These data suggest that the CCR3/eotaxin pathway is involved in the regulation of allergen-driven in situ haematopoiesis and the accumulation/mobilization of eosinophil-lineage-committed progenitor cells in the lung. Hence, targeting both IL-5 and CCR3-mediated signalling pathways may be required to control the inflammation associated with allergen-induced asthma.
Keywords: allergy, cell proliferation, eosinophils, haematopoiesis, inflammation, lung
Introduction
Allergic airway inflammation in asthma is dominated by eosinophils, which develop from CD34+ haematopoietic progenitor cells within the bone marrow (BM).1–7 Evidence increasingly suggests that in addition to the trafficking of mature eosinophils from the BM to the airways, migration of immature cells and progenitors from the BM to sites of inflammation can also occur during an allergic inflammatory response.8–11 Increased numbers of CD34+ cells in BM and airways has been reported in atopic individuals and in individuals with ongoing asthma or allergic rhinitis.12,13 To date, however, it is not clear which chemotactic factors induce the traffic of these cells to the airways during an allergic inflammatory response. It is known that the eotaxin receptor, CC chemokine receptor 3 (CCR3) is expressed on human CD34+ BM cells and that asthmatics with late responses to allergen have increased numbers of BM CD34+ CCR3+cells 24 hr after allergen challenge.14,15 These findings imply that variations in CCR3 expression on BM CD34+ cells may facilitate chemokine-mediated progenitor cell mobilization to the peripheral circulation and that eotaxins may orchestrate the homing of CD34+ cells to tissue sites of allergic inflammation. Furthermore, results from clinical studies using humanized monoclonal anti-interleukin-5 (IL-5) clearly demonstrate that eosinophils are able to reside in the tissue despite blockade of IL-5.16 These findings highlight unidentified signals that promote eosinophil survival and proliferation in vivo in response to allergen challenge and that need further investigation. Multiple lines of evidence suggest that the eotaxins produced locally at the site of allergic inflammation17–20 may influence the function and differentiation of eosinophils.21,22 Eotaxins, acting via their receptor, CCR3, may therefore not only represent an important link in the mobilization of eosinophils and their progenitors, but also play a role in haematopoiesis at sites of inflammation (i.e. in situ haematopoiesis). Therefore, we hypothesize that CD34+ CCR3+ cells are increased in the airways after allergen exposure. We further hypothesize that these cells, in addition to the classical CD34+ IL-5 receptor α subunit-positive (IL-5Rα+) eosinophil progenitor cells, have a proliferative capacity and undergo in situ proliferation in response to allergen.
In this study, the importance and potential role for these potential progenitor populations in the lung following allergen provocation were investigated in the mouse using both in vivo models (e.g. allergen provocation of wild-type and IL-5 transgenic mice as well as 5-bromo-2′-deoxyuridine (BrdU) labelling of progenitor cell populations in the lung) and ex vivo culture studies (e.g. semi-solid cultures, evaluating colony formation) to identify and characterize these cells. Moreover, the specific role of these progenitor populations in pulmonary allergen-mediated inflammatory responses was highlighted in vivo by selective depletion with a rat anti-mouse CCR3 monoclonal antibody.
Materials and methods
Animals
This study was approved by the Animal Ethics Committee in Gothenburg, Sweden. Five- to six-week-old male BALB/c mice purchased from Taconic (Ry, Denmark) were used for all in vivo experiments and the in vitro colony-forming assays. Interleukin-5 transgenic mice (line NJ.1638) were used as part of in vivo migration studies (i.e. administration of eotaxin-2) and for in vitro transmigration assays.23 These mice were kept under animal housing conditions and provided with food and water ad libitum.
Sensitization and allergen exposure
Mice were immunized twice, at an interval of 5 days, by intraperitoneal (i.p.) injections of 0·5 ml alum-precipitated antigen containing 8 μg ovalbumin [OVA; bound to 4 mg Al(OH)3, both from Sigma-Aldrich, St Louis, MO] in PBS. Eight days after the second sensitization, the mice were quickly and briefly anaesthetized with isofluorane (Baxter, Deerfield, IL) and received an intranasal administration of 100 μg OVA in 25 μl PBS on five consecutive days. In addition, one group received 25 μl PBS on five consecutive days as a control for the OVA exposure. Twenty-four hours after the final OVA exposure, the mice were killed and BM, bronchoalveolar lavage fluid (BALF) cells and lung tissue were collected.
In vivo protocol for evaluation of newly produced inflammatory cells
The BrdU (Roche Diagnostics Scandinavia AB, Bromma, Sweden) was administered to mice as a means to label newly produced cells, of which a proportion are eosinophil-lineage-committed cells. The BrdU was given at a dose of 1 mg in 250 μl PBS by i.p. injection on two occasions, 8 hr apart on day 3 and day 5 before harvesting of samples.
Cell collection and sample processing
Samples were collected 24 hr after the final OVA exposure using the protocol noted earlier. The animals were killed with a mixture of xylazin (130 mg/kg, Rompun®, Bayer, Germany) and ketamine (670 mg/kg, Ketalar®, Park-Davis, UK). When the animals were deeply anaesthetized blood was obtained by cardiac puncture of the right ventricle. Bronchoalveolar lavage (BAL) was performed by instilling 0·25 ml PBS through the tracheal cannula, followed by gentle aspiration and repeated with 0·2 ml PBS. Finally, one femur was cut at the epiphysis and the BM cells were flushed with 2 ml PBS.
Bronchoalveolar lavage fluid and bone marrow
Samples of BALF and BM were centrifuged at 300 g for 10 min at 4°. The BAL supernatant was saved for eotaxin-2 measurement and stored at − 80° until analysis. The cells were resuspended with 0·03% BSA in PBS. The total cell numbers in BAL and BM were determined using standard haematological procedures. Cytospins of BAL and BM were prepared and stained with May–Grünwald–Giemsa for differential cell counts by counting 300–500 cells using a light microscope (Zeiss Axioplan 2; Carl Zeiss, Jena, Germany). The cells were identified using standard morphological criteria, and BM mature and immature eosinophils were determined by nuclear morphology, cell size and cytoplasmic granulation.23
Lung tissue cells
The pulmonary circulation was perfused with ice-cold PBS and lungs were removed from the thoracic cavity. The lung tissue was thinly sliced and suspended RPMI-1640 (Sigma-Aldrich) complemented with 10% fetal calf serum (FCS), collagenase (5·25 mg/ml) and DNAse (3 mg/ml; Roche). After 90 min incubation in a shaking water bath (37°), any remaining intact tissue was disrupted by repeated passage through a wide-bore Pasteur pipette and filtered through a 40-μm nylon mesh (BD Biosciences, Erembodegem, Belgium). The parenchyma lung cells were diluted in Percoll (density 1·03 g/ml; Amersham Bioscience, Uppsala, Sweden) and layered on a discontinuous gradient, centrifuged at 400 g for 20 min. The cells in the top layer, mainly macrophages, dead cells and debris, were discarded. Cells at the Percoll interfaces were collected and washed in PBS complemented with 10% FCS. Total cell numbers were determined using standard haematological procedures.
