This study identifies that local ammonium supply triggers lateral root branching in Arabidopsis preferentially in the presence of AMT1;3. Ammonium thereby shapes lateral root architecture in a complementary manner to nitrate, which preferentially promotes lateral root elongation.
Abstract
Root development is strongly affected by the plant’s nutritional status and the external availability of nutrients. Employing split-root systems, we show here that local ammonium supply to Arabidopsis thaliana plants increases lateral root initiation and higher-order lateral root branching, whereas the elongation of lateral roots is stimulated mainly by nitrate. Ammonium-stimulated lateral root number or density decreased after ammonium or Gln supply to a separate root fraction and did not correlate with cumulative uptake of 15N-labeled ammonium, suggesting that lateral root branching was not purely due to a nutritional effect but most likely is a response to a sensing event. Ammonium-induced lateral root branching was almost absent in a quadruple AMMONIUM TRANSPORTER (qko, the amt1;1 amt1;2 amt1;3 amt2;1 mutant) insertion line and significantly lower in the amt1;3-1 mutant than in the wild type. Reconstitution of AMT1;3 expression in the amt1;3-1 or in the qko background restored higher-order lateral root development. By contrast, AMT1;1, which shares similar transport properties with AMT1;3, did not confer significant higher-order lateral root proliferation. These results show that ammonium is complementary to nitrate in shaping lateral root development and that stimulation of lateral root branching by ammonium occurs in an AMT1;3-dependent manner.
INTRODUCTION
In most soils, plant nutrients are not homogenously distributed, particularly if they derive from organic matter and precipitation or adsorption to the soil matrix restricts their solubility and transport to the root surface (Ettema and Wardle, 2002; Hinsinger et al., 2005). Plants may respond to a spatially restricted availability of nutrients with an alteration of the root system architecture displaying enhanced lateral root development into nutrient-rich patches. This response is nutrient specific and observed only if overall nutrient availability is limited. Early experiments with barley (Hordeum vulgare) showed that locally concentrated supplies of nitrate or phosphate, but not potassium, stimulated lateral root growth within the nutrient-rich soil patch, while lateral root formation was suppressed in nutrient-poor zones (Drew, 1975; Drew and Saker, 1975, 1978). In the case of phosphate, a low availability appears to be sensed in the root apex, where cell elongation and meristem activity become strongly reduced and thereby avoid further root development into phosphate-depleted soil zones (Svistoonoff et al., 2007; Desnos, 2008). In the case of nitrate, the role of the apical root meristem in nitrogen sensing remains unclear; however, lateral roots also elongate into nitrate-containing soil zones if the remaining root system is nitrogen deficient (Drew, 1975; Remans et al., 2006). In Arabidopsis thaliana, nitrate-driven lateral root growth depends on the expression of the MADS box transcription factor ANR1, which acts downstream of the dual-affinity nitrate transporter NRT1.1 (Zhang and Forde, 1998; Remans et al., 2006). Since an Arabidopsis mutant defective in nitrate reductase activity still responded to a local supply of nitrate, whereas a local supply of ammonium or Gln failed to elicit the same growth response, it has been concluded that local lateral root elongation is regulated by nitrate itself (Zhang et al., 1999).
Even though ammonium-dependent changes in root system architecture could not be observed in Arabidopsis before (Zhang et al., 1999), there are a few reasons why they should be expected. First, lateral root growth of barley was stimulated under local ammonium supply (Drew, 1975). Second, ammonium is less soluble than nitrate and partly adsorbed to the soil matrix so that roots growing toward the ammonium source would decrease diffusion distances (Miller et al., 2007). Third, at low external concentrations, ammonium is a preferential nitrogen source for most plants (Gazzarrini et al., 1999). Fourth, motile Chlamydomonas reinhardtii cells show mechanisms of ammonium sensing by actively moving toward a localized source of either ammonium or its nonmetabolizable substrate analog methylammonium (Ermilova et al., 2007). Finally, yeast cells sense ammonium. When grown under low ammonium supply, yeast cells produce pseudohyphae, which are nuclear-free hyphal structures that better allow foraging of a nitrogen-poor substrate (Lorenz and Heitman, 1998). Pseudohyphal differentiation depends on Mep2, which has the greatest substrate affinity of the three ammonium transporters in yeast (Marini et al., 1997) and is able to rapidly activate a protein kinase A–mediated signaling pathway (van Nuland et al., 2006). Specific point mutations in Mep2 uncoupled ammonium signaling from transport, suggesting a role as a transceptor, a protein that acts as a transporter and receptor at the same time (Lorenz and Heitman, 1998; Thevelein et al., 2005). However, AMT-type ammonium transporters from Arabidopsis were not able to substitute for a lack of Mep2 with regard to pseudohyphal differentiation in yeast (van Nuland et al., 2006).
Arabidopsis possesses six AMT-type ammonium transporters with five of these genes being expressed in roots and only AMT1;4 being expressed in pollen (Yuan et al., 2007, 2009). AMT1;1 and AMT1;3 are expressed in rhizodermal and cortical cells where they confer high-capacity and high-affinity ammonium uptake with an in vivo Km of 50 and 61 μM, respectively (Loqué et al., 2006; Yuan et al., 2007). Correlative evidence indicated that AMT1;5, which is also expressed in rhizodermal root cells, contributes only 5 to 10% of the overall ammonium uptake capacity at an even higher substrate affinity. By contrast, AMT1;2 represents a lower-affinity transporter expressed in endodermal and cortical cells that most likely plays a major role in the uptake and retrieval of ammonium from the root apoplast (Yuan et al., 2007). AMT1;1 and AMT1;2 are allosterically regulated by C-terminal phosphorylation, which trans-inhibits the activation of AMT1 subunits in a trimeric complex (Loqué et al., 2007; Neuhäuser et al., 2007). C-terminal phosphorylation of AMT1;1 is rapidly triggered by external ammonium supply and causes a decrease in ammonium uptake by roots (Lanquar et al., 2009). A working model has been set up in which the external ammonium signal is conferred to the cytosolic side either via a membrane-anchored receptor-like kinase or via AMT1;1 itself, presuming that AMT1;1 acts as a transceptor (Lanquar et al., 2009). Any relation between AMTs and root development has not yet been reported.
Against this background, we investigated changes in the lateral root architecture of Arabidopsis plants grown under local ammonium supply and observed that local ammonium stimulates lateral root initiation and leads to highly branched lateral roots, while further elongation of preemerged root initials becomes arrested. Employing different growth systems for the morphological analysis of lateral root branching and measuring in parallel the uptake of 15N-labeled ammonium, we confirmed that ammonium-induced lateral root initiation cannot be explained by a nutritional effect alone. Monitoring lateral root branching in transgenic lines with altered AMT gene expression indicated a particular involvement of AMT1;3 in ammonium-triggered lateral root formation. Moreover, a rigorous comparison of lateral root growth responses between ammonium and nitrate throughout a large set of experiments identified a complementary action of ammonium and nitrate in guiding lateral root development.
