Abstract
Dietary freeze-dried black raspberries (BRBs) inhibit N-nitrosomethylbenzylamine (NMBA)-induced tumorigenesis in the Fischer 344 rat esophagus. To determine the mechanistic basis of the anti-initiating effects of BRBs, NMBA metabolism was studied in esophageal explant cultures and in liver microsomes taken from rats fed with AIN-76A diet or AIN-76A diet containing 5% or 10% BRBs. Five percent and 10% dietary BRBs inhibited NMBA metabolism in explants (26% and20%) and in microsomes (22% and28%), but the inhibition was not dose dependent. To identify active inhibitory component(s) in BRBs, esophageal explants and liver microsomes from control rats were treated in vitro with an ethanol extract of BRBs or with individual components of BRBs [ellagic acid (EA) and two anthocyanins (cyanidin-3-glucoside and cyanidin-3-rutinoside)]. NMBA metabolism in explants was inhibited maximally by cyanidin-3-rutinoside (47%) followed by EA (33%), cyanidin-3-glucoside (23%),and the extract (11%). Similarly, in liver microsomes, the inhibition was maximal by cyanidin-3-rutinoside (47%) followed by EA (33%) and cyanidin-3-glucoside (32%). Phenylethylisothiocyanate (PEITC), a potent inhibitor of NMBA tumorigenesis in rat esophagus, was a stronger inhibitor of NMBA metabolism in vivo and in vitro than BRBs or their components. Dietary BRBs and PEITC induced glutathione S-transferase activity in the liver.
Introduction
Esophageal cancer is the third most common gastrointestinal malignancy and the sixth most frequent cause of cancer death in the world (1). Squamous cell carcinoma (SCC) is the predominant histological subtype of human esophageal cancer worldwide and is characterized by a high mortality rate (2,3). The disease has a complex etiology with a significant behavioral component. Chemoprevention can play an integral role in the overall strategy toward reducing the incidence of cancer and has emerged as a potentially viable approach to reduce the risk of esophageal cancer in high-risk individuals. For the prevention of esophageal cancer, inclusion in the diet of adequate quantities of fruits and vegetables is also important because these foodstuffs can provide inhibitory micronutrients such as polyphenols, phytohormones, vitamins, and minerals (4).
Several investigations suggest that certain nitrosamine carcinogens present in tobacco smoke and in the diet are causative agents for esophageal SCC in humans (5–7). Nitrosamine carcinogens have been shown to induce cancers of the esophagus in rodents (8). In addition, tobacco and alcohol act synergistically in the development of esophageal squamous carcinoma and possibly adenocarcinoma in humans (9,10). Factors determining individual susceptibility to esophageal cancer are still largely unclear. An imbalance between phase I enzymes, involved in carcinogen activation [for example, cytochrome P450 (CYP)], and phase II enzymes, involved in carcinogen detoxification [for example, glutathione S-transferase (GST)], may contribute to the development of the disease. It is well established that most chemical carcinogens require metabolic activation before exerting their carcinogenic effects. The activated carcinogens are usually electrophilic agents and are highly reactive toward DNA molecules. DNA modifications, especially those in oncogenes and tumor suppressor genes, are generally major driving forces for cancer development (11–14). Because CYP enzymes play key roles in the activation of most carcinogens, the inhibition of CYP-dependent carcinogen activation, especially by dietary chemicals, has been studied extensively (4).
GSTs are a family of dimeric biotransformation enzymes involved in the detoxification of (oxygen) radicals (15). They catalyze the binding of a variety of electrophiles to the sulfhydryl group of glutathione and have an important role in the detoxification of carcinogens (15). GSTs are present in all species and tissues and also in relatively large amounts in the epithelial tissues of the human gastrointestinal tract (16–19). A significant inverse correlation has been demonstrated between GST enzyme activity and tumor incidence in the mucosa along the human gastrointestinal tract, suggesting the importance of GSTs in tumor prevention (19).
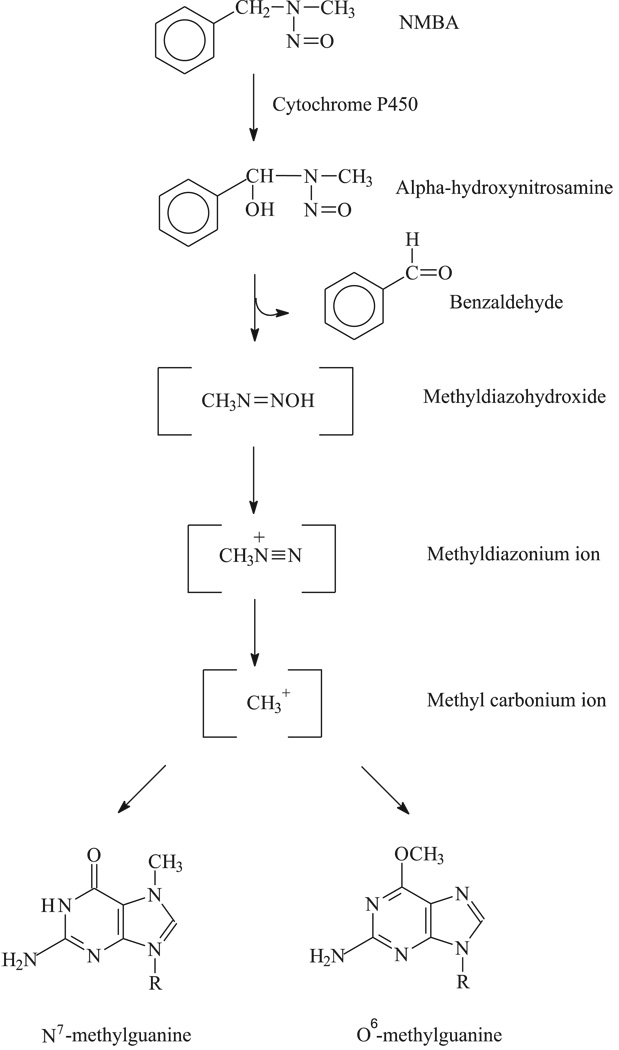
The rat esophagus in which tumors are induced by the nitrosamine carcinogen N-nitrosomethylbenzylamine (NMBA) has been used extensively for studies of the molecular mechanism(s) involved in the development of esophageal SCC as well as for the identification of chemopreventive agents for this disease (20–24). Nitrosamines are metabolically activated by CYP enzymes to exert their carcinogenic effect (5,24). The predominant pathway for nitrosamines is considered to be hydroxylation at the α-carbon atom, an activation step that ultimately yields an alkylating agent and an aldehyde in stoichiometeric amounts (25). The alkylating agent can react with tissue nucleophiles or with water to form corresponding alcohols. Like other nitrosamines, NMBA requires CYP-dependent activation. NMBA metabolism in esophageal tissue involves α-hydroxylation exclusively of the methylene carbon atom at the N-nitroso group (25). This activation results in the formation of benzaldehyde and reactive methyl diazonium ions. The reactive intermediate forms both N7 and the highly mutagenic O6 adducts on guanine (Fig. 1) (26,27). Hepatic microsomal metabolism of NMBA occurs either at the methyl carbon or the methylene carbon of the benzyl moiety (25).
Figure 1.
Hydroxylation pathway of N-nitrosomethylbenzylamine (NMBA) metabolism at the methylene carbon in the rat esophagus. NMBA metabolism involving α-hydroxylation at the methylene carbon atom at the N-nitroso group results in the formation of benzaldehyde and reactive methyl diazonium ions. The reactive intermediate forms both N7 and the highly mutagenic O6 adduct on guanine.