Immunostaining, flow cytometric analysis and gating strategy
Antibodies
Fluorescein isothiocyanate (FITC) -labelled anti-mouse CD34 (clone RAM 34; BD Bioscience), phycoerythrin (PE) or FITC-labelled anti-mouse CCR3 (clone 83101; R&D systems, Abington, UK), biotinylated anti-mouse stem cell antigen-1 (Sca-1)/Ly6 (clone 177228; R&D Systems) followed by peridinin chlorophyll protein (PerCP) -labelled streptavidin, PE-labelled anti-mouse IL-5Rα (Clone 558488; BD Bioscience), PercP-labelled anti-mouse CD45 (clone 557235; BD Bioscience), FITC-labelled BrdU (BD Bioscience) and rabbit anti-mouse major basic protein (MBP) polyclonal antibody in combination with goat anti-rabbit PE or with biotinylated swine anti-rabbit followed by streptavidin-FITC were used.
Animals were sensitized and exposed to OVA or PBS as described above. BM, blood, lung and BAL cells were collected and stained for CD34+ CCR3+ or CD34+ CCR3+ Sca-1+ and CD34+ CD45+ IL-5Rα+. Lung cells were also stained for the following combinations; CCR3+ MBP+, IL-5Rα+ CCR3+ and IL-5Rα+ MBP+ cells. Cells were pre-treated with 2% mouse serum (DAKO, Carpinteria, CA) for 15 min to prevent non-specific binding and thereafter stained with antibody or the appropriate isotype control antibody in saturating concentrations. The cells were incubated for 30 min at 4° with antibodies or isotype control, followed by two washing steps. Finally, the samples were fixed in 2% paraformaldehyde and kept at 4° until flow cytometric analysis. In experiments where cells were stained for surface marker and intracellular stained for MBP, an extended protocol was used, as per the manufacturer’s instructions (BD Cytofix/Cytoperm™ Fixation/Permeabilization Solution kit; Cat no.: 554722). In some experiments, cells were stained with 7-aminoactinomycin D (7-AAD) to exclude dead cells. In other studies, cells were also stained with anti-CD45 PerCP to exclude non leucocyte cells. The flow cytometric analysis was carried out using a FACScan flow cytometer (BD Bioscience). Twenty thousand cells were computed in list mode and analysed using the cellquest pro software. Gating was set on all intact cells and cells with the CCR3+ high side scatter (SSChigh) profile were identified as eosinophils. As eosinophil-lineage-committed progenitors are found in the mononuclear cell population,24 gating was also made on cells with an SSClow profile (Fig. 1b).
Figure 1.
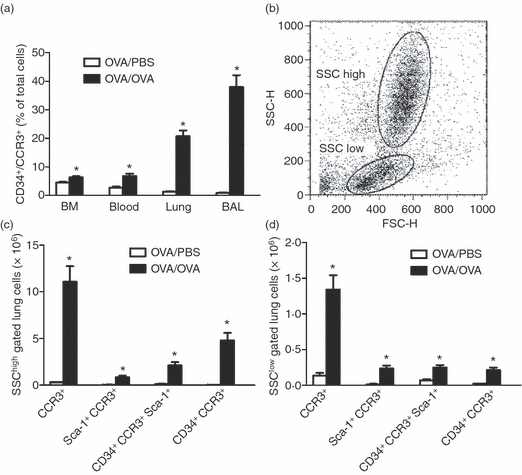
Allergen provocation increase CD34+ CCR3+ bone marrow (BM), blood, lung and bronchoalveolar lavage (BAL) cells and CD34+ CCR3+ Sca-1+ lung cells. (a) Flow cytometric analysis for CD34+ CCR3+ cells in BM, blood, lung and BAL. (b) Representative dot blot showing gating strategies. SSChigh gated population represents eosinophils, whereas eosinophil-lineage-committed progenitors are mainly found in the SSClow gated population. (c,d) Flow cytometric analysis for the number of lung CD34+ CCR3+ Sca-1+ cells, (c) gated on the SSChigh cell population and (d) gated on the SSClow cell population in the lung of ovalbumin (OVA) -sensitized/exposed BALB/c mice compared with OVA-sensitized but PBS-exposed mice. Data are shown as mean (+ SEM) (n = 3–6). *P< 0·05 between OVA-exposed and PBS-exposed groups.
Lung and BM colony assay
Animals were sensitized and exposed to OVA and lung and BM cells were harvested as described above for in vitro lung and BM colony assay. Lung CD34+ progenitor cells were enriched from the sampled Percoll fractions as described above. Enrichment of BM CD34+ cells was performed as previously described with some modification.9 Briefly, mononuclear CD3+ cells and neutrophils were depleted using biotinylated antibodies and finally CD34+ cells were enriched using the same magnetic separation method as above. Both BM and lung CD34+ cells (5 × 105) were cultured at 37° in 5% CO2 in a 12-well plate in 1 ml RPMI-1640 culture medium completed with 0·9% methylcellulose, 20% FCS, 1% penicillin-streptomycin, 2 mm l-glutamine and 0·0006%β-mercaptoethanol (all obtained from Sigma-Aldrich). Cells were seeded and divided into groups depending on cytokines added: control (no cytokines added), recombinant murine IL-5 (rmIL-5; 10 ng/ml; R&D Systems), rmEotaxin-2 (500 ng/ml; PeproTech EC, London, UK) and rmIL-5 together with rmEotaxin-2 (10 and 500 ng/ml, respectively). The BM and lung cultures were fed with 100 μl RPMI-1640 completed with penicillin-streptomycin, l-glutamine and the respective cytokines on day 6 of culture. The BM colonies were counted on day 8 of culture and lung colonies were counted on days 8–14 of culture, using an inverted light microscope as described previously.25
Flow cytometric cell cycle analysis for CD34+ CCR3+, Sca-1+ CCR3+ and IL-5Rα+ lung cells
Animals were sensitized and exposed to OVA or PBS and BrdU was administered as described above. Parenchyma lung cells were harvested as described earlier. Three different experiments were performed to determine if CD34+ CCR3+, Sca-1+ CCR3+ and IL-5Rα+ cells have a capability to proliferate locally in the airways after allergen exposure. In the first and second experiments, lung CD34+ or Sca-1+ progenitor cells were enriched from the sampled Percoll fractions by labelling the cells with a biotinylated rat anti-mouse CD34 monoclonal antibody (mAb; clone RAM34; BD Biosciences) or biotinylated rat anti-mouse Sca-1/Ly6 mAb (Clone 177228; R&D Systems). After washing, streptavidin microbeads (MACS; Miltenyi Biotec, Bergisch Gladbach, GmbH, Germany) were added, according to the manufacturer’s instructions and CD34+ or Sca-1+ cells were enriched by magnetic separation. The purity of the CD34+ cell fraction was > 75% and for Sca-1+ cells it was > 80%. The enriched fractions of lung CD34+ and Sca-1+ cells were stained for CCR3 followed by intracellular staining for BrdU and 7-AAD. In the third experiment, cells from the Percoll fractions were stained for IL-5Rα followed by an intracellular staining for BrdU and 7-AAD. Gating was set on all intact cells and eosinophils and eosinophil-lineage-committed progenitor cell populations were gated based on forward and side scatter profiles.
