Abstract
For several years, our laboratory has been evaluating the ability of lyophilized (freeze-dried) black raspberries (Rubus occidentalis, BRBs), blackberries (R. fructicosus, BBs), and strawberries (Fragaria ananasia, STRWs) to inhibit carcinogen-induced cancer in the rodent esophagus. To assure “standardized” berry preparations for study, each berry type is of the same cultivar, picked at about the same degree of ripeness, washed and frozen within 2–4 h of the time of picking, and freeze-dried under conditions that preserve the components in the berries. Some of the known chemopreventive agents in berries include vitamins A, C, and E and folic acid; calcium and selenium; β-carotene, α-carotene, and lutein; polyphenols such as ellagic acid, ferulic acid, p-coumaric acid, quercetin, and several anthocyanins; and phytosterols such as β-sitosterol, stigmasterol, and kaempferol. In initial bioassays, freeze-dried STRW, BRB, and BB powders were mixed into AIN-76A synthetic diet at concentrations of 5% and 10% and fed to Fischer 344 rats before, during, and after treatment with the esophageal carcinogen N-nitrosomethylbenzylamine (NMBA). At 25 wk of the bioassay, all three berry types were found to inhibit the number of esophageal tumors (papillomas) in NMBA-treated animals by 24–56% relative to NMBA controls. This inhibition correlated with reductions in the formation of the NMBA-induced O6-methylguanine adduct in esophageal DNA, suggesting that the berries influenced the metabolism of NMBA leading to reduced DNA damage. Studies are ongoing to determine the mechanisms by which berries influence NMBA metabolism and DNA adduct formation. BRBs and STRWs were also tested in a postinitiation scheme and were found to inhibit NMBA-induced esophageal tumorigenesis by 31–64% when administered in the diet following treatment of the animals with NMBA. Berries, therefore, inhibit tumor promotion and progression events as well as tumor initiation. In vivo mechanistic studies with BRBs indicate that they reduce the growth rate of premalignant esophageal cells, in part, through down-regulation of cyclooxygenase-2 leading to reduced prostaglandin production and of inducible nitric oxide synthase leading to reduced nitrate/nitrite levels in the esophagus. Based upon the preclinical data on rodents, we have initiated prevention trials in humans to determine if berries might exhibit chemopreventive effects in the esophagus.
Introduction
Esophageal cancer in humans occurs worldwide with a variable geographic distribution and ranks eighth in order of cancer occurrence, both sexes combined (1, 2). This cancer exists in two principal forms with distinct etiological and pathological characteristics, squamous cell carcinoma (SCC) and adenocarcinoma. More than 90% of esophageal cancers worldwide are SCCs (3,4), although adenocarcinomas are more prevalent in the United States (4–6). The principal precursor lesion for esophageal SCC is epithelial dysplasia (7). Microscopically, these lesions represent an accumulation of atypical cells with nuclear hyperchromasia, abnormally clumped chromatin, and loss of polarity. There is evidence from prospective studies that esophageal SCC probably develops through a progressive sequence from mild to severe dysplasia, carcinoma in situ, and, finally, invasive carcinoma (8–10). Most patients with cancer of the esophagus present with advanced, metastatic disease. The prognosis for these cases is poor; the overall survival rate of patients with metastatic disease is less than 10% (1,2).
The incidence of esophageal SCC shows a marked variation in its geographic distribution and occurs at very high frequencies in certain parts of China, Iran, South Africa, Uruguay, France, and Puerto Rico (7,11–14). In particular, the Henan, Shansi, and Hopei provinces in China have among the highest incidence and mortality rates for esophageal SCC in the world. Several risk factors have been identified for the disease. Excessive use of tobacco is a principal factor in the etiology of esophageal SCC, and several tobacco constituents, including nitrosamines, polycyclic hydrocarbons, aromatic amines, and various phenols and aldehydes, may be causally related to the disease (15–17). Alcohol consumption has been shown to further increase the risk of SCC in the esophagus of tobacco smokers (18). Consumption of salt-cured, salt-pickled, and moldy foods has also been implicated in the pathogenesis of the disease, particularly in high-risk areas (19). Some of these products are frequently contaminated with N-nitrosamine carcinogens and/or fungal toxins. Indeed, extensive research in China has shown that N-nitroso compounds, found in tobacco smoke and in the diet and produced from nitrites and amines in the acidic conditions of the stomach, are probable etiological factors of the disease. The detection of O6-methylguanine (O6-meGua) in the DNA of normal esophageal tissue taken from esophageal cancer patients in China further substantiates the role of methylating nitrosamines in the development of esophageal cancer (20,21). Other factors associated with an increased risk of esophageal SCC include vitamin (A, C, E, riboflavin, etc.) and trace mineral (zinc, selenium, iron, manganese, etc.) deficiencies, the consumption of hot-temperature beverages such as tea, and fungal invasion in the esophagus leading to localized inflammation and irritation of the tissues (22). Finally, human papilloma virus (HPV) may play a role in the etiology of esophageal SCC. A low frequency of HPV-16 or HPV-18 positivity has been reported in esophageal tumor samples (23).
Our laboratory has used the Fischer 344 (F344) rat for studies of the etiology, molecular biology, and chemoprevention of esophageal cancer since the late 1970s. The results of our studies are summarized in a recent review (24). Esophageal tumors are induced routinely in the rat by repeated subcutaneous (s.c.) injection of the N-nitrosamine carcinogen N-nitrosomethylbenzylamine (NMBA) (25–27). NMBA, found in the diets in China, but not in tobacco smoke, is by far the most potent of N-nitrosamine carcinogens for the rat esophagus (25). In a typical tumor bioassay, s.c. administration of NMBA at 0.25–0.5 mg/kg body weight three times a week for 5 wk or once per week for 15 wk results in 100% tumor incidence by 25 wk (25–27). Squamous papilloma is the predominant tumor histology in the F344 rat esophagus model; the incidence of SCC is rather low because the animals often succumb to the occlusive effects of large papillomas in their esophagi before carcinomas can develop. Several preneoplastic lesions, including simple hyperplasia, leukoplakia, and epithelial dysplasia, are produced in the esophagi of NMBA-treated rats. These lesions, which can be quantitated by routine histopathology, closely mimic preneoplastic lesions in the human esophagus.
In the mid-1980s, we evaluated the ability of the naturally occurring polyphenol ellagic acid to inhibit NMBA tumorigenesis in the rat esophagus when administered in the diet (28). While conducting studies with ellagic acid, we decided to identify foods in which it might be found. We evaluated a series of fruits for their content of ellagic acid and found high concentrations (630–1,500 µg/g dry weight) in black raspberries (BRBs), red raspberries, and strawberries (STRWs) (29). Ellagic acid was far more abundant in the pulp and seed of the berries; very little was found in the juice. Based upon these findings, we decided to take a “food-based” approach to cancer prevention and determine if freeze-dried berries would exhibit inhibitory effects on NMBA-tumorigenesis in the rat esophagus. The freeze-drying process is an approach to increase the concentration of putative inhibitory agents in berries because berries are composed of 85–90% water. In this article, we summarize previous studies of the ability of freeze-dried STRWs (30,31) and BRBs (32) to inhibit NMBA-tumorigenesis in the rat esophagus. We also present unpublished data on the ability of blackberries (BBs) to prevent esophageal cancer in rats. Finally, some of the mechanisms by which berries, in particular, BRBs, inhibit tumor initiation and progression in the esophagus are presented. Based upon known physiological and molecular similarities between rat and human esophagi, we anticipate that berries might be effective in the prevention of esophageal SCC in humans.
Materials and Methods
Preparation of Freeze-Dried Berries
STRWs of the Commander variety were provided by Driscoll Strawberry Associates, Inc. (Watsonville, CA) and freeze-dried in the Department of Food Science and Technology at the University of California-Davis as described (30,31). BRBs of the Jewel variety were obtained from the Dale Stokes Fruit Farm (Wilmington, OH) and freeze-dried at Van Drunen Farms (Momence, IL) as described (32). BBs of the Chester variety were obtained from Piketon Farms (Piketon, OH) and freeze-dried at Van Drunen Farms using the same procedure as described for the BRBs (32). In all cases, the berries were picked, washed with water, and frozen at −20°C on the farms within 2–4 h of the time of picking. They were then shipped frozen to the site where they were freeze-dried. The freeze-dried berries were packaged in double polyethylene bags, placed in carton boxes, and stored at −20°C. The freeze-dried berries were then shipped frozen to the Parker Food Science and Technology Building at the Ohio State University and stored frozen until use in experimental studies. Freeze-dried berries were analyzed routinely for content of certain vitamins, minerals, phenols, carotenoids, and phytosterols by Covance Laboratories, Inc. (Madison, WI) and for anthocyanin and ellagic acid content in the laboratory of Dr. Steven Schwartz, Department of Food Science and Technology, Ohio State University (33). Data from these analyses indicate that, with the exception of vitamin C, which degrades in frozen berries, the components measured in freeze-dried berries remain relatively stable for at least 1 yr when the berries are stored at −20°C.
Bioassays of Freeze-Dried Berries for Inhibitory Effects on NMBA-Induced Esophageal Tumorigenesis
Freeze-dried STRWs, BRBs, and BBs were administered in the diet at 5% and 10% before, during, and after NMBA treatment to evaluate their ability to inhibit both the initiation and progression stages of esophageal carcinogenesis (30–32) (Fig. 1A). Male F344 rats, 5–7 wk of age, were obtained from Harlan Sprague Dawley (Indianapolis, IN). For each bioassay, the rats were randomized into 5 groups of 10 to 20 animals each and were fed modified AIN-76A diet (Dyets, Inc., Bethlehem, PA) or modified AIN-76A diet containing 5% or 10% STRWs, BRBs, or BBs. AIN-76A diet was modified by reducing the starch in the diet of berry-fed animals by either 5% or 10% to maintain an isocaloric diet. Animals were maintained on their respective diets throughout the 25-wk bioassay. Two weeks after initiation of the experimental diets, rats in the positive control group and in the berry test groups received NMBA (Ash Stevens, Inc., Detroit, MI) administered s.c. at a concentration of 0.25 mg/kg body weight once per week for 15 wk. Control animals received either the vehicle for NMBA [20% dimethyl sulfoxide (DMSO):water] or the highest dietary concentration (10%) of each berry type. Weight data and food consumption data were obtained at weekly intervals throughout the bioassay. Animals were killed at 25–30 wk, complete necropsies were performed, and tissues were harvested. Esophagi were excised and fixed in 10% neutral buffered formalin, and tumors greater than 0.5 mm in diameter were counted, mapped, and sized (Fig. 2). Four hours after fixation in formalin, the tissues were transferred to a buffered saline solution where they were kept before preparation of slides for routine histopathology.