Our laboratory has demonstrated the chemopreventive effect of dietary lyophilized black raspberries (BRBs) in NMBA-induced tumorigenesis in the rat esophagus (21). The berries reduce NMBA-induced O6-methylguanine (O6-meGua) formation in esophageal DNA. Based on this observation, the present studies were conducted to determine the mechanism(s) of the anti-initiating effects of BRBs and their components against NMBA-induced esophageal carcinogenesis. The specific CYPs involved in NMBA metabolism leading to tumorigenesis in the rat esophagus have not been fully identified. CYPs that may be involved in NMBA metabolism are CYP 2A3 in rat esophagus and 2E1 in liver (28,29). Modulation of NMBA metabolism in different nontarget tissues such as the liver may also result in a reduced amount of NMBA available in the target tissue (esophagus).We propose that inhibition of NMBA-induced tumorigenesis by dietary BRBs may be due to 1) inhibition of CYPs involved in NBMA activation by BRBs components, 2) modulation of NMBA metabolism in other nontarget tissues (such as liver), and 3) induction of detoxifying enzymes (GST), leading to enhanced clearance of the reactive metabolites of NMBA from the system and a reduced level of these metabolites in the target tissue. Any or all of these effects of BRBs components would reduce NMBA-induced DNA damage in the esophagus.
Our results show that dietary BRBs and phenylethylisothiocyanate (PEITC) inhibit NMBA activation in esophageal explants and in liver microsomes of Fischer 344 (F344) rats and also induce GST activity in the liver, which could result in enhanced detoxification of NMBA. An ethanol extract of BRBs and known components of BRBs, that is, ellagic acid (EA) and anthocyanins (cyanidin-3-glucoside and cyanidin-3-rutinoside), modulated NMBA metabolism differentially in esophageal explants as well as in liver microsomes.
Materials and Methods
Chemicals
[3H]-NMBA was purchased from Moravek Biochemicals (Brea, CA) and purified by Dr. Dhimant Desai (Pennsylvania State University, Hershey, PA). An ethanol extract of BRBs was supplied by Dr. Stephen Hecht (University of Minnesota Cancer Center, Minneapolis, MN). EA and PEITC were purchased from Aldrich Chemical Company (Milwaukee, WI), and the anthocyanins cyanidin-3-glucoside and cyanidin-3-rutinoside were from Indofine Chemical Company (Hillsborough, NJ).
Ethanol Extract of BRBs
A Soxhelt extraction procedure was used to obtain the ethanol extract of lyophilized BRBs (Hecht et al., manuscript in preparation).
Animals
All experimental protocols were in accordance with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee of The Ohio State University. Four- to 5-wk-old male F344 rats, purchased from Harlan Sprague-Dawley (Indianapolis, IN), were housed three per cage under standard conditions (20 ± 2°C, 50 ± 10% relative humidity, 12-h light/dark cycles). Modified AIN-76A–purified diet (Dyets, Bethlehem, PA) and water were provided ad libitum.
Diet Preparation
Fresh frozen BRBs of the Jewel variety were purchased from the Stokes Fruit Farm (Wilmington, OH). Berries were shipped frozen from the fruit farm to Van Drunen Farms (Momence, IL) for freeze-drying. The methods for freezedrying BRBs have been described (20,21). After freeze-drying, the berries were ground into a powder, and the powder was shipped frozen to the Ohio State University where it was stored at −20°C. Diets containing 5% or 10% freeze-dried BRBs or 5 µmol PEITC were prepared weekly by mixing into modified AIN-76A diet for 20 min using a Hobart mixer and stored at 4°C until fed to the animals. The starch in the 5% and 10% BRB diet was reduced by 5% or 10% to insure an isocaloric diet. Diets were administered in glass feeding jars to allow for weekly food consumption measurements.
NMBA Metabolism in Esophageal Explant Cultures
The effect of dietary BRBs and PEITC on NMBA metabolism was studied in esophageal explant cultures and in liver microsomes prepared from BRB- and PEITC-treated rats. Rats were randomized and divided into groups of six so that they received AIN-76A diet or AIN-76A diet containing either 5% or 10% BRBs or 5 µmol PEITC. The animals were maintained on their respective diets for 3 wk, after which they were euthanized and their livers and esophagi excised. The livers were snap-frozen in liquid nitrogen and the esophagus removed aseptically. Food intake and body weight data were not taken during the 3 wk of feeding because earlier experiments with BRBs and PEITC at the same dietary levels showed no effect on these parameters (20,21,30).
The esophagi were immersed in Leibovitz’s L-15medium (GIBCO, BRL, Rockville, MD) supplemented with penicillin (100 units/ml) and streptomycin (100 µg/ml). Explant cultures were prepared as described earlier (31). Briefly, individual esophagi were opened longitudinally and cut into small explants of approximately 3 mm. The explants were placed in 60-mm tissue culture dishes with the mucosal side uppermost. Two milliliters of low-calcium PFMR-4* medium (BioSource International, Camarillo, CA) supplemented with growth factors as described before was added to each dish (30). The explants were placed in a CO2 incubator maintained at 37°C for 30min to allow the explants to adhere to the surface of the dish. The medium was removed and fresh medium containing 1 µCi/ml of [3H]NMBA was added to the explants. The explants were then placed in an incubation chamber gassed with a mixture of 50% O2, 45% N2, and 5% CO2 and maintained at 37°C with gentle rocking to allow the culture to be bathed inmedium and exposed alternately to the O2 in the chamber. After 4 h of incubation, 500 µl of medium was collected from each explant culture and filtered through an Acrodisc (0.2-µm pore size; Gilman Sciences, Ann Arbor, MI) and analyzed for unmetabolized [3H]NMBA and metabolites of NMBA by reverse-phase high-performance liquid chromatography (HPLC) as described earlier (25,31). The HPLC system used for these analyses consisted of a gradient controller, two 510 pumps, a C18 column, a 484 UV detector, an Eppendorf column heater (all from Millipore Corp., Bedford, MA), and a β-Ram radioflow detector (IN/US Systems, Inc., Tampa, FL). Fifty microliters of each sample of filtered medium was analyzed along with authentic standards for NMBA, benzyl alcohol, benzaldehyde, and benzoic acid. Samples were eluted with 2.4% acetic acid (pH 3.7) in 20% acetonitrile. The eluent flow rate was 1 ml/min, and the scintillation cocktail flow rate through the radioflow detector was 2 ml/min. Detection of eluates was by UV absorption at 254 nm and flow-through radioactivity. Major peaks of radioactivity were tagged, and the area under each peak was expressed as a percent of total radioactivity in the 50-µl sample (31). The results from three independent culture experiments were averaged.
The effects of the ethanol extract of BRBs and individual components known to be present in BRBs on NMBA metabolism were investigated in esophageal explant cultures from rats fed control AIN-76 diet. Explant cultures were prepared as described previously and pretreated with either the ethanol extract of BRBs (100 µg/ml medium), EA (100 µM), cyanidin-3-glucoside (100 µM), cyanidin-3-rutinoside (100 µM), or PEITC (100 µM) and incubated for 30 min in the rocking organ chamber as described previously. Cultures were treated with 1 µCi/ml of [3H]NMBA and incubated for an additional 4 h. NMBA metabolism was analyzed as described previously.
NMBA Metabolism in Hepatic Tissue
Livers of rats treated with BRBs and PEITC diets as described previously were used to investigate the dietary effect of BRBs and PEITC on NMBA metabolism in the liver. Microsomal and cytosolic fractions were prepared according to Smith et al. (32) and stored at −80°C until used for study. Microsomes (0.5–1.0 mg) were added to a reaction mixture containing 50 mM Tris-HCl (pH 7.4), 0.5 mM NADPH, 7.5 mM MgCl2, and 2.5 mM ethylenediaminetetraacetic acid (EDTA) in a total volume of 250 µl. The reaction was started with the addition of 1 µCi/ml of [3H]NMBA and incubated for 45 min. The reaction was terminated by the addition of 50 µl of 60% trichloroacetic acid. The reaction mixture was centrifuged at 2,000 g for 5 min, and the supernatant of each sample was filtered through 0.2-µm Nanosep filters and analyzed for NMBA and its metabolites by reverse-phase HPLC.
The effect of the ethanol extract of BRBs and individual components known to be present in BRBs on NMBA metabolism in vitro was investigated in liver microsomes from rats fed control AIN-76 diet. Microsomes (0.5–1.0 mg) were added to the reaction mixture and pre-incubated for 10 min at 37°C with either the ethanol extract of BRBs (100 µg/ml), EA (100 µM), cyanidin-3-glucoside (100 µM), or cyanidin-3-rutinoside (100 µM). To the reaction mixture 1 µCi/ml of [3H] NMBA was added, and the reaction was started by the addition of 0.5 mM NADPH. Other conditions were as described previously.