RESULTS
Lateral Root Formation Is Stimulated by Local Ammonium Supply
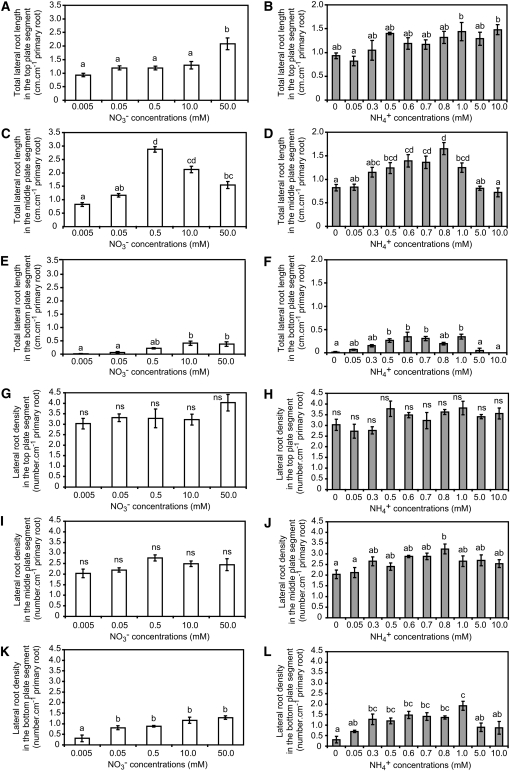
To investigate ammonium sensing responses in plants, Arabidopsis Columbia-0 (Col-0) plants were precultured under N deficiency and transferred to horizontally split agar plates (Zhang and Forde, 1998) in which ammonium or nitrate was supplemented to the middle agar segment. After 15 d of growth, the total lateral root length in the middle segment showed a dose–response curve with a threefold increase at 0.5 mM nitrate but a less than twofold increase at 0.8 mM ammonium where lateral root length found its maximum (Figures 1C and 1D). Thereby, the average length of the newly emerged lateral roots in the middle plate segment was stimulated by 132% under nitrate but only by 35% under local ammonium supply (see Supplemental Figures 1C and 1D online). By contrast, exposing the middle part of the primary root axis to increasing N concentrations increased lateral root number only by 1.4 roots plant−1 under nitrate but by 2.0 roots plant−1 under local ammonium supply (see Supplemental Figures 1I and 1J online). Since primary root length was negatively affected by elevated levels of local ammonium supply (see Supplemental Figures 1M and 1N online), we calculated lateral root densities for each segment (Figures 1G to 1L). Lateral root density is a reliable measure for lateral root number that corrects for treatment-dependent variations in the length of the parent root (Dubrovsky et al., 2009). In the middle segment, lateral root density significantly increased under local ammonium but not under local nitrate supply (Figures 1I and 1J). In the nitrogen-deficient upper root segment, lateral root length and density slightly increased with the supply of either nitrogen form but did not decrease at elevated concentrations (Figures 1A, 1B, 1G, and 1H). Lateral roots formed in the bottom segment also responded to external nitrogen supply with an increase in total or average lateral root length and density under nitrate or ammonium supply (Figures 1E, 1F, 1K, and 1L; see Supplemental Figures 1E and 1F online), which might be a consequence of nitrogen transport in the root apoplast or symplast between the middle and the bottom root segments. Nevertheless, it is important to note that the major difference between the local responses to ammonium and nitrate was the more prominent increase in lateral root number and density by ammonium, whereas lateral root length was mainly stimulated by nitrate.
Figure 1.
Lateral Root Development Is Differently Affected by Local Nitrate and Ammonium Supply.
(A) to (F) Total lateral root length per unit of primary root in the top ([A] and [B]), middle ([C] and [D]), and bottom ([E] and [F]) root compartment.
(G) to (L) Lateral root density in the top ([G] and [H]), middle ([I] and [J]), and bottom ([K] and [L]) root compartment.
Arabidopsis plants were grown for 15 d on horizontally split agar plates and supplemented with increasing concentrations of nitrogen in the form of KNO3 ([A], [C], [E], [G], [I], and [K]) or NH4Cl ([B], [D], [F], [H], [J], and [L]) only in the middle compartment. Bars represent mean values (± se) of 21 individual plants per treatment, and different letters denote significant differences among means at P < 0.05 (Tukey’s test); ns, not significant.
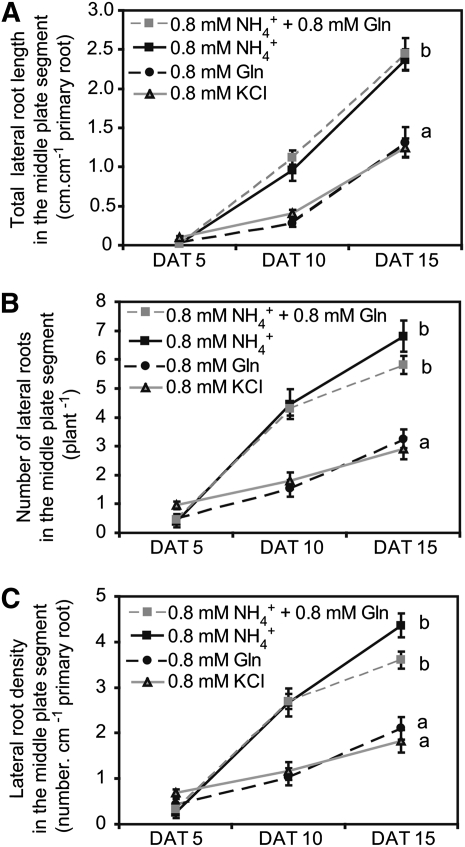
We then monitored in a time-course experiment lateral root development under local supply of Gln as an alternative reduced nitrogen source. However, neither lateral root length nor number or density in the middle segment increased above that observed in control (KCl) treatments (Figures 2A to 2C). A simultaneous supply of Gln with ammonium did not significantly alter lateral root length in the short term but after 15 d tended to repress the ammonium-stimulated increase in lateral root number and density. These observations indicated that the stimulatory effect of local ammonium supply on lateral root formation could not be mimicked by Gln. Moreover, the difference between ammonium and control treatments confirmed that ammonium preferentially stimulated lateral root number and density not only in comparison to nitrate (Figure 1) but also in comparison to nitrogen deficiency (Figure 2). Thus, the lateral root phenotype observed under ammonium was not merely caused by the absence of nitrate.
Figure 2.
Influence of Gln on Lateral Root Development under Localized Ammonium Supply.
Total lateral root length per unit of primary root (A), lateral root number (B), and lateral root density (C) in the middle root compartment. Arabidopsis plants were grown for 15 d on horizontally split agar plates and supplemented with 0.8 mM KCl, 0.8 mM NH4Cl, 0.8 mM Gln, or 0.8 mM NH4Cl + 0.8 mM Gln in the middle compartment. Roots were scanned 5, 10, or 15 d after transfer (DAT). Bars represent mean values (±se) of 21 individual plants per treatment, and different letters denote significant differences among means at P < 0.05 (Tukey’s test).
Ammonium-Induced Lateral Root Branching Is Subject to Systemic Repression
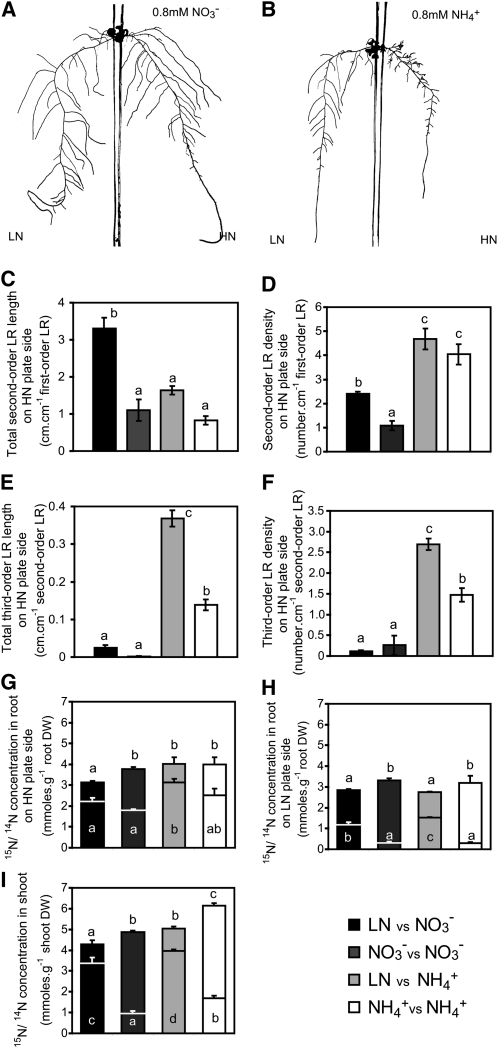
To overcome the limitations of the horizontally split plate system (i.e., restricted higher-order lateral root branching and the mixed nutritional situation in the primary root axis as it is divided into three segments with different nitrogen supplies), Arabidopsis roots were transferred to vertically split agar plates. Here, the first-order lateral root was guided into nitrogen-supplemented agar, while the remainder of the root continued to grow either on the same nitrogen concentration or under nitrogen deficiency. In agreement with the study by Remans et al. (2006), localized nitrate supply stimulated growth of second-order lateral roots as indicated by their increase in length and density on high N (HN), but both measures were strongly repressed when the other agar patch with the main root system (low N, LN plate side) was additionally supplemented with nitrate (Figures 3A, 3C, and 3D). There was no significant effect on first-order lateral root length (see Supplemental Table 1 online, HN plate side). By contrast, under local ammonium supply, second-order lateral root lengths were lower than those under nitrate supply, but second-order lateral root density and third-order lateral root development were strongly stimulated (Figures 3B to 3F; see Supplemental Table 1 online). When the remaining root system also grew in the presence of ammonium (LN plate side), third-order lateral root length and density on the HN plate side were significantly decreased, indicating that the third-order lateral root phenotype was subject to systemic repression by the nitrogen supplied to the other root fraction (Figures 3E and 3F). To verify the influence of the external nitrogen supply, lateral root formation was also assessed on the side with the primary root (see Supplemental Table 1 online, LN plate side). The lengths of the primary root and first-order laterals were hardly or not affected by additional supply of either nitrogen form, but second-order lateral root length steeply increased in the presence of nitrate. The total length of both second- and third-order lateral roots increased in the presence of ammonium, and this was associated with an increase in second-order lateral root number in the presence of either N form (see Supplemental Table 1 online). This observation confirmed that ammonium provoked a local stimulation of higher-order lateral root branching.