Eotaxin-2 measurements in BALF
The BALF eotaxin-2 in OVA-sensitized/exposed and saline-exposed animals was analysed by ELISA according to the manufacturer’s instructions (R&D Systems).
Induction of BALF eosinophilia by eotaxin-2
Interleukin-5 transgenic mice were anaesthetized using isofluorane and treated with rmEotaxin-2 (PeproTech EC, 5 μg in a total volume of 25 μl 0·1% BSA/PBS) or control vehicle (0·1% BSA/PBS) by intranasal instillation. The BAL eosinophils and CD34+ cells were measured 18 hr after the eotaxin-2 treatment.
Transmigration assay
Bone marrow and blood cells harvested from naive IL-5 transgenic mice (NJ.1638) were stained for CD34+ and CCR3+ cells before and after migration. Briefly, the migration of BM and blood CD34+ CCR3+ cells in response to eotaxins was assessed using 5-μm polycarbonate membrane transwell inserts in 24-well tissue-culture polystyrene plates (Costar, Corning, NY). The inserts were pre-incubated in medium (RPMI-1640 containing 5% FCS) for 1 hr in 37°. The BM cells (1 × 106) and blood cells (1·5 × 106) isolated from IL-5 transgenic mice and 50 ng/ml rmIL-5 in 200 μl medium were placed into the inserts. The inserts were then placed into the wells with 500 μl medium alone (control), or medium containing rmEotaxin-1 (250 ng/ml) or rmEotaxin-2 (250 ng/ml). The plates were incubated at 37° in 5% CO2 for 90 min. The cells that had migrated to the lower wells were collected, counted and stained for CD34 and CCR3 as described above for the FACS analysis. Migrated CD34+ CCR3+ cells are expressed as the relative number of migrated cells of CD34+ CCR3+ cell input.
In vivo protocol for anti-CCR3 treatment
The effects of anti-CCR3 in allergen-induced eosinophilia were investigated by pre-treatment of the animals with a depleting rat anti-mouse CCR3 mAb, (Clone 6S2-19-4; IgG2b; DNAX, Palo Alto, CA). Mice were treated i.p. with anti-CCR3 in three different doses (30–300 μg/animal in 500 μl PBS) or isotype control (100 μg/animal in 500 μl PBS, rat IgG2b, clone R35-38; BD-Bioscience Europe, Erembodegem, Belgium) 1 hr before allergen exposure on the first day of exposure.
Immunocytochemical detection of CD34 antigen together with Luxol Fast Blue counterstaining
Cytospin preparations from BM and BAL were stained for CD34 using a biotinylated rat anti-mouse CD34 mAb (clone RAM34; BD Biosciences). Bound antibodies were visualized with a Vector Red Alkaline Phosphatase Substrate kit (Vector Laboratories Inc., Burlingame, CA). The slides were also stained with Luxol Fast Blue and counterstained with Mayer’s haematoxylin (DAKO) to identify these cells as eosinophil-lineage precursors. Five hundred cells were evaluated in random fields of view.
Double immunocytochemistry for CD34 antigen and Sca-1
Cytospins from BAL were stained with a rat anti-mouse CD34 mAb (clone RAM34; BD Biosciences). A rabbit anti-rat immunoglobulin (DAKO) was used as a link antibody before incubation with alkaline phosphatase–anti-alkaline phosphatase (DAKO). Bound antibodies were visualized with the Vector Red Alkaline Phosphatase Substrate kit. Slides were then treated with a biotin blocking system (DAKO) and incubated overnight at 4° with a biotinylated rat anti-mouse Sca-1/Ly6 mAb (Clone 177228; R&D Systems). Next day, the slides were washed and incubated with streptavidin-β-galactosidase and X-Gal substrate (β-Gal staining set; Roche) and counterstained with Mayer’s haematoxylin. Four hundred cells were counted in random fields of view.
Statistical analysis
All data are expressed as mean ± SEM. Statistical analysis was carried out using a non-parametric analysis of variance (Kruskal–Wallis test) to determine the variance among more than two groups. If significant variance was found, an unpaired two-group test (Mann–Whitney U-test) was used to determine significant differences between individual groups. Wilcoxon signed rank test was used to analyse changes within the same group. P< 0·05 was considered statistically significant.
Results
FACS analysis for CD34+ CCR3+ and CD34+ CCR3+ Sca-1+ cells
Flow cytometric analysis for CD34+ CCR3+ cells in BM, blood, lung and BAL showed a significant increase of this cell population in all three compartments of OVA-sensitized/exposed animals when compared with OVA-sensitized but saline-exposed control animals (Fig. 1a). Triple staining for CD34+ CCR3+ Sca-1+ on lung cells was performed to determine if a part of the CD34+ CCR3+ cells also expressed Sca-1. Allergen exposure induced a significant increase in the number of CD34+ CCR3+ Sca-1+ lung cells both in the SSChigh gated population (i.e. eosinophils) and in the SSClow gated cell population (i.e. eosinophil-lineage-committed progenitors) when compared with saline-exposed animals (Fig. 1b). CCR3+, Sca-1+ CCR3+ and CD34+ CCR3+ cells were also increased in the SSChigh and SSClow gated cell populations in allergen-exposed mice when compared with saline-exposed mice (Fig. 1c,d).
Lung and BM colony assay
Lung CD34+ cells were cultured together with rmIL-5 or rmEotaxin-2 to examine if CD34+ cells in the lung of allergen-exposed mice form colony-forming units (CFU) in vitro. In the cultures of lung CD34+ cells (Fig. 2a), we detected 2 ± 1 CFU/well in the control culture, and 8 ± 3 versus 6 ± 2 CFU/well in cultures where either IL-5 or rmEotaxin-2 was added alone, respectively. When the combination of rmIL-5 and rmEotaxin-2 was added to the culture of lung CD34+ cells, no further significant increase in CFU/well was observed (10 ± 1 CFU/well; Fig. 2a). Interestingly, previous studies have shown that BM-derived CD34+ cells form CFU when stimulated with rmIL-5.9 Hence, BM CD34+ cells were cultured in parallel as a control for our system. In the cultures of BM CD34+ cells, we detected 1 ± 1 BM CFU/well in the control cultures (no cytokines added), whereas we found no BM CFU in the cultures where rmEotaxin-2 alone was added. In contrast, the cultures where rmIL-5 was added alone, or together with rmEotaxin-2, had 27 ± 3 and 26 ± 2 CFU/well, respectively (Fig. 2b). The optimal time for BM CFU growth was after 8 days of culture, and in lung after 8–14 days of culture. The cells were identified as eosinophils on the basis of morphologically homogeneous appearance.