Glutathione S-Transferase Activity
The dietary effect of BRBs and PEITC on GST activity in liver microsomal and cytosolic fractions was investigated. GST activity toward the substrate 1-chloro-2,4-dinitrobenzene (CDNB) was determined according to Habig et al. (33). The standard assay system in a final volume of 900 µl contained 90 mM KPO4 buffer + 1 mM EDTA at pH 6.5, 5 mM glutathione (GSH), 1 mM CDNB, and 70–100 µg probe. The reaction was started with the addition of either liver cytosol or microsomes, and the initial linear change in optical density per minute at 340λwavelength was recorded in a Spectramax M2 (Molecular Devices, Sunnywale, CA). The nonenzymatic rate was measured without the biological probe. Enzyme activity was calculated by using a molar extinction co-efficient of 9.6 µM × cm−1 for CDNB.
Statistical Analysis
Analysis of variance was applied to test for significant differences (P < 0.05) between treatment groups with log of counts per minute as the response. The least significance difference test was used to adjust for multiple comparisons.
Results
Effect of Dietary BRBs and PEITC on NMBA Metabolism in Esophageal Explant Cultures
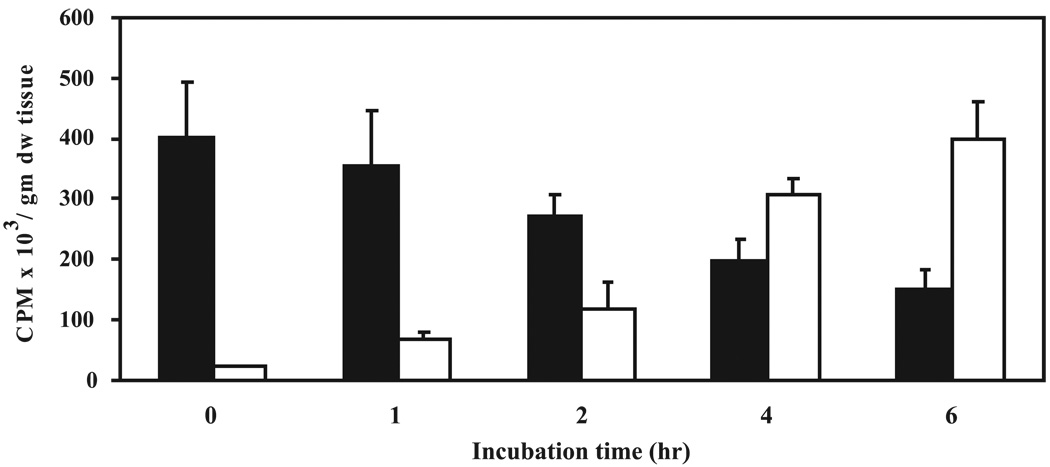
A preliminary experiment was conducted to investigate the time-dependent metabolism of NMBA in esophageal explant cultures from control rats by collecting medium at different times and analyzing by HPLC for NMBA and its metabolites. NMBA metabolism was found to be time dependent with more than 70–80% of the compound metabolized within 4–6 h. Formation of benzoic acid, the major metabolite of NMBA, in the esophagus also increased in a time-dependent manner (Fig. 2). Benzyl alcohol remained almost constant, whereas no benzaldehyde was detected in any of the medium aliquots in the study. Therefore, we routinely analyzed [3H]NMBA metabolism in our esophageal explant cultures after 4 h of incubation.
Figure 2.
Time-dependent metabolism of N-nitrosomethylbenzylamine (NMBA) by esophageal explant cultures. Esophagi from control rats were excised and aseptically removed to prepare the explant cultures. The cultures were treated with 1 µCi/ml [3H NMBA and incubated for different time periods. Medium was removed and analyzed by high-performance liquid chromatography for unmetabolized NMBA(filled bars) and benzoic acid formation (empty bars) as explained in Materials and Methods. Each bar represents mean ± SD from four rats.
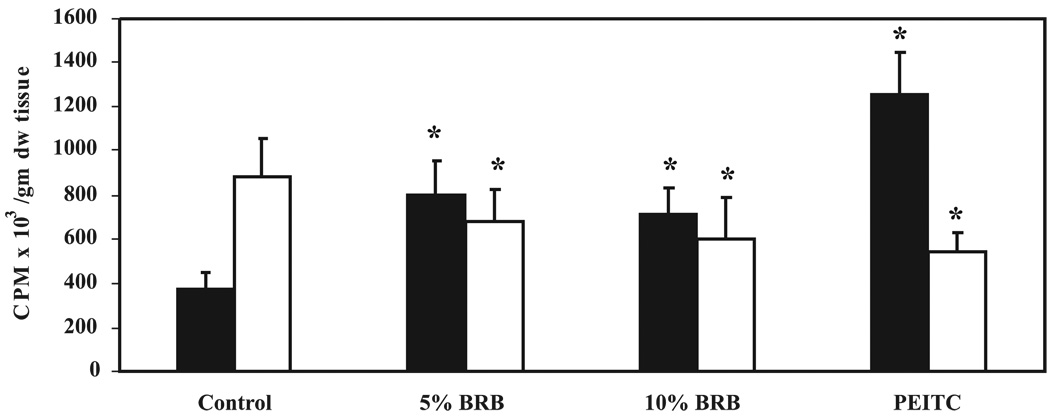
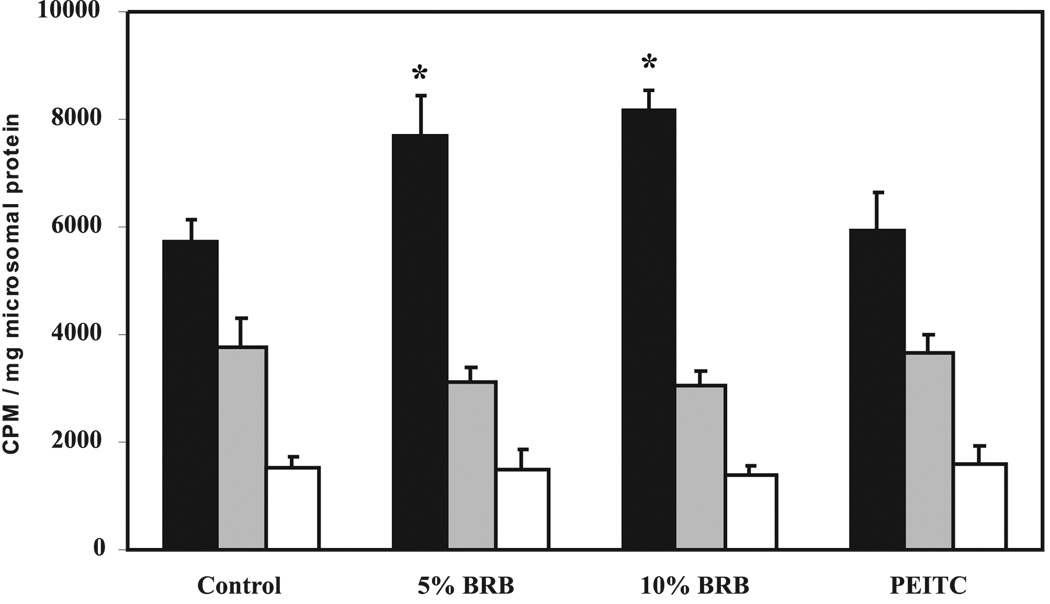
Esophageal explant cultures prepared from rats fed AIN-76A diet or AIN-76A containing either 5% or 10% BRBs or 5 µmol PEITC for 3 wk were incubated with 1 µCi/ml of [3H]NMBA for 4 h, and media aliquots were analyzed for NMBA metabolism by HPLC. Media in explant cultures prepared from control rats contained less unmetabolized NMBA than media in explant cultures from rats fed either 5% or 10% BRBs or 5 µmol PEITC. Dietary 5% and 10% BRBs or 5 µmol PEITC inhibited NMBA metabolism by 26%, 20%, and 53%, respectively, when compared with control rats (Fig. 3). The inhibition of NMBA metabolism in cultures prepared from rats treated with 5% and 10% BRBs was not dose dependent. However, the inhibition caused by both berry concentrations, as well as by PEITC, was highly significant (P < 0.001). We also detected radioactive peaks that co-eluted with the two known metabolites of NMBA, benzyl alcohol and benzoic acid. Formation of benzoic acid was consistent with changes in NMBA utilization (Fig. 3). Media from the explant cultures of animals fed either 5% or 10% BRBs or 5 µmol PEITC contained less benzoic acid, the final product of NMBA metabolism, than media from animals fed with the control diet (Fig. 3). The difference in benzoic acid formation in treated and control rats was significant (P < 0.05). The radioactive peak that co-eluted with benzyl alcohol was nearly the same in all groups (data not shown) suggesting its rapid conversion to benzoic acid. Taken together, these data suggest that 3 wk of dietary administration of 5% or 10% BRBs decreased the metabolism of NMBA in explant cultures of rat esophagus. This effect of BRBs on NMBA metabolism was not dose related.