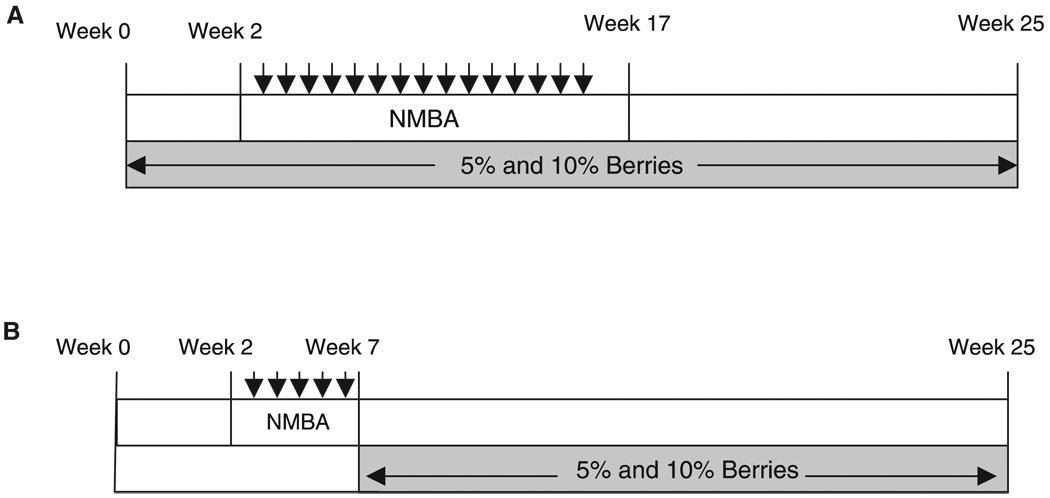
Figure 1.
(A) Experimental protocol for the complete carcinogenesis bioassay. Rats were treated with N-nitrosomethylbenzylamine (NMBA) at 0.25 mg/kg body weight once a week for 15 wk. Freeze-dried strawberries (STRWs), black raspberries (BRBs), or blackberries (BBs) were administered 2 wk prior to initial treatment with NMBA and for the duration of the 25-wk bioassay. (B) Experimental protocol for the postinitiation bioassay. Rats were treated with NMBA at 0.25mg/kg body weight three times a week for 5 wk. STRWs and BRBs were administered in the diet at 5%and 10% beginning 1 wk after NMBA treatment and for the duration of the 25-wk bioassay.
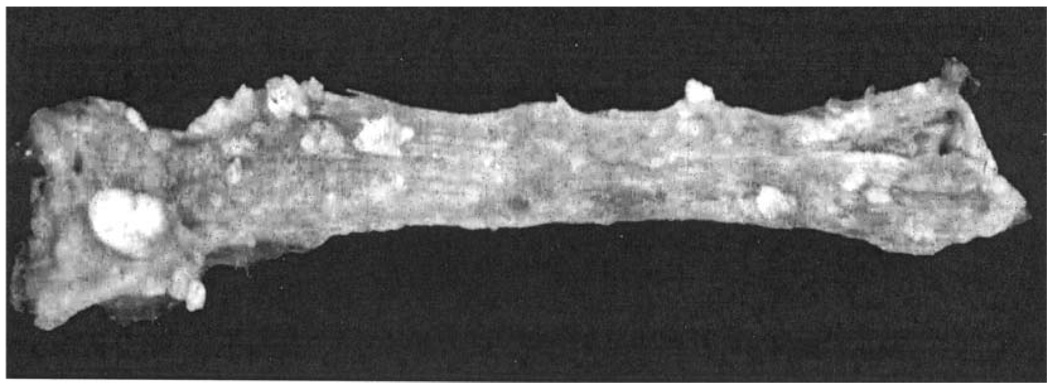
Figure 2.
Gross appearance of N-nitrosomethylbenzylamine–treated rat esophagus at the termination of a 25-wk bioassay. Note multiple papillomas on the surface of the esophagus. The large lesion on the left was found to be a carcinoma upon histological evaluation.
STRWs and BRBs, but not BBs, have also been tested for their ability to inhibit esophageal carcinogenesis when provided in the diet following treatment of rats with NMBA, that is, postinitiation (31,32) (Fig. 1B). Groups of 15–38 male F344 rats (5–7wk of age) were randomized to vehicle control, berry control (10% STRWs or BRBs), NMBA control, or NMBA plus either 5% or 10% STRWs or BRBs. NMBA groups were administered the carcinogen s.c. three times per week for 5 wk at a concentration of 0.25 mg/kg of body weight. One week after cessation of carcinogen treatment, groups of NMBA-treated rats received either STRWs or BRBs (5% or 10%) in the diet for the duration of bioassay (25 wk). The esophagi were harvested and fixed and the tumors counted and mapped. For histopathology, the esophagi from 10 animals per group were cut into thirds and paraffin embedded with the epithelium uppermost. Serial 4-µm sections were cut and mounted on slides, and the slides were stained with hematoxylin and eosin. The entire esophagus was scanned at 100× magnification, and each viewing field was classified into one of five histological categories: normal epithelium, epithelial hyperplasia, low-grade dysplasia, high-grade dysplasia, and squamous cell papilloma (Fig. 3) as described (26).
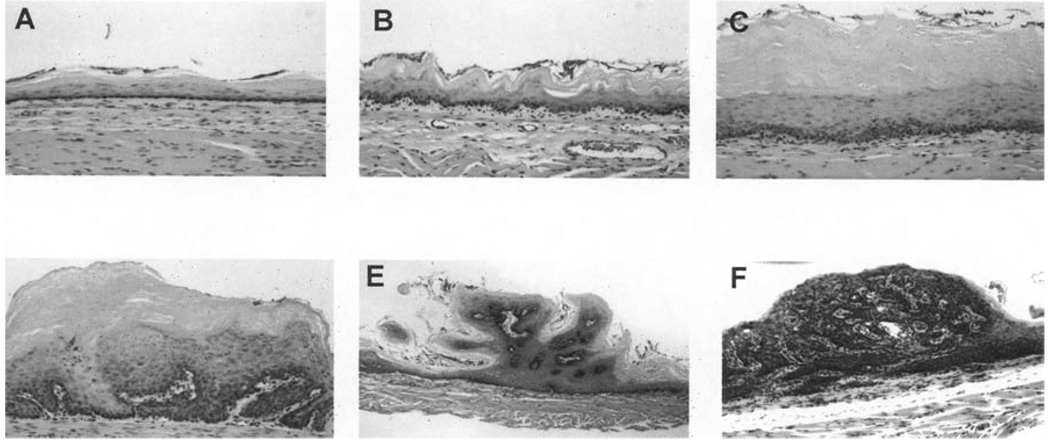
Figure 3.
Classification of microscopic histopathological and morphogenetic changes that occur during N-nitrosomethylbenzylamine–induced esophageal tumorigenesis in the Fischer 344 rat esophagus. (A) Normal esophageal epithelium, (B) epithelial hyperplasia, (C) low-grade dysplasia, (D) high-grade dysplasia, (E) squamous cell papilloma, and (F) carcinoma in situ.
O6-Methylguanine DNA Adduct Studies
Freeze-dried STRWs and BRBs, but not BBs, have been tested for their effects on the formation of O6-meGua adducts in esophageal DNA from animals treated with NMBA. Groups of 24–26 male F344 rats (7–8 wk of age) were randomized into experimental groups and placed on experimental diets. Rats were fed modified AIN-76A diet or modified AIN-76A diets containing either 5% or 10% STRWs or BRBs for 2 wk. On Day 14, rats received a single s.c. injection of NMBA at a concentration of either 0.25 mg/kg or 0.5 mg/kg body weight. Twenty-four hours after NMBA administration, the rats were killed by CO2 asphyxiation. Esophagi were harvested, split longitudinally and stripped of the underlying mucosa and submucosa, immediately frozen in liquid nitrogen, and stored at −80°C. The esophageal DNA was isolated, and O6-meGua adducts were quantitated by reverse-phase high-performance liquid chromatography as described by Morse et al. (34).
Immunohistochemical Analysis for Proliferating Cell Nuclear Antigen
Freeze-dried BRBs, but not STRWs or BBs, were examined for their effects on esophageal cell proliferation in NMBA-treated rats. The entire esophagus from five rats per group (vehicle control, 10% BRB control, NMBA control, and NMBA plus either 5% or 10% BRBs) was stained for proliferating cell nuclear antigen (PCNA) as described (32). Briefly, tissues were antigen retrieved and blocked with 3% H2O2, casein, goat serum, and avidin/biotin. Slides were then incubated with primary antibody, monoclonal mouse anti-PCNA (Biogenex, Inc., San Ramon, CA), for 30 min followed by rat-adsorbed link (biotinylated anti-immunoglobulin) for 20 min, streptavidin-horseradish peroxidase label for 20 min, and a final incubation with 3,3′-diaminobenzidine for 3.5 min to permit biomarker visualization. The percentage of cells in the basal layer of each esophagus that was stained for PCNA (labeling index, LI) was quantitated by computer-assisted image analysis as described (32). Photo-micrographs of PCNA-labeled rat esophagus using rat colon as a control are depicted in Fig. 4.