Figure 3.
Local Ammonium Supply Induces Third-Order Lateral Root Branching.
(A) and (B) Typical root morphology of wild-type plants grown on vertically split agar plates with the first lateral root growing into an HN compartment with 0.8 mM KNO3 (A) or 0.8 mM NH4Cl (B) and the remainder of the roots growing in 5 μM KNO3 (LN).
(C) to (F) Quantitative assessment of root morphology (C), second-order lateral root (LR) length, second-order lateral root density (D), third-order lateral root length (E), and third-order lateral root density (F).
(G) to (I) 15N uptake from 15N-labeled nitrate or ammonium applied to the HN side and its distribution in plants. Concentrations of total nitrogen (14N+15N, outer bars) and 15N (inner bars) in roots grown on the HN side (G), or roots grown on the LN side (H), and (I) shoots are indicated. Plants were grown on vertically split agar plates for 15 d on either local nitrogen supply (5 μM NO3− on the LN side with 0.8 mM NO3− or NH4+ on HN side; LN versus NO3−LN versus NH4+) or homogenous nitrogen supply (0.8 mM NO3− or NH4+ on both sides; NO3− versus NO3−NH4+ versus NH4+). Bars represent mean values (±se) of 10 to 20 individual plants, and different letters for total nitrogen (14N+15N, outer bars) and 15N (inner bars) denote significant differences among means at P < 0.05 (Tukey’s test). DW, dry weight.
Simultaneously, nitrogen uptake was monitored by supplementing 15N-labeled nitrate or ammonium only to the HN side. Accumulation of 15N was slightly higher in ammonium-supplied than in nitrate-supplied plants, and additional supply of N to the other agar patch did not significantly decrease root 15N accumulation from either N form (Figure 3G). As expected, 15N accumulation in roots on the low N (LN) side was lower than on the HN side, and additional 14N supply to these roots decreased 15N accumulation to a similar extent for both N forms (Figure 3H). 15N accumulation in shoots followed the same trend (Figure 3I). Referring to the total tissue N concentration, as determined by the analysis of 15N and 14N, additional supply of either N form to the LN side significantly increased N concentrations in the LN root fraction as well as in the shoot (Figures 3H and 3I). Thus, the inhibition of third-order lateral root formation on the HN side after additional ammonium supply to the other root fraction was most likely subject to systemic regulation of the N nutritional status of the root fraction on the LN side and/or the shoot.
Recently, Gln has been described as a repressor of lateral root emergence (Gifford et al., 2008). Indeed, addition of a local equimolar supply of Gln together with ammonium decreased third-order lateral root length and density, whereas Gln by itself poorly triggered third-order lateral root formation (see Supplemental Figures 2A and 2B online). The simultaneous supply of Gln significantly decreased the cumulative uptake of 15N-labeled ammonium (see Supplemental Figure 2C online). This might be due to a systemic repression, considering the proposed role of Gln as a metabolic signal for N sufficiency or to a direct downregulation of AMT1 genes by Gln (Rawat et al., 1999). To verify a systemic effect of Gln, increasing Gln concentrations were added to the LN compartment of the split-root design. Measurement of lateral root development on the HN side of the plate revealed a strong decrease in particular of third-order lateral root length and density (see Supplemental Figures 3A and 3B online). Neither 15N accumulation nor total N concentrations in ammonium-supplied roots differed significantly from control plants grown in the absence of Gln on the LN plate side (see Supplemental Figure 3C online), indicating that inhibition of third-order lateral root formation could not be explained exclusively by a Gln-mediated repression of ammonium uptake (Rawat et al., 1999). Total N concentrations in the LN root fraction or in shoots increased at the expense of ammonium-15N due to higher Gln uptake (see Supplemental Figures 3D and 3E online). Thus, Gln or Gln-derived nitrogen most likely acted as a metabolic repressor of ammonium-induced third-order lateral root formation, and this repression appeared to be independent of an inhibition of local ammonium uptake.
Ammonium and Nitrate Regulate Lateral Root Development in a Complementary Way
To investigate whether nitrate and ammonium have a differential effect on lateral root initiation or emergence, the CYCB1:β-glucuronidase (GUS) reporter line was employed, allowing the early developmental stages of lateral root primordia to be traced (Colón-Carmona et al., 1999). Taking into account preemerged lateral root primordia, the localized ammonium supply increased the density of second-order laterals as well as the length, number, and density of third-order laterals relative to the nitrate treatment (Table 1). By contrast, ammonium nitrate-grown plants had even larger numbers of lateral root primordia but resulted in a similar density of second-order laterals comparable to ammonium-supplied plants due to a concomitant increase in the length of first-order lateral roots. With regard to third-order lateral roots, ammonium nitrate enhanced the number of third-order lateral root primordia even more than ammonium supply alone, pointing to an additive effect of both N forms. Due to the lower second-order lateral root length, third-order lateral root density remained highest under ammonium. The comparison between nitrate- and ammonium nitrate–grown plants clearly showed that the higher lateral root numbers and densities conferred by ammonium supply were independent of any effects on the parent root (Table 1) and that ammonium also stimulated higher-order lateral root branching in the presence of nitrate. Thus, nitrate and ammonium promoted lateral root development in a complementary manner, in which ammonium was a dominant trigger for lateral root initiation.
Table 1.
Ammonium and Nitrate Stimulate Lateral Root Development in a Complementary Manner
| N Supply | Microscopy Analysis of First-, Second-, or Third-Order Lateral Roots | ||
| Total Lateral Root Length (cm·Plant−1) | |||
| First-Order | Second-Order | Third-Order | |
| 0.8 mM NO3− | 10.60 ± 0.45b | 35.83 ± 3.92b | 0.79 ± 0.20a |
| 0.8 mM NH4+ | 04.81 ± 0.49a | 08.01 ± 0.75a | 2.88 ± 0.26b |
| 0.4 mM NH4NO3 | 11.39 ± 0.98b | 41.04 ± 7.71b | 1.42 ± 0.29a |
| Total Number of Lateral Root Primordia Initiated (Plant−1) | |||
| First-Order | Second-Order | Third-Order | |
| 0.8 mM NO3− | nd | 33.83 ± 2.54a | 24.27 ± 2.31a |
| 0.8 mM NH4+ | nd | 25.66 ± 1.65a | 49.08 ± 3.87b |
| 0.4 mM NH4NO3 | nd | 55.88 ± 4.19b | 71.83 ± 8.41c |
| Density of Lateral Root Primordia Initiated (Number·cm−1) | |||
| First-Order | Second-Order | Third-Order | |
| 0.8 mM NO3− | nd | 3.33 ± 0.38a | 0.78 ± 0.12a |
| 0.8 mM NH4+ | nd | 5.47 ± 0.60b | 6.52 ± 0.54b |
| 0.4 mM NH4NO3 | nd | 5.14 ± 0.24b | 2.10 ± 0.42a |
Microscopy analysis of first-, second-, or third-order lateral roots length and total number or density of lateral root primordia initiated in Arabidopsis CYCB1:GUS plants grown under local supply of ammonium, nitrate, or ammonium nitrate in vertically split agar plates (for histochemical staining and determination of lateral root measures, see Methods). Values represent means (±se) of 12 to 21 individual plants per treatment, and different letters denote significant differences among nitrogen treatments at P < 0.05 (Tukey’s test); nd, not determined.
