Abstract
Our laboratory has used a rodent model of human esophageal squamous cell carcinoma to identify putative chemopreventive agents for this disease and to determine their mechanisms of action. In the present study, we treated F344 rats with the esophageal carcinogen, N-nitrosomethylbenzylamine (NMBA), thrice per week for 5 weeks. Beginning 1 week later, they were fed a synthetic diet containing 5% black raspberries (BRB) for the duration of the bioassay (25 weeks). Rats were sacrificed at weeks 9, 15, and 25. Esophageal tissues were collected, and tumor data were recorded. The expression and enzymatic activities of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) as well as the expression of c-Jun in the esophagi, were evaluated to investigate the mechanism(s) by which black raspberries modulate tumorigenesis. At week 25, BRB inhibited tumor multiplicity, the standard end point in this tumor model, from 3.78 ± 0.41 tumors per rat in NMBA-treated animals to 2.23 ± 0.21 tumors per rat in animals treated with NMBA plus BRB (P < 0.005). BRB reduced mRNA and protein expression levels of COX-2, iNOS, and c-Jun as well as the level of prostaglandin E2 in preneoplastic lesions of the esophagus at week 25. The berries inhibited mRNA expression of iNOS and c-Jun, but not COX-2, in papillomatous lesions of the esophagus. Prostaglandin E2 and total nitrite levels were also decreased by BRB in papillomas. These results suggest a novel tumor suppressive role of BRB through inhibition of COX-2, iNOS, and c-Jun.
Introduction
Cancer is the second leading cause of death after heart disease for Americans. The American Cancer Society estimates that 570,280 Americans will die of cancer in 2005. About 30% of these deaths will be related to nutritional imbalance, physical inactivity, and obesity, and thus, are preventable (1). Esophageal squamous cell carcinoma (SCC) is one of the most common malignant neoplasms worldwide. The incidence rate of this disease varies dramatically in different geographic areas of the world; the highest incidence is in the “esophageal cancer belt,” which includes areas in eastern Turkey, the former Soviet Union, Iraq, Iran, China, Japan, South Africa, and France (2). The overall 5-year survival rate for this disease is low, ranging from 5% to 15%.
Esophageal SCC has a complex etiology. In the Western world, tobacco use and alcohol consumption are the major etiologic factors. In the Far East, in addition to the use of tobacco and alcohol, the disease is associated with the intake of salty food and food contaminated with various mycotoxins, deficiencies in dietary vitamins and minerals, and thermal injuries due to the consumption of hot beverages. Nitrosamine carcinogens also seem to be important causative agents of esophageal SCC (3). Among these is the carcinogen, N-nitrosomethylbenzylamine (NMBA), which is present in the diet in China. NMBA is an asymmetrical nitrosamine that readily induces mutagenic, toxic, and carcinogenic effects in esophageal tissues and is the most potent nitrosamine carcinogen for the rat esophagus (4, 5). In Linxian, China, the region with the highest incidence of esophageal SCC in the world, NMBA was identified in pickled vegetables and its metabolism has been elucidated (6, 7).
The inducible isoform of nitric oxide synthase (iNOS) is an enzyme that catalyzes the conversion of l-arginine to citrulline resulting in the production of nitric oxide (NO; ref. 8). NO is a free radical with an unpaired electron; it can donate or accept an electron to become a nitrosonium cation (NO+) or a nitroxyl anion (NO−), which leads to nitrosative stress or oxidative stress, respectively. Nitrosative stress can lead to the formation of nitrosamine carcinogens, deamination of DNA bases, and inactivation of DNA repair proteins, all actions contributing to carcinogenesis. Similarly, oxidative stress can lead to the formation of peroxynitrite (ONOO−), which can damage DNA and lead to carcinogenesis. NO is synthesized in mammalian cells by a family of three NO synthases: nNOS, eNOS, and iNOS. nNOS and eNOS are constitutively present in neurons and endothelial cells, respectively. These isoforms are calcium-dependent and produce only a low level of NO. In contrast, iNOS is calcium- and calmodulin-independent, and when induced by cytokines or other factors, generates a high concentration of NO. Up-regulation of iNOS has been reported in several types of cancer including breast, head and neck, lung, colon, melanoma, prostate, as well as esophageal SCC (9–16).
Prostaglandin endoperoxide synthase, also called cyclooxygenase (COX), catalyzes the formation of prostanoids including prostaglandins A2, D2, E2, F2α, I2, and J2 and thromboxane A2 (17). Two COX isoforms have been cloned: COX-1 and COX-2. COX-1 is constitutively expressed in most mammalian cells and is responsible for homeostasis of normal physiologic functions. COX-2 is expressed in response to certain stimuli such as growth factors, tumor promoters, hormones, and cytokines. COX-2 is important for tumorigenesis because prostaglandins, especially prostaglandin E2 (PGE2), affect cell proliferation, differentiation, apoptosis, angiogenesis, and metastasis (18). Up-regulation of COX-2 has been detected in various cancers including colon, gastric epithelium, breast, skin, pancreas, lung, head and neck, urinary bladder, and esophageal SCC (19–27). Clinical cancer chemoprevention studies indicated that COX-2 is a valid target for cancer prevention with the COX-2 inhibitor, celecoxib. Celecoxib significantly reduced the number of colon polyps in patients with familial adenomatous polyposis compared with placebo (28). Other nonsteroidal anti-inflammatory drugs including COX-2-selective and COX-2-nonselective inhibitors, such as aspirin, piroxicam, and sulindac, also exhibit chemopreventive potential in animal and human studies (29–31).
Expression of iNOS and COX-2 is controlled by transcription factors including the activator protein (AP-1) complex. AP-1 is composed of the Jun family (c-Jun, Jun-B, and Jun-D) and the Fos family (c-Fos, Fos-B, Fra-1, and Fra-2), which regulate the expression of iNOS and COX-2 by binding to their promoter sequences (32, 33). AP-1 regulates various cellular events including cell proliferation, differentiation, and apoptosis (34). c-Jun is a major component of AP-1, it is up-regulated in transformed cell lines and human cancers and is induced by activated Ras oncogenic proteins (35–37). The Ras family of proteins (H-, N-, and K-ras) bind to GTP to form ras-GTP complexes that regulate signal transduction pathways induced by diverse extracellular signals including carcinogens that induce esophageal cancer (38).