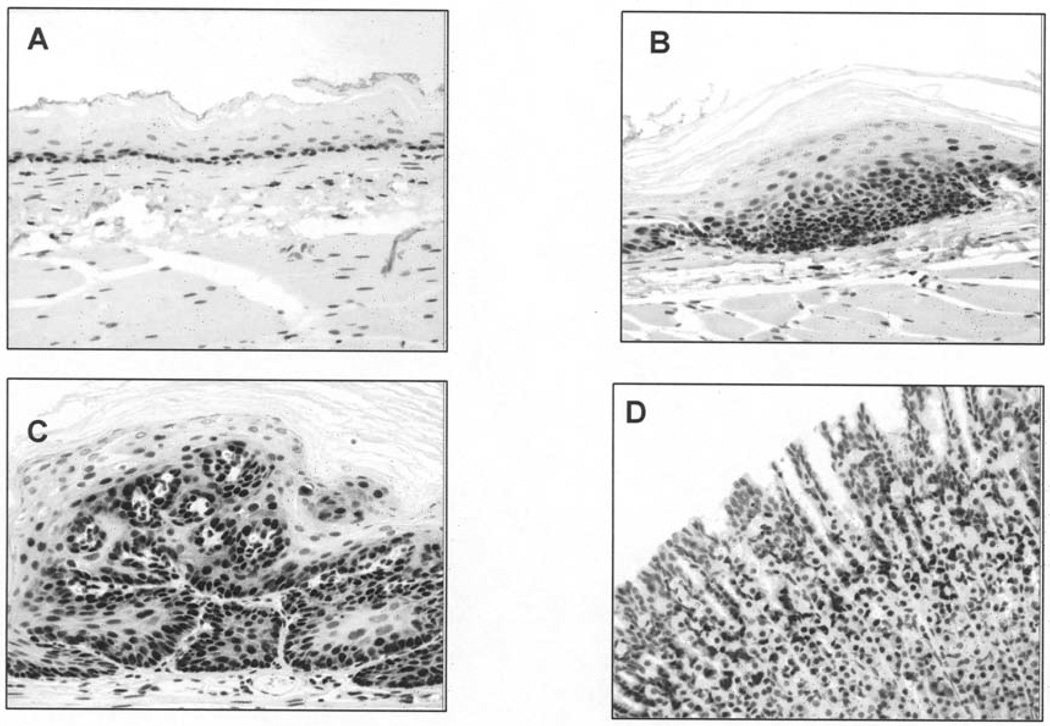
Figure 4.
Esophageal tissue sections stained with proliferating cell nuclear antigen (PCNA). (A) Low-level PCNA staining in normal esophageal epithelium; (B) increased PCNA nuclear reactivity in dysplastic esophageal epithelium; (C) intense PCNA staining in N-nitrosomethylbenzylamine–induced papilloma tissue at Week 26; and (D) positive staining in rat colon control tissue.
Quantitation of Cyclooxygenase-2 and Inducible Nitric Oxide Synthase
Freeze-dried BRBs were examined for their effects on the expression of cyclooxygenase-2(COX-2) and inducible nitric oxide synthase(iNOS) in the esophagus of NMBA-treated rats (35). The rats were treated with 5% dietary BRBs before, during, and after treatment with NMBA as described previously and depicted in Fig. 1A. At the termination of the 25-wk bioassay, the esophageal tumors were counted and sized, and one-half of the esophagus from each rat was fixed in buffered formalin for immunohistochemistry as described (32). The epithelium on the other half of the esophagus was stripped of the submucosal and muscularis layers and frozen in liquid nitrogen. All samples were stored at −80°C until analysis for expression levels of COX-2 and iNOS.
Total cellular RNA was isolated from frozen esophageal epithelium using TRIzol Reagent (GIBCO BRL, Gaithersburg, MD) according to the manufacturer’s instructions. After extraction, all RNA samples were analyzed for integrity of 18S and 28S ribosomal RNA by ethidium bromide staining of 1 µg of RNA resolved by electrophoresis on 1.2% agarose formaldehyde gels. One-step, real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed in a GeneAmp 5700 sequence detection system (Perkin-Elmer Corp., Norwalk, CT) using the QuantiTect SYBR Green RT-PCR Kit (Qiagen, Inc., Valencia, CA) as described (36). Briefly, the reaction of a 50-µl volume of total cellular RNA, QuantiTect RT Mix, QuantiTect SYBR Green RT-PCR Master Mix, and forward and reverse primers for COX-2 or iNOS were reverse transcribed, and PCR was performed after RT reaction. COX-2 and iNOS messenger RNA (mRNA) expressions were normalized against expression of the housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (HPRT). Primers for COX-2, iNOS, and HPRT were designed according to published sequences with Primer Express Software, version 2.0 (Applied Biosystems, Foster City, CA). Each individual RNA sample for each gene was assayed in triplicate. Two controls were run with every reaction: one contained RNA and QuantiTect RT Mix to detect genomic DNA and the other contained the reaction reagents without RNA to confirm that the reagents displayed no signal. After the performance of RT-PCR, all data were collected using SDS Sequence Detector Software (PE, Applied Biosystems).
Proteins were extracted from frozen esophageal epithelium, and protein concentrations were determined using the Bio-Rad assay (Bio-Rad, Hercules, CA) as recommended by the manufacturer. Fifty micrograms of protein with NuPAGE LDS Sample Buffer and NuPAGE Sample Reducing Agent (Invitrogen, Carlsbad, CA) was heated at 100°C for 1min. After cooling at room temperature for 5 min, proteins were fractioned by 7% NuPAGE Novex Tris-Acetate Gel (Invitrogen) electrophoresis. Proteins were then transferred to an Invitrolon PVDF membrane. The membrane was blocked with blocking buffer (concentrated saline and Hammersten casein solution) at 4°C overnight. The blot was probed with either COX-2 (1:500, Cayman Chemical Co., Ann Arbor, MI) or iNOS (1:200, Santa Cruz Biotechnology, Santa Cruz, CA) antibody at room temperature for 1 h. After washing extensively to eliminate nonspecific binding, the membrane was incubated with goat anti-rabbit secondary antibody labeled with alkaline phosphatase at room temperature for 1 h. The Western blots were visualized using a WesternBreeze Chromogenic Immunodetection Kit (Invitrogen). β-Actin (1:1000, Sigma, St. Louis, MO) was detected in the same sample to ensure an equal protein loading.
Cyclooxygenase-2 Activity Assay
COX-2 activity in esophageal epithelium and in papillomas was assayed by using the prostaglandin E2 (PGE2) Biotrak Enzymeimmunoassay System (Amersham Pharmacia Biotech, Piscataway, NJ) to measure PGE2 concentration. Frozen samples were homogenized in Tris-HCl buffer (pH 7.5) with 0.02 M EDTA and 5 mg/ml indomethacin. Total protein concentration for each tissue homogenate was determined using the DC Protein Assay (Bio-Rad, Hercules, CA). PGE2 was collected and purified according to the manufacturer’s instructions. The optical density was measured at 450 nm using the SpectraMax™ M2 multidetection reader (Molecular Devise Corp., Sunnyvale, CA). The PGE2 level was normalized against the protein concentration in the same sample. Each sample was assayed in triplicate.
Inducible Nitric Oxide Synthase Activity Assay
iNOS activity in esophageal epithelium was measured using a Nitrate/Nitrite Colorimetric assay kit (Cayman Chemical Co., Ann Arbor, MI) according to the manufacturer’s instructions. Frozen esophageal samples were weighed and homogenized in phosphate-buffered saline, and 80 µl of supernatant for each sample was pipetted into a 96-well optical plate and incubated with 10 µl of nitrate reductase and 10 µl of enzyme cofactor for 3 h. Griess reagents [sulfanilamide and N-(1-naphthyl)ethylenediamine] were then added, and the absorbance was measured at a wavelength of 550 nm using a SpectraMax™ M2 multidetection reader (Molecular Device Corp., Sunnyvale, CA). Standards of sodium nitrate (0–35 µM) were used to create a standard curve. The final nitrite concentration was the sum of the nitrite plus the reduced nitrate in each sample and was taken as an index of iNOS activity. Each individual sample was assayed in triplicate.
Statistical Analysis
Body weights, food consumption, tumor multiplicity (mean number of tumors/esophagus), tumor size, and preneoplastic lesion data were compared using analysis of variance (ANOVA) followed by Newman-Keuls’ multiple comparison test (P < 0.05) when appropriate. DNA adduct levels were analyzed by linear regression and ANOVA to detect differences between means and to calculate standard errors (SEs). Tumor incidence (percent of animals in each group with tumors) data were analyzed using the χ2 test. iNOS and COX-2 expression data, PGE2 concentration data, and the total nitrite and nitrate concentration results were analyzed and compared using one-way ANOVA followed by Dunnet’s multiple comparison test (P < 0.05) when appropriate. All statistical analysis was carried out using GraphPad Prism 4.0. Differences were considered statistically significant at P < 0.05.
Results
Levels of Some Nutrients and Potential Chemopreventive Agents in Berries
Table 1 lists nutrients in freeze-dried STRWs, BRBs, and BBs that we have chosen to measure on a routine basis. As indicated, all three berry types contain vitamins, carotenoids, minerals, multiple phenolic compounds including the anthocyanins, and phytosterols. The vitamin C in berries is quite labile and degrades within 2 wk when berries are stored frozen before freeze-drying. The amount listed for STRWs (371 mg/100 g dry weight) is probably accurate in that the STRWs in this analysis were freeze-dried immediately after picking. Carotenoid levels in berries are quite low, that is, from 0.01 to 1 mg/100 g dry weight. Calcium and potassium levels in berries are significant and tend to be higher in BRBs than in STRWs or BBs. As reported earlier (29), the content of ellagic acid in BRBs and BBs is higher than in STRWs. The levels of anthocyanins in BRBs are similar to those in BBs. We have not measured anthocyanins in STRWs, although they are known to be lower than in either BRBs or BBs (37). The levels of the phenolic anthocyanins are significantly higher than any of the individual polyphenols in berries, including ellagic acid. Of the phytosterols measured, the most abundant is β-sitosterol, which is higher in BRBs than in the other berry types. In our experience, the content of individual nutrients (except for vitamin C) in BRBs of the Jewel variety, obtained from the same farm and grown in the same area of the berry field for the past 7 yr, has not varied more than 20–30%.
Table 1.