Figure 2.
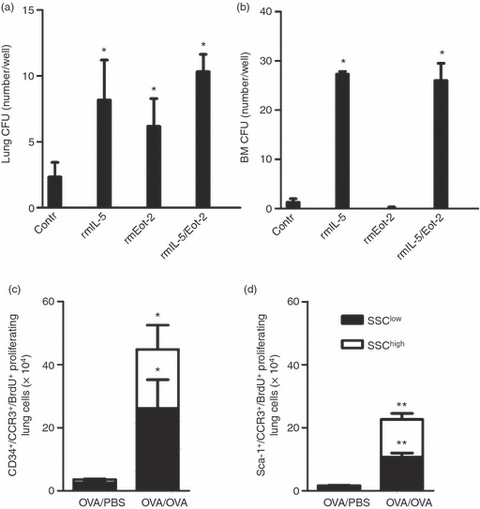
Haematopoietic activity of interleukin-5 (IL-5) and eotaxin-2 and newly produced eosinophil-lineage-committed cells proliferate after allergen exposure. (a) Colony-forming units (CFUs) after 8–14 days of culture of lung CD34+ cells and (b) 8 days of culture of bone marrow (BM) CD34+ cells harvested from ovalbumin (OVA) -sensitized/exposed mice (n = 3–6) *P< 0·05 for comparison between control and cytokine/chemokine-stimulated colonies; Wilcoxon signed rank test. The number of CCR3+ BrdU+ proliferating cells in the lung, analysed by FACS of magnetically enriched CD34+ cells (c) and Sca-1+ cells (d) from OVA-sensitized/exposed BALB/c mice compared with OVA-sensitized but PBS-exposed mice. Open bars represent cells gated on the SSChigh cell population and dark bars are cells gated on the SSClow cell population. Data are shown as mean (+ SEM) (n = 5–7). *P< 0·05 and **P< 0·01 between OVA-exposed and PBS-exposed groups.
Newly produced eosinophil-lineage-committed lung cells proliferate in vivo after allergen challenge
A multiparametric cell cycle analysis was used to assess whether the magnetically enriched CD34+ or Sca-1+ newly produced eosinophil-lineage-committed cells proliferate locally within the airways in response to allergen, by analysis of BrdU staining together with 7-AAD staining (total DNA stain). We found a significant increase in the number of CD34+ CCR3+ BrdU+ and Sca-1+ CCR3+ BrdU+ proliferating cells (i.e. cells within S phase or G2/M phase) in the allergen-exposed animals when compared with the saline exposed animals. This increase was paralleled with an increase in proliferating cells in both SSChigh and SSClow lung cell populations, representing eosinophils and their progenitors (Fig. 2c,d).
CCR3+ lung cells co-express MBP
We employed double staining of CCR3 together with MBP to further assess whether the CCR3+ cells were committed to the eosinophil lineage. Almost all of the CCR3+ cells gated on both the SSChigh and SSClow cell population co-expressed MBP (ranging between 75 and 99%) (data not shown).
CD34+ CD45+ IL-5Rα+ cells increase in the airways after allergen challenge and newly produced IL-5Rα+ lung cells proliferate in vivo
Bone marrow, lung and BAL cells were stained for CD34+ CD45+ IL-5Rα+ to evaluate the amount of CD34+ progenitors (CD34+ CD45+ cells) and the classical eosinophil progenitors (CD34+ CD45+ IL-5Rα+ cells) in our model. No differences were found in BM eosinophil progenitors of allergen-exposed animals compared with saline-exposed animals (data not shown). In contrast, lung and BAL CD34+ CD45+ IL-5Rα+ cells were significantly increased in the allergen-exposed animals compared with the saline-exposed animals (Fig. 3a). To further assess whether the IL-5Rα+ newly produced cells proliferate locally within the airways in response to allergen a multiparametric cell cycle analysis for BrdU+ cells together with 7-AAD staining (total DNA analysis) was used. A significant increase in the newly produced proliferating IL-5Rα+ cells in allergen-exposed animals compared with saline-exposed animals was found in both SSChigh and SSClow gated lung cell populations, showing that both eosinophils and their progenitors have a proliferative capacity locally in the airways in response to allergen (Fig. 3b).
Figure 3.
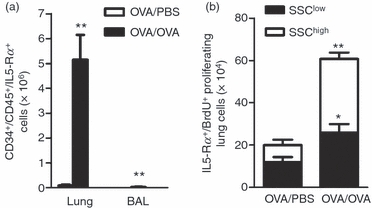
CD34+ CD45+ IL-5Rα+ cells increases in the airways and newly produced interleukin-5 receptor α subunit-positive (IL-5Rα+) lung cells proliferate after allergen challenge. (a) The number of CD34+ CD45+ IL-5Rα+ cells in the lung, analysed by FACS in ovalbumin (OVA) -sensitized/exposed BALB/c mice compared with OVA-sensitized but PBS-exposed mice. Open bars represent PBS-exposed animals and dark bar represents OVA-exposed animals. (b) Number of IL-5Rα+ BrdU+ 7-AAD+ cells in the lung, analysed by FACS in OVA-sensitized/exposed BALB/c mice compared with OVA-sensitized but PBS-exposed mice. Open bars represent cells gated on the SSChigh cell population and dark bars are cells gated on the SSClow cell population. Data are shown as mean (+ SEM) (n = 5–6). *P< 0·05 and **P< 0·01 between OVA-exposed and PBS-exposed groups.
IL-5Rα+ cells co-express CCR3
Double staining with antibodies against IL-5Rα and CCR3 was used to further evaluate if the IL-5Rα+ cells express CCR3. More than 95% of the lung IL-5Rα+ cells gated within the granulocyte population (SSChigh) stained positively for CCR3 (Fig. 4a). In contrast, only 55% of the SSClow gated IL-5Rα+ cells stained positively for CCR3 (Fig. 4b). Furthermore, SSChigh and SSClow gated CCR3 +IL-5Rα+ cells were significantly increased in the allergen-exposed animals compared with saline-exposed (Fig. 4a,b).
Figure 4.