Our laboratory reported the overexpression of iNOS and COX-2 mRNAs in preneoplastic lesions and in papillomas induced in the rat esophagus by NMBA. Overexpression of iNOS and COX-2 is associated with increases in the tissue content of nitrite/nitrate and PGE2, respectively (16, 39, 40). We also observed Ha-ras codon 12 G → A transition mutations in all papillomas during esophageal carcinogenesis in rats (41). In view of these results, we conducted the present study to determine whether dietary freeze-dried black raspberries (BRB) inhibit tumor development in the rat esophagus by modulating iNOS, COX-2, and c-Jun.
Materials and Methods
Chemicals and reagent kits
NMBA was obtained from Ash Stevens (Detroit, MI) and determined to be >98% pure by high-performance liquid chromatography. DMSO was purchased from Sigma Chemical, Co. (St. Louis, MO). The QuantiTect SYBR green reverse-transcription PCR (RT-PCR) kit was purchased from Qiagen, Inc. (Valencia, CA). The nitrate/nitrite colorimetric assay kit was purchased from Cayman Chemical Co. (Ann Arbor, MI), and the Prostaglandin E2 Biotrak Enzyme Immunoassay System from Amersham Biosciences, Corp. (Piscataway, NJ).
Animals, berries, and diets
Male F344 rats, 4 to 5 weeks old, were obtained from Harlan Sprague-Dawley (Indianapolis, IN). The animals were housed three per cage under standard conditions (20 ± 2°C, 50 ± 10% relative humidity, 12-hour light/dark cycles). Food and water were freely available. Hygienic conditions were maintained by twice-weekly cage changes and routine cleaning of the animal rooms. The animals were fed a modified American Institute of Nutrition-76A (AIN-76A) synthetic diet containing 20% casein, 0.3% d,l-methionine, 52% cornstarch, 13% dextrose, 5% cellulose, 5% corn oil, 3.5% AIN salt mixture, 1% AIN vitamin mixture, and 0.2% choline bitartrate (Dyets, Inc., Bethlehem, PA). The diet was stored routinely at 4°C before the preparation of experimental diets.
Ripened black raspberries (Jewel variety) were purchased from the Stokes Raspberry Farm (Wilmington, OH). The berries were picked mechanically, washed, and placed in a −20°C freezer within an hour of picking. They were then shipped frozen to Van Drunen Farms (Momence, IL) for freeze-drying and subsequent grinding into a powder (BRB). BRB was shipped frozen to The Ohio State University and kept at −20°C until used. It was analyzed for content of several vitamins, minerals, carotenoids, and simple polyphenols by Covance Laboratories, Inc. (Madison, WI) and for anthocyanins in the laboratory of Dr. Steven Schwartz, College of Food, Agriculture, and Environmental Sciences, The Ohio State University. In general, the contents of berries varied no more than 20% from those of BRB obtained yearly from the same source during the past 8 years.
AIN-76A diet containing 5% BRB was prepared fresh weekly and stored at 4°C. BRB were mixed into the diet (modified by reducing the concentration of cornstarch by 5% to maintain an isocaloric diet) for 25 minutes with a Hobart mixer (Troy, OH). Fresh experimental and control diets were placed in glass feeding jars weekly and fed to the rats.
Experimental procedures
After a 2-week acclimation period to the animal facility, 150 rats were randomly assigned to four experimental groups (Table 1) and placed on AIN-76A diet. Three days after the final NMBA treatment, rats in group 3 were fed the AIN-76A diet only and rats in group 4 were fed the AIN-76A diet containing 5% BRB for the duration of the bioassay. Food consumption and body weight data were recorded weekly. At 9 and 15 weeks, 5 rats from groups 1 and 2 and 10 rats from groups 3 and 4; and, at 25 weeks, 15 rats from groups 1 and 2 and 30 rats from groups 3 and 4, were euthanized by CO2 asphyxiation and subjected to gross necropsy. The esophagus of each rat was excised and opened longitudinally. Tumors >0.5 mm in a single dimension were counted, mapped, and measured (length, width, and height). Tumor volume was calculated using the formula for a prolate spheroid: length × width × height × π/6. After the tumor data were recorded, tumors were removed from the esophagus and frozen in liquid nitrogen. The epithelium was stripped of the submucosal and muscularis layers and frozen in liquid nitrogen separately. All samples were stored at −80°C until analysis.
Table 1.
Experimental design for bioassay with BRB
| Group | Treatment | No. of rats | Amount administered (ml) |
Dose administered (mg/kg body weight) |
Diet |
|---|---|---|---|---|---|
| 1 | DMSO + H2O* | 25 | 0.2 | 0 | Control AIN-76A |
| 2 | None | 25 | 0 | 0 | AIN-76A + 5% BRB |
| 3 | NMBA | 50 | 0.2 | 0.25† | Control AIN-76A |
| 4 | NMBA | 50 | 0.2 | 0.25 | AIN-76A + 5% BRB |
DMSO + H2O, vehicle for NMBA.
NMBA (0.25 mg/kg body weight) injected s.c. thrice per week for 5 weeks.
Real-time PCR
Total cellular RNA was isolated from frozen tissues using TRIzol Reagent (Life Technologies, Gaithersburg, MD) according to the manufacturer’s instructions. After extraction, all RNA samples were analyzed for integrity of 18S and 28S rRNA by ethidium bromide staining of 1 µg of RNA resolved by electrophoresis on 1.2% agarose formaldehyde gels. One-step real-time reverse-transcription PCR was done in a GeneAmp 5700 sequence detection system (Perkin-Elmer Corp., Norwalk, CT) using the QuantiTect SYBR green RT-PCR kit as described previously (33). Briefly, the reaction of a 50 µL volume of total cellular RNA, QuantiTect RT Mix, QuantiTect SYBR green RT-PCR Master Mix, and forward and reverse primers were reverse transcribed, and PCR was done after the reverse transcription reaction. The expression of iNOS, COX-2, and c-Jun mRNA was normalized against expression of the housekeeping gene, hypoxanthine-guanine phosphoribosyltransferase (HPRT). Primers for iNOS, COX-2, c-Jun, and HPRT were designed according to published sequences with Primer Express Software v2.0 (Applied Biosystems, Foster City, CA). Base sequences are shown in Table 2. Each individual RNA sample for each gene was assayed in triplicate. Two controls were run with every reaction: one contained RNA and QuantiTect RT Mix to detect genomic DNA and the other contained the reaction reagents without RNA to confirm that the reagents displayed no signal. Data were collected using SDS Sequence Detector Software (PE, Applied Biosystems).