Levels of Nutrients and Potential Chemopreventive Agents in Freeze-Dried STRWs, BRBs, and BBsa
| Components | STRWs | BRBs | BBs | |
|---|---|---|---|---|
| Vitamins | Vitamin A | 267 IU | 915 IU | 1,770 IU |
| Vitamin C | 371 | 4.4 | 85 | |
| Vitamin E | 4.95 | 15.2 | 13.6 | |
| Folic acid | 0.59 | 0.08 | 0.34 | |
| Carotenoids | α-Carotene | <0.02 | <0.02 | <0.02 |
| β-Carotene | 0.16 | <0.02 | 1.06 | |
| Zeaxanthin | <0.02 | <0.02 | 0.05 | |
| Lutein | 0.11 | <0.02 | 0.59 | |
| Minerals | Calcium | 72 | 245 | 186 |
| Magnesium | 92 | 169 | 163 | |
| Potassium | 1,110 | 1200 | 1,170 | |
| Zinc | 0.6 | 3 | 1.1 | |
| Selenium | <0.01 | <5 | <0.05 | |
| Phenols | Ellagic acid | 67 | 175 | 165 |
| Ferulic acid | 9 | 17–32 | — | |
| p-Coumaric acid | 32 | 8 | — | |
| Anthocyanins | 527–2,705 | 1,715 | ||
| Phytosterols | β-Sitosterol | 41 | 89 | 34 |
| Campesterol | <3 | 4 | 9.5 | |
| Stigmasterol | <3 | <3 | 8.2 |
Abbreviations are as follows: STRWs, strawberries; BRBs, black raspberries; BBs, blackberries. IU, international units.
General Observations in the Bioassays
During the bioassays, control animals treated with freeze-dried STRWs, BRBs, or BBs only gained 5–10% more weight than control animals fed the AIN-76A diet; however, these weight gains were not significant (data not shown). No significant differences were detected in animal body weights or food consumption between the control groups and NMBA-treated animals consuming freeze-dried STRWs, BRBs, or BBs (data not shown). Histopathological examination of sections of the liver, colon, stomach, kidney, spleen, heart, and bladder tissues from animals fed 10% STRWs or BRBs indicated that the berries did not elicit toxic effects in any of these organs (30–32). Similar results were obtained in the present study after treatment of rats with freeze-dried BBs.
Inhibition of Esophageal Tumorigenesis by STRWs, BRBs, and BBs in a Complete Carcinogenesis Bioassay
The chemopreventive effects of freeze-dried dietary STRWs, BRBs, and BBs on esophageal tumor incidence and multiplicity are summarized in Table 2. In all bioassays, the tumor incidence among groups dosed with NMBA only was 100%, and neither STRWs, BRBs, nor BBs had a significant effect on tumor incidence. Administration of 5% and 10% freeze-dried STRWs before, during, and after NMBA treatment significantly reduced tumor multiplicity to 3.1 and 1.8, respectively, compared with 4.1 in NMBA-treated controls (P < 0.05). Five percent and 10% BRB significantly reduced tumor multiplicity to 1.9 and 1.6, respectively, compared with 3.2 in NMBA-treated controls (P < 0.05). Surprisingly, 5% dietary BBs were effective in reducing tumor multiplicity, whereas 10% BBs were ineffective. There were no significant differences in tumor size among NMBA control and NMBA + 5% and NMBA + 10% berry-treated groups for all three berry types (data not shown).
Table 2.
Effects of Freeze-Dried STRWs, BRBs, and BBs on NMBA-Induced Rat Esophageal Tumorigenesis When Administered in the Diet Before, During, and After NMBA Treatmenta
| Treatment | Rats (n) | Tumor Incidence (% inhibition) | Tumor Multiplicity (% inhibition) |
|---|---|---|---|
| Vehicle control | 15 | 0 | 0.0 |
| 10% STRWs | 15 | 0 | 0.0 |
| NMBA control | 15 | 100 | 4.1 ± 0.2 |
| NMBA + 5% STRWs | 15 | 100 | 3.1 ± 1.0 (24)b |
| NMBA + 10% STRWs | 15 | 80 (20) | 1.8 ± 1.4 (56)b |
| Vehicle control | 15 | 0 | 0.0 |
| 10% BRBs | 14 | 0 | 0.0 |
| NMBA control | 13 | 100 | 3.2 ± 0.3 |
| NMBA + 5% BRBs | 14 | 79 (21) | 1.9 ± 0.4 (41)b |
| NMBA + 10% BRBs | 13 | 92 (8) | 1.6 ± 0.3 (50)b |
| Vehicle control | 10 | 0 | 0.0 |
| 10% BBs | 10 | 0 | 0.0 |
| NMBA control | 17 | 82 | 2.8 ± 0.6 |
| NMBA + 5% BBs | 20 | 75 (8) | 1.5 ± 0.4 (46)b |
| NMBA + 10% BBs | 18 | 70 (15) | 2.3 ± 0.5 (18) |
Inhibition of O6-Methylguanine Adducts
The effects of dietary administration of freeze-dried STRWs and BRBs on NMBA-induced DNA adduct formation in the F344 rat esophagus are shown in Table 3. Levels of O6-meGua were not detectable in the esophagi of rats that were treated with vehicle alone. O6-meGua levels were 4.4 pmol/mg DNA in rats treated with NMBA at 0.25 mg/kg of body weight. Administration of 5% and 10% STRWs prior to treatment with NMBA significantly inhibited esophageal O6-meGua levels by 68% and 57%, respectively, whereas treatment with the same concentrations of BRBs reduced adduct levels by 73% and 80%, respectively. These data clearly indicate that STRWs and BRBs reduce the level of genetic damage in the esophagus of rats treated with NMBA. BBs were not tested for their effects on the formation of O6-meGua adducts in the esophagus of NMBA-treated rats.
Table 3.
Effects of Dietary Administration of Freeze-Dried STRWs and BRBs on NMBA-Induced DNA Adduct Formation in the Esophagus of Fischer 344 Ratsa
| Treatment | pmol O6-meGua/mg DNA | % Inhibition |
|---|---|---|
| NMBA (0.25 mg/kg) | 4.4 ± 0.9 | — |
| NMBA + 5% STRWs | 1.4 ± 0.1b | 68 |
| NMBA + 10% STRWs | 1.9 ± 0.7b | 57 |
| NMBA + 5% BRBs | 1.2 ± 0.3b | 73 |
| NMBA + 10% BRBs | 0.9 ± 0.2b | 80 |
Abbreviations are as follows: STRWs, strawberries; BRBs, black raspberries; NMBA, N-nitrosomethylbenzylamine; O6-meGua, O6-methylguanine. Rats were fed 5% or 10% freeze-dried STRWs or BRBs for 14 days and then given a single subcutaneous injection of NMBA at 0.25 mg/kg body weight. The esophageal DNA was isolated and O6-meGua measured as described in Materials and Methods. STRW data are taken from Ref. 31 and BRB data are from Ref. 32.
Significantly different from NMBA controls (P < 0.05).
Inhibition by Freeze-Dried Strawberries and Black Raspberries in Postinitiation Bioassays
Freeze-dried STRWs and BRBs were evaluated for their ability to inhibit NMBA-induced tumorigenesis in the rat esophagus when administered in the diet after completion of NMBA treatment (Table 4). BBs were not evaluated for potential inhibitory effects in a postinitiation protocol. At 5% and 10% of the diet, freeze-dried STRWs significantly reduced tumor multiplicity by 38% and 31%, respectively, when compared with NMBA controls (P < 0.05). Interestingly, 5% STRWs appeared to be more effective than 10% STRWs, and there was no difference in tumor multiplicity between the NMBA + 5% STRW group and the NMBA + 10% STRW group. Similarly, 5% and 10% dietary BRBs significantly reduced tumor multiplicity by 64% and 43%, respectively, when compared with NMBA controls (P < 0.05). Again, 5% BRBs appeared to be more effective than 10% BRBs. Thus, when administered in the diet following NMBA treatment, STRWs and BRBs effectively reduced tumor number in the rat esophagus, but these reductions were not dose related.
Table 4.
Postinitiation Effects of Dietary STRWs and BRBs on NMBA-Induced Esophageal Tumorigenesis in the Fischer 344 Rata
| Tumor Multiplicity | ||||
|---|---|---|---|---|
| Treatment | Rats (n) |
Tumor Incidence (%) |
Tumors/Rat (mean ± SE) |
% Inhibition |
| Vehicle control | 10 | 0 | 0 | 0 |
| 10% STRWs | 10 | 0 | 0 | 0 |
| NMBA control | 14 | 100 | 4.8 ± 0.6 | 0 |
| NMBA + 5% STRWs | 15 | 100 | 3.0 ± 0.3b | 38 |
| NMBA + 10% STRWs | 15 | 100 | 3.3 ± 0.4b | 31 |
| Vehicle control | 10 | 0 | 0 | 0 |
| 10% BRBs | 10 | 0 | 0 | 0 |
| NMBA control | 15 | 87 | 1.4 ± 0.3 | 0 |
| NMBA + 5% BRBs | 15 | 40b | 0.5 ± 0.2b | 64 |
| NMBA + 10% BRBs | 15 | 47b | 0.8 ± 0.3b | 43 |
Table 5 shows the effects of freeze-dried STRWs and BRBs on the frequency of preneoplastic lesions in NMBA-treated rat esophagus when the berries were provided in the diet postinitiation. The addition of 10% STRWs to the diet of NMBA-treated animals significantly reduced the occurrence of hyperplastic lesions as well as low- and high-grade dysplastic lesions. At 5% of the diet, STRWs reduced the occurrence of low- and high-grade dysplastic lesions. The effects of BRBs on the occurrence of preneoplastic lesions were less pronounced in that they reduced the occurrence of high-grade dysplastic lesions only. However, it should be mentioned that these lesions were evaluated by two individuals in separate experiments. These individuals may have scored them differently as indicated by the observation that hyperplastic and low-grade dysplastic lesions were identified in the esophagus of control (vehicle and 10% BRB) rats in one study but not in the other study.
Table 5.