Figure 3.
Effect of dietary 5%or 10% black raspberry (BRB) or 5 µmol phenylethylisothiocyanate (PEITC) on the N-nitrosomethylbenzylamine (NMBA) metabolism in esophageal explant cultures. Explant cultures of esophagi from rats fed with control diet or diets containing 5% or 10% BRBs or 5 µmol PEITC for 3 wk were prepared. The cultures were treated with 1µCi/ml [3H]NMBA and incubated for 4 h as described in Materials and Methods. Medium was analyzed for the unmetabolized NMBA (filled bars) and benzoic acid formation (empty bars) as described in Fig. 1. Each bar represents mean values ± SD (n = 10 rats) from three independent experiments. *Statistically significant difference from control group (P < 0.05).
Effect of Different Components of BRBs on the Metabolism of NMBA in Esophageal Explant Cultures
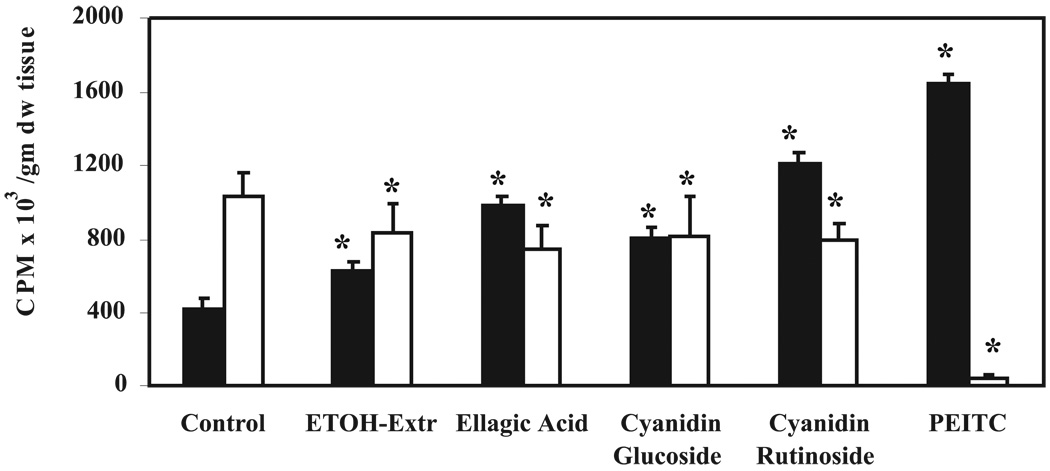
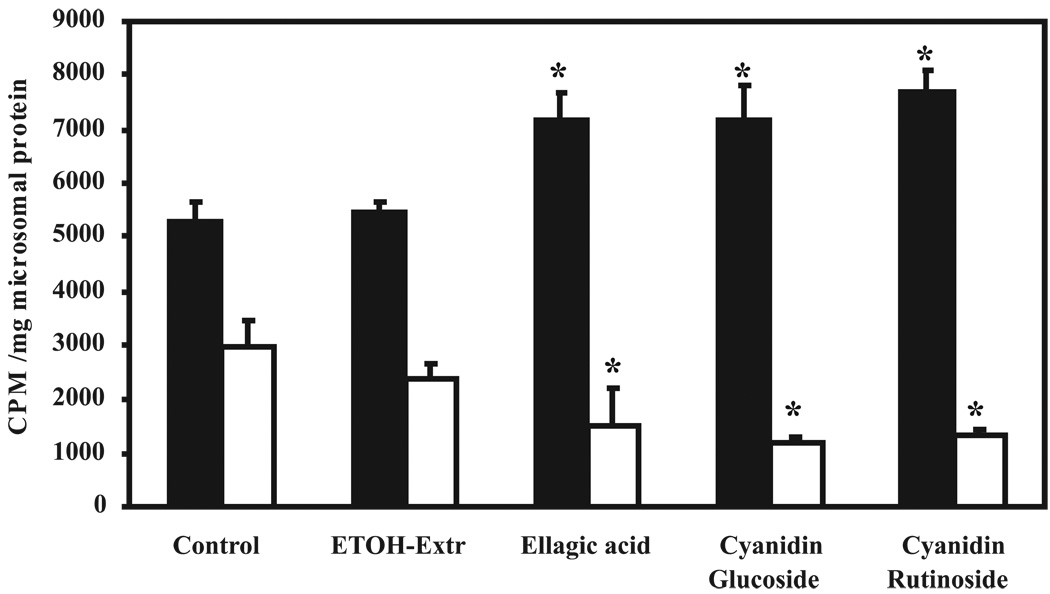
To identify the active inhibitory component(s)of BRBs that influence NMBA metabolism, another series of experiments was performed. Esophageal explants from control rats were cultured and treated in vitro with either an ethanol extract of BRBs (100 µg/ml), EA (100 µM), cyanidin-3-glucoside (100 µM), cyanidin-3-rutinoside (100 µM), or PEITC (100 µM) and incubated with 1 µCi/ml [3H]NMBA for 4 h. As observed previously, 75% of [3H]NMBA was metabolized in untreated esophageal explants. [3H]NMBA metabolism was highly significantly (P<0.001) inhibited in explants treated with PEITC (72%) followed by cyanidin-3-rutinoside (47%), EA (33%), cyanidin-3-glucoside (23%), and the ethanol extract of BRBs (11%) (Fig. 4).The production of benzoic acid correlated with the utilization of NMBA (Fig. 4).
Figure 4.
Effect of isolated components known to be present in black raspberry (BRBs) on N-nitrosomethylbenzylamine (NMBA) metabolism in esophageal explant cultures. Esophagi from control rats were excised and aseptically removed to prepare explant cultures. The cultures were treated with the ethanol extract of BRBs (100 µg/ml), ellagic acid (100 µM), cyaniding-3-glucoside (100 µM), cyaniding-3-rutinoside (100 µM), or phenylethylisothiocyanate (100 µM) and incubated for 30 min. Other conditions are as explained in Fig. 2. Medium was analyzed for unmetabolized NMBA (filled bars) and benzoic acid formation (empty bars). Each bar represents mean values ± SD(n = 9 rats) from three independent experiments. *Statistically significant difference from control group (P < 0.05).
Effect of Dietary 5% and 10% BRBs and 5 mol PEITC on the Metabolism of NMBA by Liver Microsomes
Although liver is a nontarget organ of NMBA carcinogenesis, hepatic metabolism of NMBA has been reported (34). Because NMBA is administered subcutaneously to induce esophageal tumorigenesis, much of the dose likely encounters hepatic cells prior to delivery to the esophagus. Although liver metabolizes NMBA, it does not do so as efficiently as the esophagus. Modulation of hepatic metabolism of NMBA, therefore, may lead to different levels of carcinogen in the target organ, thereby affecting the induction of tumorigenesis.