Table 2.
Nucleotide sequences of the primers used to assay gene expression by real-time reverse transcription PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| iNOS | 5′-GCTCGAGATGTCATGAAGGAGAT-3′ | 5′-AGCAGGTCAGCAAAGAACTTATAGC-3′ |
| COX-2 | 5′-CAGCCATGCAGCAAATCCTT-3′ | 5′-CCAACCCATGTCAAAACCGTG GTGAAT-3′ |
| c-Jun | 5′-TGCAAAGATGGAAACGACCTT-3′ | 5′-CCACTCTCGGACTGGAGGAA-3′ |
| HPRT | 5′-GCTCGAGATGTCATGAAGGAGAT-3′ | 5′-AGCAGGTCAGCAAAGAACTTATAGC-3′ |
Western blot analysis
Proteins were extracted from frozen esophageal epithelium. Protein concentration was determined using the Bio-Rad assay (Bio-Rad, Hercules, CA) according to the manufacturer’s recommendations. Fifty micrograms of protein with NuPAGE LDS Sample Buffer and NuPAGE sample reducing agent (Invitrogen, Carlsbad, CA) were heated at 100°C for 1 minute. After cooling at room temperature for 5 minutes, proteins were fractioned by 7% NuPAGE Novex Tris-acetate gel electrophoresis (Invitrogen). Proteins were then transferred to Invitrolon polyvinylidene difluoride membrane. The membrane was blocked with blocking buffer (concentrated saline and Hammersten casein solution) at 4°C overnight. The blot was probed with iNOS (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), COX-2 (1:500, Cayman Chemical), or c-Jun (1:200, Santa Cruz Biotechnology) polyclonal antibody at room temperature for 1 hour. After being washed extensively to eliminate nonspecific binding, the membrane was incubated with goat anti-rabbit secondary antibody labeled with alkaline phosphatase at room temperature for 1 hour. The Western blots were visualized using Western Breeze chromogenic immunodetection kit (Invitrogen). β-Actin (1:1,000; Sigma) was detected in the same sample to ensure an equal protein loading.
Nitrate/nitrite colorimetric assay
As described previously (39), frozen esophagi were weighed, homogenized in PBS, and centrifuged. iNOS activity in the supernatant was measured using a nitrate/nitrite colorimetric assay kit according to the manufacturer’s instructions. Briefly, 80 µL supernatant for each sample was transferred to a 96-well optical plate and incubated with 10 µL nitrate reductase and 10 µL enzyme cofactor for 3 hours. Griess reagent [sulfanilamide and N-(1-naphthyl)ethylenediamine] was added and the absorbance was measured at 550 nm using a SpectraMax M2 multidetection reader (Molecular Devise Corp., Sunnyvale, CA). Standards of sodium nitrate (0–35 µmol/L) were used to create a standard curve. The final nitrite concentration was the sum of the nitrite plus the reduced nitrate in each sample and was taken as an index of iNOS activity as described previously. Each sample was assayed in triplicate.
PGE2 enzyme immunoassay
COX-2 activity in esophageal epithelium and papillomas were assayed by using the Biotrak Enzyme Immunoassay System (Amersham Biosciences) to measure PGE2 concentration. In brief, frozen samples were homogenized in Tris-HCl buffer (pH 7.5) with 0.02 mol/L EDTA and 5 mg/mL indomethacin. Total protein concentration for each tissue homogenate was determined using the DC Protein Assay (Bio-Rad). PGE2 was collected and purified according to the manufacturer’s instructions. Absorbance was measured at 450 nm using the SpectraMax M2 multidetection reader (Molecular Devise). PGE2 concentration was normalized against protein concentration in the same sample. Each sample was assayed in triplicate.
Statistical analysis
Body weight, food consumption, tumor multiplicity (mean number of tumors/esophagus), and tumor volume data were collected for all rats fed control or experimental diets. These data—the iNOS, COX-2, and c-Jun expression data; total nitrite and nitrate data; and PGE2 data—were analyzed and compared using one-way ANOVA followed by Dunnet’s multiple comparison test to identify individual differences among groups when the ANOVA was significant. Tumor incidence (percentage of animals in each group with tumors) data were analyzed using the χ2 test. All statistical analysis was carried out using GraphPad Prism 4.0. Differences were considered statistically significant at P < 0.05. All P values were two-sided.
Results
General observations
The mean body weights and food consumption in all rats were not significantly different throughout the bioassay (data not shown). No observable gross or histopathologic changes occurred in the lungs, liver, kidneys, small intestine, and colon of rats treated with BRB only. All tumor specimens removed from the esophagus at necropsy were found to be papillomas by histopathologic examination.
BRB inhibits tumor development
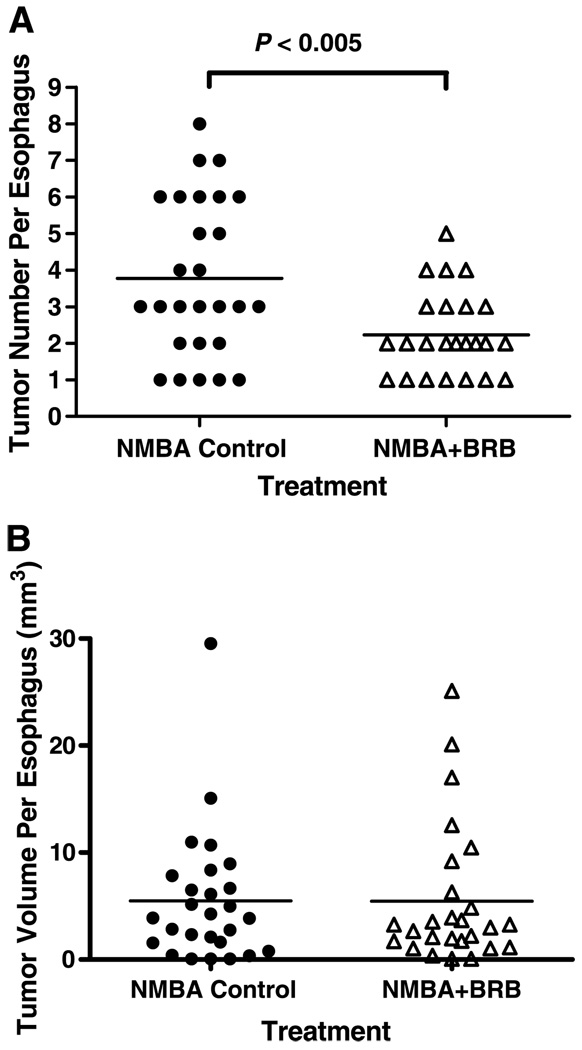
At weeks 9 and 15 of the bioassay, <10% to 20% of the esophagi, respectively, had tumors (papillomas). The tumor responses (incidence, multiplicity, and volume) at these time points were too low to determine if BRB treatment produced any inhibitory effects. At the end of the bioassay (week 25), none of the DMSO-treated rats (group 1) or the rats fed 5% BRB (group 2) developed tumors. In rats treated with NMBA, however, BRB reduced the incidence of esophageal tumors from 96% in NMBA controls (group 3) to 89% in rats treated with NMBA + 5% BRB (group 4). This reduction was not significant (P > 0.05). In contrast, BRB significantly reduced tumor multiplicity, the standard end point in this tumor model, from 3.78 ± 0.41 tumors per esophagus in group 3 to 2.23 ± 0.21 tumors per esophagus in group 4 (P < 0.005; Fig. 1A). The difference in tumor volume between groups 3 and 4 was not significant at 25 weeks (Fig. 1B).