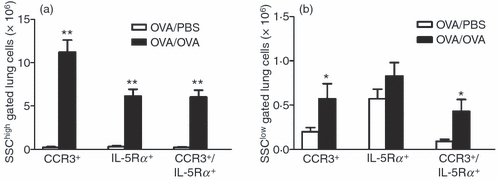
Number of CCR3+-IL-5Rα+ cells in the lung. (a) The number of CCR3+, interleukin-5 receptor α subunit-positive (IL-5Rα+)and CCR3+ IL-5Rα+ cells in the lung, analysed by FACS and gated on SSChigh cell population and (b) gated on SSClow cell population in ovalbumin (OVA) -sensitized/exposed BALB/c mice compared with OVA-sensitized but PBS-exposed mice. Open bars represent PBS-exposed animals and dark bar represents OVA-exposed animals. Data are shown as mean (+ SEM) (n = 5–6). *P< 0·05 and **P< 0·01 between OVA-exposed and PBS-exposed groups.
Eotaxin-2 in BALF
Eotaxin-2 levels were measured in BALF to further investigate the interplay between BALF eotaxin-2 levels and the accumulation of eosinophils and their progenitors in the airways in OVA-exposed animals. BALF eotaxin-2 levels significantly increased in the OVA-exposed animals compared with the sensitized but saline-exposed control animals (694 ± 157 versus 27 ± 8 pg/ml, P< 0·01).
Eotaxin-2 induces CD34+ eosinophil recruitment to the airways
We have previously shown that IL-5 transgenic mice have an increased number of CD34+ cells in blood10 and administration of eotaxin-2 to the airway lumen of different IL-5 transgenic mouse strains result in traffic of eosinophils to the airways.26,27 Therefore we used an IL-5 transgenic mouse strain to further elucidate the role of eotaxin-2 in the in vivo recruitment of CD34+ cells to the airways. Eotaxin-2 treatment significantly induced CD34+ cell recruitment to the airways in IL-5 transgenic mice when compared with animals treated with vehicle (0·1% BSA/PBS) (Fig. 5a).
Figure 5.
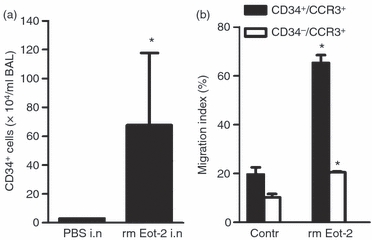
Eotaxin-2 induces migration of CD34+ CCR3+ cells. (a) The effect of local airway administered eotaxin-2 (Eot-2) to interleukin-5 (IL-5) transgenic mice (NJ.1638) on bronchoalveolar lavage (BAL) CD34+ eosinophil-lineage-committed cells compared with IL-5 transgenic mice administered vehicle. Data are shown as mean (+ SEM) (n = 3), *P< 0·05. (b) In vitro transmigration assay of CD34+ CCR3+ and CD34− CCR3+ blood cells in response to eotaxin-2. Data are expressed as migration index (% of migrated cells of cell input) and shown as mean (+ SEM) (n = 3).
Eotaxin-1 and eotaxin-2 induce migration of CD34+ BM and blood eosinophils in vitro
Bone marrow and blood CD34+ CCR3+ eosinophils migrated in response to eotaxin-1 and eotaxin-2. Eotaxin-1 was the most effective chemokine of the two on BM CD34+ CCR3+ eosinophil migration (data not shown), whereas eotaxin-2 was the most potent in blood CD34+ CCR3+ eosinophil migration (Fig. 5b). Also the CD34− CCR3+ blood eosinophils migrated in response to eotaxin-2, but to a lesser extent (Fig. 5b).
Effects of systemic anti-CCR3 treatment
Intraperitoneal treatment with anti-CCR3 resulted in a significant reduction in BAL eosinophils compared with the isotype control-treated group (Table 1).The inhibitory effect of the anti-CCR3 antibody on BAL eosinophils was paralleled with a reduction in CD34+ eosinophil-lineage-committed cells in BAL regardless of dose administered (Fig. 6a). Furthermore, systemically administered anti-CCR3 treatment resulted in a significant reduction of BAL CD34+ Sca-1+ cell compared with the isotype control-treated group (Fig. 6b). In contrast, a non-significant reduction in BM eosinophils was found in the group treated with the highest dose of anti-CCR3 compared with the isotype control-treated group (Table 1). However, eosinophil-lineage-committed cells positive for CD34 (BM CD34+ cells) was significantly reduced after the anti-CCR3 treatment when compared with the control-treated group (Fig. 6c).
Table 1.
Eosinophils in bone marrow and bronchoalveolar lavage (BAL) after anti-CCR3 treatment
| Bone marrow eosinophils (% of total cells) | BAL eosinophils (× 104/ml) | |||
|---|---|---|---|---|
| Treatment | Immature | Mature | Total | |
| IgG2b | 7·2 (0·9) | 12·4 (1·2) | 19·6 (1·6) | 121·4 (15·6) |
| aCCR3 (30 μg i.p.) | 5·7 (1·1) | 10·2 (2·1) | 15·9 (3·0) | 48·7 (13·4)* |
| aCCR3 (100 μg i.p.) | 7·9 (1·1) | 11·6 (1·3) | 19·5 (2·5) | 44·7 (12·5)* |
| aCCR3 (300 μg i.p.) | 5·7 (0·7) | 8·5 (0·8) | 14·2 (1·0) | 13·3 (3·2)** |
Effect of in vivo treatment with isotype control (IgG2b) or anti-CCR3 in ovalbumin (OVA) -sensitized/exposed mice on bone marrow and BAL eosinophilia. Data are shown as mean (SEM) (n = 5–9, *P< 0·05 and **P< 0·01 for comparison between control and aCCR3 treatment; Mann–Whitney U-test).
Figure 6.
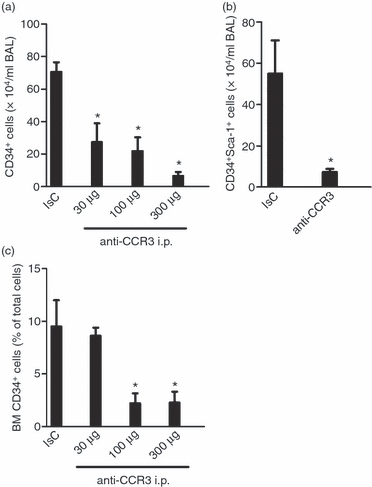
Effects of in vivo treatment with anti-CCR3 monoclonal antibody. Effect of in vivo treatment with the anti-CCR3 monoclonal antibody versus its isotype control (rat IgG2b) antibody, on (a) bronchoalveolar lavage (BAL) CD34+, (b) BAL CD34+ Sca1+ cells and (c) bone marrow (BM) CD34+ cells in ovalbumin (OVA) -sensitized/exposed BALB/c mice. The antibody was administered intraperitoneally (30–300 μg) 1 hr before exposure on the first day of exposure. Data are shown as mean (+ SEM) (n = 6–9). *P< 0·05 compared with the rat IgG2b-treated group.