Effects of Freeze-Dried STRWs and BRBs on NMBA-Induced Preneoplastic Lesions in the Rat Esophagusa
| No. Lesions/Rat (mean ± SE) | ||||
|---|---|---|---|---|
| Treatment | Rats (n) | Epithelial Hyperplasia | Low-Grade Dysplasia | High-Grade Dysplasia |
| Vehicle control | 7 | 0 | 0 | 0 |
| 10% STRWs | 15 | 0 | 0 | 0 |
| NMBA control | 12 | 43.3 ± 2.4 | 9.6 ± 2.4 | 11.6 ± 1.8 |
| NMBA + 5% STRWs | 14 | 40.6 ± 2.6 | 20.6 ± 3.5b | 5.6 ± 0.9b |
| NMBA + 10% STRWs | 15 | 33.5 ± 2.1b | 19.3 ± 2.8b | 4.1 ± 0.5b |
| Vehicle control | 10 | 18.8 ± 2.4 | 2.0 ± 0.8 | 0 |
| 10% BRBs | 10 | 20.8 ± 2.9 | 2.7 ± 1.0 | 0 |
| NMBA control | 15 | 44.7 ± 1.7 | 9.5 ± 0.8 | 7.2 ± 0.9 |
| NMBA + 5% BRBs | 15 | 40.6 ± 2.0 | 8.3 ± 0.9 | 4.1 ± 0.6b |
| NMBA + 10% BRBs | 15 | 41.7 ± 2.8 | 8.7 ± 1.0 | 4.9 ± 0.8b |
Proliferating Cell Nuclear Antigen
The effect of BRBs on the PCNA LI in the esophagus of rats treated with NMBA at 25 wk of the bioassay is shown in Table 6. The PCNA LI in vehicle controls and in rats treated with 10% BRB only was 21.3% and 20.0%, respectively. In rats treated with NMBA (0.25 mg/kg body weight) the PCNA LI was increased to 32.3%, and the dietary administration of both 5% and 10% BRBs significantly reduced the LI in NMBA-treated rats to 22.7% and 25.7%, respectively. Thus, the berries reduced the PCNA LI in NMBA-treated animals almost to the level seen in non-NMBA–treated controls; however, this inhibition was not related to the dietary concentration of berries.
Table 6.
Effects of Dietary Administration of BRBs on PCNA LI in the Rat Esophagusa
| Treatment | PCNA LI (%) | % Reduction |
|---|---|---|
| Vehicle control | 21.3 | 0 |
| 10% BRBs | 20.0 | 0 |
| NMBA control | 32.2 | 0 |
| NMBA + 5% BRBs | 22.7b | 30 |
| NMBA + 10% BRBs | 25.7b | 20 |
Abbreviations are as follows: BRBs, black raspberries; PCNA, proliferating cell nuclear antigen; LI, labeling index; NMBA, N-nitrosomethylbenzylamine.
Significantly different from NMBA controls (P < 0.05). Data are taken from Ref. 32.
Cyclooxygenase-2 and Inducible Nitric Oxide Synthase mRNA Expression
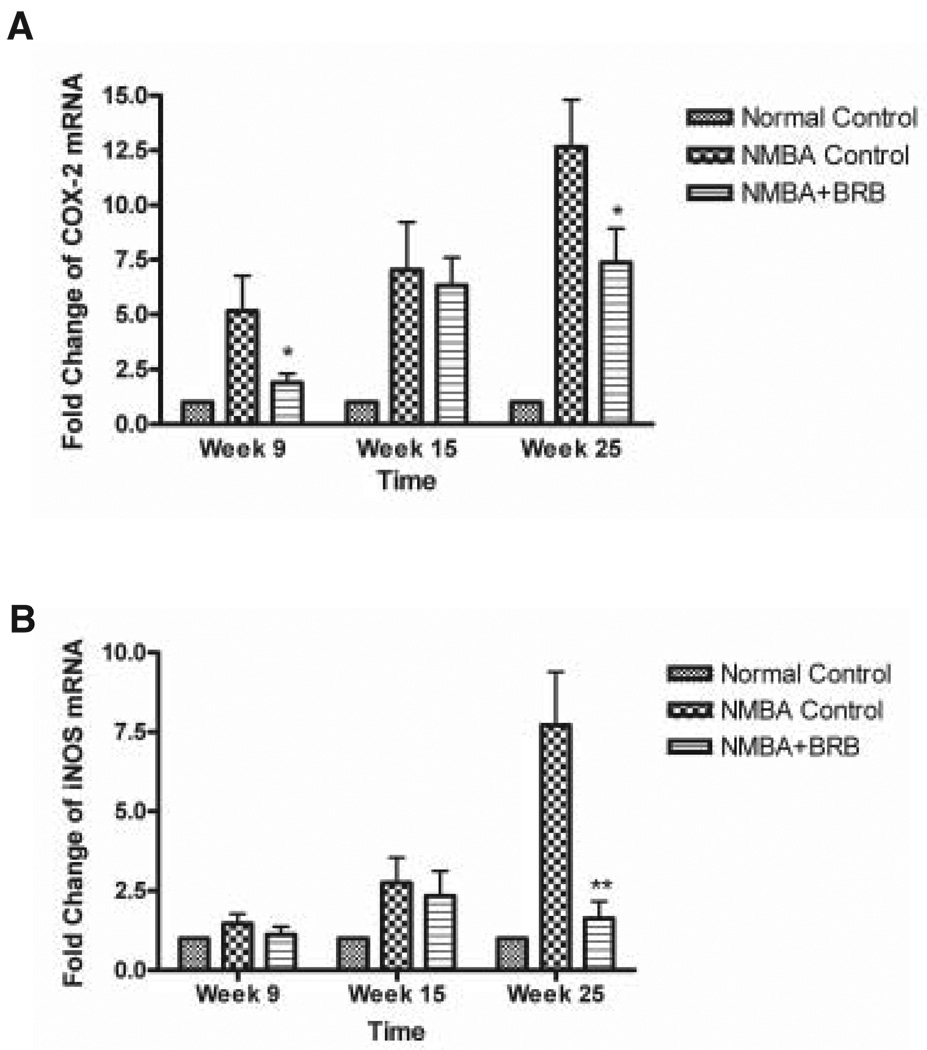
BRBs, at 5% of the diet, were evaluated for their effects on the expression of COX-2 and iNOS mRNAs in the esophagus of NMBA-treated rats at 9, 15, and 25 wk of a postinitiation bioassay (Fig. 5). The berries significantly reduced the expression levels of COX-2 in the esophagus at Weeks 9 and 25 of the bioassay (P < 0.05). They also caused some reduction in COX-2 expression at 15 wk, but this reduction was not significant. BRBs significantly reduced the expression level of iNOS mRNA in the esophagus of NMBA-treated rats at Week 25 (P < 0.05). At 9 and 15 wk, they caused some reduction in iNOS expression, but these reductions were not significant.
Figure 5.
(A) Effect of 5% freeze-dried black raspberries (BRBs) on the messenger RNA (mRNA) expression levels of cyclooxygenase-2 at Weeks 9, 15, and 25 of the postinitiation bioassay. (B) Effect of 5% freeze-dried BRBs on the mRNA expression levels of inducible nitric oxide synthase at the same time points. The BRBs were administered in the diet beginning at Week 7 and for the duration of the 25-wk bioassay. The values are relative densitometric intensity expressed as a mean; bars, ±SD. *P < 0.05 and **P < 0.005 as determined by analysis of variance compared with the N-nitrosomethylbenzylamine control group.
Cyclooxygenase-2 and Inducible Nitric Oxide Synthase Protein Expression
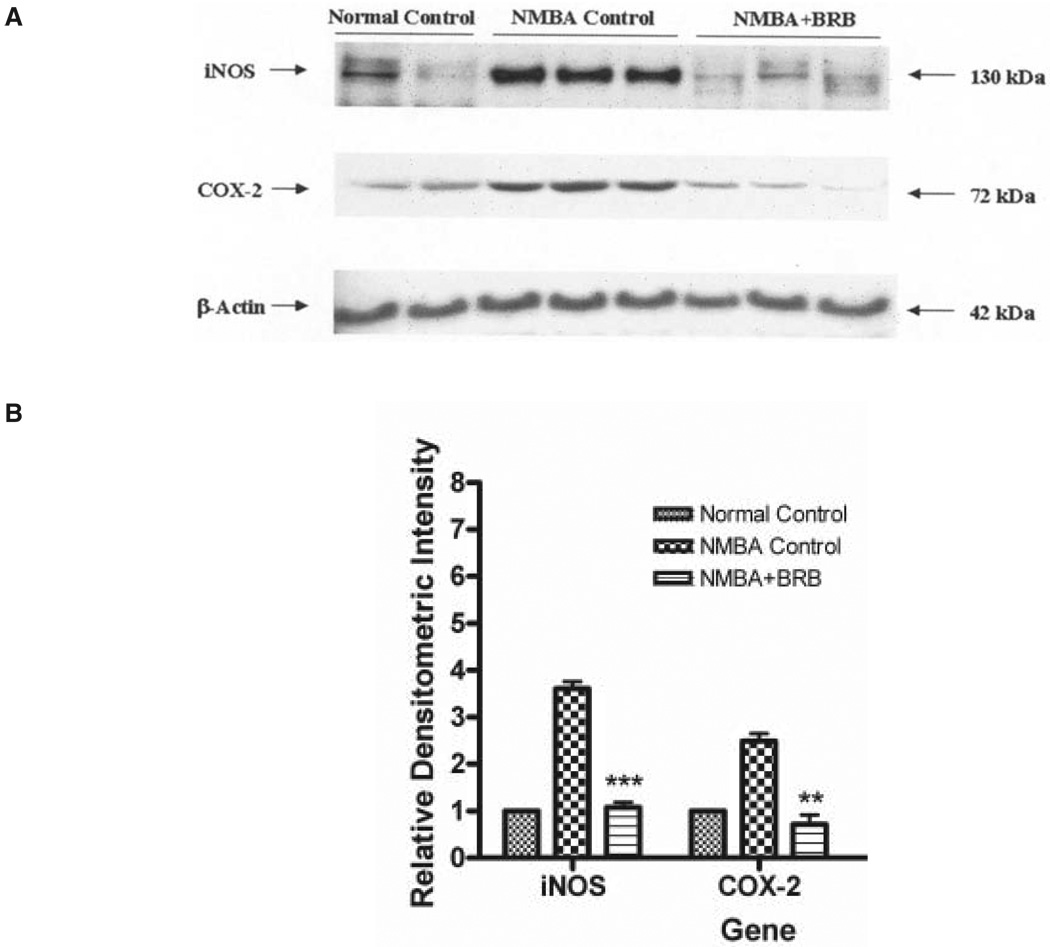
Western blot analysis was used to examine the potential effects of BRBs on protein expression in the esophagus of NMBA-treated rats at 25 wk of the bioassay. As shown in Fig. 6, NMBA induced the overexpression of both COX-2 and iNOS proteins. The dietary administration of 5% freeze-dried BRBs significantly reduced the levels of both COX-2 and iNOS proteins in NMBA-treated animals to those seen in vehicle-treated controls (P < 0.05).