Figure 1.
Effect of BRB on tumor development in NMBA-treated rat esophagus at 25 weeks. Rats treated with NMBA + BRB had fewer tumors than rats treated with NMBA only (A); however, there was no significant difference in tumor volume between the two groups of rats (B). ●, NMBA control; △, NMBA+BRB.
BRB down-regulates mRNA expression of iNOS, COX-2, and c-Jun
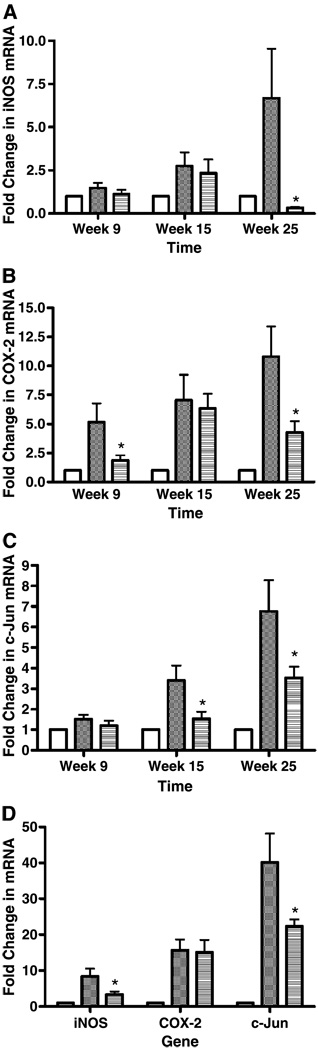
We determined if the inhibition of tumor development in NMBA-treated rat esophagus by BRB was associated with modulation of iNOS, COX-2, and c-Jun mRNA expression. Real-time reverse transcription-PCR was done on samples of esophageal epithelium collected from 10 animals per group at each of weeks 9 and 15, and on samples of esophageal epithelium and papillomas collected from 28 to 29 animals per group at week 25. By histopathologic analysis, all esophagi collected from solvent-treated rats were classified as normal and those from NMBA-treated rats, after the removal of papillomas, were classified as preneoplastic (i.e., exhibiting extensive hyperplasia and foci of dysplastic change). Exophytic lesions on the surface of the esophagus that were >0.5 mm in a single dimension were counted as tumors, and all of these tumors were found to be papillomas on histopathologic analysis. The papillomas were removed from the esophagus and stored separately. As shown in Fig. 2A, BRB suppressed the mRNA expression of iNOS in preneoplastic lesions at week 25 (95%, P < 0.05) but not at weeks 9 and 15. In contrast, BRB inhibited the mRNA expression of COX-2 in preneoplastic lesions by 64% (P < 0.05) and 60% (P < 0.05) at both weeks 9 and 25, respectively (Fig. 2B). BRB down-regulated the mRNA expression of c-Jun in preneoplastic lesions by 55% (P < 0.05) at week 15 and by 48% (P < 0.05) at week 25 (Fig. 2C). At 25 weeks, BRB suppressed the mRNA expression of iNOS and c-Jun by 60% (P < 0.05) and 44% (P < 0.05) in papillomas, respectively, but did not reduce the mRNA expression of COX-2 in papillomas (Fig. 2D).
Figure 2.
Fold changes for iNOS (A), COX-2 (B), and c-Jun (C) mRNA expression in rat esophageal preneoplastic lesions at weeks 9, 15, and 25; and in papillomas at week 25 (D). Columns, means; bars, ± SE; *, P < 0.05 as determined by ANOVA when compared with the control diet group. □, Normal control;  , NMBA control;
, NMBA control;  , NMBA+BRB.
, NMBA+BRB.
BRB inhibits protein expression of iNOS, COX-2, and c-Jun
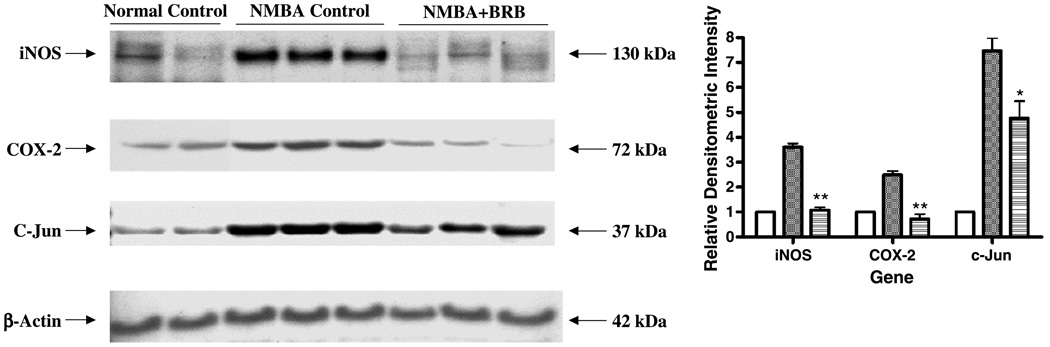
Western blot analysis was used to determine whether BRB inhibited iNOS and COX-2 protein in preneoplastic lesions at 25 weeks and, if so, whether this inhibition was associated with modulation of c-Jun. As shown in Fig. 3, the NMBA-induced overexpression of iNOS and COX-2 was decreased from 3.6-fold and 2.5-fold in rats fed the control diet to 1.1-fold (P < 0.005) and 0.72-fold (P < 0.005) in rats fed the BRB diet. BRB also inhibited NMBA-induced expression of c-Jun by 36% relative to the control diet (P < 0.05).