Liver microsomes from rats fed AIN-76A diet containing 5% or 10% BRBs or 5 µmol PEITC for 3 wk were prepared and incubated with 1 µCi/ml [3H]NMBA (Fig. 5). Microsomes from control rats metabolized about 50% of the available NMBA in vitro. Dietary 5% and 10% BRBs inhibited NMBA metabolism in the microsomes by 22% and 28%, respectively, whereas PEITC had no effect on NMBA metabolism (Fig. 5). Unlike esophageal explants, the major metabolite of NMBA formed by liver microsomes was benzyl alcohol and not benzoic acid. Formation of benzoic acid remained constant in all treatments, whereas production of benzyl alcohol correlated with the utilization of NMBA.
Figure 5.
Effect of dietary 5% or 10% black raspberry (BRB) or 5 µmol phenylethylisothiocyanate (PEITC) on N-nitrosomethylbenzylamine (NMBA) metabolism in liver microsomes. Liver microsomes from rats fed with AIN-76A diet or AIN-76A containing 5% or 10% BRBs or 5 µmol PEITC for 3 wk were incubated with 1 µCi/ml [3H]NMBA for 1 h, and a metabolic profile of NMBA (filled bars), benzyl alcohol (shaded bars), and benzoic acid (empty bars) was determined by high-performance liquid chromatography. Data are mean values ± SD (n = 9) from three independent experiments. *Statistically significant difference from control group (P < 0.05).
Effect of Different Components of BRBs on NMBA Metabolism by Liver Microsomes
Liver microsomes from control rats were treated in vitro with either the ethanol extract of BRBs (100 µg/ml), EA (100 µM), cyanidin-3-glucoside (100 µM), or cyanidin-3-rutinoside (100 µM) and incubated with 1 µCi/ml [3H]NMBA. As observed previously, 50% of [3H]NMBA was metabolized by untreated microsomes. NMBA metabolism was inhibited maximally by microsomes treated with cyanidin-3-rutinoside (47%) followed by EA (33%), cyanidin-3-glucoside (32%), and the ethanol extract of BRBs (11%) compared with untreated microsomes (Fig. 6). Inhibition by cyanidin-3-rutinoside, EA, and cyanidin-3-glucoside was significant (P < 0.05). The increase in NMBA metabolism resulted in an increased formation of benzyl alcohol (Fig. 6).
Figure 6.
Effect of isolated components known to be present in black raspberry (BRB) on N-nitrosomethylbenzylamine (NMBA) metabolism in liver microsomes. Microsomes from control rats were pre-incubated for 10 min with the ethanol extract of BRBs (100 µg/ml), ellagic acid (100 µM), cyanidin-3-glucoside (100 µM), or cyanidin-3-rutinoside (100 µM). Reaction was started with 1 µCi/ml [3H]NMBA and incubated for 1 h at 37°C. NMBA (filled bars) and benzyl alcohol (empty bars) were analyzed as explained in Fig. 5. Data are mean ± SD (n = 9) from three separate experiments. *Statistically significant difference from control group (P < 0.05).
Effect of Dietary BRBs and PEITC on Glutathione S-Transferase Activity in the Liver of F344 Rats
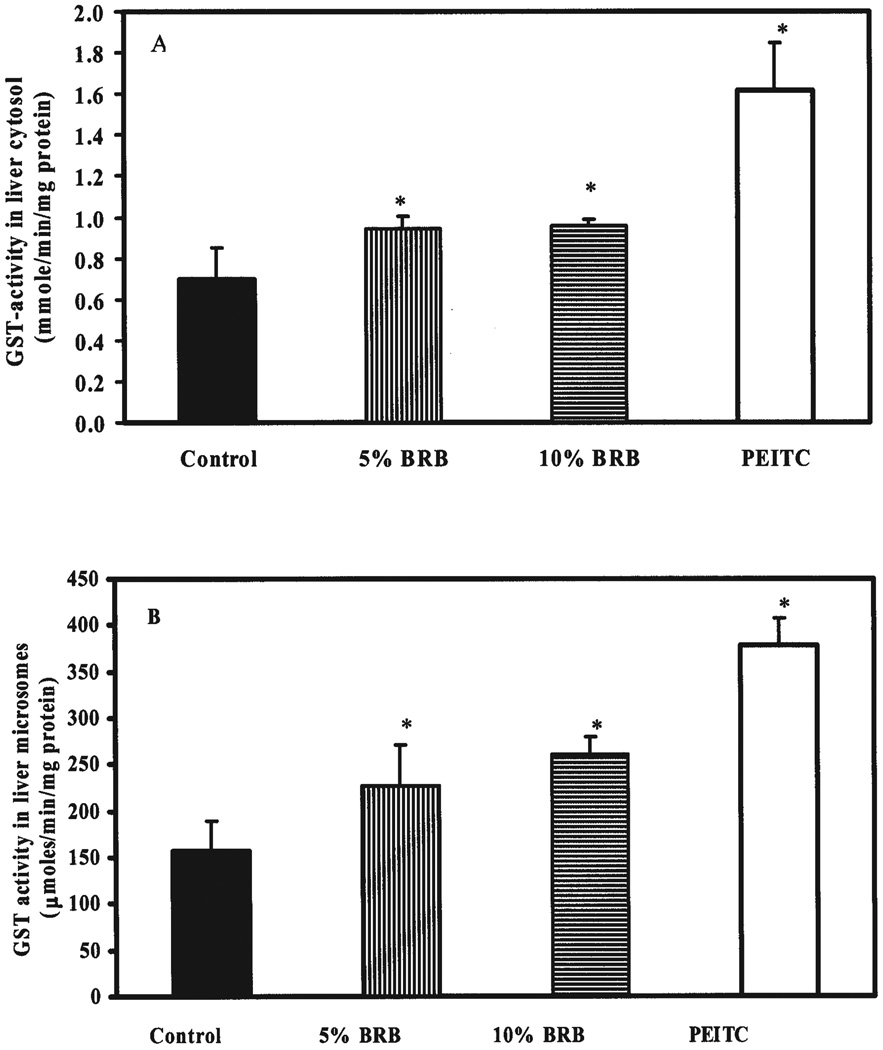
The role of phase II enzymes in the metabolism of NMBA has not been fully determined. Although phase II conjugates do not appear to be major metabolites, there have been reports in the literature of the formation of both glucuronide and glutathione conjugates of metabolites generated by NMBA activation pathways (35). Therefore, we studied the enzymatic activity of the phase II enzyme GST using the substrate CDNB in both liver cytosol and microsomes from rats fed either AIN-76A diet or AIN-76A containing 5% or 10% BRBs or 5 µmol PEITC. Results indicate that the enzymatic activity of GST was induced maximally by dietary PEITC (147%) followed by 10% BRBs (47%) and 5% BRBs (45%) in liver cytosols (Fig. 7A) and by 155%, 67%, and 46% in liver microsomes, respectively (Fig. 7B).
Figure 7.
Effect of dietary black raspberry (BRB) and phenylethylisothiocyanate (PEITC) on glutathione S-transferase (GST) activity in the liver. Enzymatic activity of GST was estimated in (A) cytosolic and (B) microsomal fractions of liver from rats fed AIN-76A diet or AIN-76A containing 5% or 10% BRBs or 5 µmol PEITC for 3 wk, as explained in Materials and Methods. Each bar represents mean ± SD (n = 9) from three separate experiments. *Statistically significance difference (P < 0.05) from control group.