Discussion
The present study provides evidence for a role of the CCR3/Eotaxin pathway in local proliferation and mobilization of CD34+ cells in the airways after allergen exposure. We have determined that CD34+ CCR3+ cells increase in BM, blood and airways after allergen exposure, and further demonstrated that allergen-induced newly produced eosinophil-lineage-committed (CD34+ CCR3+ BrdU+) lung cells have the capacity to proliferate in situ after allergen exposure. Significantly, IL-5 and eotaxin-2 each alone stimulated in vitro CFUs of lung CD34+ cells but not BM CD34+ cells. Moreover, delivery of eotaxin-2 to the airways of IL-5 transgenic mice resulted in a substantial increase of CD34+ cells in BAL and in vitro transmigration assays show that BM and blood CD34+ CCR3+ cells migrate in response to eotaxin-2. These data, together with our observations showing that systemic treatment with a depleting anti-CCR3 antibody abolished both CD34+ and Sca-1+ cells in airways to levels similar to control animals, suggest a role of this chemokine receptor in lung progenitor cells.
The present study showed that allergen-sensitized and allergen-exposed animals displayed a significant increase in CD34+ CCR3+ cells (relative to allergen-sensitized but saline-exposed animals) in not only the BM, but also in blood and airways. We further demonstrate that a proportion of the CD34+ CCR3+ cells in the airways stain positively for Sca-1, which confirms that some of these cells are likely to be haematopoietic stem cells. That is, Sca-1 is considered to be a stem cell marker, and has recently been shown to be involved in regulating the repopulation ability of haematopoietic stem cells in mice.28,29 Previously it had been shown that both immature and mature BM eosinophils express CCR3 and that the expression is higher in BM from patients with atopic asthma compared with controls, suggesting that there is an increased pool of CCR3+ immature and mature eosinophils available for rapid mobilization.14,30,31 In addition, the expression of CCR3 has been shown to be up-regulated during maturation of CD34+ cells to circulating eosinophils, suggesting a role in the trafficking of metamyelocytes to inflamed tissue.31 Furthermore, an increase in CD34+ cells in sputum has been reported in atopic asthmatic patients as well as in nasal polyp tissue.32 The increase of CD34+ cells in the nasal mucosa of patients during a pollen season, suggests that progenitors are recruited into the local airway tissue by allergen-dependent mechanisms; here they may differentiate into more mature cells within the site of allergic inflammation (i.e. in situ haematopoiesis).13,22,33–35 These parallel phenomena in allergic mice and asthmatics imply that the mouse model has relevance to the human disease in relation to eosinophil maturation and trafficking.
We employed a multiparametric cell cycle analysis assessed by FACS to assess whether the CD34+ newly produced eosinophil-lineage-committed cells proliferate locally within the airways in response to allergen. A significant increase of newly produced proliferating CD34+ eosinophil-lineage-committed cells in vivo after allergen exposure (compared with the saline-exposed animals) was identified. It is noteworthy that almost all lung cells that stained positively for CCR3 also co-expressed MBP, which further argues for the eosinophil-lineage commitment of these CD34+ CCR3+ cells. In addition, we cultured CD34+ lung cells to assess their capacity to form CFUs in vitro after incubation with rmIL-5 alone, rmEotaxin-2 alone, or with the combination of rmIL-5 and rmEotaxin-2. Surprisingly, a significant increase in CFUs compared with control was found in all three groups, arguing that eotaxin-2 itself can function as an eosinophilopoietic factor in the lung, expanding the previous findings that lung progenitors can produce IL-5-dependent CFUs in vitro.9,24 Studies in humans have suggested a role of eotaxin-1 in the differentiation of CD34+ cells towards eosinophils because cord-blood-derived CD34+ cells cultured in the presence of eotaxin-1 differentiate into eosinophils.21 Furthermore, we have previously shown that CD34+ cells release markedly more IL-5 compared with the CD34− eosinophils, suggesting that the airway CD34+ cells may play an autocrine role in their final maturation to eosinophils.9 In contrast, we were unable to detect any colony formation of BM CD34+ cells that were incubated with eotaxin-2 alone, suggesting that this chemokine only has haematopoietic function outside the BM. Taken together, these findings suggest that allergen-induced haematopoietic events do occur in the lung during allergen exposure, and that eotaxin-2 has haematopoietic effects alone or together with IL-5, primarily within the airways, whereas IL-5 has haematopoietic effects in the BM as well as in the lung.
CD34+ progenitors that co-express IL-5Rα are considered to be the earliest eosinophil-lineage-committed progenitor cell.4 CD34+ IL-5Rα+ cell numbers are increased in the mucosa of patients with atopic asthma compared with controls and CD34+ IL-5Rα+ as well as CD34+ CCR3+ cells have been shown to increase in BM, circulation and induced sputum in patients with allergic asthma compared with controls.4,12–14,36,37 The present study show that CD34+ CD45+ IL-5Rα+ eosinophil progenitors are increased in the airways after allergen exposure, confirming previous published data in mice and humans.4,22,36,37 However, we also demonstrate a significant increase in the proliferating IL-5Rα+ cells in vivo in the lung after allergen challenge. It is important to note that most of the IL-5Rα+ cells in the airways of allergen-exposed mice also co-expressed CCR3, which implies that these receptors may have complementary functions in the lung CD34+ cells. Additionally, we found a significant reduction in CD34+ Sca-1+ BAL cells in the animals treated with a CCR3-depleting antibody compared with the isotype control-treated group, confirming the role of CCR3 in the accumulation of airway CD34+ cells. Our finding of airway cells with stem cell markers such as CD34 and Sca-1 after allergen exposure, together with evidence of proliferation of lung CD34+ and Sca-1+ cells, further argues that eosinophilopoiesis can occur locally in the lung after allergen exposure.
A significant reduction in the CD34+ BM cells was found with the CCR3 antibody treatment, further verifying a role of the CCR3 receptor on CD34+ BM eosinophil-lineage-committed cells. Previously, it has been shown that combined systemic and local airway administration of this depleting anti-CCR3 mAb, abolish eosinophils from the airway lumen after allergen exposure38 and CCR3-deficient mice have a greatly reduced eosinophilic inflammatory response to allergen.39,40 A recent study shows that anti-CCR3 mAb treatment inhibits the migration and differentiation of mouse BM CD34+ cells in vitro.41 However, in the same study they used a depleting anti-CCR3 mAb, which induced antibody-mediated killing42 without any additional antagonistic activities, casting doubt on the conclusions noted in this paper.41
In conclusion, our study argues that the CCR3/eotaxin pathway is involved in both the regulation of allergen-driven in situ haematopoiesis as well as the accumulation of eosinophil-lineage-committed progenitor cells in the lung. These data further suggest that the development of therapeutic strategies directly targeting in situ lung eosinophilopoiesis may represent a novel approach in the treatment of asthma. Targeting CCR3, or alternatively eotaxin-1 and/or eotaxin-2, may be effective in reducing tissue progenitor cell proliferation and mobilization in allergen-induced airway eosinophilia.