Figure 3.
Effect of BRB on the protein expression of iNOS, COX-2, and c-Jun in preneoplastic lesions at week 25. The indicated proteins were detected by Western blot analyses. Representative blots are shown, similar results were obtained from triplicate experiments. Columns, mean relative densitometric intensities; bars, ± SE; *, P < 0.05; **, P < 0.005 as determined by ANOVA when compared with the control diet group. □, Normal control;  , NMBA control;
, NMBA control;  , NMBA+BRB.
, NMBA+BRB.
BRB reduces total nitrite and PGE2 levels
We also evaluated the effects of dietary BRB on iNOS and COX-2 activities in esophageal tissues collected at 25 weeks by measuring total nitrate and nitrite levels and PGE2 levels, respectively, by enzyme immunoassay (Table 3). BRB decreased total nitrate and nitrite levels from 4.40 ± 0.52 to 4.17 ± 0.83 µmol/L (not significant) in preneoplastic lesions, and from 7.66 ± 1.65 to 4.04 ± 1.24 µmol/L in papillomas (47% reduction; P < 0.05). PGE2 levels were reduced in preneoplastic lesions from 3.05 ± 0.59 µg/mg protein in rats treated with NMBA only to 1.22 ± 0.13 µg/mg protein in rats treated with NMBA + BRB (60% reduction; P < 0.05). In addition, BRB treatment suppressed PGE2 production in papillomas from 18.10 ± 4.40 µg/mg esophageal protein in rats treated with NMBA only to 6.98 ± 1.68 µg/mg esophageal protein in rats treated with NMBA + BRB (61% reduction; P < 0.05).
Table 3.
Modulation of iNOS and COX-2 activities in the esophagus of NMBA-treated rats by BRB at 25 weeks
| Diet | Preneoplasia | Papilloma | ||
|---|---|---|---|---|
| iNOS (mean ± SE)* | COX-2 (mean ± SE)† | iNOS (mean ± SE) | COX-2 (mean ± SE) | |
| Control | 4.40 ± 0.52 | 3.05 ± 0.59 | 7.66 ± 1.65 | 18.10 ± 4.40 |
| 5% BRB | 4.17 ± 0.83 | 1.22 ± 0.13‡ | 4.04 ± 1.24‡ | 6.98 ± 1.68‡ |
iNOS activity is expressed as µmol/L nitrite.
COX-2 activity is expressed as µg/mg protein.
Significantly lower than rats fed control diets (P < 0.05).
Discussion
Epidemiologic studies have revealed the protective effects of raw fruit and vegetable consumption on the development of several types of cancer in humans. However, the mechanisms of this inhibition by food components are not fully understood. The present study is part of a large-scale investigation of the chemopreventive effects of black raspberries on gastrointestinal cancers in animals and in humans. Our data suggest that black raspberries inhibit events associated with tumor progression in rats pretreated with NMBA, at least in part by reducing the expression and activities of iNOS and COX-2. This reduction is associated with an inhibitory effect of BRB on the transcription factor, c-Jun. These results may be relevant for human esophageal SCC because these genes are frequently overexpressed in this disease (15, 27).
In the NMBA model of rat esophageal tumorigenesis, the principal end point for assessing the inhibitory effects of chemopreventive agents is tumor multiplicity. Reductions in tumor incidence occur only with very potent chemopreventive agents because virtually all NMBA-treated rats develop multiple tumors at 25 weeks. With less active chemopreventive agents, large numbers of animals per group (60–100) are required to show an inhibitory effect on tumor incidence, which makes routine studies cost prohibitive. Similarly, tumor volume data are of limited use in chemoprevention studies because the variability in esophageal tumor volume among individual animals is significant. Thus, as for tumor incidence data, a large number of animals per group (60–100) is required to show the significant inhibitory effect of chemopreventive agents on tumor volume.
Black raspberries, a small black berry native to North America, are an abundant source of flavonoid compounds (e.g., ellagic acid, ferulic acid, coumaric acid, quercetin, and the anthocyanins), vitamins (e.g., vitamins A, C, and E and folic acid), minerals (e.g., calcium, potassium, selenium, and zinc), and phytosterols (e.g., β-sitosterol, campesterol, and stigmasterol). The chemical composition of BRB has not been fully determined. However, recent data suggest that the anthocyanins are most abundant compounds in BRB representing ~ 5% to 6% of their dry weight. Many of the known compounds have antioxidant and anti-inflammatory activities and protect against cancer in animal models. For example, ellagic acid protects against chemically induced cancer in multiple organ sites including the rat esophagus (42). It reduces the metabolic activation of procarcinogens by inhibition of cytochrome P450 enzymes, and it enhances phase II enzyme activities resulting in more efficient detoxification of carcinogens. Cytochrome P450s, 2E1 and 1A1, for example, are major enzymes involved in the bioactivation of procarcinogens such as NMBA and benzo(a)pyrene (43, 44). Ellagic acid decreased the activation of these two enzymes and inhibited carcinogenesis in the rat esophagus and mouse skin (45). Ascorbic acid is another chemopreventive agent in black raspberries. It suppressed the in vivo nitrosation of primary and secondary amines in the stomach from nitrite, thus, inhibiting the formation of nitrosamine carcinogens including NMBA (46). Calcium protects against the development of colon cancer in animals (47), and β-sitosterol has chemopreventive effects against carcinogen-induced mammary cancer in rodents (48). These are only a few examples of the multiple known chemopreventive agents in black raspberries. By freeze-drying the berries, these components are concentrated ~ 10-fold because black raspberries are ~ 90% water by weight. Ascorbic acid is the only component known to degrade substantially during storage and before freeze-drying the berries. The other components seem to be well preserved even when BRB are stored at 4°C for several months. Biodirected fractionation studies are under way to identify the most active inhibitory components in BRB against NMBA-induced rat esophageal tumorigenesis.