Discussion
Chemoprevention is a pharmacological approach of intervention to prevent, inhibit, or reverse the process of carcinogenesis (36). Natural products derived from plants have been shown to have great potential for chemoprevention of various types of cancers, which forms the basis for the discovery of many chemopreventive agents. Many compounds, especially those of dietary origin, have been shown to inhibit P450-dependent activation of carcinogens and to inhibit tumorigenesis induced by these carcinogens. Other mechanisms, such as the induction of phase II detoxification enzymes trapping the reactive carcinogenic species, also contribute to the inhibitory activity of these compounds. Isothiocyanates [PEITC and benzyl isothiocyanate (BITC)] from cruciferous vegetables such as cabbage, cauliflower, and broccoli are potent and selective inhibitors of carcinogenesis induced by a variety of chemical carcinogens. These effects are mediated through favorable modification of both phase I and II enzymes involved in carcinogen metabolism. PEITC and BITC inhibit N-nitrosodiethylamine–, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone–, and benzo[a]pyrene–induced fore-stomach and lung tumors in mice (37,38). These tobacco carcinogens are known to require CYPs for their activation. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenols has been reported (39). EA, a naturally occurring plant polyphenol present in various fruits and nuts regularly consumed by humans, modulates activities of phase I and II enzymes (40,41). It possesses numerous anticarcinogenic and antimutagenic properties toward a variety of different carcinogens including nitrosamines, azoxymethane, mycotoxins, and polycyclic aromatic hydrocarbons (41–43). Our laboratory has demonstrated the chemopreventive effect of dietary freeze-dried BRBs and strawberries against NMBA-induced tumorigenesis in the rat esophagus (21,44). The berries reduce NMBA-induced O6-meGua formation in esophageal DNA, and they reduce the growth rate of premalignant esophageal epithelial cells. Based on the reduction in adduct formation, the present studies were conducted to determine the mechanism(s) of the anti-initiating effects of BRBs and their components against NMBA-induced esophageal carcinogenesis.
NMBA is the most potent of the nitrosamines in inducing esophageal cancer in rats and is used as a model carcinogen in rats for the study of esophageal tumorigenesis. NMBA metabolism produces methyl adducts in DNA and preferentially in epithelial cells of the esophagus (38). Similar to many nitrosamines, NMBA requires metabolic activation to exert its carcinogenic effect. NMBA metabolism in esophageal tissue involves α-hydroxylation exclusively of the methylene carbon atom at the N-nitroso group by CYPs (34). The activation results in the formation of benzaldehyde and reactive methyl diazonium ions. The reactive intermediate forms both N7 and the highly mutagenic O6 adducts on guanine. Two known nitrosamine metabolizing enzymes in the rat are CYP2A3 (28,29), which is found in the esophagus (and lung and nasal mucosa), and CYP2E1, found primarily in the liver (29). In the present study, 3 wk of dietary administration of 5% or 10% BRBs or 5 µmol PEITC inhibited NMBA metabolism in esophageal explant cultures. As in previous studies (31), NMBA was 75% metabolized by untreated esophageal explants. Formation of benzoic acid, the major metabolite of NMBA in esophageal tissue, was consistent with changes in NMBA metabolism. Inhibition of NMBA metabolism by dietary BRBs was not dose dependent. Although the reason(s) for this are unknown, it is possible that the uptake of berry compounds from the diet into the esophagus reaches a threshold beyond which no additional amounts in the diet are absorbed. Our metabolism data are consistent with earlier studies in which it was found that the inhibition of NMBA-induced esophageal tumorigenesis in rats by BRBs was not dose related (21).
BRBs were less active than PEITC in inhibiting NMBA metabolism in esophageal explants. PEITC is a very potent inhibitor of CYP-dependent monooxygenase activity and of NMBA-induced esophageal tumorigenesis (37,45–47). Its inhibitory effect is likely due to covalent binding of isothiocyanates (or their metabolites) to P450 apoproteins (37). BRBs components may also act as competitive inhibitors of P450s but have lower binding affinities to the enzymes than PEITC. An early study from our laboratory (21), evaluating the mechanism(s) by which BRBs inhibit O6-meGua adduct formation by NMBA, found that inhibition of adduct formation was greater at an NMBA dose of 0.25 mg/kg than at 0.5 mg/kg. This suggests the involvement of competitive inhibition because it could be overcome by an increasing concentration of NMBA.
Because NMBA is administered subcutaneously, much of the dose likely encounters hepatic cells prior to delivery to the esophagus. Hepatic microsomal metabolism of NMBA occurs either at the methyl carbon or the methylene carbon of the benzyl moiety (25). The dietary effect of 5% or 10% BRBs or 5 µmol PEITC on the metabolism of NMBA in liver microsomes was investigated. NMBA was less efficiently metabolized by liver microsomes than by esophageal explants. This is consistent with previous investigations from our laboratory in which we observed that the NMBA dosing regimen used resulted in lower levels of O6-meGua in liver compared with the esophagus (48,49). Interestingly, dietary BRBs, but not PEITC, inhibited NMBA metabolism in liver microsomes. These results are in agreement with an earlier report in which it was found that PEITC administered in vivo inhibited the metabolism of the rat esophageal carcinogen N-nitrosomethylamylamine (NMAA) and the formation of O6-meGua DNA adducts from NMAA in the rat esophagus but not in the liver (50). Inhibition of carcinogen metabolism in the liver can result in reduced first-pass clearance of many xenobiotics and thus result in greater delivery of the carcinogen to extrahepatic tissues.
BRBs are rich in nutrients as well as nonnutritive phytochemicals, which may act individually or in combination to inhibit carcinogenic processes. Although similar to strawberries in composition, BRBs generally have higher levels of phenolics (for example, ellagic and ferulic acids and the anthocyanins), sterols, calcium, iron, zinc, and antioxidant activity (43,44),which may influence their chemopreventive potential. Earlier work by Daniel et al. (40) found strawberries to be rich in EA, a plant polyphenol known to have antimutagenic and anticarcinogenic properties. EA inhibits carcinogenesis through multiple mechanisms, against various classes of chemical carcinogens, and in different target organs, including the rat esophagus (40–44). However, the inhibitory effect of freeze-dried strawberries on NMBA-induced tumorigenesis in the esophagus of F344 rats is not due solely to EA (21). Methanol and ethanol extracts of berries contain numerous compounds, several of which are at higher levels than EA, especially the anthocyanins (40,51). Anthocyanins have strong free radical scavenging and antioxidant activities, and, at the molecular level, they contribute to cancer prevention by 1) inhibiting cell transformation through targeting the mitogen-activated protein kinase pathway and activator protein-1, 2) suppressing inflammation and carcinogenesis through targeting the nuclear factor kappa B pathway and cyclooxygenase-2, and 3) apoptotic induction of cancer cells through reactive oxygen species/c-Jun NH2-terminal kinase–mediated caspase activation (52).
This knowledge, coupled with epidemiological data associating increased fruit consumption with decreased esophageal cancer risk, led to the present investigation to identify the active inhibitory component(s) of BRBs. The principal anthocyanins detected in methanol and ethanol extracts of BRBs are cyanidin-3-glucoside, cyanidin-3-sambubio-side, cyanidin-3-rutinoside, and cyanidin-3-(2G-xylosylrutinoside) (53). To our knowledge, only cyanidin-3-glucoside and cyanidin-3-rutinoside are available commercially. We found that NMBA metabolism by esophageal explants was maximally inhibited by PEITC followed by cyanidin-3-rutinoside, EA, cyanidin-3-glucoside, and the ethanol extract of BRBs; in liver microsomes, NMBA metabolism was maximally inhibited by cyanidin-3-rutinoside followed by EA, cyanidin-3-glucoside, and the ethanol extract of BRBs. The amount of PEITC and EA used was based on previous studies, and the amounts of anthocyanins used were comparable with EA. The ethanol extract of BRBs was the least effective in inhibiting NMBA metabolism, perhaps because the contents of anthocyanins and EA present in the extract are significantly lower than the amounts of the individual anthocyanins and EA used for study. Inhibition of NMBA metabolism was consistent with formation of its metabolites, benzoic acid in esophageal explants and benzyl alcohol in liver microsomes.
Drug metabolizing, detoxifying, and antioxidant enzymes are important cellular defenses against carcinogenesis. Phase II enzymes, such as GST, conjugate the endogenous nucleophile, GSH, to a large array of electrophilic compounds and thus provide a safeguard against chemical carcinogenesis. The role of phase II enzymes in the metabolism of NMBA is somewhat unclear. Phase II conjugates do not appear to be major metabolites of NMBA; however, there are reports of the formation of both glucuronide and glutathione conjugates of NMBA-derived metabolites (35). EA has been found to scavenge electrophilic intermediates of carcinogens and to increase the levels of GST as well as glutamate-cysteine ligase severalfold in rat liver (54). This ligase is involved in the synthesis of glutathione. EA also induces the expression of a battery of genes, including those coding for the phase II enzymes GST-Ya as well as NAD(P)H:quinone reductase (35,54,55). Therefore, we examined the effect of dietary BRBs and PEITC on GST activity in liver cytosol and microsomes. GST activity was induced by both dietary BRBs as well as PEITC. Induction of GST activity by PEITC was about three times higher than either concentration of dietary BRBs. The combined effect of BRBs components, such as EA, to inhibit CYPs involved in NMBA metabolism and to induce phase II enzymes such as GST in rat liver could contribute to an enhanced clearance of NMBA from the body.