Acknowledgments
In particular, the authors acknowledge DNAX, Palo Alto, CA for the rat anti-mouse CCR3 monoclonal antibody used in this study. The study was supported by the Swedish Medical Research Council (K2001-71X-13492-02B), the Swedish Heart Lung Foundation, and the Vårdal Foundation. Prof. Jan Lötvall is funded by the Herman Krefting’s foundation against Asthma/Allergy and AB from EAACI Research Fellow Exchange Scholarship.
Glossary
Abbreviations:
- 7-AAD
7-aminoactinomycin D
- BALF
bronchoalveolar lavage fluid
- BM
bone marrow
- IL-5Rα
interleukin-5 receptor α subunit
- MBP
major basic protein
- Sca-1
stem cell antigen-1
Disclosures
The authors have no financial conflict of interest.
References
- 1.Bousquet J, Chanez P, Lacoste JE, et al. Eosinophilic inflammation in asthma. N Engl J Med. 1990;323:1033–9. doi: 10.1056/NEJM199010113231505. [DOI] [PubMed] [Google Scholar]
- 2.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–63. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 3.Bradley BL, Azzawi M, Jacobson M, et al. Eosinophils, T-lymphocytes, mast cells, neutrophils and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. 1991;88:661–74. doi: 10.1016/0091-6749(91)90160-p. [DOI] [PubMed] [Google Scholar]
- 4.Sehmi R, Wood LJ, Watson R, Foley R, Hamid Q, O′Byrne PM, Denburg JA. Allergen-induced increases in IL-5 receptor α-subunit expression on bone marrow-derived CD34+ cells from asthmatic subjects A novel marker of progenitor cell commitment towards eosinophilic differentiation. J Clin Invest. 1997;100:466–75. doi: 10.1172/JCI119789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomaki M, Zhao LL, Lundahl J, Sjöstrand M, Jordana M, Linden A, O′Byrne PM, Lötvall J. Eosinophilopoiesis in a murine model of allergic airway eosinophilia: involvement of bone marrow IL-5 and IL-5 receptor α. J Immunol. 2000;165:4040–50. doi: 10.4049/jimmunol.165.7.4040. [DOI] [PubMed] [Google Scholar]
- 6.Palframan RT, Collins PD, Severs NJ, Rothery S, Williams TJ, Rankin SM. Mechanisms of acute eosinophil mobilization from the bone marrow stimulated by interleukin-5: the role of specific adhesion molecules and phosphatidylinositol 3-kinase. J Exp Med. 1998;188:1621–32. doi: 10.1084/jem.188.9.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanchet MR, McNagny KM. Stem cells, inflammation and allergy. Allergy Asthma Clin Immunol. 2009;5:13. doi: 10.1186/1710-1492-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inman MD, Ellis R, Wattie J, Denburg JA, O′Byrne PM. Allergen-induced increases in airway responsiveness, airway eosinophilia, and bone-marrow eosinophil progenitors in mice. Am J Respir Cell Mol Biol. 1999;21:473–9. doi: 10.1165/ajrcmb.21.4.3622. [DOI] [PubMed] [Google Scholar]
- 9.Sergejeva S, Johansson AK, Malmhäll C, Lötvall J. Allergen exposure-induced differences in CD34+ cell phenotype; relationship to eosinophilopoietic responses in different compartments. Blood. 2004;103:1270–7. doi: 10.1182/blood-2003-05-1618. [DOI] [PubMed] [Google Scholar]
- 10.Johansson AK, Sergejeva S, Sjöstrand M, Lee JJ, Lötvall J. Allergen-induced traffic of bone marrow eosinophils, neutrophils and lymphocytes to airways. Eur J Immunol. 2004;11:3135–45. doi: 10.1002/eji.200425043. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Satio H, Crawford L, Inman MD, Cyr MM, Denburg JA. Haemopoietic mechanisms in murine allergic upper and lower airway inflammation. Immunology. 2005;114:386–96. doi: 10.1111/j.1365-2567.2005.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson DS, Damia R, Zeibecoglou K, Molet S, North J, Yamada T, Kay AB, Hamid Q. CD34+/Interleukin-5Rα messenger RNA+ cells in the bronchial mucosa in asthma: potential airway eosinophil progenitors. Am J Respir Cell Mol Biol. 1999;20:9–13. doi: 10.1165/ajrcmb.20.1.3449. [DOI] [PubMed] [Google Scholar]
- 13.Sergejeva S, Malmhäll C, Lötvall J, Pullerits T. Increased number of CD34+ cells in nasal mucosa of allergic rhinitis patients: inhibition by a local corticosteroid. Clin Exp Allergy. 2005;35:34–8. doi: 10.1111/j.1365-2222.2004.02038.x. [DOI] [PubMed] [Google Scholar]
- 14.Sehmi R, Dorman S, Baatjes A, et al. Allergen-induced fluctuation in CC chemokine receptor 3 expression on bone marrow CD34+ cells from asthmatic subjects: significance for mobilization of haemopoietic progenitor cells in allergic inflammation. Immunology. 2003;109:536–46. doi: 10.1046/j.1365-2567.2003.01686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorman S, Babirad CI, Post J, Watson RM, Foley R, Jones GL, O’Byrne PM, Sehmi R. Progenitor egress from the bone marrow after allergen challenge: role of stromal cell-derived factor 1α and eotaxin. J Allergy Clin Immunol. 2005;115:501–7. doi: 10.1016/j.jaci.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Menzies-Gow A, Flood-Page P, Sehmi R, Burman J, Hamid Q, Robinson DS, Kay AB, Denburg J. Anti-IL-5 (mepolizumab) therapy induces bone marrow eosinophil maturational arrest and decreases eosinophil progenitors in the bronchial mucosa of atopic asthmatics. J Allergy Clin Immunol. 2003;111:714–9. doi: 10.1067/mai.2003.1382. [DOI] [PubMed] [Google Scholar]
- 17.Ying S, Meng Q, Zeibecoglou K, Robinson DS, Macfarlane A, Humbert M, Kay AB. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3) and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and non-atopic (Intrinsic) asthmatics. J Immunol. 1999;163:6321–9. [PubMed] [Google Scholar]
- 18.Lamkhioued B, Renzi PM, Abi-Younes S, et al. Increased expression of eotaxin in bronchoalveolar lavage and airways of asthmathics contributes to the chemotaxis of eosinophils to the sites of inflammation. J Immunol. 1997;159:4593–601. [PubMed] [Google Scholar]