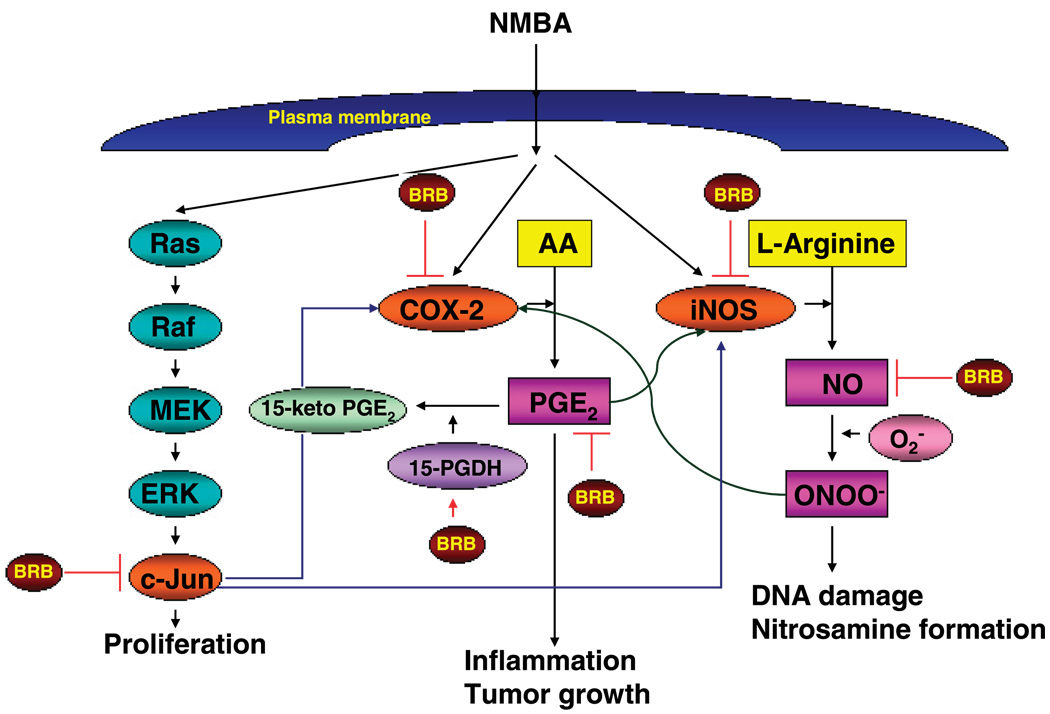
The precise mechanism by which black raspberries inhibit tumor development in the esophagus of rats that have been treated with NMBA is not fully understood. However, in the present study, black raspberry powder was found to inhibit both mRNA expression levels and activities of iNOS and COX-2, two enzymes previously shown in our laboratory to be up-regulated in rat esophageal tumorigenesis (16, 40). Moreover, BRB were found to down-regulate the expression of c-Jun, a component of the transcription factor, AP-1, which controls the expression of multiple genes. Thus, we depict the possible mechanisms for the prevention of tumor development by BRB in Fig. 4.
Figure 4.
Possible mechanisms for the prevention of tumor development in rat esophagus by BRB.
The data presented in Fig. 2A–C indicate that the expression levels of COX-2, iNOS, and c-Jun were modulated significantly by berries only when they exceeded the levels in normal tissue by at least a factor of 4. Thus, COX-2 expression was down-regulated as early as week 9, c-Jun at week 15, and iNOS only at week 25. The COX-2 results are not surprising in that we have reported that COX-2 overexpression is an early event in rat esophageal carcinogenesis (40). The lack of an inhibitory effect on COX-2 expression at week 15 is surprising and this result is under further investigation. The significant down-regulation of c-Jun at week 15 is probably related to its higher expression level at this time point than at 9 weeks. In this regard, we have reported that essentially all NMBA-induced papillomas in the rat esophagus exhibit a G → A transition mutation in the H-ras gene, and that the percentage of premalignant dysplastic foci in the esophagus that exhibit mutant ras increases with time after NMBA treatment (41, 49). Because c-Jun is downstream of ras, its expression levels might be related to the percentage of esophagus cells that exhibit mutant ras. Thus, the berries may have been effective at down-regulating c-Jun only in tissues that had a sufficient number of ras-activated cells. Inhibition of the expression of iNOS was observed in the esophagus only at 25 weeks. Again, this was probably related to the higher expression levels of this gene at this time point, which is in agreement with our immunohistochemical studies showing higher levels of iNOS protein in more advanced lesions (16).
The present study is of potential importance to humans because both iNOS and COX-2 are overly expressed in dysplastic lesions of human esophagus and esophageal SCC. In endoscopic surveys of high-risk populations in China, dysplastic lesions exhibited over-expression of iNOS and COX-2 and ~ 70% of patients with esophageal dysplasia are later diagnosed with esophageal SCC (50, 51). Because our results showed that black raspberry powder inhibits iNOS and COX-2 during the progression stage of esophageal carcinogenesis, using black raspberries for chemoprevention of this disease could be an effective strategy for its prevention in high-risk populations.
Interestingly, in the current investigation, we found that BRB significantly inhibit both COX-2 expression and COX-2-derived PGE2 levels in precancerous tissues, but only PGE2 and not COX-2 levels in papillomas. Although the reason(s) for this are unknown, recent studies indicate that an enzyme, 15-hydroxyprostaglandin dehydrogenase (15-PGDH), is a prostaglandin-degrading enzyme that catalyzes the oxidization of the 15(S)-hydroxyl group of PGE2 to produce inactive 15-keto PGE2 (52). The level of PGE2 depends on the rates of COX-2-dependent biosynthesis and 15-PGDH-dependent degradation. In NMBA-induced papillomas, BRB did not inhibit COX-2 expression, but they did reduce PGE2 levels. This could be due to an induction of 15-PGDH by BRB.
In conclusion, the present study suggests mechanisms for the chemopreventive action of black raspberries. The outcome of this study is of great interest and has implications for the prevention of human esophageal cancer. Natural food products such as BRB may offer a relatively nontoxic alternative to the prevention of esophageal cancer in humans. In this regard, a phase I clinical trial of freeze-dried BRB recently conducted in our laboratory indicated that black raspberries were well tolerated by humans when administered p.o. at 45 g of powder per day for 7 days (53). In addition, in a trial involving subjects with Barrett’s esophagus, black raspberry powder was well tolerated when administered at 32 and 45 g/d for 6 months (54). Thus, black raspberries may be a useful alternative to chemopreventive drugs for the prevention of human esophageal cancer.