In conclusion, our studies indicate that dietary BRBs inhibit NMBA metabolism in esophageal explant cultures as well as in liver microsomes. Among the isolated components known to be present in BRB, cyanidin-3-rutinoside was the most potent inhibitor of NMBA metabolism followed by EA and cyanidin-3-glucoside. Dietary BRBs and PEITC also induced GST activity in the liver, which could result in enhanced detoxification of NMBA. In-depth mechanistic studies are currently ongoing to determine the mechanisms involved in the inhibition of NMBA metabolism by dietary BRBs and PEITC.
Acknowledgments and Notes
We thank Dr. Stephen S. Hecht for providing the ethanol extract of BRBs, Dr. Dennis Pearl for statistical analysis, and Drs. Hyejeong Hwang and Nancy Zikri for technical assistance. Supported by NCI Grant CA 103180.
References
- 1.Blot WJ, Mclaughlin JK. The changing epidemiology of esophageal cancer. Semin Oncol. 1999;26:2–8. [PubMed] [Google Scholar]
- 2.Stoner GD, Rustgi AK. Biology of esophageal squamous cell carcinoma. Gastroint Cancers Biol Diagn Ther. 1995;8:141–146. [Google Scholar]
- 3.Beer DG, Stoner GD. Clinical models of chemoprevention for the esophagus. Hematol Oncol Clin North Am. 1998;12:1055–1077. doi: 10.1016/s0889-8588(05)70041-1. [DOI] [PubMed] [Google Scholar]
- 4.Block DG, Patterson B, Subhar A. Fruits, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1998;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 5.Hecht SS. Biochemistry, biology and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 6.Jalas JR, Ding X, Murphy SE. Comparative metabolism of the tobacco-specific nitrosamines 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol by rat cytochrome P450 2A3 and human cytochrome P450 2A13. Drug Metab Dispos. 2003;31:1199–1202. doi: 10.1124/dmd.31.10.1199. [DOI] [PubMed] [Google Scholar]
- 7.Shimada T, Martin MV, Pruess-Schwartz D, Marnett LJ, Guengerich FP. Roles of individual cytochrome P450 enzymes in bioactivation of benzo(a)pyrene, 7,8-dihydroxy-7,8-dihydrobenzo(a)pyrene and other dihydrodiol derivatives of polycyclic aromatic hydrocarbons. Cancer Res. 1989;49:6304–6312. [PubMed] [Google Scholar]
- 8.Preussmann R, Stewart BW. N-nitroso carcinogens. In: Searle CE, editor. Chemical Carcinogens. Washington, DC: American Chemical Society; 1984. pp. 643–828. (ACS Monograph 182) [Google Scholar]
- 9.Gray MR, Donnelly RJ, Kingsnorth AN. The role of smoking and alcohol in metaplasia and cancer risk in Barrett’s columnar-lined oesophagus. Gut. 1993;34:727–731. doi: 10.1136/gut.34.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menke-Pluymers MBE, Hop WCJ, Dees J, van Blankenstein M, Tilanus HW. Risk factors for the development of an adenocarcinoma in columnar-lined Barrett’s esophagus. Cancer. 1993;72:1155–1158. doi: 10.1002/1097-0142(19930815)72:4<1155::aid-cncr2820720404>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Adesnik M, Atchison M. Genes for cytochrome P450 and their regulation. Crit Rev Biochem. 1986;19:247–305. doi: 10.3109/10409238609084657. [DOI] [PubMed] [Google Scholar]
- 12.Gonzales FJ. Molecular genetics of the P450 superfamily. Pharmacol Ther. 1990;45:1–38. doi: 10.1016/0163-7258(90)90006-n. [DOI] [PubMed] [Google Scholar]
- 13.Guengerich FP, Shimada T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P450 enzymes. Chem Res Toxicol. 1991;4:391–407. doi: 10.1021/tx00022a001. [DOI] [PubMed] [Google Scholar]
- 14.Ding X, Kaminsky LS. Human extrahepatic cytochromes P450: function in xenobioticmetabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts. Annu Rev Pharmacol Toxicol. 2003;43:149–173. doi: 10.1146/annurev.pharmtox.43.100901.140251. [DOI] [PubMed] [Google Scholar]
- 15.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and contribution of the isoenzymes to cancer chemoprevention and drug resistance. Crit Rev Biochem Mol Biol. 1995;31:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 16.van Lieshout EM, Roelofs HM, Dekker S, Mulder CJ, Wobbes T, et al. Polymorphic expression of the glutathione S-transferase P1 gene and its susceptibility to Barrett’s esophagus and esophageal carcinoma. Cancer Res. 1999;59:586–589. [PubMed] [Google Scholar]
- 17.Peters WH, Kock L, Nagengast FM, Kremers PG. Biotransformation enzymes in human intestine: critical levels in the colon? Gut. 1991;32:408–412. doi: 10.1136/gut.32.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters WH, Nagengast FM, van Tongeren JH. Glutathione S-transferase, cytochrome P450, and uridine 59-diphosphate-glucuronosyltransferase in human small intestine and liver. Gastroenterology. 1989;96:783–789. [PubMed] [Google Scholar]
- 19.Peters WH, Roelofs HM, Hectors MP, Nagengast FM, Jansen JB. Glutathione and glutathione S-transferases in Barrett’s epithelium. Br J Cancer. 1993;67:1413–1417. doi: 10.1038/bjc.1993.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoner GD, Kresty LA, Carlton PS, Siglin JC, Morse MA. Isothiocyanates and freeze-dried strawberries as inhibitors of esophageal cancer. Toxicol Sci. 1999;52:S95–S100. doi: 10.1093/toxsci/52.2.95. [DOI] [PubMed] [Google Scholar]
- 21.Kresty LA, Morse MA, Morgan C, Carlton PS, Lu J, et al. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001;61:6112–6119. [PubMed] [Google Scholar]
- 22.Chen T, Stoner GD. Inducible nitric oxide synthase expression in N-nitrosomethylbenzylamine (NMBA)-induced rat esophageal tumorigenesis. Mol Carcinog. 2004;40:232–240. doi: 10.1002/mc.20035. [DOI] [PubMed] [Google Scholar]
- 23.Chen T, Nines R, Peschke SM, Kresty LA, Stoner GD. Chemopreventive effects of a selective nitric oxide synthase inhibitor on carcinogen-induced rat esophageal tumorigenesis. Cancer Res. 2004;64:3714–3717. doi: 10.1158/0008-5472.CAN-04-0302. [DOI] [PubMed] [Google Scholar]
- 24.Lai DY, Arcos J. Dialkynitrosamine bioactivation and carcinogenesis. Life Sci. 1980;27:2149–2165. doi: 10.1016/0024-3205(80)90379-3. [DOI] [PubMed] [Google Scholar]
- 25.Morse MA, Lu J, Stoner GD, Murphy SE, Peterson LA. Metabolism of N-nitrosobenzylmethylamine by human cytochrome P-450 enzymes. J Toxicol Environ Health A. 1999;58:397–411. doi: 10.1080/009841099157133. [DOI] [PubMed] [Google Scholar]
- 26.Morse MA, Amin SG, Hecht SS, Chung FL. Effects of aromatic isothiocyanates on tumorigenicity, O6-methylguanine formation, and metabolism of the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in A/J mouse lung. Cancer Res. 1987;49:2894–2897. [PubMed] [Google Scholar]