The Ammonium Transporter-Defective Mutant qko Shows Decreased Ammonium-Triggered Lateral Root Branching
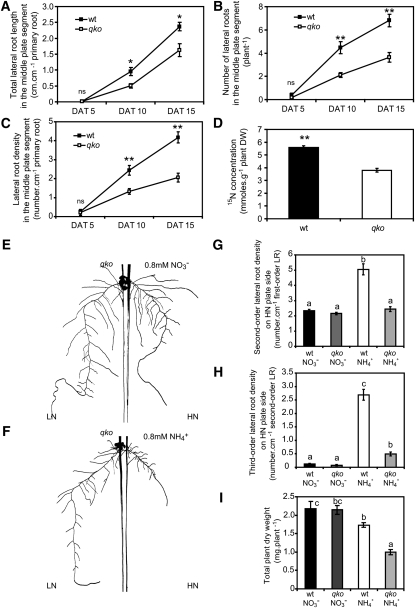
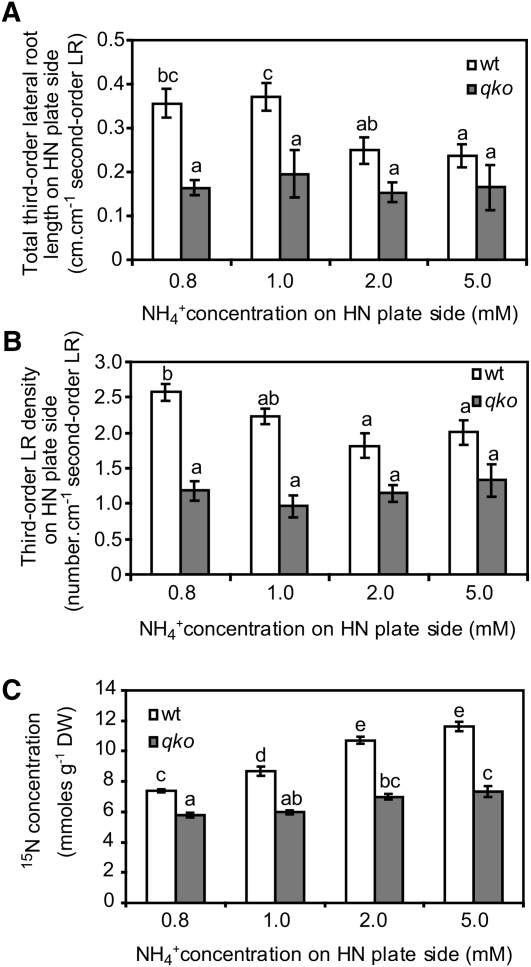
We then investigated the role of ammonium uptake in lateral root branching, employing the Arabidopsis qko mutant, which is defective in four root-expressed AMMONIUM TRANSPORTER (AMT) genes (amt1;1, amt1;2, amt1;3, and amt2;1) and shows a 90 to 95% reduction of high-affinity ammonium influx in short-term uptake studies (Yuan et al., 2007). Fifteen days after transfer to horizontally split agar plates, first-order lateral root length in qko was 32% lower than in the wild type, whereas the visible number or density of first-order lateral roots formed in response to ammonium decreased by 48 or 53%, respectively (Figures 4A to 4C). The analysis of 15N concentrations in entire seedlings after 15 d of growth in the presence of 15N-labeled ammonium in the middle segment revealed a 32% decrease in cumulative ammonium uptake in qko (Figure 4D). The comparison between wild-type and qko plants thus confirmed that local ammonium supply exerted a stronger stimulation of lateral root initiation than of elongation and that the ammonium-induced changes in root architecture depended on AMT-type ammonium transport proteins or their uptake capacity. Under local ammonium supply on vertically split agar plates, we observed that second-order and third-order lateral root formation in qko was strongly reduced (Figures 4F to 4H), which went along with a weaker biomass production (Figure 4I), whereas the growth response to nitrate was similar to the wild type (Figures 4E, 4G, and 4H). Since the loss of lateral root branching in qko under localized ammonium supply (Figures 4C and 4F to 4H) coincided with lower cumulative ammonium uptake (Figure 4D), a nutritional effect of ammonium-derived nitrogen had to be considered. In this case, a higher concentration of locally supplied ammonium should restore lateral root branching in qko due to ammonium uptake via low-affinity transport systems (Loqué and von Wirén, 2004). However, increasing external ammonium supply showed that even 5 mM ammonium did not restore wild-type levels of either the length or density of third-order lateral roots in qko (Figures 5A and 5B). In wild-type plants, third-order lateral root development decreased under high local ammonium supplies probably due to ammonium-induced systemic inhibition (Figure 3). At 5 mM ammonium supply, cumulative 15N uptake in qko was 21% higher than under 0.8 mM. This was almost at the same level as in wild-type plants supplied with 0.8 mM (Figure 5C) where they showed a vigorous third-order lateral root development. Thus, higher local ammonium supplies could not restore lateral root formation in qko, indicating that lateral root formation depends on the presence of AMT proteins rather than on the quantity of absorbed ammonium.
Figure 4.
Third-Order Lateral Root Branching Induced by Local Ammonium Supply Requires AMT Genes.
(A) to (D) Time course of lateral root development on horizontally split agar plates. Lateral root length (A), lateral root number (B), lateral root density (C), and 15N concentration (D) in roots from 15N-labeled ammonium supplied to the middle root compartment. Wild-type (wt) and qko (amt1;1 amt1;2 amt1;3 amt2;1) plants were grown for 15 d on horizontally split agar plates. Bars represent mean values (±se), and asterisks denote significant differences between lines at *P < 0.05 or **P < 0.001 (Student’s t test); n = 10 to 12 individual plants per treatment; ns, not significant. DAT, d after transfer; DW, dry weight.
(E) to (H) Root morphology of qko plants grown on vertically split agar plates with the first lateral root growing into 0.8 mM NO3− (E) or 0.8 mM NH4+ (F) and the remainder of the roots growing in 5 μM NO3− (LN). Second-order lateral root density (G) and third-order lateral root density (H) of roots grown in the HN compartment.
(I) Total plant dry weight of plants grown for 12 d under localized nitrate or ammonium supply. Bars represent mean values (±se) of 12 to 20 individual plants per treatment. Different letters denote significant differences among means at P < 0.05 (Tukey’s test).
Figure 5.
Millimolar Ammonium Supply Cannot Restore Third-Order Lateral Root Development in qko.
Third-order lateral root (LR) length (A), third-order lateral root (LR) density (B), and 15N concentrations (C) in roots from 15N-labeled ammonium supplied to the HN compartment. Wild-type (wt) and qko (amt1;1 amt1;2 amt1;3 amt2;1) plants were grown on vertically split agar plates with a localized supply of increasing ammonium concentrations. Bars represent means (±se), and different letters denote significant differences at P < 0.05 (Tukey’s test); n = 10 to 15. DW, dry weight.
The Role of AMT1;3 in Lateral Root Branching
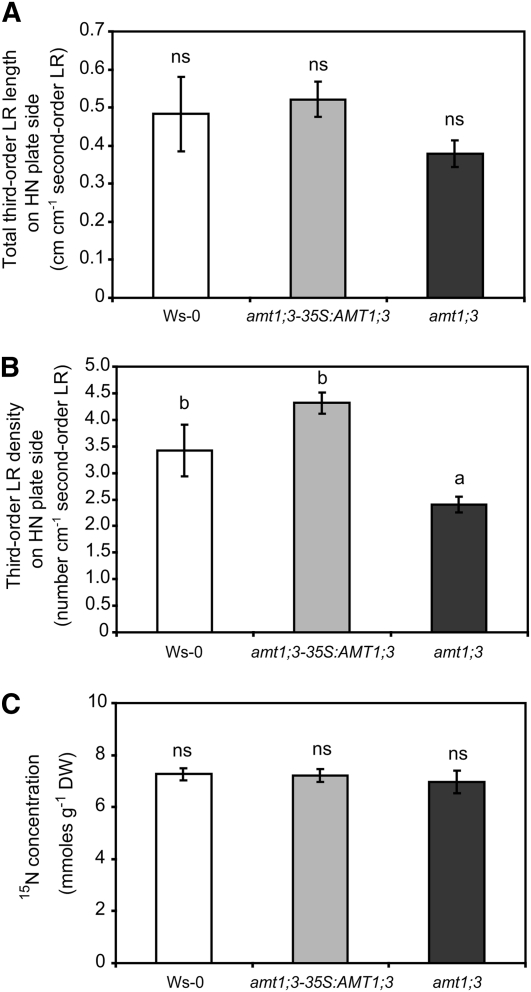
Considering that Arabidopsis plants express five AMT-type ammonium transporters in roots (Yuan et al., 2007) and that AMT1;5, which is still functional in qko, plays only a minor role in lateral root branching (Figure 4), we investigated the role of the four remaining AMTs in third-order lateral root formation by phenotyping the corresponding single T-DNA– or transposon-tagged insertion lines. With respect to third-order lateral root length, none of the single insertion lines differed from their corresponding wild-type backgrounds (Figures 6A to 6D). Only the amt1;3-1 T-DNA insertion line yielded a significantly lower third-order lateral root density compared with its wild-type Wassilewskija (Ws-0) background. In fact, lateral root density in amt1;3-1 decreased by 30% and thus still remained at a higher relative level than that of qko plants, which was 50 to 75% below wild-type levels (Figures 4 and 5). To verify an effect of AMT1;3, amt1;3-1 plants expressing a 35S:AMT1;3 construct (Loqué et al., 2006) were assayed and observed to restore wild-type levels of third-order lateral root density completely (Figures 7A and 7B). Interestingly, ammonium-derived 15N accumulation was similar in all lines (Figure 7C), supporting the notion that the lower third-order lateral root density in amt1;3-1 was not related to impaired acquisition of ammonium-derived N.