Figure 6.
(A) Effects of black raspberries on the expression of inducible nitric oxide synthase and cyclooxygenase-2 proteins at Week 25 of the bioassay as determined by Western blot analysis. Similar results were obtained from triplicate experiments. (B) Values are relative densitometric intensity expressed as a mean; bars, ±SD. **P < 0.005 and ***P < 0.0005 as determined by analysis of variance when compared with the N-nitrosomethylbenzylamine control group.
Levels of Prostaglandin E2 and Nitrite in Esophageal Tissues
The effects of 5% dietary freeze-dried BRBs on COX-2 and iNOS activities in esophageal tissues at 25 wk of the bioassay were determined by measuring total PGE2 and nitrite levels, respectively, by enzyme immunoassay (Table 7). BRBs reduced total PGE2 levels in the esophagus from 9.89 ± 2.56 µg/mg protein in rats treated with NMBA only to 4.10 ± 1.05 µg/mg protein in rats treated with NMBA + 5% BRB (P < 0.05). The berries reduced nitrite levels from 5.90 ± 0.90 µM in rats treated with NMBA only to 4.12 ± 0.68 µM in rats treated with NMBA + 5% BRB (P < 0.05).
Table 7.
Modulation of iNOS and COX-2 Activities in NMBA-Treated Rat Esophagus by 5% Dietary BRBsa
| NMBA | Diet | Nitriteb (mean ± SE) |
PGE2c (mean ± SE) |
|---|---|---|---|
| − | AIN-76A | 0.48 ± 0.77 | 1.27 ± 0.75 |
| + | AIN-76A | 5.90 ± 0.90 | 9.89 ± 2.56 |
| + | AIN-76A + BRBs | 4.12 ± 0.68d | 4.10 ± 1.05d |
Abbreviations are as follows: iNOS, inducible nitric oxide synthase; COX-2, cyclooxygenase-2; NMBA, N-nitrosomethylbenzylamine; BRBs, black raspberries; PGE2, prostaglandin E2. Data are from Ref. 35.
iNOS activity is expressed as micromolar concentration of nitrite in esophageal protein.
COX-2 activity is expressed as micrograms of PGE2 per milligram of esophageal protein.
Significantly lower than in rats treated with NMBA and fed AIN-76A diet (P < 0.05).
Discussion
For more than 2 decades, our laboratory has focused research efforts on cancer chemoprevention using NMBA-induced tumors in the rat esophagus as a model for SCC of the esophagus (24). In this model, the principal end points for assessing the inhibitory effects of most chemopreventive agents are tumor multiplicity, as assessed by surface counts of esophageal lesions, and reductions in preneoplastic lesions (hyperplasia and dysplasia), as determined by histopathology. Reductions in tumor incidence occur only with very potent chemopreventive agents such as certain isothiocyanates (26,27,42) because virtually all NMBA-treated rats develop multiple tumors at 25 wk. The use of lower doses of NMBA to reduce the tumor number generally results in an unacceptable number of animals that have either one tumor per animal or no tumors at all. Under these circumstances, with less-active chemopreventive agents, large numbers of animals per group (60–100) are required to demonstrate an inhibitory effect on tumor incidence, which makes routine studies cost prohibitive. Similarly, tumor volume data are of limited use in this model because the variability in esophageal tumor volume among individual animals is significant. Thus, as for tumor incidence data, large numbers of animals per group (60–100) are required to demonstrate a significant inhibitory effect of chemopreventive agents on tumor volume.
Initially, we demonstrated the protective effect of the naturally occurring polyphenol ellagic acid on NMBA-tumorigenesis in the rat esophagus (28). Ellagic acid was most effective in inhibiting esophageal carcinogenesis when provided in the diet before, during, and after administration of the carcinogen; it was only modestly active in a postinitiation protocol (38). For this reason, and because we had demonstrated that ellagic acid has poor solubility and bioavailability (data not published), we decided to abandon studies with this chemopreventive agent. Nevertheless, when studies with ellagic acid were ongoing, we decided to determine where it might be found in the diet. Guided by a report demonstrating the presence of ellagitannins in loganberry wine (39), we examined a series of fruits for their contents of both free- and glucose-bound ellagic acid and found that the highest contents of total ellagic acid were in berries (29). Interestingly, the ellagic acid in berries was detectable in the pulp and seed and not in the juice. Because the water in berries accounts for about85–90% of their wet weight, we reasoned that removal of the water from berries under conditions that would preserve other berry components would result in about a 10-fold concentration of the ellagic acid. Studies were then undertaken with “ellagic acid–enriched”, that is, freeze-dried, berries to determine if they might protect against NMBA-induced esophageal carcinogenesis when provided in the diet. Initial studies were undertaken with STRWs, and they were found to produce an approximate 50% inhibition of NMBA-tumorigenesis in the rat esophagus when provided at 10% of a synthetic diet (Table 2) (30,31). This inhibition was similar to that seen in earlier experiments with pure ellagic acid, which initially led us to believe that the ellagic acid in the berries was responsible for the inhibitory effect. Analysis of the 10% STRW diet indicated, however, that the ellagic acid content in the diet was less than one-fifth of that used in the earlier studies with pure ellagic acid. Moreover, most of the ellagic acid in the STRW diet was in the form of ellagitannins, which have limited bioavailability in animals (40). Thus, it became apparent that other components in berries, including several of those listed in Table 1, probably contribute substantially to their cancer-inhibitory effects. Perhaps the most important among these components are the anthocyanins, which, collectively, are the most abundant of the polyphenols in berries. Recently, using biodirected fractionation, we and our collaborators have shown that the anthocyanins in BRBs are the most effective components in down-regulating the expression of benzo[a]pyrene diol epoxide–induced AP-1 and NF-κB luciferase reporter genes in JB-6 clone 41 mouse epidermal cells (41). It remains to be determined, however, whether this activity of the anthocyanins is related to their anticarcinogenic potential.
STRWs and BRBs appeared to be equally effective in inhibiting the induction, by NMBA, of tumors in the rat esophagus when provided in the diet before, during, and after carcinogen treatment (Table 2). At 5% of the diet, BBs were as effective as either STRWs or BRBs, but, surprisingly, they were ineffective in reducing the tumor response when fed at 10% of the diet. We have no explanation for this result, although the somewhat higher variability in the tumor response to NMBA among animals in the BB study may be partially responsible. We intend to repeat the BB study before making final conclusions. All berry types were effective in reducing tumor multiplicity but not tumor incidence. This result was not unexpected because only phenethyl and phenylpropyl isothiocyanates have been shown to reduce tumor incidence in the esophagus of NMBA-treated rats (26,42). This remarkable inhibitory effect of the isothiocyanates appears to be due to their ability to inhibit the metabolic activation of NMBA to DNA-damaging species (43).
NMBA is a procarcinogen that requires metabolic activation to produce its carcinogenic effect. Its activation is mediated by cytochrome P450s forming benzaldehyde and a methylating species, which preferentially methylate the N7- and O6-positions of guanine (17,44). Although N7-meGua is the predominant adduct formed in DNA, O6-meGua is critical for carcinogenesis because it is poorly repaired (45). Studies indicate that formation of the O6-meGua adduct and its persistence are closely linked to esophageal tumor induction in rats (45). Our studies indicate that the feeding of STRWs and BRBs in the diet of rats reduces the formation of NMBA-induced O6-meGua adducts in esophageal DNA in a dose-responsive manner (Table 3). Thus, berries contain compounds that influence the metabolism of NMBA leading to reduced genetic damage. To determine the mechanisms of the anti-initiation effects of berries, we are currently examining the effects of berries on the expression levels and activities of phase I cytochrome P450 enzymes and phase II detoxifying enzymes in NMBA-treated rat esophagus, using both conventional biochemical assays and DNA array analysis. Some of the results of these studies are summarized in another publication in this series (46) and will not be repeated here. It is conceivable that berry components could also influence the activities of enzymes involved in DNA repair, and this possibility is also under investigation.
Chemopreventive agents have been classified by Wattenberg as either “blocking” (anti-initiation) agents or “suppressing” (antipromotion/progression) agents (47). In the NMBA-rat esophagus model, suppressing agents are identified by the addition of putative inhibitors to the diet following treatment of the animals with NMBA (Fig. 1B). NMBA is administered s.c. in an abbreviated period of 5 wk after which the esophageal epithelium is found to contain significant areas of hyperplasia as well as foci of dysplastic change with varying degrees of severity (48). The histopathological appearance of the rat esophagus at this time point is not unlike what is observed by endoscopy in the esophagus of subjects residing in high-risk areas for esophageal SCC (7–10). The results of our studies indicate that both STRWs and BRBs inhibit postinitiation tumorigenic events as evidenced by reductions in tumor multiplicity and in the development of preneoplastic lesions (Tables 4 and 5) (31,32). It was gratifying that both berry types were found to possess suppressing activity because multiple chemopreventive agents have been inactive in the rat esophagus when tested in a postinitiation scheme (42,49,50). The observation that both berry types were more effective when administered at 5% of the diet than at 10% suggests that there may be some antagonistic effects of berry components at the higher dose level and that “more is not necessarily better”. BBs were not tested for suppressing activity; however, it seems likely that they would also be effective.
Cell proliferation is known to play a role in esophageal tumorigenesis (51–53). Our study with BRBs suggests that at least one mechanism by which the berries suppress esophageal tumor development in pre-initiated rats is to reduce the rate of esophageal cell proliferation almost to levels seen in control animals (Table 6). This is probably responsible for the observed reduction in the development of dysplastic lesions in NMBA-treated rat esophagus. We have shown that the down-regulation of esophageal cell proliferation by berries is not related to the overexpression of cyclin D1 and probably not to the overexpression of either transforming growth factor-α or epidermal growth factor receptor because all three biomarkers are expressed predominately in the papilloma stage of tumor development and to a much lesser extent in dysplastic lesions (54,55).