- 19.Ying S, Robinson DS, Meng Q, et al. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol. 1997;27:3507–16. doi: 10.1002/eji.1830271252. [DOI] [PubMed] [Google Scholar]
- 20.Rådinger M, Johansson AK, Sitkuaskiene B, Sjöstrand M, Lötvall J. Eotaxin-2 regulates newly produced and CD34+ airway eosinophils after allergen exposure. J Allergy Clin Immunol. 2004;113:1109–16. doi: 10.1016/j.jaci.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Lamkhioued B, Abdelilah SG, Hamid Q, Mansour N, Delespesse G, Renzi PM. The CCR3 receptor is involved in eosinophil differentiation and is up-regulated by Th2 cytokines in CD34+ progenitor cells. J Immunol. 2003;170:537–47. doi: 10.4049/jimmunol.170.1.537. [DOI] [PubMed] [Google Scholar]
- 22.Rådinger M, Lötvall J. Eosinophil progenitors in allergy and asthma – do they matter? Pharmacol Ther. 2009;121:174–84. doi: 10.1016/j.pharmthera.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Lee NA, McGarry MP, Larson KA, Horton MA, Kristensen AB, Lee JJ. Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J Immunol. 1997;158:1332–44. [PubMed] [Google Scholar]
- 24.Southam DS, Widmer N, Ellis R, Hirota JA, Inman MD, Sehmi R. Increased eosinophil-lineage committed progenitors in the lung of allergen-challenged mice. J Allergy Clin Immunol. 2005;115:95–102. doi: 10.1016/j.jaci.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 25.Kuo HP, Wang CH, Lin HC, Hwang KS, Liu SL, Chung KF. Interleukin-5 in growth and differentiation of blood eosinophil progenitors in asthma: effect of glucocorticoids. Br J Pharmacol. 2001;134:1539–47. doi: 10.1038/sj.bjp.0704389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang M, Hogan SP, Mahalingam S, et al. Eotaxin-2 and IL-5 cooperate in the lung to regulate IL-13 production and airway eosinophilia and hyperreactivity. J Allergy Clin Immunol. 2003;112:935–43. doi: 10.1016/j.jaci.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 27.Crosby JR, Shen HH, Borchers MT, Justice JP, Ansay T, Lee JJ, Lee NA. Ectopic expression of IL-5 identifies an additional CD4+ T cell mechanism of airway eosinophil recruitment. Am J Physiol Lung Cell Mol Physiol. 2002;282:99–108. doi: 10.1152/ajplung.2002.282.1.L99. [DOI] [PubMed] [Google Scholar]
- 28.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 29.Ito CY, Li CY, Bernstein A, Dick JE, Stanford WL. Hematopoietic stem cell and progenitor defects in Sca-1/Ly-6A-null mice. Blood. 2003;101:517–23. doi: 10.1182/blood-2002-06-1918. [DOI] [PubMed] [Google Scholar]
- 30.Zeibecoglou K, Ying S, Yamanda T, et al. Increased mature and immature CCR3 messenger RNA+ eosinophils in bone marrow from patients with atopic asthma compared with atopic and non-atopic control subjects. J Allergy Clin Immunol. 1999;103:99–106. doi: 10.1016/s0091-6749(99)70532-4. [DOI] [PubMed] [Google Scholar]
- 31.Robinson DS, North J, Zeibecoglou K, et al. Eosinophil development and bone marrow and tissue eosinophils in atopic asthma. Int Arch Allergy Immunol. 1999;118:98–100. doi: 10.1159/000024039. [DOI] [PubMed] [Google Scholar]
- 32.Sehmi R, Howie K, Sutherland DR, Schragge W, O′Byrne PM, Denburg JA. Increased levels of CD34+ hemopoietic progenitor cells in atopic subjects. Am J Respir Cell Mol Biol. 1996;15:645–55. doi: 10.1165/ajrcmb.15.5.8918371. [DOI] [PubMed] [Google Scholar]
- 33.Wood LJ, Inman MD, Denburg JA, O′Byrne PM. Allergen challenge increases cell traffic between bone marrow and lung. Am J Respir Cell Mol Biol. 1998;18:759–67. doi: 10.1165/ajrcmb.18.6.3006. [DOI] [PubMed] [Google Scholar]
- 34.Denburg JA. Bone marrow in atopy and asthma: hematopoietic mechanisms in allergic inflammation. Immunol Today. 1999;20:111–3. doi: 10.1016/s0167-5699(98)01423-6. [DOI] [PubMed] [Google Scholar]
- 35.Cameron L, Christodoulopoulos P, Lavinge F, et al. Evidence for local eosinophil differentiation within allergic nasal mucosa: inhibition with soluble IL-5 receptor. J Immunol. 2000;164:1538–45. doi: 10.4049/jimmunol.164.3.1538. [DOI] [PubMed] [Google Scholar]
- 36.Dorman SC, Sehmi R, Gauvreau GM, et al. Kinetics of bone marrow eosinophilopoiesis and associated cytokines after allergen inhalation. Am J Respir Crit Care Med. 2004;169:565–72. doi: 10.1164/rccm.200307-1024OC. [DOI] [PubMed] [Google Scholar]
- 37.Dorman SC, Efthimiadis A, Babirad I, Watson RM, Denburg JA, Hargreave FE, O′Byrne PM, Sehmi R. Sputum CD34+ IL-5Rα+ increase after allergen: evidence for in situ eosinophilopoiesis. Am J Respir Crit Care Med. 2004;169:573–7. doi: 10.1164/rccm.200307-1004OC. [DOI] [PubMed] [Google Scholar]
- 38.Justice JP, Borchers MT, Crosby JR, et al. Ablation of eosinophils leads to a reduction of allergen-induced pulmonary pathology. Am J Physiol Lung Cell Mol Physiol. 2003;284:L169–78. doi: 10.1152/ajplung.00260.2002. [DOI] [PubMed] [Google Scholar]
- 39.Humbles AA, Lu B, Friends DS, et al. The murine CCR3 receptor regulated both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci USA. 2002;99:1479–84. doi: 10.1073/pnas.261462598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pope SM, Zimmermann N, Stringer K, Karow M, Rothenberg ME. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol. 2005;175:5341–50. doi: 10.4049/jimmunol.175.8.5341. [DOI] [PubMed] [Google Scholar]
- 41.Ben S, Li X, Xu F, et al. Treatment with anti-CC chemokine receptor 3 monoclonal antibody or dexamethasone inhibits the migration and differentiation of bone marrow CD34+ progenitor cell in an allergic mouse model. Allergy. 2008;63:1164–76. doi: 10.1111/j.1398-9995.2008.01747.x. [DOI] [PubMed] [Google Scholar]
- 42.Grimaldi JC, Yu NX, Gruning G, et al. Depletion of eosinophils in mice through the use of antibodies specific for C-C chemokine receptor 3 (CCR3) J Leukoc Biol. 1999;65:846–53. doi: 10.1002/jlb.65.6.846. [DOI] [PubMed] [Google Scholar]