Figure 6.
Third-Order Lateral Root Development in Single amt Mutants on the Ammonium-Containing Plate Side.
(A) to (D) Total third-order lateral root (LR) length.
(E) to (H) Third-order lateral root density of plants grown for 15 d on vertically split agar plates with a localized supply of 0.8 mM ammonium.
(A) and (E) Col-gl and amt1;1-1.
(B) and (F) Col-0 and amt1;2-1.
(C) and (G) Ws-0 and amt1;3-1.
(D) and (H) Col-gl and amt2;1-1.
Bars represent means (±se), and asterisks denote significant differences at P < 0.05 (Student’s t test); ns, not significant; n = 12 to 20.
Figure 7.
Third-Order Lateral Root Development in the 35S:AMT1;3-Transformed amt1;3-1 Insertion Line.
Total third-order lateral root (LR) length (A), third-order lateral root density (B), and 15N concentrations (C) in roots from 15N-labeled ammonium supplied to the HN compartment. Ws-0, amt1;3-1, and amt1;3-1-35S:AMT1;3 plants were grown for 15 d on vertically split agar plates with a localized supply of 0.8 mM ammonium. Bars represent means (±se), and different letters denote significant differences at P < 0.05 (Tukey’s test); ns, not significant; n = 12 to 20. DW, dry weight.
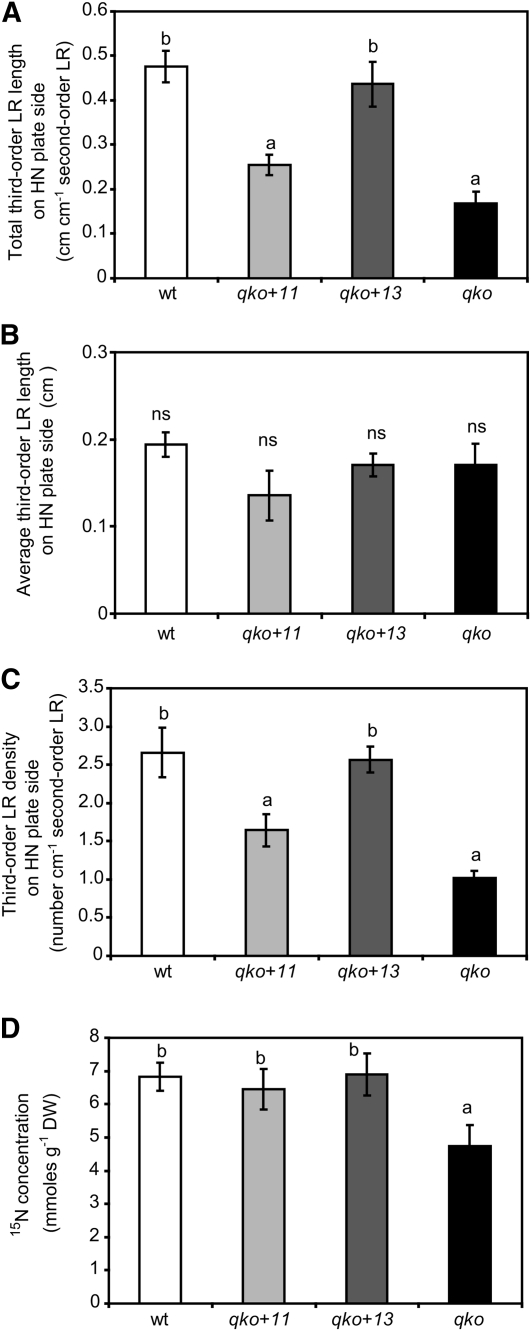
Since Ws-0 plants showed a slightly higher third-order lateral root formation than other Arabidopsis accession lines (Figure 6), the role of AMT1;3 was assessed in the Col-0 background employing the qko line with reconstituted expression of AMT1;3 (qko+13) and compared against qko+11 expressing AMT1;1, which is the only other AMT transporter showing an overlapping cell type–specific expression pattern in roots and comparable biochemical transport properties (Loqué et al., 2006; Yuan et al., 2007). Third-order lateral root length and third-order lateral root density of qko+13 plants achieved wild-type levels, whereas qko+11 plants did not restore third-order lateral root formation (length and density) and remained similar to qko plants (Figures 8A and 8C). By contrast, there was no significant effect of either AMT on average third-order lateral root length (Figure 8B). In agreement with their similar ammonium uptake capacities as determined in short-term transport studies and the same biomass production as wild-type plants on ammonium-based medium (Yuan et al., 2007), qko+11 and qko+13 both accumulated ammonium-derived N in roots to the same extent (Figure 8D), clearly exceeding the 15N accumulation by qko plants. Thus, although both transporters conferred a similar ammonium uptake capacity, only AMT1;3, but not AMT1;1, was able to trigger third-order lateral root proliferation, and this action was independent of the genetic background. We also verified whether pruning of the primary root affects higher-order lateral root branching as it favors the comparison of two more balanced root fractions (Remans et al., 2006). However, similar results were obtained showing that AMT1;3 is able to restore third-order lateral root formation (see Supplemental Table 2 online), indicating that primary root pruning had minimal impact on higher-order lateral root branching.
Figure 8.
Reconstituted Expression of AMT1;3 but Not of AMT1;1 Restores Third-Order Lateral Root Development in qko.
Total third-order lateral root (LR) length (A), average third-order lateral root length (B), third-order lateral root density (C), and 15N concentrations (D) in roots from 15N-labeled ammonium supplied to the high N compartment. Wild-type, qko (amt1;1 amt1;2 amt1;3 amt2;1), qko+11 (amt1;2 amt1;3 amt2;1), or qko+13 plants (amt1;1 amt1;2 amt2;1) were grown for 15 d on vertically split agar plates with a localized supply of 0.8 mM ammonium. Bars represent means (±se), and different letters denote significant differences at P < 0.05 (Tukey’s test); ns, not significant; n = 15 to 20. DW, dry weight.