In recent studies, however, we have identified two biomarkers that are overly expressed in the preneoplastic stages of esophageal tumor development in rats, that is, COX-2 and iNOS (50,56). As is the case for many normal tissues, COX-2 expression in normal rat esophagus is very minimal; however, its expression increases progressively with tumor development. This increase is accompanied by increases in COX-2 activity as indicated by the detection of elevated levels of PGE2 in the esophagus (50). In view of the importance of PGE2 and of other products of COX-2 activity in stimulating cell proliferation, the observed down-regulation of COX-2 by BRBs in the esophagus of NMBA-treated rats (Fig. 5) in the present study would appear to be an important mechanism by which berries inhibit cell proliferation in preneoplastic esophageal tissues and, ultimately, tumor development. Recently, we reported that a specific COX-2 inhibitor (L-748706, obtained from Merck, Inc., West Point, PA) reduced esophageal tumor development in NMBA-treated rats when added to the diet in a postinitiation protocol (57). Inhibition of tumor development by L-748706 was dependent upon reducing PGE2 levels in NMBA-treated esophagus almost to levels detected in normal esophagus. Although dietary 5% BRBs were not as effective in reducing PGE2 levels in NMBA-treated esophagus as L-748706, the approximate 50% reduction observed in berry-treated esophagus was significant (P < 0.05). Up-regulation of COX-2 has been detected in many human cancers including esophageal SCC (58). Thus, it is possible that dietary berries might exhibit inhibitory effects on the development of esophageal SCC in humans, in part, by down-regulation of COX-2.
iNOS is an enzyme that catalyzes the conversion of l-arginine to citrulline resulting in the production of nitric oxide (NO) (59). NO is a free radical with an unpaired electron; therefore, it can denote or accept an electron to become a nitrosonium cation (NO+) or a nitroxyl anion (NO−), which leads to nitrosative stress and oxidative stress, respectively. Nitrosative stress can lead to the formation of nitrosamine carcinogens, deamination of DNA bases, and inactivation of DNA repair proteins, all actions contributing to carcinogenesis. Similarly, oxidative stress contributes to the formation of peroxynitrite, which can damage DNA leading to carcinogenesis and which can be catalyzed by tissue peroxidase to end products that activate COX-2 (60). Thus, it is not surprising that iNOS inhibitors reduce COX-2 activity (61). Studies have shown that iNOS is overly expressed in many human cancers including esophageal SCC (62), and, recently, we reported its overexpression in NMBA-treated rat esophagus (56). The observed down-regulation of iNOS mRNA expression and reduction in nitrite levels in NMBA-treated rat esophagus by 5% freeze-dried BRBs in the present study indicates that this is another mechanism by which berries inhibit tumor development. By inhibiting iNOS and NO production, berries could reduce nitrosative and oxidative stress, inflammation, and COX-2 activity in rat esophagus, all actions that interact in carcinogenesis and tumor development. Their ability to down-regulate both COX-2 and iNOS is important in that both genes are commonly overly expressed in individual human esophageal SCCs (63,64).
In summary, our data indicate that freeze-dried berries inhibit both initiation and postinitiation events in rat esophageal carcinogenesis as evidenced by decreases in tumor multiplicity, reduction in adduct formation, reduced proliferative indices, inhibition of preneoplastic lesion formation, and down-regulation of COX-2 and iNOS. Although the down-regulation of two key genes such as COX-2 and iNOS is likely to be important for the preventative effects of berries, recent unpublished DNA array data indicate that berries down-regulate a large number of other genes in NMBA-treated esophagus that are associated with multiple cellular processes including apoptosis, angiogenesis, matrix formation, cell cycle control, and so forth. Undoubtedly, the combined effects of these actions are involved in the overall protective effect of berries. Utilization of a food-based approach to cancer prevention may provide a midpoint on the chemopreventive continuum with the use of individual drugs representing one end of the spectrum and nutritional interventions the other. On the basis of an average daily consumption of 1,900 cal among females and 2,700 cal among males, approximately 1.4–2 cups of berries would be required to consume a diet of 5% fresh berries (32). Because berries are 85–90% water, 1.4 and 2.0 cups of fresh berries are equivalent to about 30 and 40 g of freeze-dried berries. Although the level found to be inhibitory is larger than a standard serving of fruit, it would appear to be behaviorally achievable. Thus, the concept of using freeze-dried berries, and perhaps other freeze-dried fruits and vegetables, may serve as a viable alternative for cancer chemoprevention.
Acknowledgments and Notes
This research was supported by the Ohio Department of Agriculture and National Institutes of Health RO1 grants CA961130 and CA103180. We thank Drs. Qingguo Tian and Steven Schwartz for the analysis of anthocyanins in berries.
References
- 1.World Cancer Research Fund and American Institute for Cancer Research. Food, Nutrition and the Prevention of Cancer: A Global Perspective. Washington, DC: American Institute for Cancer Research; 1997. pp. 188–129. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Geneva, Switzerland: World Health Organization; The World Health Report. 1997
- 3.Stoner GD, Rustgi AK. Biology of esophageal squamous cell carcinoma. Gastrointest Cancers Biol Diagn Ther. 1995;8:141–146. [Google Scholar]
- 4.Beer DG, Stoner GD. Clinical models of chemoprevention for the esophagus. Hematol Oncol Clin North Am. 1998;12:1055–1077. doi: 10.1016/s0889-8588(05)70041-1. [DOI] [PubMed] [Google Scholar]
- 5.Cameron AJ, Ott BJ, Payne WS. The incidence of adenocarcinoma in columnar-lined (Barrett’s) esophagus. N Engl J Med. 1985;74:857–859. doi: 10.1056/NEJM198510033131404. [DOI] [PubMed] [Google Scholar]
- 6.Blot WJ, Devesa SS, Kneller RW, Fraumeni JF. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 7.Krasna MJ, Wolfer RS. Esophageal carcinoma: diagnosis, evaluation and staging. In: Aisner J, Arriagada R, Green MR, Martini N, Perry MC, editors. Comprehensive Textbook of Thoracic Oncology. Baltimore, MD: Williams & Wilkins; 1996. pp. 563–584. [Google Scholar]
- 8.Anani PA, Gardiol D, Savary M, Monnier P. An extensive morphological and comparative study of clinically early and obvious squamous cell carcinoma of the esophagus. Pathol Res Pract. 1991;187:214–219. doi: 10.1016/S0344-0338(11)80774-0. [DOI] [PubMed] [Google Scholar]
- 9.Kuwano H, Watanabe M, Sadanaga N, Ikebe M, Mori M, et al. Squamous epithelial dysplasia associated with squamous cell carcinoma of the esophagus. Cancer Lett. 1993;72:141–147. doi: 10.1016/0304-3835(93)90120-x. [DOI] [PubMed] [Google Scholar]
- 10.Shu YJ, Uan XQ, Jin SP. Further investigation of the relationship between dysplasia and cancer of the esophagus. Chin Med. 1981;6:39–41. [Google Scholar]
- 11.Schottenfeld D. Epidemiology of cancer of the esophagus. Semin Oncol. 1984;11:92–100. [PubMed] [Google Scholar]
- 12.Sons HU. Etiologic and epidemiologic factors of carcinoma of the esophagus. Surg Gynecol Obstet. 1987;165:183–190. [PubMed] [Google Scholar]
- 13.Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40:2633–2640. [PubMed] [Google Scholar]
- 14.Rose E. Esophageal cancer in Transkei: 1955–69. JNCI. 1983;51:7–16. doi: 10.1093/jnci/51.1.7. [DOI] [PubMed] [Google Scholar]
- 15.Wynder EL, Bross IJ. A study of etiological factors in cancer of the esophagus. Cancer. 1961;14:389–401. doi: 10.1002/1097-0142(196103/04)14:2<389::aid-cncr2820140220>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Tuyns AJ. Epidemiology of esophageal cancer in France. In: Pfeifer CJ, editor. Cancer of the Esophagus. Boca Raton, FL: CRC Press; 1982. pp. 3–22. [Google Scholar]
- 17.Hecht SS, Stoner GD. Lung and esophageal carcinogenesis. In: Aisner J, Arriagada R, Green MR, Martini N, Perry MC, editors. Comprehensive Textbook of Thoracic Oncology. Baltimore, MD: Williams & Wilkins; 1996. pp. 25–50. [Google Scholar]
- 18.Tuyns AJ. Recherches concernant les facteurs etiologiques du cancer de l’oesophage dans l’ouest de la France. Bull Cancer. 1980;67:15–28. [PubMed] [Google Scholar]
- 19.Ribeiro U, Jr, Posner MC, Safatle-Ribeiro AV, Reynolds JC. Risk factors for squamous cell carcinoma of the esophagus. Br J Surg. 1996;83:1174–1185. [PubMed] [Google Scholar]
- 20.Yang WX, Pu J, Lu SH, Li FM, Guo LP. Studies on the exposure level of nitrosamines in the gastric juice and its inhibition in high risk areas of esophageal cancer. Chin J Oncol. 1992;14:407–410. [PubMed] [Google Scholar]
- 21.Umbenhauer D, Wild CP, Montesano R, Saffhill R, Boyle JM, et al. O6-methyldeoxyguanosine in oesophageal DNA among individuals at high risk of oesophageal cancer. Int J Cancer. 1985;36:661–665. doi: 10.1002/ijc.2910360607. [DOI] [PubMed] [Google Scholar]
- 22.Li MN, Cheng SJ. Etiology of carcinoma of the esophagus. In: Huang GJ, Kai WY, editors. Carcinoma of the Esophagus and Gastric Cardia. Berlin: Springer-Verlag; 1984. pp. 26–51. [Google Scholar]