Acknowledgments
Grant support: National Cancer Institute grants R01 CA96130 and CA103180.
References
- 1.American Cancer Society. Cancer facts and figures, 2005. Atlanta: American Cancer Society; 2005. p. 1. [Google Scholar]
- 2.Muñoz N. Epidemiological aspects of esophageal cancer. Endoscopy. 1993;23:609–612. doi: 10.1055/s-2007-1010415. [DOI] [PubMed] [Google Scholar]
- 3.Neurath GB, Dunger M, Pein FG, Ambrosius D, Schreiber O. Primary and secondary amines in the human environment. Food Chem Toxicol. 1977;15:275–282. doi: 10.1016/s0015-6264(77)80197-1. [DOI] [PubMed] [Google Scholar]
- 4.Peto R, Gray R, Brantom P, Grasso P. Effects on 4080 rats of chronic administration of N-nitrosodiethylamine or N-nitrosodimethylamine: a detailed dose-response study. Cancer Res. 1991;51:6415–6451. [PubMed] [Google Scholar]
- 5.Hodgson RM, Wiessler M, Kleihues P. Preferential methylation of target organ DNA by the oesophageal carcinogen N-nitrosomethylbenzylamine. Carcinogenesis. 1980;1:861–866. doi: 10.1093/carcin/1.10.861. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Li M. Roussin red methylester, a tumor promoter isolated from pickled vegetables. Carcinogenesis. 1989;10:617–620. doi: 10.1093/carcin/10.3.617. [DOI] [PubMed] [Google Scholar]
- 7.Labuc GE, Archer MC. Esophageal and hepatic microsomal metabolism of N-nitroso-N-methylbenzylamine and dimethylnitrosamine in the rat. Cancer Res. 1982;42:3181–3186. [PubMed] [Google Scholar]
- 8.Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- 9.Tschugguel W, Schneeberger C, Unfried G, et al. Expression of inducible nitric oxide synthase in human breast cancer depends on tumor grade. Breast Cancer Res Treat. 1999;56:145–151. doi: 10.1023/a:1006288526311. [DOI] [PubMed] [Google Scholar]
- 10.Gallo O, Masini E, Morbidelli L, et al. Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J Natl Cancer Inst. 1998;90:587–596. doi: 10.1093/jnci/90.8.587. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Wang C, Chen T, Lin H, Yu C, Kuo H. Increased level of exhaled nitric oxide and up-regulation of nitric oxide synthase in patients with primary lung cancer. Br J Cancer. 1998;78:534–541. doi: 10.1038/bjc.1998.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambs S, Merriam WG, Bennett WP, et al. Frequent NOS-2 expression in human colon adenocarcinomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–341. [PubMed] [Google Scholar]
- 13.Andrew PJ, Harant H, Lindley IJ. Nitric oxide regulates IL-8 expression in melanoma cells at the transcriptional level. Biochem Biophys Res Commun. 1995;214:949–956. doi: 10.1006/bbrc.1995.2378. [DOI] [PubMed] [Google Scholar]
- 14.Klotz T, Bloch W, Volberg C, Engelmann U, Addicks K. Selective expression of iNOS in human prostate carcinoma. Cancer. 1998;82 1897-03. [PubMed] [Google Scholar]
- 15.Tanaka H, Kijima H, Tokunaga T, et al. Frequent expression of inducible nitric oxide synthase in esophageal squamous cell carcinomas. Int J Oncol. 1999;14:1069–1073. doi: 10.3892/ijo.14.6.1069. [DOI] [PubMed] [Google Scholar]
- 16.Chen T, Stoner GD. Inducible nitric oxide synthase expression in N-nitrosomethylbenzylamine (NMBA)-induced rat esophageal tumorigenesis. Mol Carcinog. 2004;40:232–240. doi: 10.1002/mc.20035. [DOI] [PubMed] [Google Scholar]
- 17.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part I) J Natl Cancer Inst. 1998;90:1529–1536. doi: 10.1093/jnci/90.20.1529. [DOI] [PubMed] [Google Scholar]
- 18.Dubois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 19.Sinicrope FA, Gill S. Role of COX-2 in colorectal carcinogenesis. Cancer Metastasis Rev. 2004;23:63–72. doi: 10.1023/a:1025863029529. [DOI] [PubMed] [Google Scholar]
- 20.Restiäki A, Honkanen N, Jänkälä H, Sipponen P, Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997;57:1276–1280. [PubMed] [Google Scholar]
- 21.Parrett ML, Harris RE, Joarder FS, Ross MS, Clausen KP, Robertson FM. Cyclooxygenase gene expression in human breast cancer. Int J Oncol. 1997;10:503–507. doi: 10.3892/ijo.10.3.503. [DOI] [PubMed] [Google Scholar]
- 22.Müller-Decker K, Kopp-Schneider A, Marks F, Seibert K, Fürstenberger G. Localization of prostaglandin H synthase isozymes in murine epidermal tumors: suppression of skin tumor promotion by inhibition of prostaglandin H synthase-2. Mol Carcinog. 1998;23:36–44. doi: 10.1002/(sici)1098-2744(199809)23:1<36::aid-mc5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Tucker ON, Dannenberg AJ, Yang EK, et al. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999;59:987–990. [PubMed] [Google Scholar]
- 24.Hida T, Yatabe Y, Achiwa H, et al. Increased expression of cyclooxygenase-2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- 25.Chan G, Boyle JO, Yang EK, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 26.Mohammed SI, Knapp DW, Bostwick DG, et al. Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res. 1999;59:5647–5650. [PubMed] [Google Scholar]
- 27.Ratnasinghe D, Tangrea J, Roth MJ, et al. Expression of cyclooxygenase-2 in human squamous cell carcinoma of the esophagus; an immunohistochemical survey. Anticancer Res. 1999;19:171–174. [PubMed] [Google Scholar]
- 28.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 29.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 30.Jacoby RF, Cole CE, Tutsch K, et al. Chemopreventive efficacy of combined piroxicam and difluoromethylornithine treatment of Apc mutant Min mouse adenomas, and selective toxicity against Apc mutant embryos. Cancer Res. 2000;60:1864–1870. [PubMed] [Google Scholar]