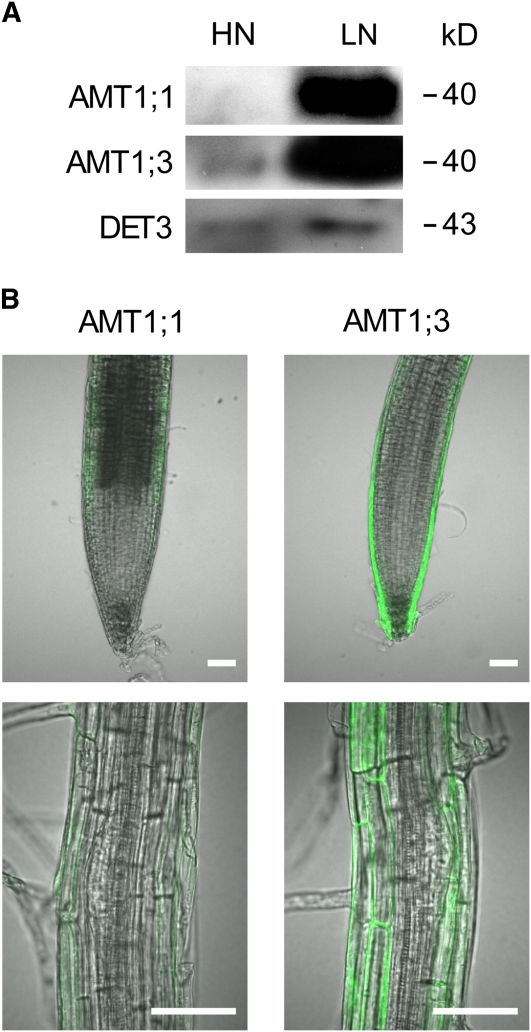
We then verified expression levels of the two proteins by protein gel blot analysis and observed that both proteins were highly expressed on the LN side of vertically split agar plates (Figure 9A). On the HN side supplemented with 0.8 mM ammonium, AMT1;3 protein was detected although the level was low, and AMT1;1 protein was hardly detectable or almost absent from that root fraction. Since uptake studies indicated a contribution of either AMT1 to 15N-ammonium uptake from the HN side (Figure 8D), we further verified the presence and relative abundance of the AMT1;1 and AMT1;3 proteins in plants expressing their promoter-gene-green fluorescent protein (GFP) fusion constructs (Loqué et al., 2006). The fluorescence images showed a very low protein accumulation of AMT1;1 in outer root cells in ammonium-supplied subapical root zones and in mature root zones developing lateral roots (Figure 9B). By contrast, AMT1;3 protein expression was particularly prominent in lateral root cap and epidermal cells of lateral root tips as well as in the cortex and rhizodermis of root zones where lateral root primordia emerge (Figure 9B). This localization pattern was conserved in first- and second-order lateral roots.
Figure 9.
AMT1;1 and AMT1;3 Are Differently Regulated under Localized Ammonium Supply.
(A) Protein accumulation of AMT1;1 and AMT1;3 in roots from the LN compartment or the HN compartment supplemented with 0.8 mM ammonium. Wild-type plants were grown on vertically split agar plates for 15 d. Gel blots from microsomal membrane fractions were probed with anti-AMT1;1, anti-AMT1;3, and anti-DET3.
(B) Localization of AMT1;1 and AMT1;3. AMT1;1 promoter:AMT1;1 gene:GFP and AMT1;3 promoter:AMT1;3 gene:GFP plants were grown on vertically split agar plates, and GFP-derived fluorescence on the HN compartment was observed under a confocal microscope. The top and bottom panels show first-order lateral root tips and the differentiation zone of second-order lateral roots with third-order lateral root primordia, respectively. Bars = 50 μm.
DISCUSSION
A Complementary Role for Ammonium and Nitrate in Lateral Root Development
This study shows that not only nitrate but also ammonium is a locally sensed nitrogen form that regulates lateral root development. Under nitrogen-deficient growth conditions, local ammonium supply enhanced lateral root formation in particular via a higher density of first- and higher-order lateral roots (Figures 1 and 3) and thereby provoked a highly branched phenotype in lateral root architecture (Figure 3B). This phenotype is in agreement with the bushy appearance of lateral roots of barley grown within a horizontal zone that was supplied locally with ammonium (Drew, 1975). Ammonium-triggered lateral root branching became more apparent on vertically split than on horizontally split agar plates (Figures 3D to 3F versus Figures 1I and 1J) and was expressed in a much smaller increase in total or average lateral root length than that of nitrate-triggered branching (Figures 1C and 1D; see Supplemental Figures 1C and 1D online), which might explain why the stimulating effect of ammonium on lateral root development in Arabidopsis has remained uncovered in previous studies (Zhang et al., 1999). Based on our and previous results (Zhang and Forde, 1998; Remans et al., 2006), we propose a different action of ammonium and nitrate on lateral root development. In contrast with nitrate, which supports mainly the elongation of lateral roots (Zhang et al., 1999; Remans et al., 2006), localized ammonium supply poorly stimulates lateral root elongation (Figures 1 and 3, Table 1). As is evident by microscopy analysis of the CYCB1:GUS reporter line, ammonium increased the total number of preemerged and emerged lateral root primordia, thus triggering primarily the initiation rather than the emergence of lateral roots (Table 1). This corroborates the recent finding that different nitrogen signals predominantly regulate lateral root establishment versus elongation (Gifford et al., 2008). With regard to the stimulation of lateral root initiation, the reduced nitrogen source Gln could not substitute for ammonium (Figure 2; see Supplemental Figure 2 online). Replacing part of the nitrate nutrition by ammonium strongly enhanced lateral root number and length not only beyond the level of nitrate- but also of ammonium-supplied plants (Table 1), emphasizing that ammonium and nitrate shape lateral root structure in a complementary manner. In ecological terms, this complementary regulation might reflect an adaptation of lateral root development to the different mobility of these nitrogen forms in soils. While in most soils ammonium is highly adsorbed to negatively charged residues in the soil matrix (Miller et al., 2007), where a highly branched root system can efficiently cut diffusion distances of ammonium to the root surface, nitrate is highly mobile and may be better exploited with far-reaching lateral roots. Since ammonium is the substrate for nitrification and nitrate does not occur as an exclusive nitrogen source in soils (Miller et al., 2007), a minimum of lateral root branching is always assured even at low ammonium levels.
Several lines of evidence indicated that ammonium-triggered lateral root branching cannot be explained simply by a nutritional effect but is based on a sensing event: (1) addition of ammonium or Gln to the LN plate side in vertically split agar plates suppressed ammonium-mediated third-order lateral root branching, indicating a systemic downregulation of this morphological response to ammonium (Figure 3; see Supplemental Figure 3 online); (2) millimolar ammonium supplies to the ammonium uptake-defective line qko could not restore wild-type levels of third-order lateral root formation (Figure 5), although cumulative ammonium uptake was at levels sufficient to allow for third-order lateral root formation in the wild type; and (3) in transgenic lines with deregulated AMT gene expression, third-order lateral root formation did not correlate with the cumulative uptake of 15N-labeled ammonium (Figures 7 and 8). Thus, ammonium appears to act as an external morphological trigger that is locally sensed by plant roots. In vertically split agar plates, ammonium addition to the main root system (LN root fraction) suppressed third-order lateral root formation on the HN side (Figures 3E and 3F) and caused a higher N nutritional status in the LN root fraction and the shoot (Figures 3H and 3I), indicating that ammonium-mediated lateral root formation was subject to systemic repression. Likewise, Gln supply to the main root system effectively repressed ammonium-dependent lateral root branching (see Supplemental Figure 3 online). In agreement with the study by Gifford et al. (2008) showing that Gln/Glu is a dominant signal repressing nitrate-responsive lateral root emergence, we propose that Gln or a product of its metabolism might also act as a metabolic repressor for the systemic downregulation of ammonium-triggered lateral root initiation.
The Possible Function of AMT1;3 in Ammonium-Triggered Lateral Root Development
An almost complete loss of higher-order lateral root branching in qko (Figure 4) and the failure to restore lateral root formation by millimolar supplies of external ammonium (Figure 5) indicated that one or several of the four defective AMT transporters in Arabidopsis roots should be involved in ammonium sensing. We therefore monitored third-order lateral root formation in single amt insertion lines and found that only the amt1;3-1 mutant had a significant decrease in lateral root density (Figure 6). This decrease was fully reversed by expression of AMT1;3 in the amt1;3-1 background without significantly affecting the cumulative uptake of 15N-labeled ammonium (Figure 7), indicating that the amount of ammonium-N accumulating via AMT1;3 in roots is not of primary relevance for the recovery of third-order lateral root formation. However, in absolute numbers, the third-order lateral root density in amt1;3-1 was not as low as in qko (Figures 4 and 5), suggesting that their different genetic background might account for this difference or that other AMTs might also contribute to ammonium-stimulated lateral root branching. To rule out possible effects of genetic backgrounds, qko lines with reconstituted expression of AMT1;1 or AMT1;3 were directly compared against qko. Although reconstituted expression of either AMT1 yielded the same cumulative uptake of ammonium-15N as wild-type plants (Figure 8D), which is in agreement with the observation that both reconstituted lines achieved the same short-term uptake capacity or biomass when grown on ammonium as a sole nitrogen source (Yuan et al., 2007), only qko+13 lines, but not qko+11 lines, developed third-order lateral roots with length and density comparable to wild-type plants (Figures 8A and 8C; see Supplemental Table 2 online). It was therefore unlikely that quantitative differences in the ammonium transport capacity or in the N nutritional status of AMT1;1- or AMT1;3-reconstituted lines account for the stimulated lateral root branching in the presence of AMT1;3. Since average third-order lateral root length remained unchanged (Figure 8B), AMT1;3 did not promote the elongation of third-order laterals. Assessing cell type–specific expression in promoter-gene-GFP lines revealed that under local ammonium supply, the AMT1;3 protein accumulated mainly in outer cell layers of the root tips as well as in rhizodermal and cortical cells of root zones that develop lateral root primordia (Figure 9B). This cell type–specific expression pattern of AMT1;3 was similar to that observed in previous studies in N-deficient plants (Loqué et al., 2006). Whereas AMT1;3 expression in the root tip might be relevant for lateral root formation at a rather early stage (e.g., when auxin pulses initiate pericycle founder cells) (De Smet et al., 2007), AMT1;3 may promote lateral root formation also at a later stage (e.g., when lateral root initials begin to emerge) (Péret et al., 2009). In both root zones, the AMT1;3 protein expression was more prominent than that of AMT1;1; this was also reflected in protein gel blot analyses that still showed detection of AMT1;3 in the ammonium-induced root fraction (Figure 9A). Taken together, all these observations are in agreement with a dominant function of the AMT1;3 protein in ammonium sensing and higher-order lateral root branching that was not fulfilled by AMT1;1. The failure of AMT1;1 to confer lateral root branching could not be explained by an absence of its expression under local ammonium supply as it substantially contributed to 15N-ammonium uptake from the HN side (Figure 8D). Besides the extent of temporal or spatial differences in gene and protein expression levels, transport properties or structural protein features may also account for the observed differences in phenotype, which may require further investigation. In addition, as evident from the difference between the third-order lateral root densities of amt1;3 mutant and qko (Figures 6 and 8), the function of AMT1;3 is not always expected to be exclusive among the AMT1 family members, although its function alone is sufficient for ammonium sensing particularly in the absence of other AMTs (Figures 8A and 8C; see Supplemental Table 2 online).