- 23.Togawa K, Jaskiewicz K, Takahashi H, Meltzer SJ, Rustigi AK. Human papillomavirus DNA sequences in esophageal squamous cell carcinoma. Gastroenterology. 1994;107:128–136. doi: 10.1016/0016-5085(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 24.Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22:1737–1746. doi: 10.1093/carcin/22.11.1737. [DOI] [PubMed] [Google Scholar]
- 25.Siglin JC, Khare L, Stoner GD. Evaluation of dose and treatment duration on the esophageal tumorigenicity of N-nitrosomethylbenzylamine in rats. Carcinogenesis. 1995;16:259. doi: 10.1093/carcin/16.2.259. [DOI] [PubMed] [Google Scholar]
- 26.Stoner GD, Morrissey DT, Heur YH, Daniel EM, Galati AJ, et al. Inhibitory effects of phenethyl isothiocyanate on N-nitroso methylbenzylamine carcinogenesis in the rat esophagus. Cancer Res. 1991;51:2063–2070. [PubMed] [Google Scholar]
- 27.Stoner GD, Adams C, Kresty LA, Desai D, Hecht SS, et al. Inhibition of N′-nitrosonornicotine-induced esophageal tumorigenesis by 3-phenylpropyl isothiocyanate. Carcinogenesis. 1998;19:2139–2143. doi: 10.1093/carcin/19.12.2139. [DOI] [PubMed] [Google Scholar]
- 28.Mandal S, Stoner GD. Inhibition of N-nitrosobenzylmethylamine induced esophageal tumorigenesis in rats by ellagic acid. Carcinogenesis. 1990;11:55–61. doi: 10.1093/carcin/11.1.55. [DOI] [PubMed] [Google Scholar]
- 29.Daniel EM, Krupnick AS, Heur Y-H, Blinzler JA, Nims RW, et al. Extraction, stability and quantitation of ellagic acid in various fruits and nuts. J Food Comp Anal. 1989;2:338–349. [Google Scholar]
- 30.Stoner GD, Kresty LA, Carlton PS, Siglin JC, Morse MA. Isothiocyanates and freeze-dried strawberries as inhibitors of esophageal cancer. Toxicol Sci. 1999;52:95–100. doi: 10.1093/toxsci/52.2.95. [DOI] [PubMed] [Google Scholar]
- 31.Carlton PS, Kresty LA, Siglin JC, Morse MA, Lu J, et al. Inhibition of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus by dietary freeze-dried strawberries. Carcinogenesis. 2001;22:441–446. doi: 10.1093/carcin/22.3.441. [DOI] [PubMed] [Google Scholar]
- 32.Kresty LA, Morgan C, Carlton PS, Lu J, Gupta A, et al. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001;61:6112–6119. [PubMed] [Google Scholar]
- 33.Tian Q, Aziz RM, Stoner GD, Schwartz SJ. Anthocyanin determination in black raspberry (Rubus occidentalis) and biological specimens using liquid chromatography-electrospray ionization tandem mass spectrometry. Food Chem Toxicol. 2005;70:43–47. [Google Scholar]
- 34.Morse MA, LaGreca SD, Amin SG, Chung FL. Effects of indole-3 carbinol on lung tumorigenesis and DNA methylation induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and on the metabolism and disposition of NNK in A/J mice. Cancer Res. 1990;50:2613. [PubMed] [Google Scholar]
- 35.Chen T, Rose ME, Hwang H, Nines RG, Stoner GD. Chemopreventive effects of black raspberries in a rodent model of esophageal cancer: down regulation of COX-2, iNOS and c-Jun. Cancer Res. 2006;66:2853–2859. doi: 10.1158/0008-5472.CAN-05-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 37.Tian Q, Giusti M, Stoner GD, Schwartz SJ. Screening for anthocyanins using high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry with precursor-ion analysis, product-ion analysis, common-neutral-loss analysis, and selected reaction monitoring. J Chromatog A. 2005;1091:72–82. doi: 10.1016/j.chroma.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 38.Siglin JC, Barch DH, Stoner GD. Effects of dietary phenethyl isothiocyanate, ellagic acid, sulindac and calcium on the induction and progression of N-nitrosomethylbenzylamine-induced esophageal carcinogenesis in rats. Carcinogenesis. 1995;16:1101–1106. doi: 10.1093/carcin/16.5.1101. [DOI] [PubMed] [Google Scholar]
- 39.Bate-Smith EC. Plant phenolics in foods. In: Fairbairn JW, editor. The Pharmacology of Plant Phenolics. New York: Academic Press; 1959. pp. 133–147. [Google Scholar]
- 40.Daniel EM, Ratnayake S, Kinstle T, Stoner GD. The effects of pH and rat intestinal contents on the liberation of ellagic acid from purified and crude ellagitannins. J Nat Prod. 1991;54:946–952. doi: 10.1021/np50076a004. [DOI] [PubMed] [Google Scholar]
- 41.Hecht SS, Huang C, Stoner GD, Li J, Kenney PMJ, et al. Identification of cyanidin glycosides as constituents of freeze-dried black raspberries which inhibit anti-benzo[a]pyrene 7,8 diol-9, 10-epoxide induced NFκB and AP-1 activity. Carcinogenesis. doi: 10.1093/carcin/bgi366. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson JT, Morse MA, Kresty LA, Stoner GD. Effect of alkyl chain length on inhibition of N-nitrosomethylbenzylamine-induced esophageal tumorigenesis and DNA methylation by isothiocyanates. Carcinogenesis. 1995;16:1011–1015. doi: 10.1093/carcin/16.5.1011. [DOI] [PubMed] [Google Scholar]
- 43.Nakajima M, Yoshida R, Shimada N, Yamazaki H, Yokoi T. Inhibition and inactivation of human cytochrome P450 isoforms by phenethyl isothiocyanate. Drug Metab Dispos. 2001;29:1110–1113. [PubMed] [Google Scholar]
- 44.Craddock VM, Henderson AR. Effect of N-nitrosamines carcinogenic for oesophagus on O6-alkyl-guanine-DNA-methyl transferase in rat oesophagus and liver. J Cancer Res Clin Oncol. 1986;111:229–236. doi: 10.1007/BF00389238. [DOI] [PubMed] [Google Scholar]
- 45.Pegg A. Methylation of the O6-position of guanine in DNA is the most likely initiating event in carcinogenesis by methylating agents. Cancer Invest. 1984;2:223–231. doi: 10.3109/07357908409104376. [DOI] [PubMed] [Google Scholar]
- 46.Reen RK, Nines R, Stoner GD. Modulation of N-nitrosomethylbenzylamine (NMBA) metabolism by black raspberries (BRB) in the esophagus and liver of Fischer 344 rats. Nutr Cancer. 2006;54:47–57. doi: 10.1207/s15327914nc5401_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wattenberg LW. Chemoprevention and cancer. Cancer Res. 1985;45:1–8. [PubMed] [Google Scholar]
- 48.Liston BW, Gupta A, Nines R, Carlton PS, Kresty LA, et al. Incidence and effects of Ha-ras codon 12 G→A transition mutations in preneoplastic lesions induced by N-nitrosomethylbenzylamine in the rat esophagus. Mol Carcinog. 2001;32:1–8. doi: 10.1002/mc.1058. [DOI] [PubMed] [Google Scholar]
- 49.Liston BW, Nines R, Carlton PS, Gupta A, Aziz R, et al. Perillyl alcohol as a chemopreventive agent in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis. Cancer Res. 2003;63:2399–2403. [PubMed] [Google Scholar]
- 50.Carlton PS, Gopalakrishnan R, Gupta A, Liston BA, Habib S, et al. Piroxicam is an ineffective inhibitor of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus. Cancer Res. 2002;62:4376–4382. [PubMed] [Google Scholar]
- 51.Bahl R, Arora S, Nath N, Mathur M, Shukla NK, et al. Novel polymorphism in p21 (waf1/cip1) cyclin dependent kinase inhibitor gene: association with human esophageal cancer. Oncogene. 2000;19:323–328. doi: 10.1038/sj.onc.1203325. [DOI] [PubMed] [Google Scholar]
- 52.Cox LS. Who binds wins: competition for PCNA rings out cell-cycle changes. Trends Cell Biol. 1997;7:493–498. doi: 10.1016/S0962-8924(97)01170-7. [DOI] [PubMed] [Google Scholar]
- 53.Sherr CJ. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- 54.Wang Q-S, Sabourin CLK, Wang H, Stoner GD. Overexpression of cyclin D1 and cyclin E in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis. Carcinogenesis. 1996;17:1583–1588. doi: 10.1093/carcin/17.8.1583. [DOI] [PubMed] [Google Scholar]
- 55.Wang Q-S, Sabourin CLK, Bijur GN, Robertson FM, Stoner GD. Alterations in transforming growth factor-alpha and epidermal growth factor receptor expression during rat esophageal tumorigenesis. Mol Carcinog. 1996;15:144–153. doi: 10.1002/(SICI)1098-2744(199602)15:2<144::AID-MC7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 56.Chen T, Stoner GD. Inducible nitric oxide synthase expression in N-nitrosomethylbenzylamine (NMBA)-induced rat esophageal tumorigenesis. Mol Carcinog. 2004;40:232–240. doi: 10.1002/mc.20035. [DOI] [PubMed] [Google Scholar]
- 57.Stoner GD, Qin H, Nines RG, Aziz RM, Dixit R, et al. The effects of L-748706, a selective cyclooxygenase-2 inhibitor, on N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis. Carcinogenesis. 2005;26:1590–1595. doi: 10.1093/carcin/bgi111. [DOI] [PubMed] [Google Scholar]
- 58.Ratnasinghe D, Tangrea J, Roth MJ, Dawsey S, Hu N, et al. Expression of cyclooxygenase-2 in human squamous cell carcinoma of the esophagus; an immunohistochemical study. Anticancer Res. 1999;19:171–174. [PubMed] [Google Scholar]
- 59.Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- 60.Landino LM, Crews BC, Timmons MD, Morrow JD, Marnett LJ. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc Natl Acad Sci USA. 1996;93:15069–15074. doi: 10.1073/pnas.93.26.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rao CV, Indranie C, Simi B, Manning PT, Connor JR, et al. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res. 2002;62:165–170. [PubMed] [Google Scholar]
- 62.Tanaka H, Kijima H, Tokunaga T, Himeno S, Kenmochi T, et al. Frequent expression of inducible nitric oxide synthase in esophageal squamous cell carcinomas. Int J Oncol. 1999;14:1069–1073. doi: 10.3892/ijo.14.6.1069. [DOI] [PubMed] [Google Scholar]
- 63.Jin Y, Zhang W, Liu B, Wang H, Han Z, et al. Expression of inducible nitric oxide synthase in human esophageal biopsies from carcinoma and precancerous lesions. Zhongguo Yi Ke Xue Yuan Xue Bao. 2000;22:570–572. [PubMed] [Google Scholar]
- 64.Yu HO, Xu SQ, Liu LY, Cai XK, Lu WH, et al. Cyclooxygenase-2 expression in squamous dysplasia and squamous cell carcinoma of the esophagus. Cancer Lett. 2003;198:193–201. doi: 10.1016/s0304-3835(03)00340-9. [DOI] [PubMed] [Google Scholar]