- 27.Hodgson RM, Wiessler M, Kleihues P. Preferential methylation of target organ DNA by the oesophageal carcinogen N-nitrosomethylbenzylamine. Carcinogenesis. 1980;1:861–866. doi: 10.1093/carcin/1.10.861. [DOI] [PubMed] [Google Scholar]
- 28.Gopalakrishnan R, Morse MA, Lu J, Weghorst CM, Sabourin CL, et al. Expression of cytochrome P450 2A3 in rat esophagus: relevance to N-nitrosobenzylmethylamine. Carcinogenesis. 1999;20:885–891. doi: 10.1093/carcin/20.5.885. [DOI] [PubMed] [Google Scholar]
- 29.Pinto RL, Moraes E, Albano RM, Silva MC, Godoy W, et al. Rat esophageal cytochrome P450 (CYP) monooxygenase system: comparison to the liver and relevance in N-nitrosodiethylamine carcinogenesis. Carcinogenesis. 2001;22:1877–1883. doi: 10.1093/carcin/22.11.1877. [DOI] [PubMed] [Google Scholar]
- 30.Stoner GD, Morrissey DT, Heur YH, Daniel EM, Galati AJ, et al. Inhibitory effects of phenethyl isothiocyanate on N-nitrosobenzylmethylamine carcinogenesis in the rat esophagus. Cancer Res. 1991;51:2063–2068. [PubMed] [Google Scholar]
- 31.Morse MA, Lu J, Gopalakrishnan R, Peterson LA, Wani G, et al. Mechanism of enhancement of esophageal tumorigenesis by 6-phenylhexyl isothiocyanate. Cancer Lett. 1997;112:119–125. doi: 10.1016/s0304-3835(96)04556-9. [DOI] [PubMed] [Google Scholar]
- 32.Smith TJ, Liao AL, Wang LD, Yang GY, Starcic S, et al. Characterization of xenobiotic-metabolizing enzymes and nitrosamine metabolism in the human esophagus. Carcinogenesis. 1998;19:667–672. doi: 10.1093/carcin/19.4.667. [DOI] [PubMed] [Google Scholar]
- 33.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 34.Lubec GE, Archer MC. Esophageal and hepatic microsomal metabolism of N-nitrosomethylbenzylamine and N-nitrosodimethylamine in the rat. Cancer Res. 1982;42:3181–3186. [PubMed] [Google Scholar]
- 35.Wiessler M, Rossnagel G. Glucuronides of N-nitrosomethylbenzylamine. IARC Sci Publ. 1987;84:170. [PubMed] [Google Scholar]
- 36.Kelloff GJ. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res. 2000;78:199–334. doi: 10.1016/s0065-230x(08)61026-x. [DOI] [PubMed] [Google Scholar]
- 37.Goosen TC, Mills DE, Hollenberg PF. Effects of benzyl isothiocyanate on rat and human cytochromes P450: identification of metabolites formed by P450 2B1. J Pharmacol Exp Ther. 2001;296:198. [PubMed] [Google Scholar]
- 38.Craddock VM, Henderson AR. Effect of N-nitrosamines carcinogenic for oesophagus on O6-alkyl-guanine-DNA-methyl transferase in rat oesophagus and liver. J Cancer Res Clin Oncol. 1986;111:229–236. doi: 10.1007/BF00389238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Y, Ho CT, Amin SG, Han C, Chung FL. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 1992;52:3875–3879. [PubMed] [Google Scholar]
- 40.Daniel EM, Krupkick AS, Heur Y, Blinzler JA, Nims RW, et al. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J. Food Comp Anal. 1989;2:338–349. [Google Scholar]
- 41.Ahn D, Putt D, Kresty L, Stoner GD, Fromm D, et al. The effects of dietary ellagic acid on rat hepatic and esophageal mucosal cytochromes P450 and phase II enzymes. Carcinogenesis. 1996;17:821–828. doi: 10.1093/carcin/17.4.821. [DOI] [PubMed] [Google Scholar]
- 42.Castonguay A, Gali HU, Perchellet EM, Gao XM, Boukharta M, et al. Antitumorigenic and antipromoting activities of ellagic acid, ellagitannins and oligomeric anthocyanin and procyanidin. Int J Oncol. 1997;10:367–373. [PubMed] [Google Scholar]
- 43.Barch DH, Fox CC. Selective inhibition of methylbenzylnitrosamine-induced formation of O6-methylguanine by dietary ellagic acid in rats. Cancer Res. 1988;48:7088–7092. [PubMed] [Google Scholar]
- 44.Carlton PS, Kresty LA, Siglin JC, Morse MA, Lu J, et al. Inhibition of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus by dietary freeze-dried strawberries. Carcinogenesis. 2001;22:441–446. doi: 10.1093/carcin/22.3.441. [DOI] [PubMed] [Google Scholar]
- 45.Narayanan BA, Geoffroy O, Willingham MC, Re GG, Nixon DW. p53/p21 (WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999;136:215–221. doi: 10.1016/s0304-3835(98)00323-1. [DOI] [PubMed] [Google Scholar]
- 46.Wilkinson JT, Morse MA, Kresty LA, Stoner GD. Effect of alkyl chain length on inhibition of N-nitrosomethylbenzylamine-induced esophageal tumorigenesis and DNA methylation by isothiocyanates. Carcinogenesis. 1995;16:1011. doi: 10.1093/carcin/16.5.1011. [DOI] [PubMed] [Google Scholar]
- 47.Ishizaki H, Brady JF, Ning SM, Yang CS. Effect of phenyl isothiocyanate on microsomal N-nitrosodimethylamine metabolism and other monooxygenase activities. Xenobiotica. 1990;20:255–264. doi: 10.3109/00498259009046845. [DOI] [PubMed] [Google Scholar]
- 48.Morse MA, Zu H, Galati AJ, Schmidt CJ, Stoner GD. Dose-related inhibition by dietary phenethyl isothiocyanate of esophageal tumorigenesis and DNA methylation induced by N-nitrosobenzylmethylamine in rats. Cancer Lett. 1993;72:103–110. doi: 10.1016/0304-3835(93)90018-5. [DOI] [PubMed] [Google Scholar]
- 49.Siglin JC, Khare L, Stoner GD. Evaluation of dose and treatment duration on the esophageal tumorigenicity of N-nitrosomethylbenzylamine in rats. Carcinogenesis. 1995;16:259. doi: 10.1093/carcin/16.2.259. [DOI] [PubMed] [Google Scholar]
- 50.Huang Q, Lawson TA, Chung FL, Morris CR, Mirvish SS. Inhibition by phenylethyl and phenylhexyl isothiocyanate on metabolism of and DNA methylation by N-nitrosomethylamylamine in rats. Carcinogenesis. 1993;14:749–754. doi: 10.1093/carcin/14.4.749. [DOI] [PubMed] [Google Scholar]
- 51.Clifford MN. Anthocyaninsnature, occurrence and dietary burden. J Sci Food Agric. 2000;80:1063–1072. [Google Scholar]
- 52.Hou DX, Fujii M, Terahara N, Yoshimoto M. Molecular mechanisms behind the chemopreventive effects of anthocyanidins. J Biomed Biotechnol. 2004;5:321–325. doi: 10.1155/S1110724304403040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tian Q, Aziz RM, Stoner GD, Schwartz SJ. Anthocyanin determination in black raspberry (Rubus occidentalis) and biological specimens using liquid chromatography-electrospray ionization tandem mass spectrometry. J Food Sci. 2005;70:43–47. [Google Scholar]
- 54.Shepherd AG, Manson MM, Ball HW, McLellan LI. Regulation of rat glutamate-cysteine ligase (gamma-glutamylcysteine synthetase) subunits by chemopreventive agents and in aflatoxin B(1)-induced preneoplasia. Carcinogenesis. 2000;21:1827–1834. doi: 10.1093/carcin/21.10.1827. [DOI] [PubMed] [Google Scholar]
- 55.Barch DH, Rundhaugen LM, Pillay NS. Ellagic acid induces transcription of the rat glutathione S-transferase-Ya gene. Carcinogenesis. 1995;16:665–668. doi: 10.1093/carcin/16.3.665. [DOI] [PubMed] [Google Scholar]