Based on the inability of nrt1;1 mutant plants to proliferate lateral root growth into nitrate-rich patches and the functional involvement of NRT1;1 in ANR1-dependent nitrate signaling, the dual-affinity nitrate transporter NRT1;1/CHL1 has been characterized as a nitrate sensor or as a facilitator of nitrate influx into nitrate-sensing cells (Remans et al., 2006). More recently, a nitrate sensing function of NRT1;1 was corroborated, as substrate-induced gene expression of the high-affinity nitrate transporter NRT2;1 was also found to be dependent on NRT1;1 (Ho et al., 2009). In the case of nitrate-induced lateral root formation, two microRNA-dependent regulatory modules have been identified. While the miRNA167/ARF8 module regulates the ratio between initiating and emerging lateral roots by inserting nitrogen responses at the level of auxin signaling (Gifford et al., 2008), the miRNA393/AFB3 regulatory module confers nitrate regulation of an auxin receptor to regulate primary and lateral root growth by modifying auxin perception in roots cultured under high nitrate supplies (Vidal et al., 2010). Our study now indicates the complementary action of ammonium and nitrate in lateral root development and raises the question whether the sensing machinery for ammonium employs similar regulatory modules as those suggested for nitrate sensing.
METHODS
Plant Material and Plant Culture
The following lines were used: AMT1;1-promoter-ORF:GFP, AMT1;3-promoter-ORF:GFP, amt1;1-1, amt1;3-1, and amt1;3-1-35S:AMT1;3 (Loqué et al., 2006); amt1;2-1, amt2;1-1, qko (amt1;1 amt1;2 amt1;3 amt2;1), qko+11, and qko+13 (Yuan et al., 2007); and CYCB1:GUS (Ferreira et al., 1994). Col-0, Ws-0, and C. glabra (Col-gl) served as wild types, but if not otherwise stated, the Arabidopsis thaliana ecotype Col-0 was used as a wild type. Seeds were surface sterilized with 70% ethanol and germinated on MGRL medium (Fujiwara et al., 1992) containing 5 mM MES (Sigma-Aldrich; pH 5.7), 0.5% sucrose, and 1% Difco Agar (Becton Dickinson and Company). For transfer to vertically split agar plates (Remans et al., 2006), plants were supplemented with 0.5 mM nitrate, whereas for transfer to horizontally split agar plates (Zhang and Forde, 1998), plants were precultured in the absence of nitrogen. In one experiment (see Supplemental Table 2 online), primary roots were pruned prior to transfer to vertically split agar plates as described by Remans et al. (2006). Plants were precultured on vertically oriented plates for 10 d and transferred to fresh plates containing the same solid medium supplemented locally with NH4Cl or KNO3 at indicated concentrations and KCl to balance Cl− or K+ concentrations in all segments of the plate. The medium was supplemented with 5 to 10 mM MES according to the increasing concentrations of NH4Cl or KNO3 supplied to the HN plate side under vertically split agar plates to avoid changes in the pH, which was kept constant at 5.7. Root system architecture was assessed after 15 d of growth in a growth chamber with a 10/14-h day/night regime at 22°/19°C and a light intensity of 120 μmol photons m−2 s−1 on horizontally or vertically split agar plates. Supply of 15N-labeled NH4Cl or KNO3 (1% labeled by 15N) to the agar medium was followed by analysis of accumulated 15N in roots or shoots by isotope-ratio mass spectrometry (Yuan et al., 2007). To investigate a local effect of Gln on lateral root growth, 0.8 mM Gln was added to the ammonium-containing root compartment (see Supplemental Figure 2 online), and to investigate a systemic effect, Gln was added to the remainder of the roots growing in the absence of ammonium (see Supplemental Figure 3 online).
Root Growth Measurements
Root systems were scanned at a resolution of 300 dpi (Epson Expression 10000XL scanner), and the background noise was removed from images using Adobe Photoshop version 5.0 LE (Adobe Systems). Root growth was determined by analyzing scanned images by WinRHIZO version Pro2007d (Regents Instruments). Total lateral root length (cm cm−1 parental root) was calculated by adding up the lengths of all individual roots of one class, divided by the length of the parental root. Average third-order lateral root length (cm) was determined by dividing total lateral root length by their number. Lateral root density (number cm−1 parental root) was calculated by dividing the total number of lateral roots of one class by the length of their parental root. The number of emerged lateral roots (>0.5 mm) was determined by image analysis, except for experiments with CYCB1:GUS plants (Table 1) where analysis required histochemistry and microscopy. All the experiments were performed at least twice and yielded similar results.
Histochemical Analysis and Microscopy
For histochemical studies, CYCB1:GUS (cycb1:uid; Ferreira et al., 1994) reporter plants were incubated overnight at 37°C in GUS reaction buffer (1 mg/mL of 5-bromo-4-chloro-3-indolyl-β-d-glucuronide in 100 mM sodium phosphate, pH 7, and the stained seedlings were cleared according to Malamy and Benfey (1997). Fixed (6 h in 4% formaldehyde in phosphate or PBS buffer, pH 6.5) roots expressing the cycb1:uid marker were analyzed by a light microscope (Zeiss Axiovert 200 M) to determine the total number of primordia initiated, by summing the numbers of all organs at various developmental stages (preemerged primordia, emerged primordial, and lateral roots <0.5 mm, and macroscopically visible lateral roots >0.5 mm). Fluorescence of GFP in AMT1;1 promoter:AMT1;1 gene:GFP lines (AMT1;1-promoter-ORF:GFP; Loqué et al., 2006) and AMT1;3 promoter:AMT1;3 gene:GFP lines (AMT1;3-promoter-ORF:GFP; Loqué et al., 2006) was observed under a FV500 confocal laser scanning microscope (Olympus). GFP was detected by a 505- to 525-nm band-pass filter. Several independent lines were observed on the same day under the same settings of the laser scanning system.
Protein Gel Blot Analysis
For the preparation of microsomal membrane fractions, Arabidopsis roots were harvested separately from the HN and LN segments of vertically split agar plates. Protein gel blot analysis was performed exactly as described by Loqué et al. (2006).
Accession Numbers
Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are At4g13510 (AMT1;1), At1g64780 (AMT1;2), At3g24300 (AMT1;3), and At2g38290 (AMT2;1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Primary and Lateral Root Development under Local Nitrate and Ammonium Supply.
Supplemental Figure 2. Gln Represses Ammonium-Induced Third-Order Lateral Root Branching.
Supplemental Figure 3. Systemic Repression of Ammonium-Induced Third-Order Lateral Root Branching by Gln.
Supplemental Table 1. The Presence of Ammonium and Nitrate Triggers Lateral Root Development.