- 31.Giardiello FM, Yang VW, Hylind LM, et al. Primary chemoprevention of familial adenomatous polyposis with sulindac. N Engl J Med. 2002;346:1054–1059. doi: 10.1056/NEJMoa012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Chen X, Teng X, Snead C, Catravas JD. Molecular cloning and analysis of the rat inducible nitric oxide synthase gene promoter in aortic smooth muscle cells. Biochem Pharmacol. 1998;55:1873–1880. doi: 10.1016/s0006-2952(98)00078-1. [DOI] [PubMed] [Google Scholar]
- 33.Chen LC, Chen BK, Chang JM, Chang WC. Essential role of c-Jun induction and coactivator p300 in epidermal growth factor-induced gene expression of cyclooxygenase-2 in human epidermoid carcinoma A431 cells. Biochim Biophys Acta. 2004;1683:38–48. doi: 10.1016/j.bbalip.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 35.Bohmann D, Bos TJ, Admon A, Nishimura T, Vigt PK, Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987;238:1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- 36.Tessari G, Ferrara C, Poletti A, et al. The expression of proto-oncogene c-Jun in human pancreatic cancer. Anticancer Res. 1999;19:863–867. [PubMed] [Google Scholar]
- 37.Westwick JK, Cox AD, Der CJ, et al. Oncogenic Ras activates c-Jun via a separate pathway from the activation of extracellular signal-regulated kinases. Proc Natl Acad Sci U S A. 1994;91:6030–6034. doi: 10.1073/pnas.91.13.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsao AS, Kim ES, Hong WK. Chemoprevention of cancer. CA Cancer J Clin. 2004;54:150–180. doi: 10.3322/canjclin.54.3.150. [DOI] [PubMed] [Google Scholar]
- 39.Chen T, Nines RG, Peschke SM, Kresty LA, Stoner GD. Chemopreventive effects of a selective nitric oxide synthase inhibitor on carcinogen-induced rat esophageal tumorigenesis. Cancer Res. 2004;64:3714–3717. doi: 10.1158/0008-5472.CAN-04-0302. [DOI] [PubMed] [Google Scholar]
- 40.Carlton PS, Gopalakrishnan R, Gupta A, et al. Piroxicam is an ineffective inhibitor of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus. Cancer Res. 2002;62:4376–4382. [PubMed] [Google Scholar]
- 41.Liston BW, Gupta A, Nines R, et al. Incidence and effects of Ha-ras codon 12 G → A transition mutations in preneoplastic lesions induced by N-nitrosomethylbenzylamine in the rat esophagus. Mol Carcinog. 2001;32:1–8. doi: 10.1002/mc.1058. [DOI] [PubMed] [Google Scholar]
- 42.Mandal S, Stoner GD. Inhibition of N-nitrosomethylbenzylamine-induced esophageal tumorigenesis in rats by ellagic acid. Carcinogenesis. 1990;11:55–61. doi: 10.1093/carcin/11.1.55. [DOI] [PubMed] [Google Scholar]
- 43.Guengerich FP, Shimada T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P450 enzymes. Chem Res Toxicol. 1991;4 doi: 10.1021/tx00022a001. 391-07. [DOI] [PubMed] [Google Scholar]
- 44.Xue H, Aziz RM, Sun N, et al. Inhibition of cellular transformation by berry extracts. Carcinogenesis. 2001;22:351–356. doi: 10.1093/carcin/22.2.351. [DOI] [PubMed] [Google Scholar]
- 45.Stoner GD, Mukhtar H. Polyphenols as cancer chemopreventive agents. J Cell Biochem Suppl. 1995;22:169–180. doi: 10.1002/jcb.240590822. [DOI] [PubMed] [Google Scholar]
- 46.Bosetti C, La Vecchia C, Talamini R, et al. Food groups and risk of squamous cell esophageal cancer in northern Italy. Int J Cancer. 2000;87:289–294. [PubMed] [Google Scholar]
- 47.Slattery ML, Neuhausen SL, Hoffman M, et al. Dietary calcium, vitamin D, VDR genotypes and colorectal cancer. Int J Cancer. 2004;111:750–756. doi: 10.1002/ijc.20330. [DOI] [PubMed] [Google Scholar]
- 48.Awad AB, Chen YC, Fink CS, Hemmessey T. Beta-sitosterol inhibits HT-29 human colon cancer cell growth and alters membrane lipids. Anticancer Res. 1996;16:2797–2804. [PubMed] [Google Scholar]
- 49.Wang Y, You M, Reynolds SH, Stoner GD, Anderson MW. Mutational activation of the cellular Harvey ras oncogene in rat esophageal papillomas induced by methylbenzylnitrosamine. Cancer Res. 1990;50:1591–1595. [PubMed] [Google Scholar]
- 50.Jin Y, Zhang W, Liu B, Wang H, Han Z, Wang L. Expression of inducible nitric oxide synthase in human esophageal biopsies from carcinoma and precancerous lesions. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2000;22:570–572. [PubMed] [Google Scholar]
- 51.Yu HP, Xu SQ, Liu L, et al. Cyclooxygenase-2 expression in squamous dysplasia and squamous cell carcinoma of the esophagus. Cancer Lett. 2003;198:193–201. doi: 10.1016/s0304-3835(03)00340-9. [DOI] [PubMed] [Google Scholar]
- 52.Tai HH, Ensor CM, Tong M, Zhou H, Yan F. Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat. 2002;68–69:483–493. doi: 10.1016/s0090-6980(02)00050-3. [DOI] [PubMed] [Google Scholar]
- 53.Stoner GD, Sardo C, Apseloff G, et al. Pharmacokinetic study of anthocyanins and ellagic acid in healthy volunteers fed 45 g of freeze-dried black raspberries daily for 7 days. J Clin Pharmacol. 2005;45:1153–1164. doi: 10.1177/0091270005279636. [DOI] [PubMed] [Google Scholar]
- 54.Kresty LA, Frankel W, Hammond C, et al. Transitioning from preclinical to clinical chemopreventive assessments of lyophilized black raspberries: interim results show berries modulate markers of oxidative stress in Barrett’s esophagus patients. Nutr Cancer. 2006 doi: 10.1207/s15327914nc5401_15. In press. [DOI] [PubMed] [Google Scholar]