Supplemental Table 2. Reconstituted Expression of AMT1;3 Restores Third-Order Lateral Development in qko Also When Primary Roots Are Pruned.
Supplementary Material
Acknowledgments
We thank Anne Bohner, Carolin Stahl, Melanie Ruff, Lisa Unrath (University of Hohenheim, Germany), and Mayu Miyamoto (RIKEN Plant Science Center, Japan) for excellent technical assistance, Klaus Harter (University of Tübingen, Germany) for providing the CYCB1:GUS reporter line, Lixing Yuan for valuable discussions, and Ben Gruber for critically reading the manuscript. This research was supported by the Deutsche Forschungsgemeinschaft (WI1728/13-1). S.K. received a Grant-in-Aid for Young Scientists (A) (21688006) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. H.T. received grants from Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and Bio-Oriented Technology Research Advancement Institution of Japan.
References
- Colón-Carmona A., You R., Haimovitch-Gal T., Doerner P. (1999). Technical advance: Spatio-temporal analysis of mitotic activity with a labile cyclin-GUS fusion protein. Plant J. 20: 503–508 [DOI] [PubMed] [Google Scholar]
- De Smet I., et al. (2007). Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134: 681–690 [DOI] [PubMed] [Google Scholar]
- Desnos T. (2008). Root branching responses to phosphate and nitrate. Curr. Opin. Plant Biol. 11: 82–87 [DOI] [PubMed] [Google Scholar]
- Drew M.C. (1975). Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol. 75: 479–490 [Google Scholar]
- Drew M.C., Saker L.R. (1975). Nutrient supply and the growth of the seminal root system in barley. II. Localized, compensatory increases in lateral root growth and rates of nitrate uptake when nitrate supply is restricted to only part of the root system. J. Exp. Bot. 26: 79–90 [Google Scholar]
- Drew M.C., Saker L.R. (1978). Nutrient supply and the growth of the seminal root system in barley. III. Compensatory increases in growth of lateral roots, and in the rates of phosphate uptake, in response to a localized supply of phosphate. J. Exp. Bot. 29: 435–451 [Google Scholar]
- Dubrovsky J.G., Soukup A., Napsucialy-Mendivil S., Jeknic Z., Ivanchenko M.G. (2009). The lateral root initiation index: An integrative measure of primordium formation. Ann. Bot. (Lond.) 103: 807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermilova E.V., Nikitin M.M., Fernández E. (2007). Chemotaxis to ammonium/methylammonium in Chlamydomonas reinhardtii: The role of transport systems for ammonium/methylammonium. Planta 226: 1323–1332 [DOI] [PubMed] [Google Scholar]
- Ettema C.H., Wardle D.A. (2002). Spatial soil ecology. Trends Ecol. Evol. 17: 177–183 [Google Scholar]
- Ferreira P.C., Hemerly A.S., Engler J.D., van Montagu M., Engler G., Inzé D. (1994). Developmental expression of the arabidopsis cyclin gene cyc1At. Plant Cell 6: 1763–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Hirai M.Y., Chino M., Komeda Y., Naito S. (1992). Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol. 99: 263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzarrini S., Lejay L., Gojon A., Ninnemann O., Frommer W.B., von Wirén N. (1999). Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell 11: 937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford M.L., Dean A., Gutierrez R.A., Coruzzi G.M., Birnbaum K.D. (2008). Cell-specific nitrogen responses mediate developmental plasticity. Proc. Natl. Acad. Sci. USA 105: 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsinger P., Gobran G.R., Gregory P.J., Wenzel W.W. (2005). Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. New Phytol. 168: 293–303 [DOI] [PubMed] [Google Scholar]
- Ho C.H., Lin S.H., Hu H.C., Tsay Y.F. (2009). CHL1 functions as a nitrate sensor in plants. Cell 138: 1184–1194 [DOI] [PubMed] [Google Scholar]
- Lanquar V., Loqué D., Hörmann F., Yuan L., Bohner A., Engelsberger W.R., Lalonde S., Schulze W.X., von Wirén N., Frommer W.B. (2009). Feedback inhibition of ammonium uptake by a phospho-dependent allosteric mechanism in Arabidopsis. Plant Cell 21: 3610–3622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loqué D., Lalonde S., Looger L.L., von Wirén N., Frommer W.B. (2007). A cytosolic trans-activation domain essential for ammonium uptake. Nature 446: 195–198 [DOI] [PubMed] [Google Scholar]
- Loqué D., von Wirén N. (2004). Regulatory levels for the transport of ammonium in plant roots. J. Exp. Bot. 55: 1293–1305 [DOI] [PubMed] [Google Scholar]
- Loqué D., Yuan L., Kojima S., Gojon A., Wirth J., Gazzarrini S., Ishiyama K., Takahashi H., von Wirén N. (2006). Additive contribution of AMT1;1 and AMT1;3 to high-affinity ammonium uptake across the plasma membrane of nitrogen-deficient Arabidopsis roots. Plant J. 48: 522–534 [DOI] [PubMed] [Google Scholar]
- Lorenz M.C., Heitman J. (1998). The MEP2 ammonium permease regulates pseudohyphal differentiation in Saccharomyces cerevisiae. EMBO J. 17: 1236–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy J.E., Benfey P.N. (1997). Dow and out in Arabidopsis: The formation of lateral roots. Trends Plant Sci. 2: 390–396 [Google Scholar]
- Marini A.M., Soussi-Boudekou S., Vissers S., André B. (1997). A family of ammonium transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 17: 4282–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.J., Fan X., Orsel M., Smith S.J., Wells D.M. (2007). Nitrate transport and signalling. J. Exp. Bot. 58: 2297–2306 [DOI] [PubMed] [Google Scholar]
- Neuhäuser B., Dynowski M., Mayer M., Ludewig U. (2007). Regulation of NH4+ transport by essential cross talk between AMT monomers through the carboxyl tails. Plant Physiol. 143: 1651–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B., Larrieu A., Bennett M.J. (2009). Lateral root emergence: A difficult birth. J. Exp. Bot. 60: 3637–3643 [DOI] [PubMed] [Google Scholar]
- Rawat S.R., Silim S.N., Kronzucker H.J., Siddiqi M.Y., Glass A.D.M. (1999). AtAMT1 gene expression and NH4+ uptake in roots of Arabidopsis thaliana: Evidence for regulation by root glutamine levels. Plant J. 19: 143–152 [DOI] [PubMed] [Google Scholar]
- Remans T., Nacry P., Pervent M., Filleur S., Diatloff E., Mounier E., Tillard P., Forde B.G., Gojon A. (2006). The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc. Natl. Acad. Sci. USA 103: 19206–19211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svistoonoff S., Creff A., Reymond M., Sigoillot-Claude C., Ricaud L., Blanchet A., Nussaume L., Desnos T. (2007). Root tip contact with low-phosphate media reprograms plant root architecture. Nat. Genet. 39: 792–796 [DOI] [PubMed] [Google Scholar]
- Thevelein J.M., et al. (2005). Nutrient sensing systems for rapid activation of the protein kinase A pathway in yeast. Biochem. Soc. Trans. 33: 253–256 [DOI] [PubMed] [Google Scholar]
- van Nuland A., Vandormael P., Donaton M., Alenquer M., Lourenço A., Quintino E., Versele M., Thevelein J.M. (2006). Ammonium permease-based sensing mechanism for rapid ammonium activation of the protein kinase A pathway in yeast. Mol. Microbiol. 59: 1485–1505 [DOI] [PubMed] [Google Scholar]
- Vidal E.A., Araus V., Lu C., Parry G., Green P.J., Coruzzi G.M., Gutiérrez R.A. (2010). Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 107: 4477–4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Graff L., Loqué D., Kojima S., Tsuchiya Y.N., Takahashi H., von Wirén N. (2009). AtAMT1;4, a pollen-specific high-affinity ammonium transporter of the plasma membrane in Arabidopsis. Plant Cell Physiol. 50: 13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Loqué D., Kojima S., Rauch S., Ishiyama K., Inoue E., Takahashi H., von Wirén N. (2007). The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. Plant Cell 19: 2636–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Forde B.G. (1998). An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279: 407–409 [DOI] [PubMed] [Google Scholar]
- Zhang H., Jennings A., Barlow P.W., Forde B.G. (1999). Dual pathways for regulation of root branching by nitrate. Proc. Natl. Acad. Sci. USA 96: 6529–6534 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.